Abstract
Nkx2-1 (Nkx homeobox-1 gene), also known as TTF-1 (thyroid transcription factor-1), is a tissue-specific transcription factor of the thyroid, lung, and ventral forebrain. While it has been shown to play a critical role in lung development and lung cancer differentiation and morphogenesis, molecular mechanisms mediating Nkx2-1 cell- and tissue-specific expression in normal and cancerous lungs have yet to be fully elucidated. The recent identification of prognostic biomarkers in lung cancer, particularly in lung adenocarcinoma (ADC), and the different reactivity of patients to chemotherapeutic drugs have opened new avenues for evaluating patient survival and the development of novel effective therapeutic strategies. The function of Nkx2-1 as a proto-oncogene was recently characterized and the gene is implicated as a contributory factor in lung cancer development. In this review, we summarize the role of this transcription factor in the development, diagnosis, and prognosis of lung cancer in the hope of providing insights into the utility of Nkx2-1 as a novel biomarker of lung cancer.
Keywords: Nkx2-1, TTF-1, Lung cancer, Biomarker, Diagnosis, Prognosis, Therapy
1. Introduction
During organogenesis, the lung buds are derived from the lateral-esophageal sulcus that evaginates into the thyroid and the stomach along with the foregut endoderm. This process is mediated by a dynamic profile of organ-specific gene expression. Nkx2-1 (Nkx homeobox-1 gene), a member of the Nkx2 family of homeodomain-containing transcription factors, is one of the key genes expressed. Also known as TTF-1 (thyroid transcription factor-1), T/EBP (thyroid-specific enhancer-binding protein), or TITF (thyroid transcription factor-1 gene), Nkx2-1 was identified in 1989 in the rat thyroid cell line FRTL-5, and shown to be a transcriptional regulator of the rat thyroglobulin promoter (Civitareale et al., 1989). Subsequent studies identified the high sequence homology of the homeodomain region with the Drosophila Nkx2 gene family. Currently, Nkx2-1 is known for its transcriptional activity in thyroid, lung, and ventral forebrain (Bingle, 1997). In the lung, Nkx2-1 regulates normal development and morphogenesis, especially of lung epithelial cell differentiation and perinatal respiratory development. A more recent study (Weir et al., 2007; Kwei et al., 2008) showed that Nkx2-1 can also function as a proto-oncogene and contribute to the pathogenesis of lung cancer. Since then, Nkx2-1 has emerged as a useful molecular marker for lung cancer diagnosis and prognosis.
2. Structure of the Nkx2-1 gene and protein, and its biological functions in the lung
The Nkx2-1 gene is comprised of three exons and two introns, and is located on chromosome 14q13.3. The messenger RNA (mRNA) and protein expressions of Nkx2-1 are different in normal lung tissues and lung carcinomas (Bai and Shen, 2008). Missense and synonymous mutations of the Nkx2-1 gene have been found in 16% of lung cancers. The NH2-terminal harbors binding sites for co-activators, while the transactivation domain of the Nkx2-1 gene is located in the COOH-terminal, and the DNA-binding homeodomain is located centrally. This structure facilitates Nkx2-1 interactions with multiple transcription factors, by which the protein is able to regulate gene expression driving lung formation and biological functions (Fig. 1). In particular, the NH2-terminus has been shown to bind to the following transcriptional coactivators: cyclic-adenosine monophosphate (AMP) response element binding protein (CBP) (Naltner et al., 2000a; 2000b; Yi et al., 2002), steroid receptor coactivator-1 (SRC-1) (Naltner et al., 2000a; 2000b; Yi et al., 2002; Yang et al., 2004), and transcriptional coactivator with PDZ-binding motif (TAZ) (Park et al., 2004). The centralized homeodomain is known to bind to other transcription factors, including retinoic acid receptors (RARs) (Yan et al., 2001), zinc finger GATA transcription-6 (GATA-6) (Liu et al., 2002; Weidenfeld et al., 2002), and nuclear factor of activated T cells (NFAT) (Dave et al., 2004). Finally, the COOH-terminus functionally interacts with the DNA repair protein thymine DNA glycosylase (TDG) (Missero et al., 2001), other transcriptional coactivators such as p300 (Bachurski et al., 2003; Grasberger et al., 2005), SRC-3, SRC-2, BR22 (amino acid 6–206) (Yang et al., 2001; 2003), poly(adenosine diphosphate-ribose) polymerase-1 (PARP-1), PARP-2 (Maeda et al., 2006), and other transcription factors such as nuclear factor κB (NF-κB) (Islam and Mendelson, 2006), signal transducers and activators of transcription 3 (STAT3) (Yan et al., 2002), Drosophila mothers against decapentaplegic 3 (SMAD3) (Li et al., 2002), nuclear factor I (NFI) (Missero et al., 2001), and ezrin-radixin-moesin (ERM) (Lin et al., 2006).
Fig. 1.
Structure of the Nkx2-1 protein
Nkx2-1 has three domains: N-terminal, coactivator binding domain; center, DNA-binding homeodomain; C-terminal, transactivation domain. Different regions interact with different transcription factors to regulate the formation and biological functions of the lungs. CBP: cyclic-AMP response element binding protein; SRC-1: steroid receptor coactivator; TAZ: transcriptional coactivator with PDZ-binding motif; RAR: retinoic acid receptor; GATA-6: zinc finger GATA transcription-6; TDG: thymine DNA glycosylase
In human, calf, rat, and mouse, Nkx2-1 protein has been characterized as a single polypeptide chain of between 371 and 378 amino acids (aa) and with a molecular mass ranging from 38–42 kDa. The orthologues between the species have up to 98% sequence similarity. An alternative splice isoform, composed of three exons and encoding a 30-aa extension at the N-terminus, has been found in various mammalian species and has a molecular mass of 44 kDa (Hamdan et al., 1998). The two Nkx2-1 transcripts appear to have different functions in vivo (Li et al., 2000). It is well known that the 42-kDa human Nkx2-1 is a critical regulator of lung type II cell differentiation (Kolla et al., 2006); however, whether the 44-kDa variant is expressed or functions in the human lung remains unknown.
Expression of Nkx2-1 in cells is dependent upon fibroblast growth factor (FGF) signaling, a key component of lung formation (Serls et al., 2005). Nkx2-1, as a pivotal gene itself in lung development and morphogenesis, directly binds to and activates the expression of several lung-specific genes, including surfactant protein B (SPB) (Bohinski et al., 1994), SPC (Kelly et al., 1996), SPA (Bruno et al., 1995), Clara cell secretory protein (CCSP) (Toonen et al., 1996), and adenosine triphosphate (ATP)-binding-cassette transporter 3 (ABCA3) (Besnard et al., 2007). More importantly, Nkx2-1 is also strongly associated with expression of cancer-related genes in the lung, such as LAMP3 (lysosomal-associated membrane protein 3) and CEACAM6 (carcinoembryonic antigen-related cell adhesion molecule 6) (Kolla et al., 2006), and has been shown to be influenced by lung cancer growth.
3. Clinical application of Nkx2-1 in lung cancer
3.1. Normal tissue expression
Nkx2-1 has been detected in mammalian samples of fetal lung, thyroid, and diencephalon. After birth, Nkx2-1 is expressed in subsets of epithelial cells, including alveolar type II cells, Clara cells, and bronchial basal cells. Deletion of Nkx2-1 in the mouse resulted in malformations of the forebrain, thyroid, and lung, consistent with its function as a transcriptional regulator of several important thyroid-specific and lung-specific genes. In normally developing epithelial cells of the lung, Nkx2-1 is synthesized in the cytoplasm and transported into the nucleus. In human lung cancer cells, however, Nkx2-1 accumulates in the cytoplasm, as evidenced by low nuclear and high cytoplasmic immunostaining with Nkx2-1 antibody (Fujita et al., 2003). However, the function and significance of cytoplasmic-restricted Nkx2-1 remain unknown (Table 1).
Table 1.
Nkx2-1 expression in normal and cancerous lung tissues
| Tissue and Disease | Disease status | Nkx2-1 expression profile | Reference |
| Normal tissue | Fetal lung | Epithelial cells | Stahlman et al., 1996 |
| Adult lung | Alveolar type II cells, Clara cells, and bronchial basal cells | Ikeda et al., 1995; Holzinger et al., 1996 | |
| Thyroid | Kimura et al., 1996 | ||
| Diencephalon | Restricted regions of the brain | Stahlman et al., 1996; Kimura et al., 1996 | |
| Lung disease | Acute inflammation, oedema, haemorrhage, atelectasis | Reduced or absent | Stahlman et al., 1996 |
| Respiratory dysfunction and recurrent infection | Perturbed expression due to gene deletions, missense mutations or nonsense mutations | Devriendt et al., 1998; Iwatani et al., 2000; Krude et al., 2002; Pohlenz et al., 2002; Doyle et al., 2004 | |
| Lung cancer | Adenocarcinoma | High expression | Myong et al., 2003; Tan et al., 2003 |
| Squamous carcinoma | Low expression or absent | Myong et al., 2003; Tan et al., 2003 | |
| Large cell lung cancer | Low expression or absent | Nakamura et al., 2002 | |
| Small cell lung cancer | High expression | Kwei et al., 2008; Tanaka et al., 2007 |
3.2. Lung cancer expression
Nkx2-1 has different expression profiles in different types of lung cancer. For example, Nkx2-1 expression is high in lung adenocarcinomas (ADCs) and small cell lung cancers (SCLCs), but low or completely absent in large cell lung cancers (LCLCs) and lung squamous cell cancers (SCCs) (Table 1).
Tan et al. (2003) reported distinctive Nkx2-1 expression levels in different histological subtypes of 126 stages I–III non-small cell lung cancer (NSCLC) patients. Among those, 68% of ADCs (51 of 75) were positive but only 21% of squamous cell carcinomas (9 of 43) were positive. In a previous study, the positive expression of Nkx2-1 was found in 25%–76% of ADC subtypes (Bejarano et al., 1995; Fabbro et al., 1996; Holzinger et al., 1996; di Loreto et al., 1997; Bohinski et al., 1998; di Loreto et al., 1998; Harlamert et al., 1998; Kaufmann and Dietel, 2000) and 0%–38% of SCCs (Kaufmann and Dietel, 2000; Ordonez, 2000c; Pelosi et al., 2001; Haque et al., 2002). Several studies have evaluated the expression of Nkx2-1 in SCLCs; however, whether this can be used as a specific marker for SCLC remains unclear (Ordonez, 2000c). Nkx2-1-positive ADC was strongly associated with female sex, nonsmoking status, negative p53 staining, and preserved expression of p27 in NSCLC patients (Yatabe et al., 2002). Pelosi et al. (2001) found that Nkx2-1 immunoreactivity in ADC was associated with tumor sizes of <3 cm (P=0.08).
Recently, a clinical analysis of 371 tumors to determine single nucleotide polymorphisms associated with lung cancer found that over-expression of Nkx2-1 occurred in 12% of lung ADCs (Weir et al., 2007). Furthermore, amplification of the Nkx2-1 gene was the single most common focal genetic event detected by high-resolution copy-number analysis of lung ADC. Another study (Zhang et al., 2009) of 404 NSCLCs and 28 benign pulmonary diseases (BPDs), which detected Nkx2-1 expression by the immunohistochemical EnVision two-step method, revealed that Nkx2-1 expression was negative in BPDs, mild in SCCs and tumor-adjacent normal alveolar epithelial cells (AECs) and bronchus tissue, and high in female, non-smoking, and asymptomatic patients. The sensitivity of Nkx2-1 for well or moderately differentiated ADC was reported as 84.0% and specificity as 89.8%.
3.3. Role of Nkx2-1 in diagnosis of lung cancer
Nkx2-1 acts as a lung development gene, is a proto-oncogene, and has different efficacy in different biological sites. The histopathologic significance of defining the primary source of metastatic tumors is well recognized. Nkx2-1 was found to be expressed in about 60%–70% of lung ADCs and showed high sensitivity and specificity for diagnosis of primary lung cancer. Nkx2-1 was also characterized as a tissue specific marker, capable of distinguishing morphologically similar metastatic ADCs of lung origin from other unknown primary sites (except thyroid tumor metastases).
Stenhouse et al. (2004) confirmed that positive Nkx2-1 was a useful lineage marker for tumors arising from the peripheral airway of the alveolar epithelium. In this study, none of the 106 non-lung ADCs was found to express Nkx2-1, and only three out of 37 lung non-ADCs expressed Nkx2-1. However, 75% of 128 lung ADCs strongly expressed Nkx2-1. Mucinous and poorly differentiated lung ADCs showed reduced or no expression of Nkx2-1. Nkx2-1 has also been confirmed in clinical samples of atypical adenomatous hyperplasia, small cell carcinomas, and other neuroendocrine tumors (di Loreto et al., 1997; Agoff et al., 2000; Ordonez, 2000c; Cheuk et al., 2001; Oliveira et al., 2001; Stenhouse et al., 2004). On the contrary, Oliveira et al. (2001) and Ordonez (2000b) found that Nkx2-1 was mostly confined to cells arising from the lung and was absent from the neuroendocrine tissues.
Nkx2-1 is considered a highly specific marker for lung ADC. Gomez-Fernandez et al. (2002) examined body cavity fluids from 113 patients with lung ADC on the basis of cytology and found that Nkx2-1 was expressed in 54% of lung ADC, but was completely absent in other types. In another study using immunohistochemical staining to detect Nkx2-1, Nkx2-1, along with cytokeratin 20 (CK20) and neurofilaments (NF), was found to be effective for distinguishing Merkel cell carcinoma (MCC) from SCLC (Bobos et al., 2006). In addition, none of 138 MCC samples expressed Nkx2-1, while about 53%–100% of SCLC samples expressed Nkx2-1.
Lotan et al. (2009) determined an optimized molecular panel for distinguishing the most likely primary site of invasive micropapillary carcinoma (IMC), which included uroplakin, CK20, Nkx2-1, estrogen receptor (ER), Wilms tumor protein-1 (WT-1) and/or paired box protein 8 (PAX8), and mammaglobin. Use of Nkx2-1 was based on its uniformly positive expression in lung IMC cases, as detected by immunostaining. Nkx2-1 mRNA (Jiang et al., 2008) had the best diagnostic performance in pleural effusions of primary pulmonary ADC (PPA): the sensitivity 93.0%, the specificity 100.0%, and the accuracy 96.6%. The mRNA expression rate of pleural effusions was higher in patients with PPA (93.0%) than that in either metastatic pulmonary ADC (0%) or primary pulmonary squamous cell carcinoma (12.5%). Moreover, Nkx2-1 proved to be a useful marker for distinguishing primary lung ADC from pleural mesothelioma (Ordonez, 2000a). Cytology specimens are known to be particularly challenging for distinguishing ADC of the lung from non-pulmonary ADC or malignant mesothelioma. However, Khoor et al. (2011) showed that, in 26 pulmonary ADCs, 26 non-pulmonary ADCs (13 breast, 5 ovarian, 2 gastric, 2 prostatic, 1 esophageal, 1 colonic, 1 pancreatic, and 1 renal) and 4 malignant mesothelioma patients, Nkx2-1 had high sensitivity (73%) and specificity (100%) in differentiating lung ADC.
Caudal-related homeobox 2 (CDX-2) and Nkx2-1 were also capable of differentiating gastrointestinal from pulmonary carcinoids (Saqi et al., 2005), and Nkx2-1 was proposed as a potentially useful marker to distinguish pulmonary from gastrointestinal or pancreatic well-differentiated neuroendocrine tumors. CDX-2 and Nkx2-1 (Lin et al., 2007; Strickland-Marmol et al., 2007) were characterized as highly specific for identifying the original site of intestinal and lung tumors from metastatic neuroendocrine neoplasms. An immunohistochemical staining panel for distinguishing the primary site of metastatic well-differentiated neuroendocrine tumors (WDNETs) from the gastrointestinal tract, pancreas and lung included CDX-2, pancreatic duodenal homeobox-1 (PDX-1), neuroendocrine secretory protein-55 (NESP-55) and Nkx2-1 (Srivastava and Hornick, 2009).
Nkx2-1, Nkx2-8 (Nkx homeobox-8 gene), and PAX9 (paired box gene 9), the three transcription factors known to mediate lung development and maturation, were recently characterized as cooperating oncogenes located together on chromosome 14q13 (Hsu et al., 2009). NSCLC patients with early stage tumors that expressed both Nkx2-1 and Nkx2-8 had poor survival. In addition, KRAS and epidermal growth factor receptor (EGFR) mutations were correlated with tumors having coactivated PAX9 and Nkx2-1, or Nkx2-8 and Nkx2-1. This study also suggested that coactivation of Nkx2-1 and Nkx2-8 was associated with a potentially aggressive phenotype of NSCLC, and that the underlying molecular mechanism was independent of the rat sarcoma (RAS) pathway.
3.4. Role of Nkx2-1 in progression of lung cancer
In lung ADC, Nkx2-1 was shown to promote carcinogenesis by increasing the formation of new vessels (Pelosi et al., 2001) and enhancing the growth of cell proliferation (Puglisi et al., 1999). Kwei et al. (2008) showed that small interfering RNA (siRNA)-mediated knockdown of Nkx2-1 reduced cell proliferation and increased apoptosis in lung cancer. Nkx2-1, Nkx2-8, and PAX9 (Kendall et al., 2007) synergistically promote proliferation of immortalized human lung epithelial cells, as evidenced by over-expression of any combination of the three genes. In another study, Nkx2-1 over-expression was shown to be independent of Nkx2-8 (Harris et al., 2011). We have found that over-expressing Nkx2-1 leads to inhibition of lung ADC cell proliferation and prompts necrosis (unpublished data). Epithelial-to-mesenchymal transition (EMT), which can be elicited by transforming growth factor-β (TGF-β), facilitates cancer cell invasion and metastasis. Satio et al. (2009) determined that Nkx2-1 could inhibit the EMT process through down-regulation of TGF-β, thereby preventing metastasis of lung cancer. Meanwhile, in a recent study in our lab, we found that high expression of Nkx2-1 can diminish the migration ability of lung ADC cells, suggesting that lung cancer cells with low expression of Nkx2-1 have strong metastasis and invasion capabilities. Both of these properties involve down-regulation of TGF-β. A study by Winslow et al. (2011) suggested that Nkx2-1 might play a dual role of oncogenic and suppressive functions in a single tumor type. Thus, the true roles of Nkx2-1 in metastasis and migration of lung cancer cells are not yet fully understood and require further study.
3.5. Therapeutic role of Nkx2-1 in lung cancer
To date, very few studies have investigated the relation between Nkx2-1 and patient sensitivity to the epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) or to antineoplastic agents. Wislez et al. (2010) studied bronchioloalveolar carcinoma (BAC) patients and found that 95% of non-mucinous tumors expressed Nkx2-1, compared to only 27% of mucinous tumors. Moreover, the non-mucinous tumors appeared to be more sensitive to EGFR-TKIs. However, whether over-expression of Nkx2-1 truly reflects an increased sensitivity to EGFR-TKIs in lung cancer patients remains to be determined. The mitotic inhibitor chemotherapeutic agent paclitaxel was more effective at treating mucinous BAC tumors than at treating non-mucinous tumors (West et al., 2005). Coactivation of Nkx2-1 and Nkx2-8 in human lung squamous carcinoma cells (NCI-H2170) was shown to elicit resistance to the alkylating chemotherapeutic agent cisplatin (Hsu et al., 2009). In a study performed in our lab, we found that high expression of Nkx2-1 in the lung adenocarinoma cell line NCI-H1299 produced increased sensitivity specifically to the nucleoside analogue gemcitabine, but did not affect sensitivity to cisplatin or the antimitotic docetaxel. We believe that new and improved therapeutic strategies for NSCLC can be designed based on the relationship between Nkx2-1 and subtypes of NSCLC and/or the status of EGFR, and that expression of Nkx2-1 might play a role in evaluating sensitivity to various anticancer drugs.
3.6. Prognostic role of Nkx2-1 in lung cancer
While the 5-year survival rate of all lung cancer patients has increased by 3%–5% in recent years (Coleman et al., 2011), those patients with NSCLC have not experienced this benefit. Thus, it is very important to specifically evaluate the survival of NSCLC patients in response to established and new therapeutic approaches. This type of focused clinical study will likely provide information that will help in the design of optimal therapeutic schedules based on currently available treatments. Furthermore, by continuing experimental studies to determine the molecular mechanisms underlying NSCLC and disease progression and severity, manipulable biological factors, such as transcription factors like Nkx2-1, may be identified and targeted for molecular therapeutic intervention.
A multitude of studies have aimed to determine the role of Nkx2-1 in lung cancer prognosis (Table 2). Yet, the results are largely conflicting. While nine studies have reported a positive correlation between Nkx2-1 over-expression and survival, two studies found a negative correlation and four found that Nkx2-1 was not significantly (NS) associated with lung cancer survival. Puglisi et al. (1999) published the first study on the potential of Nkx2-1 as a prognostic indicator of NSCLC, and used a multivariate analysis approach. A total of 89 patients with stages I–IIIB NSCLCs were categorized into three groups according to the extent of tumor immunoreactivity for Nkx2-1. Positive Nkx2-1 patients were associated with a poor prognosis (P=0.009). We should point out that this study has some limitations; for example, the performance status was low and the study relied solely on a polyclonal antibody, instead of a monoclonal antibody. Results from another study (Pelosi et al., 2001) found no relationship between Nkx2-1 immunoreactivity and patient survival. Two other studies (Haque et al., 2002; Tan et al., 2003), evaluated stages I–IIIA NSCLCs with long-term follow-up and found that patients with strong Nkx2-1 expression had significantly better survival than patients with no Nkx2-1 (P=0.0067). A meta-analysis (Berghmans et al., 2006) using ten related studies published in the lung cancer literature showed that Nkx2-1 positivity was indeed significantly associated with better survival in NSCLC patients, and especially with early and locally advanced stages and in ADC.
Table 2.
Studies aimed at assessing the prognostic role of Nkx2-1 on survival of patients with lung cancer
| Reference | Specimen source | Diagnosis | Stage | n | Correlation with survival |
| Yoon et al., 2011 | Peripheral blood | NSCLC | I–III | 79 | Negative |
| Martins et al., 2009 | Histopathology | NSCLC | IIIB–IV | 51 | Positive |
| Barletta et al., 2009 | Histopathology | ADC | I–IV | 89 | Positive |
| Wang et al., 2007 | Histopathology | BAC | III | 81 | Positive |
| Berghmans et al., 2006 | Ten-published articles | Meta-analysis | I–IV | 10 | Positive |
| Barlesi et al., 2005 | Histopathology | ADC | I–IV | 106 | Positive |
| Stenhouse et al., 2004 | Histopathology | Lung tumor | Unknown | 165 | NS |
| Au et al., 2004 | Histopathology | NSCLC | Unknown | 284 | NS |
| Shah et al., 2004 | Histopathology | NSCLC | I–III | 63 | NS |
| Saad et al., 2004 | Histopathology | ADC | I | 100 | Positive |
| Myong, 2003 | Histopathology | NSCLC | I–III | 65 | Positive |
| Tan et al., 2003 | Histopathology | NSCLC | I–III | 126 | Positive |
| Haque et al., 2002 | Histopathology | NSCLC | I–III | 57 | Positive |
| Pelosi et al., 2001 | Histopathology | NSCLC | I | 222 | NS |
| Puglisi et al., 1999 | Histopathology | NSCLC | I–III | 88 | Negative |
NS: not significant; n: number of patients
Still another study of 89 patients with lung ADC (Barletta et al., 2009) showed that patients with high Nkx2-1 expression but with no Nkx2-1 gene amplification had improved overall survival (P<0.001). Patients with high gene expression of Nkx2-1 had a higher risk of death, almost three times higher than patients with low Nkx2-1 gene expression. Thus, the authors suggested that prognosis was related to Nkx2-1 gene activity and not Nkx2-1 protein levels, but this hypothesis has not yet been confirmed.
Martins et al. (2009) assessed the prognostic ability of Nkx2-1 and matrix metalloproteinases-9 (MMP-9). MMP-9 is a protease that is commonly expressed in NSCLC and has recognized angiogenic and metastatic potential. They found that the expression of these factors was associated with the outcomes of stage IIIB or IV lung ADC patients treated with platinum regimens. High Nkx2-1 combined with low MMP-9 was associated with good survival (median survival: 127.6 weeks) and was designated as a low risk prognostic indicator. Either low Nkx2-1 or high MMP-9 alone was associated with a median survival of 39.0 weeks and was designated as an intermediate risk prognostic indicator. Low Nkx2-1 combined with high MMP-9 was associated with poor survival (median survival: 16.4 weeks) and was designated as a high risk prognostic indicator. Likewise, Wang et al. (2007) reported that the survival time of patients with positive Nkx2-1 was longer than those without Nkx2-1 in non-mucinous type and stage III BAC patients.
In another study (Yoon et al., 2011), the mRNA levels of Nkx2-1 and CK19 in circulating tumor cells (CTCs) were investigated in 79 surgically resected NSCLC patients by using nested real-time reverse transcription polymerase chain reaction (RT-PCR) assay. Nkx2-1 mRNA was more highly expressed in NSCLC patients’ CTCs than was CK19. Comparing expression in patient-matched CTCs prior to and after resection revealed that 36.1% of patients were Nkx2-1-positive before surgery and 37.5% after surgery. Post-surgery Nkx2-1-positive patients experienced shorter progression-free survival (P=0.004) compared to those with Nkx2-1-negative status. Moreover, patients who were pre-surgery Nkx2-1-negative and post-surgery Nkx2-1-positive also had shorter progression-free survival (P<0.001). This study suggested that Nkx2-1 mRNA-expressing CTC could be used as a surrogate predictor prior to clinical manifestations of observable symptoms. Monitoring Nkx2-1(+) CTCs status after surgery was also proposed as useful for identifying high-risk patients among the surgically resected NSCLC population. In the advanced tumor stage, negative Nkx2-1 expression was an independent and significant marker for poor prognosis (Hiramatsu et al., 2010). Several other studies also evaluated the prognostic role of Nkx2-1 expression in NSCLC (Haque et al., 2002; Au et al., 2004; Berghmans et al., 2006), especially in lung ADC (Stenhouse et al., 2004; Barlési et al., 2005).
Thus, a definitive conclusion on the efficacy of Nkx2-1 as a prognostic indicator of lung cancer outcomes, or that of NSCLC in particular, has not been reached. We recently found that over-expression of Nkx2-1 not only inhibited the migration and proliferation of lung cancer cells but also promoted necrosis, indicating that strong expression of Nkx2-1 would have anticancer protective effects. Thus, Nkx2-1 may yet become established as a good prognostic marker for survival in NSCLC, especially of the ADC subtype.
4. Conclusions
Nkx2-1 interacts with multiple transcription factors in a cell/tissue-specific manner to regulate a multitude of differentiation and developmental functions (Fig. 2). In the lung, Nkx2-1 plays a pivotal role in normal lung development and also in that of tumors, especially contributing to tumor cell differentiation and morphogenesis processes. Nkx2-1 over-expression has been clinically detected in patient samples of NSCLC ADC subtype and SCLC, while low or no expression has been found in LCLCs or lung SCCs. Nkx2-1 has been successfully used to distinguish the metastatic and morphologically similar ADCs of pulmonary origin from those of other unknown primary sites. Furthermore, Nkx2-1 expression is strongly associated with the prognosis and efficacy of several commonly used therapeutic agents. Understanding the prognostic factors underlying different types of lung cancer, particularly of lung ADC, and their influence on tumor response to chemotherapeutic drugs will facilitate more in-depth evaluation of the survival of lung cancer patients and help to develop novel effective therapeutic strategies. Furthermore, the recently recognized inhibitory property of Nkx2-1 on mucous cell metaplasia and T helper 2 (Th2) inflammation (Maeda et al., 2011), suggests that a better understanding of Nkx2-1 molecular interactions will provide insights into new therapeutic targets to treat cancer-related and inflammation-related lung diseases.
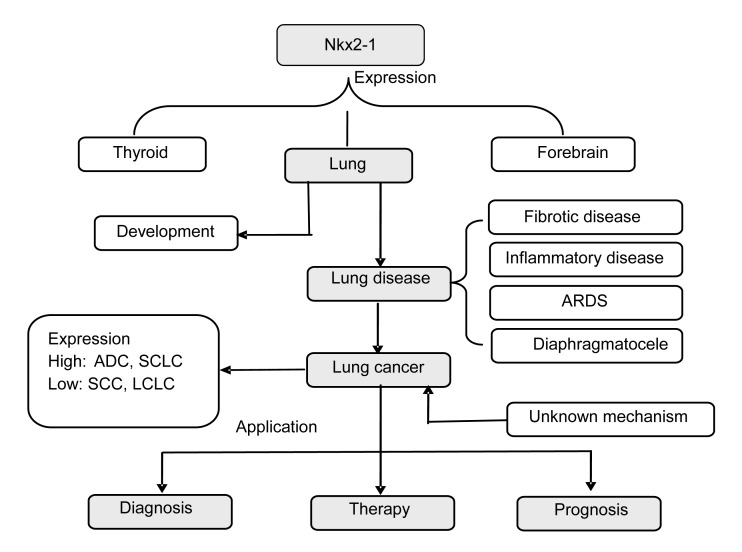
Fig. 2.
A schematic diagram to summarize the role of Nkx2-1 in the lung
Nkx2-1 activates the expression of selected genes in the thyroid, lung, and ventral forebrain, which have a close relationship with the development of the fetal and postnatal lung. Nkx2-1 mutations can induce idiopathic pulmonary fibrosis (IPF), pulmonary infection, acute respiratory distress syndrome (ARDS), and diaphragmatic hernia, among other disease processes. Nkx2-1 is highly expressed in lung cancer, especially in ADC and SCLC, while lower expression is found in SCC and LCLC. Nkx2-1 is clinically applicable for lung cancer diagnosis, and as an indicator for therapy type and of prognosis. Understanding the role of Nkx2-1 in lung cancer may open new avenues of research to improve the evaluation of the survival of the lung cancer patients and to develop novel effective therapeutic strategies
Acknowledgments
We thank Medjaden Bioscience Limited (Room 2001-4, China Insurance Group Building, 141 Des Voeux Road Central, Hong Kong, China) for assisting in the preparation of this manuscript.
Footnotes
Project supported by the Zhejiang Provincial Natural Science Foundation of China (No. LQ12H01001), and the Zhejiang Provincial Medicine & Health Scientific Foundation of China (No. 2012KYA143)
References
- 1.Agoff SN, Lamps LW, Philip AT, Amin MB, Schmidt RA, True LD, Folpe AL. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonarry neuroendocrine tumors. Mod Pathol. 2000;13(3):238–242. doi: 10.1038/modpathol.3880044. [DOI] [PubMed] [Google Scholar]
- 2.Au NH, Cheang M, Huntsman DG, Yorida E, Coldman A, Elliott WM, Bebb G, Flint J, English J, Gilks CB, et al. Evaluation if immunohistochemical markers in non-small cell lung cancer by unsupervised hierarchical clustering analysis: a tissue microarray study of 284 cases and 18 markers. J Pathol. 2004;204(1):101–109. doi: 10.1002/path.1612. [DOI] [PubMed] [Google Scholar]
- 3.Bachurski CJ, Yang GH, Currier TA, Gronostajski RM, Hong D. Nuclear factor I/thyroid transcription factor 1 interactions modulate surfactant protein C transcription. Mol Cell Biol. 2003;23(24):9014–9024. doi: 10.1128/MCB.23.24.9014-9024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai XY, Shen H. Mutational analysis of thyroid transcription factor-1 gene (TTF-1) in lung carcinomas. In Vitro Cell Dev Bio Anim. 2008;44(1-2):17–25. doi: 10.1007/s11626-007-9062-0. [DOI] [PubMed] [Google Scholar]
- 5.Barletta JA, Perner S, Iafrate AJ, Yeap BY, Weir BA, Johnson LA, Johnson BE, Meyerson M, Rubin MA, Travis WD, et al. Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med. 2009;13(8b):1977–1986. doi: 10.1111/j.1582-4934.2008.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlési F, Pinot D, Legoffic A, Doddoli C, Chetaille B, Torre JP, Astoul P. Positive thyroid transcription factor 1 staining strongly correlates with survival of patients with adenocarcinoma of the lung. Br J Cancer. 2005;93(4):450–452. doi: 10.1038/sj.bjc.6602717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bejarano PA, Baughman RP, Biddinger PW, Miller MA, Fenoglio-Preiser C, Al Kafaji B, di Lauro R, Whitsett JA. Surfactant proteins and thyroid transcription factor-1 in pulmonary and breast carcinomas. Mod Pathol. 1995;9(4):445–452. [PubMed] [Google Scholar]
- 8.Berghmans T, Paesmans M, Mascaux C, Martin B, Meert AP, Haller A, Lafitte JJ, Sculier JP. Thyroid transcription factor 1—a new prognostic factor in lung cancer: a meta-analysis. Ann Oncol. 2006;17(11):1673–1676. doi: 10.1093/annonc/mdl287. [DOI] [PubMed] [Google Scholar]
- 9.Besnard V, Xu Y, Whitsett JA. Sterol response element binding protein and thyroid transcription factor-1 (Nkx2.1) regulate Abca3 gene expression. Am J Physiol Lung Cell Mol Physiol. 2007;293(6):L1395–L1405. doi: 10.1152/ajplung.00275.2007. [DOI] [PubMed] [Google Scholar]
- 10.Bingle CD. Thyroid transcription factor-1. Int J Biochem Cell Biol. 1997;29(12):1471–1473. doi: 10.1016/S1357-2725(97)00007-1. [DOI] [PubMed] [Google Scholar]
- 11.Bobos M, Hytiroglou P, Kostopoulos I, Karkavelas G, Papadimitriou CS. Immunohistochemical distinction between Merkel cell carcinoma and small cell carcinoma of the lung. Am J Dermatopathol. 2006;28(2):99–104. doi: 10.1097/01.dad.0000183701.67366.c7. [DOI] [PubMed] [Google Scholar]
- 12.Bohinski RJ, di Lauro R, Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol Cell Biol. 1994;14(9):5671–5681. doi: 10.1128/MCB.14.9.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohinski RJ, Bejarano PA, Balko G, Warnick RE, Whitset JA. Determination of lung as the primary site of cerebral metastatic adenocarcinomas using monoclonal antibody to thyroid transcription factor-1. J Neuro-oncol. 1998;40(3):227–231. doi: 10.1023/A:1006102607697. [DOI] [PubMed] [Google Scholar]
- 14.Bruno MD, Bohinski RJ, Huelsman KM, Whitsett JA, Korfhagen TR. Lung cell-specific expression of the murine surfactant protein A (SP-A) gene is mediated by interactions between the SP-A promoter and thyroid transcription factor-1. J Biol Chem. 1995;270(12):6531–6536. doi: 10.1074/jbc.270.12.6531. [DOI] [PubMed] [Google Scholar]
- 15.Cheuk W, Kwan MY, Suster S, Chan JK. Immunostaining for thyroid transcription factor-1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med. 2001;125(2):228–231. doi: 10.5858/2001-125-0228-IFTTFA. [DOI] [PubMed] [Google Scholar]
- 16.Civitareale D, Lonigro R, Sinclair AJ, di Lauro R. A thyroid-specific nuclear protein essential for tissue-specific expression of the thyroglobulin promoter. EMBO J. 1989;8(9):2537–2542. doi: 10.1002/j.1460-2075.1989.tb08391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, Nur U, Tracey E, Coory M, Hatcher J, et al. Cancer survival in Australin, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the international Cancer Benchmarking Partnership): an analysis of population-based cancer registry date. Lancet. 2011;377(9760):127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dave V, Childs T, Whitsett JA. Nuclear factor of activated T cells regulates transcription of the surfactant protein D gene (Sftpd) via direct interaction with thyroid transcription factor-1 in lung epithelial cells. J Biol Chem. 2004;279(33):34578–34588. doi: 10.1074/jbc.M404296200. [DOI] [PubMed] [Google Scholar]
- 19.Devriendt K, Vanhole C, Matthijs G, de Zegher F. Deletion of thyroid transcription factor-1 gene in an infant with neonatal thyroid dysfunction and respiratory failure. N Engl J Med. 1998;338(18):1317–1318. doi: 10.1056/NEJM199804303381817. [DOI] [PubMed] [Google Scholar]
- 20.di Loreto C, di Lauro V, Puglisi F, Damante G, Fabbro D, Betrami CA. Immunocytochemical expression of tissue-specific transcription factor-1 in lung carcinoma. J Clin Pathol. 1997;50(1):30–32. doi: 10.1136/jcp.50.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.di Loreto C, Puglisi F, di Lauro V, Damante G, Beltrami CA. TTF-1 protein expression in pleural malignant mesotheliomas and adenocarcinomas of the lung. Cancer Lett. 1998;124(1):73–78. doi: 10.1016/S0304-3835(97)00466-7. [DOI] [PubMed] [Google Scholar]
- 22.Doyle DA, Gonzalez I, Thomas B, Scavina M. Autosomal dominant transmission of congenital hypothyroidism, neonatal respiratory distress, and ataxia caused by a mutation of NKX2-1 . J Pediatr. 2004;145(2):190–193. doi: 10.1016/j.jpeds.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Fabbro D, di Loreto C, Stamerra O, Beltrami CA, Lonigro R, Damante G. TTF-1 gene expression in human lung tumors. Eur J Cancer. 1996;32(3):512–517. doi: 10.1016/0959-8049(95)00560-9. [DOI] [PubMed] [Google Scholar]
- 24.Fujita J, Ohtsuki Y, Bandoh S, Ueda Y, Kubo A, Tojo Y, Yamaji Y, Ishida T. Expression of thyroid transcription factor-1 in 16 human lung cancer cell lines. Lung Cancer. 2003;39(1):31–36. doi: 10.1016/S0169-5002(02)00390-2. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Fernandez C, Jorda M, Delgado PI, Ganjei-Azar P. Thyroid transcription factor 1: a marker for lung adenocarcinoma in body cavity fluids. Cancer. 2002;96(5):289–293. doi: 10.1002/cncr.10743. [DOI] [PubMed] [Google Scholar]
- 26.Grasberger H, Ringkananont U, Lefrancois P, Abramowicz M, Vassart G, Refetoff S. Thyroid transcription factor 1 rescues PAX8/p300 synergism impaired by a natural PAX8 paired domain mutation with dominant negative activity. Mol Endocrinol. 2005;19(7):1779–1791. doi: 10.1210/me.2004-0426. [DOI] [PubMed] [Google Scholar]
- 27.Hamdan H, Liu H, Li C, Jones C, Lee M, de Lemos R, Minoo P. Structure of the human Nkx2.1 gene. Biochim Biophy Acta. 1998;1396(3):336–348. doi: 10.1016/S0167-4781(97)00210-8. [DOI] [PubMed] [Google Scholar]
- 28.Haque AK, Syed S, Lele SM, Freeman DH, Adegboyega PA. Immunohistochemical study of thyroid transcription factor-1 and HER2/neu in non-small cell lung cancer: strong thyroid transcription factor-1 expression predicts better survival. Appl Immunohistochem Mol Morphol. 2002;10(2):103–109. doi: 10.1097/00022744-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Harlamert HA, Mira J, Bejarano PA, Baughman RP, Miller MA, Whitsett JA, Yassin R. Thyroid transcription factor-1 cytokeratins 7 and 20 in pulmonary and breast carcinomas. Acta Cytol. 1998;42(6):1382–1388. doi: 10.1159/000332172. [DOI] [PubMed] [Google Scholar]
- 30.Harris T, Pan Q, Sironi J, Lutz D, Tian J, Sapkar J, Perez-Soler R, Keller S, Locker J. Both gene amplification and allelic loss occur at 14q13.3 in lung cancer. Clin Cancer Res. 2011;17(4):690–699. doi: 10.1158/1078-0432.CCR-10-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiramatsu M, Ninomiya H, Inamura K, Nomura K, Takeuchi K, Satoh Y, Okumura S, Nakagawa K, Yamori T, Matsuura M, et al. Activation status of receptors tyrosine kinase downstream pathways in primary lung adenocarcinoma with reference of KRAS and EGFR mutations. Lung Cancer. 2010;70(1):94–102. doi: 10.1016/j.lungcan.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Holzinger A, Dingle S, Bejarano PA, Miller MA, Weaver TE, di Lauro R, Whitsett JA. Monoclonal antibody to thyroid transcription factor-1: production, characterization, and usefulness in tumor diagnosis. Hybridoma. 1996;15(1):49–53. doi: 10.1089/hyb.1996.15.49. [DOI] [PubMed] [Google Scholar]
- 33.Hsu DS, Acharya CR, Balakumaran BS, Riedel RF, Kim MK, Stevenson M, Tuchman S, Mukherjee S, Barry W, Dressman HK, et al. Characterizing the developmental pathways TTF-1, NKX2-8, and PAX9 in lung cancer. PNAS. 2009;106(13):5312–5317. doi: 10.1073/pnas.0900827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda K, Clark JC, Shaw-White JR, Stahlman MT, Boutell CJ, Whitsett JA. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem. 1995;270(14):8108–8114. doi: 10.1074/jbc.270.14.8108. [DOI] [PubMed] [Google Scholar]
- 35.Islam KN, Mendelson CR. Permissive effects of oxygen on cyclic AMP and interleukin-1 stimulation of surfactant protein A gene expression are mediated by epigenetic mechanisms. Mol Cell Biol. 2006;26(8):2901–2912. doi: 10.1128/MCB.26.8.2901-2912.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwatani N, Mabe H, Devriendt K, Kodama M, Miike T. Deletion of NKX2.1 gene encoding thyroid transcription factor-1 in two siblings with hypothyroidism and respiratory failure. J Pediatr. 2000;137(2):272–276. doi: 10.1067/mpd.2000.107111. [DOI] [PubMed] [Google Scholar]
- 37.Jiang B, Wu GP, Zhao YJ, Wang SC. Transcription expression and clinical significance of TTF-1 mRNA in pleural effusion of patients with lung cancer. Diagn Cytopathol. 2008;36(12):849–854. doi: 10.1002/dc.20926. [DOI] [PubMed] [Google Scholar]
- 38.Kaufmann O, Dietel M. Thyroid transcription factor-1 is the superior immunohistochemical marker for pulmonary adenocarcinoma and large cell carcinoma compared to surfactant proteins A and B. Histopathplogy. 2000;36(1):8–16. doi: 10.1046/j.1365-2559.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 39.Kelly SE, Bachurski CJ, Burhans MS, Glasser SW. Transcription of the lung-specific surfactant protein C gene is mediated by thyroid transcription factor 1. J Biol Chem. 1996;271(12):6881–6888. doi: 10.1074/jbc.271.12.6881. [DOI] [PubMed] [Google Scholar]
- 40.Kendall J, Liu Q, Bakleh A, Krasnitz A, Nguyen KC, Lakshmi B, Gerald WL, Powers S, Mu D. Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. PNAS. 2007;104(42):16663–16668. doi: 10.1073/pnas.0708286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khoor A, Byrd-Gloster AL, Nicosia SV. Expression of thyroid transcription factor-1 in malignant pleural effusions. Pathol Oncol Res. 2011;17(2):263–267. doi: 10.1007/s12253-010-9308-0. [DOI] [PubMed] [Google Scholar]
- 42.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, pituitary. Genes Dev. 1996;10(1):60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 43.Kolla V, Gonzales LW, Gonzales J, Wang P, Angampalli S, Feinstein SI, Ballard PL. Thyroid transcription factors in differentiating type II cells: regulation, isoforms, and target genes. Am J Respir Cell Mol Biol. 2006;36(2):213–225. doi: 10.1165/rcmb.2006-0207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krude H, Schutz B, Biebermann H, von Moers A, Schnabel D, Neitzel H, Tonnies H, Weise D, Lafferty A, Schwarz S, et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J Clin Invest. 2002;109(4):475–480. doi: 10.1172/JCI200214341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwei KA, Kim YH, Girard L, Kao J, Pacyna-Gengelbach M, Salari K, Lee J, Choi YL, Sato M, Wang P, et al. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene. 2008;27(25):3635–3640. doi: 10.1038/sj.onc.1211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Cai J, Pan Q, Minoo P. Two functionally distinct forms of Nkx2.1 protein are expressed in the pulmonary epithelium. Biochem Biophys Res Commun. 2000;270(2):462–468. doi: 10.1006/bbrc.2000.2443. [DOI] [PubMed] [Google Scholar]
- 47.Li C, Zhu NL, Tan RC, Ballard PL, Derynck R, Minoo P. Transforming growth factor-β inhibits pulmonary surfactant protein B gene transcription through SMAD3 interactions with NKX2.1 and HNF-3 transcription factors. J Biol Chem. 2002;277(41):38399–38408. doi: 10.1074/jbc.M203188200. [DOI] [PubMed] [Google Scholar]
- 48.Lin S, Perl AK, Shannon JM. Erm/thyroid transcription factor 1 interactions modulate surfactant protein C transcription. J Biol Chem. 2006;281(24):16716–16726. doi: 10.1074/jbc.M602221200. [DOI] [PubMed] [Google Scholar]
- 49.Lin X, Saad RS, Luckasevic TM, Silverman JF, Liu Y. Diagnostic value of CDX-2 and TTF-1 expression in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15(4):407–414. doi: 10.1097/01.pai.0000210416.53493.0f. [DOI] [PubMed] [Google Scholar]
- 50.Liu C, Glasser SW, Wan H, Whitsett JA. GATA-6 and thyroid transcription factor-1 directly interact and regulate surfactant protein-C gene expression. J Biol Chem. 2002;277(6):4519–4525. doi: 10.1074/jbc.M107585200. [DOI] [PubMed] [Google Scholar]
- 51.Lotan TL, Ye H, Melamed J, Wu XR, Shih IeM, Epstein JI. Immunohistochemical panel to identify the primary site of invasive micropapillary carcinoma. Am J Surg Pathol. 2009;33(7):1037–1041. doi: 10.1097/PAS.0b013e3181962dcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maeda Y, Hunter TC, Loudy DE, Dave V, Schreiber V, Whitsett JA. PARP-2 interacts with TTF-1 and regulates expression of surfactant protein-B. J Biol Chem. 2006;281(14):9600–9606. doi: 10.1074/jbc.M510435200. [DOI] [PubMed] [Google Scholar]
- 53.Maeda Y, Chen G, Xu Y, Haitchi HM, Du L, Keiser AR, Howarth PH, Davies DE, Holgate ST, Whitsett JA. Airway epithelial transcription factor NKX2-1 inhibits mucous cell metaplasia and Th2 inflammation. Am J Respir Crit Care Med. 2011;184(4):421–429. doi: 10.1164/rccm.201101-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martins SJ, Takagaki TY, Silva AG, Gallo CP, Silva FB, Capelozzi VL. Prognostic relevance of TTF-1 and MMP-9 expression in advanced lung adenocarcinoma. Lung Cancer. 2009;64(1):105–109. doi: 10.1016/j.lungcan.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 55.Missero C, Pirro MT, Simeone S, Pischetola M, di Lauro R. The DNA glycosylase T:G mismatch-specific thymine DNA glycosylase represses thyroid transcription factor-1-activated transcription. J Biol Chem. 2001;276(36):33569–33575. doi: 10.1074/jbc.M104963200. [DOI] [PubMed] [Google Scholar]
- 56.Myong NH. Thyroid transcription factor-1 (TTF-1) expression in human lung carcinomas: its prognostic implication and relationship with expressions of p53 and Ki-67 proteins. J Korea Med Sci. 2003;18(4):494–500. doi: 10.3346/jkms.2003.18.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura N, Miyagi E, Murata S, Kawaoi A, Katoh R. Expression of thyroid transcription factor-1 in normal and neoplastic lung tissues. Mod Pathol. 2002;15(10):1058–1067. doi: 10.1097/01.MP.0000028572.44247.CF. [DOI] [PubMed] [Google Scholar]
- 58.Naltner A, Ghaffari M, Whitsett JA, Yan C. Retinoic acid stimulation of the human surfactant protein B promoter is thyroid transcription factor 1 site-dependent. J Biol Chem. 2000;275(1):56–62. doi: 10.1074/jbc.275.1.56. [DOI] [PubMed] [Google Scholar]
- 59.Naltner A, Wert S, Whitsett JA, Yan C. Temporal/spatial expression of nuclear receptor coactivators in the mouse lung. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1066–L1074. doi: 10.1152/ajplung.2000.279.6.L1066. [DOI] [PubMed] [Google Scholar]
- 60.Oliveira AM, Tazelaar HD, Myers JL, Erickson LA, Lloyd RV. Thyroid transcription factor-1 distinguishes metastatic pulmonary from well-differentiated neuroendocrine tumors of other sites. Am J Surg Pathol. 2001;25(6):815–819. doi: 10.1097/00000478-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 61.Ordonez NG. Thyroid transcription factor-1 is a marker of lung and thyroid carcinomas. Adv Anat Pathol. 2000;7(2):123–127. doi: 10.1097/00125480-200007020-00007. [DOI] [PubMed] [Google Scholar]
- 62.Ordonez NG. Value of thyroid transcription factor-1, E-cadherin, BG8, WT1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and nonpulmonary adenocarcinoma. Am J Surg Pathol. 2000;24(4):598–606. doi: 10.1097/00000478-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 63.Ordonez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinoma from other small cell carcinomas. Am J Surg Pathol. 2000;24(9):1217–1223. doi: 10.1097/00000478-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Park KS, Whitsett JA, di Palma T, Hong JH, Yaffe MB, Zannini M. TAZ interacts with TTF-1 and regulates expression of surfactant protein-C. J Biol Chem. 2004;279(17):17384–17390. doi: 10.1074/jbc.M312569200. [DOI] [PubMed] [Google Scholar]
- 65.Pelosi G, Fraggetta F, Pasini F, Maisonneuve P, Sonzogni A, Iannucci A, Terzi A, Bresaola E, Valduga F, Lupo C, et al. Immunoreactivity for thyroid transcription facter-1 in stage I non-small cell carcinomas of the lung. Am J Surg Pathol. 2001;25(3):363–372. doi: 10.1097/00000478-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Pohlenz J, Dumitrescu A, Zundel D, Martine U, Schonberger W, Koo E, Weiss RE, Cohen RN, Kimura S, Refetoff S. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109(4):469–473. doi: 10.1172/JCI200214192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puglisi F, Barbone F, Damante G, Bruckbauer M, di Lauro V, Beltrami CA, di Loreto C. Prognostic value of thyroid transcription factor-1 in primary, resected, non-small cell lung carcinoma. Mod Pathol. 1999;12(3):318–324. [PubMed] [Google Scholar]
- 68.Saad RS, Liu YL, Han H, Landreneau RJ, Silverman JF. Prognostic significance of thyroid transcription factor-1 expression in both early-stage conventional adenocarcinoma and bronchioloalveolar carcinoma of the lung. Hum Pathol. 2004;35(1):3–7. doi: 10.1016/j.humpath.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Saqi A, Alexis D, Remotti F, Bhagat G. Usefulness of CDX2 and TTF-1 in differentiating gastrointestinal from pulmonary carcinoids. Am J Clin Pathol. 2005;123(3):394–404. doi: 10.1309/UKN6PVRKXHG422DA. [DOI] [PubMed] [Google Scholar]
- 70.Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132(1):35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- 71.Shah L, Walter KL, Borczuk AC, Kawut SM, Sonett JR, Gorenstein LA, Ginsburg ME, Steinglass KM, Powell CA. Expression of syndecan-1 and expression of epidermal growth factor receptor are associated with survival in patients with non small cell lung carcinoma. Cancer. 2004;101(7):1632–1638. doi: 10.1002/cncr.20542. [DOI] [PubMed] [Google Scholar]
- 72.Srivastava A, Hornick JL. Immunohistochemical staining for CDX-2, PDX-1, NESP-55 and TTF-1 can help distinguish gastrointestinal carcinoid tumors from pancreatic endocrine and pulmonary carcinoid tumors. Am J Surg Pathol. 2009;33(4):626–632. doi: 10.1097/PAS.0b013e31818d7d8b. [DOI] [PubMed] [Google Scholar]
- 73.Stahlman MT, Gray ME, Whitsett JA. Expression of thyroid transcription factor-1 (TTF-1) in fetal and neonatal human lung. J Histochem Cytochem. 1996;44(7):673–678. doi: 10.1177/44.7.8675988. [DOI] [PubMed] [Google Scholar]
- 74.Stenhouse G, Fyfe N, King G, Chapman A, Kerr KM. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J Clin Pathol. 2004;57(4):383–387. doi: 10.1136/jcp.2003.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strickland-Marmol LB, Khoor A, Livingston SK, Rojiani A. Utility of tissue-specific transcription factors 1 and Cdx2 in determining the primary site of metastatic adenocarcinomas to the brain. Arch Pathol Lab Med. 2007;131(11):1686–1690. doi: 10.5858/2007-131-1686-UOTTFT. [DOI] [PubMed] [Google Scholar]
- 76.Tan D, Li Q, Deeb G, Ramnath N, Slocum HK, Brooks J, Cheney R, Wiseman S, Anderson T, Loewen G. Thyroid transcription factor-1 expression prevalence and its clinical implications in non-small cell lung cancer: a high-throughput tissue microarray and immunohistochemistry study. Hum Pathol. 2003;34(6):597–604. doi: 10.1016/S0046-8177(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka H, Yanagisawa K, Shinjo K, Taguchi A, Maeno K, Tomida S, Shimada Y, Osada H, Kosaka T, Matsubara H, et al. Lineage-specific dependency of lung adenocarcinomas on the lung development regulator TTF-1. Cancer Res. 2007;67(13):6007–6011. doi: 10.1158/0008-5472.CAN-06-4774. [DOI] [PubMed] [Google Scholar]
- 78.Toonen RF, Gowan S, Bingle CD. The lung enriched transcription factor TTF-1 and the ubiquitously expressed proteins Sp1 and Sp3 interact with elements located in the minimal promoter of the rat Clara cell secretory protein gene. Biochem J. 1996;316(Pt 2):467–473. doi: 10.1042/bj3160467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang CL, Yue DS, Zhang ZF, Zhan ZL, Sun LN. Value of thyroid transcription factor-1 in identification of the prognosis of bronchioloalveolar carcinoma. Zhonghua Yi Xue Za Zhi. 2007;87(33):2350–2354. (in Chinese) [PubMed] [Google Scholar]
- 80.Weidenfeld J, Shu W, Zhang L, Millar SE, Morrisey EE. The WNT7b promoter is regulated by TTF-1, GATA6, and Foxa2 in lung epithelium. J Biol Chem. 2002;277(23):21061–21070. doi: 10.1074/jbc.M111702200. [DOI] [PubMed] [Google Scholar]
- 81.Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R, Lin WM, Province MA, Kraja A, Johnson LA, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450(7171):893–898. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.West HL, Crowley JJ, Vance RB, Franklin WA, Livingston RB, Dakhil SR, Giguere JK, Rivkin SE, Kraut M, Chansky K, et al. Advanced bronchioloalveolar carcinoma: a phase II trial of paclitaxel by 96-hour infusion (SWOG 9714): a Southwest Oncology Group study. Ann Oncol. 2005;16(7):1076–1080. doi: 10.1093/annonc/mdi215. [DOI] [PubMed] [Google Scholar]
- 83.Winslow MM, Dayton TL, Verhaak RG, Kim-Kiselak C, Snyder EL, Feldser DM, Hubbard DD, DuPage MJ, Whittaker CA, Hoersch S, et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature. 2011;473(7345):101–104. doi: 10.1038/nature09881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wislez M, Antoine M, Baudrin L, Poulot V, Neuville A, Pradere M, Longchampt E, Isaac-Sibille S, Lebitasy MP, Cadranel J. Non-mucinomous and mucinous subtypes of adenocarcinoma with bronchioloalveolar carcinoma features differ by biomarker expression and in the response to gefitinib. Lung Cancer. 2010;68(2):185–191. doi: 10.1016/j.lungcan.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 85.Yan C, Naltner A, Conkright J, Ghaffari M. Protein-protein interaction of retinoic acid receptor α and thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem. 2001;276(24):21686–21691. doi: 10.1074/jbc.M011378200. [DOI] [PubMed] [Google Scholar]
- 86.Yan C, Naltner A, Martin M, Naltner M, Fangman JM, Gurel O. Transcriptional stimulation of the surfactant protein B gene by STAT3 in respiratory epithelial cells. J Biol Chem. 2002;277(13):10967–10972. doi: 10.1074/jbc.M109986200. [DOI] [PubMed] [Google Scholar]
- 87.Yang L, Yan D, Bruggeman M, Du H, Yan C. Mutation of a lysine residue in a homeodomain generatesdominant negative thyroid transcription factor 1. Biochemistry. 2004;43(39):12489–12497. doi: 10.1021/bi049283o. [DOI] [PubMed] [Google Scholar]
- 88.Yang MC, Wang B, Weissler JC, Margraf LR, Yang YS. BR22, a 26 kDa thyroid transcription factor-1 associated protein (TAP26), is expressed in human lung cells. Eur Respir J. 2003;22(1):28–34. doi: 10.1183/09031936.03.00117702. [DOI] [PubMed] [Google Scholar]
- 89.Yang YS, Yang MC, Wang B, Weissler JC. BR22, a novel protein, interacts with thyroid transcription factor-1 and activates the human surfactant protein B promoter. Am J Respir Cell Mol Biol. 2001;24(1):30–37. doi: 10.1165/ajrcmb.24.1.4050. [DOI] [PubMed] [Google Scholar]
- 90.Yatabe Y, Mitsudomi T, Takahashi T. TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol. 2002;26(6):767–773. doi: 10.1097/00000478-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 91.Yi M, Tong GX, Murry B, Mendelson CR. Role of CBP/p300 and SRC-1 in transcriptional regulation of the pulmonary surfactant protein-A (SP-A) gene by thyroid transcription factor-1 (TTF-1) J Biol Chem. 2002;277(4):2997–3005. doi: 10.1074/jbc.M109793200. [DOI] [PubMed] [Google Scholar]
- 92.Yoon SO, Kim YT, Jung KC, Jeon YK, Kim BH, Kim CW. TTF-1 mRNA-positive circulating tumors cells in the peripheral blood predict poor prognosis in surgically resected non-small cell lung cancer patients. Lung Cancer. 2011;71(2):209–216. doi: 10.1016/j.lungcan.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 93.Zhang P, Han Y, Huang L, Li Q, Ma D. Expression and clinical significance of TTF-1 and P63 in NSCLC. Chin J Lung Cancer. 2009;12(9):995–999. doi: 10.3779/j.issn.1009-3419.2009.09.010. (in Chinese) [DOI] [PubMed] [Google Scholar]