Abstract
Objective: To evaluate the effects of tetrandrine citrate, a novel tetrandrine salt with high water solubility, on the growth of imatinib (IM)-resistant chronic myeloid leukemia (CML) in vitro and in vivo, and reveal action molecular mechanisms. Methods: Cell viability in vitro was measured using methyl thiazolyl tetrazolium (MTT) assay. CML cell growth in vivo was assessed using a xenograft model in nude mice. Bcr-Abl and β-catenin protein levels were determined using Western blotting. Bcr-Abl messenger RNA (mRNA) was measured by reverse transcription polymerase chain reaction (RT-PCR). Flow cytometry (FCM) was used to determine cell cycle status. Results: Tetrandrine citrate inhibited the growth of IM-resistant K562 cells, primary leukemia cells, and primitive CD34+ leukemia cells, and their inhibition concentration that inhibited 50% of target cells (IC50) ranged from 1.20 to 2.97 μg/ml. In contrast, tetrandrine citrate did not affect normal blood cells under the same conditions, and IC50 values were about 10.12–13.11 μg/ml. Oral administration of tetrandrine citrate caused complete regression of IM-resistant K562 xenografts in nude mice without overt toxicity. Western blot results revealed that treatment of IM-resistant K562 cells with tetrandrine citrate resulted in a significant decrease of both p210Bcr-Abl and β-catenin proteins, but IM did not affect the Bcr-Abl protein levels. Proteasome inhibitor, MG132, did not prevent tetrandrine-mediated decrease of the p210Bcr-Abl protein. RT-PCR results showed that tetrandrine treatment caused a decrease of Bcr-Abl mRNA. FCM analysis indicated that tetrandrine induced gap 1 (G1) arrest in CML cells. Conclusions: Tetrandrine citrate is a novel orally active tetrandrine salt with potent anti-tumor activity against IM-resistant K562 cells and CML cells. Tetrandrine citrate-induced growth inhibition of leukemia cells may be involved in the depletion of p210Bcr-Abl mRNA and β-catenin protein.
Keywords: Chronic myeloid leukemia, Imatinib-resistance, Tetrandrine citrate, Bcr-Abl protein, β-catenin protein
1. Introduction
Philadelphia chromosome positive (Ph+) chronic myeloid leukemia (CML) is a common malignant clonal disorder of hematopoietic stem cells caused by the oncogenic Bcr-Abl tyrosine kinase. Since the development of Abl tyrosine kinase inhibitors (TKIs) (e.g., imatinib (IM), dasatinib, and nilotinib), targeted therapy with these TKIs has markedly improved the outcome of Ph+ CML. However, most patients ceasing TKI therapy invariably relapse due to drug resistance caused by the mutation of the Bcr-Abl kinase, and the insensitivity of the TKIs to leukemia stem cells (LSCs) (Li and Li, 2007; Konig et al., 2008; Stuart et al., 2009), creating a need for more potent and safer therapies against Abl kinase activity-independent targets.
Several potential targets have been proposed for the treatment of Ph+ CML. These targets include the β-catenin in the Wnt/β-catenin pathway (Hu et al., 2009), nuclear factor-κB (NF-κB) pathway (Guzman et al., 2005), CD44 (Krause et al., 2006), Hedgehog pathway (Dierks et al., 2008), Alox5 (Chen et al., 2009a), and PTEN (phosphatase and tensin homolog) (McCubrey et al., 2008). The β-catenin in the Wnt/β-catenin signaling pathway is essential for the self-renewal of Bcr-Abl+ CML cells (Coluccia et al., 2007) and the survival of LSCs (Hu et al., 2009), and its protein stability is largely dependent on the protein levels of the Bcr-Abl kinase in Ph+ CML cells (Coluccia et al., 2007). These data strongly suggest that the Bcr-Abl/β-catenin axis may be an attractive, yet unrealized treatment option. As current TKIs mainly target the activity of the Bcr-Abl kinase, rather than its protein level, we proposed that a strategy that disrupts the Bcr-Abl/β-catenin axis by down-regulating the protein level of the Bcr-Abl kinase could have better therapeutic effects for two reasons. First, the depletion of the Bcr-Abl kinase protein could override TKI resistance to kinase mutation. Second, disrupting the Bcr-Abl/β-catenin axis causes instability and subsequent degradation of β-catenin protein essential for survival of LSCs, and renders LSCs more vulnerable to eradication, thereby improving the outcome of Ph+ CML.
Tetrandrine is a long-established drug that has been widely used to treat silicosis and arthritis for centuries in China (Lai, 2002), and previous studies showed that it has anti-tumor activity for several tumor cell lines in vitro (Lee et al., 2002; Wang et al., 2005; Ng et al., 2006). However, tetrandrine free base is hydrophobic alkaloids with very low solubility in water, which affects its antitumor activity in vivo. In this study, we first investigated the effects of tetrandrine citrate, a novel tetrandrine salt with high water solubility, on the growth of IM-resistant K562 cells, primary CML samples, and normal blood cell samples in vitro and in vivo, and then explored potential molecular mechanisms responsible for its anti-leukemia activity.
2. Materials and methods
2.1. Reagents and antibodies
Tetrandrine free base was purchased from China’s National Institute for the Control of Pharmaceutical and Biological Products (Beijing) and its structure is shown in Fig. 1. IM was generously provided by Dr. Ding, and was directly dissolved in water at a concentration of 10 mg/ml. c-abl and β-catenin antibodies were purchased from Santa Cruz Biotechnology (CA, USA). CD34 antibody-R conjugated to phycoerithrin was purchased from Invitrogen (Carlsbad, CA). β-actin antibody was from Sigma Chemical (St. Louis, MO, USA).
Fig. 1.
Chemical structure of tetrandrine
2.2. Generation of tetrandrine citrate with high water solubility
Because tetrandrine free base is a hydrophobic alkaloid with very low solubility in water, we generated a novel tetrandrine salt, tetrandrine citrate, with high water solubility. Tetrandrine citrate was generated by mixing tetrandrine free base and citrate acid in a 4:1 ratio, and dissolved in double distilled water. The solubility of tetrandrine citrate in water was determined to be up to 500 mg/ml.
2.3. Human CML cell lines and culture
Human CML cell lines, including IM-resistant K562 and K562 cell lines (CML), were obtained from the Cancer Institute of Zhejiang University. Leukemia cells were grown in RPMI-1640 (roswell park memorial institute) supplemented with 10% (v/v) fetal calf serum (FCS) at 37 °C in a 95% air, 5% CO2 humidified incubator.
2.4. Isolation and culture of primary leukemia cells and normal hematopoietic cells
Primary leukemia specimens were obtained from patients who had given informed consent. Normal blood cells were also obtained from peripheral blood of healthy volunteer donors. Mononuclear cells were isolated from the samples using Ficoll-Plaque density gradient separation (Sigma-Aldrich, St. Louis, MO, USA). Primary leukemia cells and normal blood cells were cultured in RPMI-1640 medium with 10% FCS at 37 °C in a 95% air, 5% CO2 humidified incubator.
2.5. Cell viability determination
Cells were cultured in the presence of various concentrations of tetrandrine citrate for 48 h, and cell viability was measured by methyl thiazolyl tetrazolium (MTT) (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay as previously described (Xu et al., 2006).
2.6. Methylcellulose colony-forming assay
We used methylcellulose colony assay to assess the survival of tetrandrine citrate-treated CML leukemic primitive stem/progenitor cells. Four primary CML specimens were treated with tetrandrine citrate (2.0 or 6.0 μg/ml; 14 d), and the leukemia colony-forming units (CFUs) were scored after 14 d of culture. Methylcellulose colony-forming assay with MethoCult GF H4434 was used to measure leukemia colony forming cells according to the manufacturer’s protocol (Stem Cell Technologies Inc.). Colonies were scored via microscopy after 14 d of culture.
2.7. Immunofluorescent staining of CD34+ leukemia cells
CD34+ cells in CML CFUs were identified by immunofluorescent staining. Briefly, CML CFUs in primary CML specimens were cultured in tetrandrine (2.0 μg/ml) using methylcellulose colony-forming assay for 14 d, and then stained with CD34 antibody-R conjugated to phycoerithrin. Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), and imaged using fluorescence microscopy with a Zeiss fluorescent microscope (Carl Zeiss, Jena, Germany).
2.8. Western blot analysis
Cellular protein was extracted using the Mammalian Protein Extraction Reagent (Thermo Scientific, IL, USA) for Western blotting as previously described (Xu et al., 2006).
2.9. IM-resistant K562 leukemia xenograft model and treatment
All animal procedures were approved by the Institution’s Ethics Committee of Zhejiang Chinese Medical University. Female nude mice (nu−/−; 6–7-week-old) were injected subcutaneously in the left or right flank with 2×107 log-phase IM-resistant K562 cells in a 0.2-ml suspension. When the tumors reached volumes of 50 to 100 mm, the mice were randomly assigned to two groups (treatment and control groups). In the treatment group, tetrandrine citrate or IM was administered orally at a dose of 100 mg/kg (in 0.4 ml) three times a day, at 8:00 AM, 2:00 PM, and 8:00 PM, for 10 consecutive days. The mice in the control group were given equal volumes of water at the same times and duration as the treatment group. Mouse weight and tumor volumes were measured every five days. Tumor weight was measured at the end of experiments (Day 35).
2.10. Flow cytometric analysis of cell cycle status
The cell cycle status was evaluated by flow cytometry (FCM). The percentages of cells in the gap 1 (G1), synthesis (S), and gap 2 (G2)–mitosis (M) phases were calculated using Multicycle software (Phoenix Flow Systems, San Diego, CA).
2.11. Assessment of Bcr-Abl mRNA levels
Briefly, total cellular RNA was extracted using Trizol reagent (Invitrogen, USA), and then 2 μg of RNA was reverse-transcribed with oligo(dT)15 by Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega, USA) at 37 °C for 60 min. Thermal cycle conditions for p210Bcr-Abl were as follows: 95 °C for 5 min and cycling for 30 cycles between 95 °C for 30 s and 60 °C for 30 s, 72 °C for 1 min 30 s, 72 °C for 5 min. The amplified product was 468 bp in size. β-actin complementary DNA (cDNA) was used as an internal control. Thermal cycle conditions: 95 °C for 2 min and cycling for 26 cycles between 95 °C for 20 s and 57 °C for 15 s, 72 °C for 1 min 45 s, 72 °C for 5 min. Polymerase chain reaction (PCR) products were electrophoresed through a 1.5% (0.015 g/ml) agarose gel and visualized using ethidium bromide staining. The sequences amplified from cDNAs were confirmed by sequencing with a PE-377 DNA sequencer (PE Applied Biosystems, Foster City, USA). The primers sequences were as follows: p210Bcr-Abl forward: 5′-ggcaagagttacacgttcctgatc-3′; reverse: 5′-gtgattatagcctaagacccggag-3′.
2.12. Statistical analysis
Results are expressed as means±standard deviation (SD). Differences were evaluated by t-test and P values less than 0.05 were considered statistically significant.
3. Results
3.1. Tetrandrine citrate preferentially inhibits growth of CML and IM-resistant K562 cells
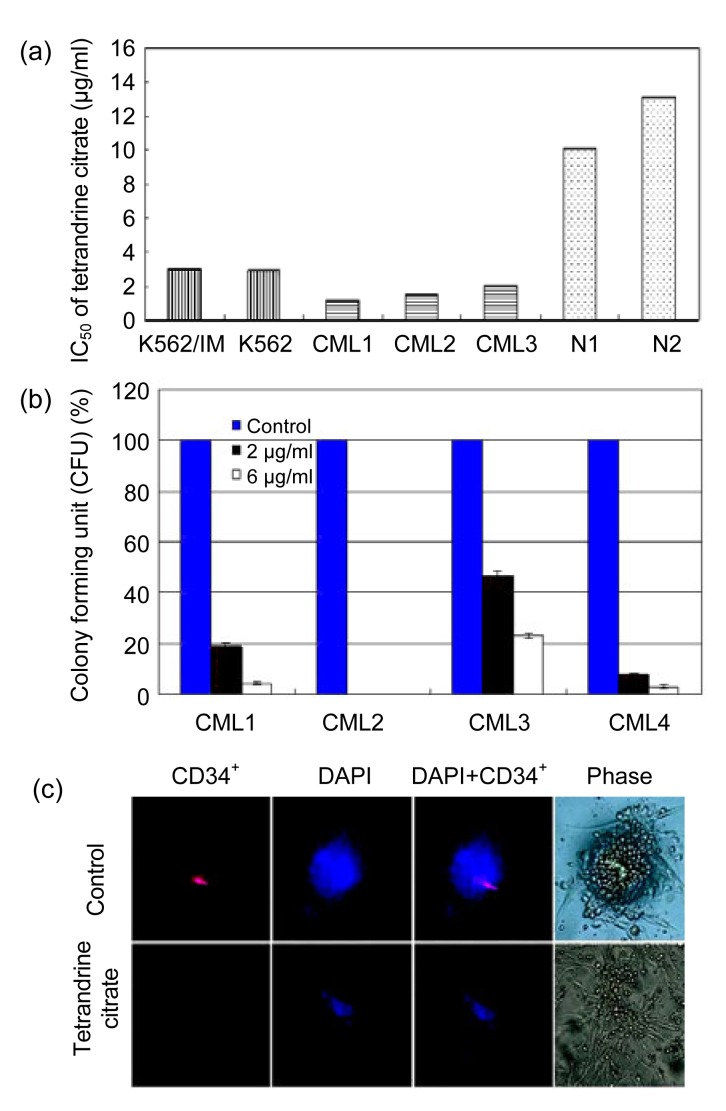
The inhibition concentration that inhibited 50% of target cells (IC50) was calculated from dose-response curves. Proliferation of both of K562, IM-resistant K562 CML cells was significantly inhibited by tetrandrine citrate (IC50 values were 2.94 and 2.97 μg/ml, respectively) (Fig. 2a) (P>0.05), indicating that tetrandrine citrate can override IM-resistance of CML cells. Similarly, primary leukemia cells from three CML patients also showed a strong cytotoxic response to tetrandrine citrate (IC50 values were 1.20, 1.48, and 2.00 μg/ml) (Fig. 2a). In contrast, normal hematopoietic cells from the peripheral blood of two healthy donors showed a less cytotoxic response to tetrandrine citrate (IC50 values were 10.12 and 13.11 μg/ml, respectively). Thus, tetrandrine citrate is much more cytotoxic to CML cells than to normal blood cells, suggesting that tetrandrine citrate preferentially inhibits the growth of CML cells.
Fig. 2.
Effects of tetrandrine citrate on the growth of CML cells and normal blood cells
(a) Cell viabilities of the indicated CML cell lines, primary CML cells, and normal blood cells (N) measured by MTT assay. Cells were incubated with tetrandrine citrate at a range of concentrations for 48 h; (b) Average percent of CFUs formed after primary CML cells were seeded at a low cell density and incubated with tetrandrine citrate (0, 2.0, or 6.0 μg/ml) for 14 d. The averages are normalized to untreated control (100%); (c) Untreated or tetrandrine citrate-treated (2.0 μg/ml, 14 d) primary CML cells immunofluorescently stained with anti-CD34+ antibodies (red) or stained with DAPI (blue)
3.2. Tetrandrine citrate treatment affects survival of primary CML leukemic stem/progenitor cells
The number of CML CFUs was reduced by tetrandrine citrate treatment (Fig. 2b). To further confirm ablation of the CML stem/progenitor cells, we investigated the effect of tetrandrine citrate on CML CD34+ CFUs by immunofluorescent staining. Tetrandrine citrate treatment resulted in a dramatic inhibition of the CML CFUs and loss of CD34+ cells (Fig. 2c). Together, these results demonstrate that tetrandrine citrate can induce death of both proliferating-leukemia cells and primitive leukemia stem/progenitor cells.
3.3. Tetrandrine citrate causes durable complete regression of IM-resistant CML xenografts in nude mice
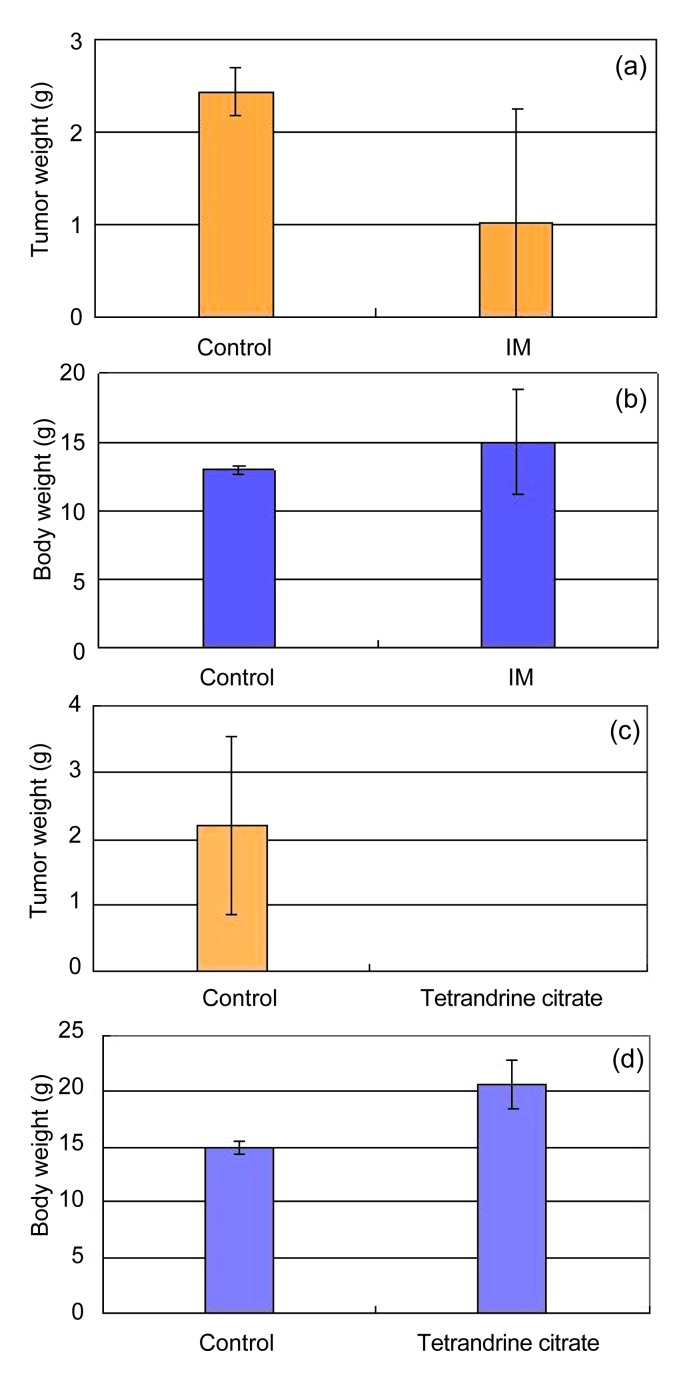
Tumor growth was inhibited by treatment with IM, but 4 of 7 tumors exhibited regrowth (Fig. 3a). Tumor weights in the control and IM-treated groups were (2.44±0.25) and (1.02±1.23) g, respectively, while body weights were (13.00±0.25) and (15.00±3.78) g, respectively (Fig. 3b). These results indicate that IM can partially inhibit the growth of the IM-resistant K562 xenografts, but cannot eradicate the tumor completely.
Fig. 3.
Comparison of in vivo anti-leukemia activities of tetrandrine citrate and IM against IM-resistant K562 xenografts in nude mice
Tetrandrine citrate or IM was administered at 100 mg/kg body weight orally three times daily (8:00 AM, 2:00 PM, and 8:00 PM) for 10 consecutive days. Effects of IM on the growth of IM-resistant K562 xenografts (a, b), and body weight of tumor-bearing mice; (c, d) Effects of tetrandrine citrate on the growth of IM-resistant K562 xenografts, and body weight of tumor-bearing mice
We then evaluated the in vivo activity of tetrandrine citrate as a single anti-leukemia agent for IM-resistant K562 xenografts in nude mice. All mice in the control group developed tumors within 15 d, with an average tumor weight of (2.20±0.77) g at 35 d (Fig. 3c). In contrast, no tetrandrine citrate-treated animals developed tumors within 15 d, and all were tumor free at the end of the experiment (Day 35) (Fig. 3c). Tetrandrine citrate toxicity was limited. The weights of tumor-bearing mice treated by tetrandrine citrate increased, but decreased in untreated mice with the development of tumors (Fig. 3d). No gross abnormalities were observed in the tetrandrine citrate treated group at the end of experiment.
3.4. Tetrandrine citrate depletes Bcr-Abl protein in IM-resistant K562 leukemia cells
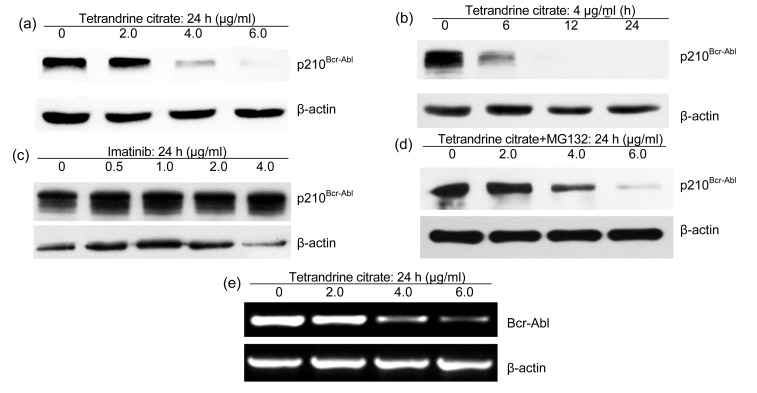
We next determined whether tetrandrine citrate can affect expression of p210Bcr-Abl protein in IM-resistant K562 leukemia cells using Western blotting. IM-resistant K562 cells were treated with tetrandrine citrate or IM at various concentrations for indicated time, and total cellular proteins were extracted for Western blotting analysis of p210Bcr-Abl protein. Experimental results showed that tetrandrine citrate treatment markedly decreased p210Bcr-Abl protein levels of IM-resistant K562 cells in a dose- and time-dependent manner (Figs. 4a and 4b), but IM treatment did not affect the Bcr-Abl total protein levels in IM-resistant leukemia cells (Fig. 4c).
Fig. 4.
Effects of tetrandrine citrate and IM on expression of p210Bcr-Abl protein in IM-resistant K562 leukemia cells
(a, b) Tetrandrine citrate decreased Bcr-Abl protein levels of IM-resistant K562 leukemia cells in dose- and time-dependent manners. The cells were treated with tetrandrine citrate at the indicated concentrations for the indicated time, followed by Western blot analysis for Bcr-Abl; (c) IM did not affect Bcr-Abl protein in IM-resistant K562 cells. The cells were treated with IM (0–4.0 μg/ml) for 24 h, followed by Western blot analysis for Bcr-Abl. β-actin was used as a loading control; (d) Proteasome inhibitor MG132 did not prevent degradation of p210Bcr-Abl protein induced by tetrandrine; (e) Bcr/abl fusion transcripts were reduced by tetrandrine with dose-dependence. Leukemia cells were treated with tetrandrine, and total cellular RNAs were extracted with Trizol. Bcr-Abl fusion transcripts were analyzed with RT-PCR
To determine whether depletion of p210Bcr-Abl protein by tetrandrine is involved in proteasome-mediated degradation, we used the proteasome inhibitor, MG132, to inhibit the degradation of p210Bcr-Abl protein in tetrandrine-treated cells. We found that MG132 did not significantly prevent p210Bcr-Abl protein degradation (Fig. 4d), suggesting that tetrandrine-mediated p210Bcr-Abl protein reduction may be due to transcription inhibition of Bcr-Abl fusion gene.
To validate this hypothesis, we determined Bcr-Abl transcripts of leukemia cells after exposure to tetrandrine at different concentrations for 24 h using reverse transcription PCR (RT-PCR). As expected, Bcr-Abl transcript levels of leukemia cells were markedly reduced by tetrandrine with dose dependence (Fig. 4e).
3.5. Tetrandrine citrate down-regulates β-catenin protein level of IM-resistant K562 leukemia cells
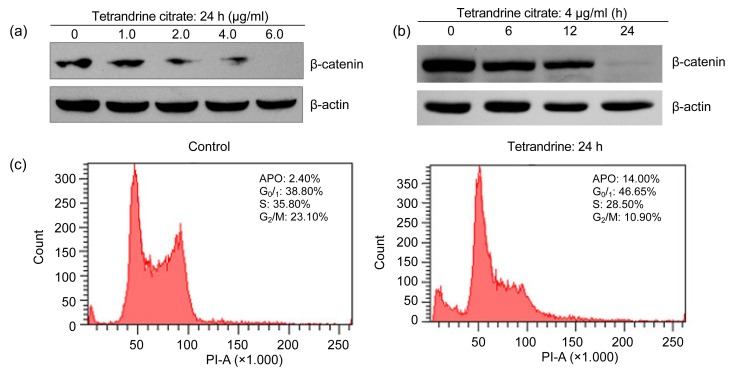
β-catenin in the Wnt/β-catenin signaling pathway, which is stabilized by the Bcr-Abl protein level (Coluccia et al., 2007), has been linked to the survival and self-renewal of IM-resistant LSCs (Hu et al., 2009). Therefore, we assessed the effect of tetrandrine citrate on the β-catenin protein levels of IM-resistant K562 cells. IM-resistant K562 cells were treated with tetrandrine citrate at various concentrations for indicated time, and total cellular proteins were extracted for Western blotting analysis of β-catenin protein. The Western blot results indicated that tetrandrine citrate treatment caused a decrease of β-catenin protein levels of leukemia cells in dose- and time-dependent manners (Figs. 5a and 5b). These results indicate that tetrandrine citrate can also reduce β-catenin protein levels in leukemia cells. These data suggest that anti-leukemia mechanism of tetrandrine citrate is distinct to that of tyrosine kinase inhibitors (Guzman et al., 2005; Hu et al., 2009).
Fig. 5.
Effect of tetrandrine citrate on β-catenin protein expression and cell cycles of IM-resistant K562 cells
The cells were treated with tetrandrine citrate at the indicated concentrations (0, 1.0, 2.0, 4.0, and 6.0 μg/ml) (a) and times (0, 6, 12, and 24 h) (b), followed by Western blot analysis for β-catenin protein, and β-actin was used as a loading control; (c) Tetrandrine caused G1 arrest in IM-resistant K562 leukemia cells. APO: apoptotic cells
3.6. Tetrandrine citrate induces G1 arrest in CML cells
To determine the phase of the cell cycle at which tetrandrine exerts its growth inhibition effect, exponentially growing IM-resistant K562 leukemia cells were treated with tetrandrine at 4 μg/ml for 24 h and analyzed by FCM (Fig. 5c). FCM analysis showed that G1 phase cells increased from 38.80% to 46.65%, whereas S phase cells decreased from 35.80% to 28.50%. In addition, we also observed an increase of apoptotic cells from 2.40% to 14.40% (Fig. 5c). These results indicate that tetrandrine inhibits the growth of CML cells by causing G1 phase arrest, apoptosis and decreasing S phase cells.
4. Discussion
It has been shown that the inhibition of Bcr-Abl kinase activity by tyrosine kinase inhibitors, which is the standard treatment for Ph+ CML, is insufficient to cure this disease completely due to the presence of LSCs insensitive to kinase inhibition (Li and Li, 2007; Konig et al., 2008; Hu et al., 2009). Thus, it is necessary for curative treatment of CML to identify agents that specifically target the kinase activity-independent pathways. Since Bcr-Abl oncogene is a master causative factor in leukemogenesis, and Bcr-Abl protein plays a crucial role in stabilizing the β-catenin in Wnt/β-catenin signaling pathway essential for survival of LSCs (Hu et al., 2009), we propose that Bcr-Abl/β-catenin axis is a valid target for developing small molecules superior to TKIs, and disrupting Bcr-Abl/β-catenin axis and therefore represents a potentially useful treatment strategy for CML.
In this study, we demonstrated that tetrandrine citrate inhibited the growth of IM-resistant K562 cells, primary leukemia cells and primitive CD34+ leukemia stem/progenitor cells, and other leukemia cell lines but did not affect the growth of normal blood cells under the same conditions. Animal results showed that oral administration of tetrandrine citrate caused complete regression of IM-resistant K562 xenografts in nude mice without overt toxicity. Molecular mechanism studies revealed that treatment of IM-resistant K562 cells with tetrandrine citrate resulted in depletion of both p210Bcr-Abl and β-catenin proteins, whereas, IM treatment did not affect Bcr-Abl total protein levels in IM-resistant leukemia cells. These observations indicate that tetrandrine citrate is a novel orally active tetrandrine salt that can eradicate IM-resistant Ph+ CML cells in vitro and in vivo by depleting Bcr-Abl and β-catenin proteins and disrupting the Bcr-Abl/β-catenin axis. At least two advantages of this strategy are noted: (1) depletion of Bcr-Abl protein may offer the potential to override resistance to TKIs caused by Bcr-Abl kinase mutations, which is supported by a recent study (Chen et al., 2009b); (2) depletion of Bcr-Abl and β-catenin proteins results in the disruption of the Bcr-Abl/β-catenin axis that is essential for LSC survival and self-renewal, and thus provides a better therapeutic approach to CML. Indeed, our results indicate that this compound can effectively ablate the primitive LSCs (CD34+ leukemia cells), which are exceptionally refractory to be killed by the TKIs or conventional chemotherapeutic agents (Holyoake et al., 1999; Elrick et al., 2005; Holtz et al., 2005; Barnes and Melo, 2006). Additionally, we found that tetrandrine induced G1 arrest in CML cells, which is consistent with Meng’s observations that tetrandrine causes G1 arrest in colon cancer cells (Meng et al., 2004).
Tetrandrine is a natural bisbenzylisoquinoline and has been shown to be active against a variety of tumors, such as colon cancer (Meng et al., 2004), glioma (Chen and Tseng, 2010), ovarian cancer (Zhang et al., 2011), and hepatocellular carcinoma (Liu et al., 2011). Notably, tetrandrine inhibits Wnt/β-catenin signaling and suppresses tumor growth of human colorectal cancer (He et al., 2011). In this study, we provide evidence to show that tetrandrine citrate is a novel orally active tetrandrine salt that has potent anti-tumor activity against both CML and IM-resistant CML cells. Tetrandrine citrate-induced growth inhibition of leukemia cells might be involved in the depletion of p210Bcr-Abl and β-catenin proteins that are essential for survival and self-renewal of LSCs. These findings suggest that tetrandrine citrate may be a useful agent for treating IM-resistant CML.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30672381, 30873095, and 81070420), and the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents, and the Zhejiang Provincial Natural Science Foundation of China (Nos. Y206238, Y2080570, and Y2080210)
References
- 1.Barnes DJ, Melo JV. Primitive, quiescent and difficult to kill: the role of non-proliferating stem cells in chronic myeloid leukemia. Cell Cycle. 2006;5(24):2862–2866. doi: 10.4161/cc.5.24.3573. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Tseng SH. The potential of tetrandrine against gliomas. Anticancer Agents Med Chem. 2010;10(7):534–542. doi: 10.2174/187152010793498609. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Hu Y, Zhang H, Peng C, Li S. Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nat Genet. 2009;41(7):783–792. doi: 10.1038/ng.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Hu Y, Michaels S, Segal D, Brown D, Li S. Inhibitory effects of omacetaxine on leukemic stem cells and BCR-ABL-induced chronic myeloid leukemia and acute lymphoblastic leukemia in mice. Leukemia. 2009;23(8):1446–1454. doi: 10.1038/leu.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coluccia AM, Vacca A, Duñach M, Mologni L, Redaelli S, Bustos VH, Benati D, Pinna LA, Gambacorti-Passerini C. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26(5):1456–1466. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, Manley P, Trussell C, Schmitt-Graeff A, Landwerlin K, Veelken H, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14(3):238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Elrick LJ, Jorgensen HG, Mountford JC, Holyoake TL. Punish the parent not the progeny. Blood. 2005;105(5):1862–1866. doi: 10.1182/blood-2004-08-3373. [DOI] [PubMed] [Google Scholar]
- 8.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105(11):4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim SH, Huang E, Gao Y, Yang K, Wagner ER, et al. Tetrandrine inhibits Wnt/β-catenin signaling and suppresses tumor growth of human colorectal cancer. Mol Pharmacol. 2011;79(2):211–219. doi: 10.1124/mol.110.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtz MS, Forman SJ, Bhatia R. Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia. 2005;19(6):1034–1041. doi: 10.1038/sj.leu.2403724. [DOI] [PubMed] [Google Scholar]
- 11.Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94(6):2056–2064. [PubMed] [Google Scholar]
- 12.Hu Y, Chen Y, Douglas L, Li S. β-Catenin is essential for survival of leukemia stem cells in sensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemia. Leukemia. 2009;23(1):109–116. doi: 10.1038/leu.2008.262. [DOI] [PubMed] [Google Scholar]
- 13.Konig H, Holtz M, Modi H, Manley P, Holyoake TL, Forman SJ, Bhatia R. Enhanced BCR-ABL kinase inhibition does not result in increased inhibition of downstream signaling pathways or increased growth suppression in CML progenitors. Leukemia. 2008;22(4):748–755. doi: 10.1038/sj.leu.2405086. [DOI] [PubMed] [Google Scholar]
- 14.Krause DS, Lazarides K, von Andrian UH, van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12(10):1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 15.Lai JH. Immunomodulatory effects and mechanisms of plant alkaloid tetrandrine in autoimmune diseases. Acta Pharmacol Sin. 2002;23(12):1093–1101. [PubMed] [Google Scholar]
- 16.Lee JH, Kang GH, Kim KC, Kim KM, Park DI, Choi BT, Kang HS, Lee YT, Choi YH. Tetrandrine-induced cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Int J Oncol. 2002;21(6):1239–1244. [PubMed] [Google Scholar]
- 17.Li S, Li D. Stem cell and kinase activity-independent pathway in resistance of leukaemia to BCR-ABL kinase inhibitors. J Cell Mol Med. 2007;11(6):1251–1262. doi: 10.1111/j.1582-4934.2007.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Gong K, Mao X, Li W. Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma. Int J Cancer. 2011;129(6):1519–1531. doi: 10.1002/ijc.25817. [DOI] [PubMed] [Google Scholar]
- 19.McCubrey JA, Steelman LS, Abrams SL, Bertrand FE, Ludwig DE, Bäsecke J, Libra M, Stivala F, Milella M, Tafuri A, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008;22(4):708–722. doi: 10.1038/leu.2008.27. [DOI] [PubMed] [Google Scholar]
- 20.Meng LH, Zhang H, Hayward L, Takemura H, Shao RG, Pommier Y. Tetrandrine induces early G1 arrest in human colon carcinoma cells by down-regulating the activity and inducing the degradation of G1-S-specific cyclin-dependent kinases and by inducing p53 and p21Cip1. Cancer Res. 2004;64(24):9086–9092. doi: 10.1158/0008-5472.CAN-04-0313. [DOI] [PubMed] [Google Scholar]
- 21.Ng LT, Chiang LC, Lin YT, Lin CC. Antiproliferative and apoptotic effects of tetrandrine on different human hepatoma cell lines. Am J Chin Med. 2006;34(1):125–135. doi: 10.1142/s0192415x06003692. [DOI] [PubMed] [Google Scholar]
- 22.Stuart SA, Minami Y, Wang JY. The CML stem cell: evolution of the progenitor. Cell Cycle. 2009;8(9):1338–1343. doi: 10.4161/cc.8.9.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang FP, Wang L, Yang JS, Nomura M, Miyamoto K. Reversal of P-glycoprotein-dependent resistance to vinblastine by newly synthesized bisbenzylisoquinoline alkaloids in mouse leukemia P388 cells. Biol Pharm Bull. 2005;28(10):1979–1982. doi: 10.1248/bpb.28.1979. [DOI] [PubMed] [Google Scholar]
- 24.Xu R, Dong Q, Yu Y, Zhao X, Gan X, Wu D, Lu Q, Xu X, Yu XF. Berbamine: a novel inhibitor of bcr/abl fusion gene with potent anti-leukemia activity. Leuk Res. 2006;30(1):17–23. doi: 10.1016/j.leukres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Wang C, Wang H, Wang K, Du Y, Zhang J. Combination of Tetrandrine with cisplatin enhances cytotoxicity through growth suppression and apoptosis in ovarian cancer in vitro and in vivo. Cancer Lett. 2011;304(1):21–32. doi: 10.1016/j.canlet.2011.01.022. [DOI] [PubMed] [Google Scholar]