Abstract
Background and objective: ST13, is the gene encoding the HSP70 interacting protein (HIP). Previous research has shown that ST13 mRNA and protein levels are down-regulated in colorectal cancer (CRC) tissues compared with adjacent normal tissues. This study aims at the role of ST13 in the proliferation and migration of CRC cells. Methods: The transcript level of ST13 in different CRC cell lines was evaluated by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). ST13-overexpressed and ST13-knockdown CRC cells were constructed respectively by lentiviral transduction, followed by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay, plate colony formation, cell-cycle analysis, and migration assays to evaluate the influence of ST13 on proliferation and migration in vitro. Moreover, a mouse xenograft study was performed to test in vivo tumorigenicity of ST13-knockdown CRC cells. Results: Lentivirus-mediated overexpression of ST13 in CRC cells inhibited cell proliferation, colony formation, and cell migration in vitro. In contrast, down-regulation of ST13 by lentiviral-based short hairpin RNA (shRNA) interference in CRC cells significantly increased cell proliferation and cloning efficiency in vitro. In addition, down-regulation of ST13 expression significantly increased the tumorigenicity of CRC cells in vivo. Conclusions: ST13 gene is a proliferation regulator that inhibits tumor growth in CRC and may affect cell migration.
Keywords: Colorectal cancer, ST13, Proliferation, Colony formation, Cell cycle, Migration
1. Introduction
Heat-shock proteins (HSPs) are a highly conserved molecular chaperone family, some of which are induced by sublethal cellular stresses including temperature elevation, oxidative damage, and hypoxia (Young et al., 2004; Mahalingam et al., 2009). In addition, they are known to facilitate folding of nascent polypeptides, induce solubilisation of loose protein aggregates, afford protection against protein aggregation, participate in refolding of proteins which have been damaged, and sequester damaged proteins and target them for degradation (Hartl, 1996; Csermely, 1997; Söti and Csermely, 2002; Soo et al., 2008). HSPs are generally classified by their molecular sizes, e.g., HSP100, HSP90, HSP70, HSP60, HSP40, and small HSPs (sHSPs) (Powers and Workman, 2007). They have been found to be overexpressed in many human cancers, including both solid tumours and haematological malignancies (Ciocca et al., 1993; Kimura et al., 1993; Chant et al., 1995; Ralhan and Kaur, 1995; Takayama et al., 2003; Whitesell and Lindquist, 2005; Neckers, 2007) and play important roles in tumor initiation and progression, such as rendering cancer cells refractory to anti-proliferative signals (Hollstein et al., 1991; Lane et al., 1993) and avoiding apoptosis (Lanneau et al., 2008). HSPs are potentially ideal therapeutic targets in cancer treatment.
ST13 is the gene encoding the HSP70 interacting protein (HIP), a co-chaperone of the 70-kDa HSPs (HSC/HSP70) (Höhfeld et al., 1995; Shi et al., 2007). We originally identified the ST13 gene by subtraction hybridization with normal mucosal tissue and colorectal cancers (Zheng et al., 1997). The ST13 gene, which is located on chromosome 22q13 (Zhang et al., 1998), has shown frequent loss of heterozygosity in colorectal, ovarian, and breast cancers. However, the precise location of ST13 (22q13.2) is apparently outside the minimal region of deletion that is common for both colorectal and breast cancers according to a detailed human chromosome map, the Human Genome Project (Castells et al., 2000). ST13 protein (HIP) is composed of an N-terminal region, a central tetratricopeptide repeat (TPR) domain followed by a highly charged region, and a C-terminal region containing glycine-glycine-methionine-proline (GGMP) repeats and a Sti1 motif (Prapapanich et al., 1996a; 1998; Irmer and Höhfeld, 1997). HIP may facilitate the chaperone function of HSC/HSP70 in controlling the activities of regulatory proteins such as steroid receptors and regulators of proliferation or apoptosis, and in protein folding and repair (Prapapanich et al., 1996a; 1996b; Höhfeld and Jentsch, 1997; Irmer and Höhfeld, 1997; Shi et al., 2007).
In previous studies, we have proved that ST13 mRNA and protein levels were lower in colorectal cancer tissues compared with adjacent normal tissues (Mo et al., 1996; Zheng et al., 1997; Dong et al., 2005; Wang et al., 2005). Moreover, increased ST13 protein expression suppressed proliferation of colorectal cancer cells and induced apoptosis in colorectal cancer cell lines (Yang et al., 2008; Yu et al., 2009). In the present study, we employed a lentiviral system to overexpress or knock down ST13 gene in colorectal cancer cells and examined the roles of ST13 in colorectal cancer cell growth both in vitro and in vivo. We also tested functions of ST13 in migration.
2. Materials and methods
2.1. Cell lines and cell culture
Human colorectal cancer cell lines (RKO, HT29, SW480, SW620, LOVO, LS174T, and HEK293) were purchased from the American Type Culture Collection (Manassas, VA) and cultured under recommended conditions.
2.2. Isolation of RNA and real-time reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
Total RNA from cell line was extracted from subconfluent cells in the exponential phase of growth using Trizol reagent (Invitrogen, Carlsbad, CA), according to the manufacture’s instructions. Real-time PCR primers were ST13 (5′-CGGAGAAAGTATGAGCGAAAA-3′ and 5′-AAGCCACCTGGAAAAGAGCC-3′) and β-actin (5′-TTCCAGCCTTCCTTCCTGGG-3′ and 5′-TTGCGCTCAGGAGGAGCAAT-3′). Transcript level of ST13 was determined by real-time PCR using the Applied Biosystems StepOne Real-Time PCR system (Applied Biosystems, Carlsbad, CA). The real-time PCR was carried out in a total volume of 20 μl per well containing SYBR® master mix reagent kit (Applied Biosystems, Carlsbad, CA) in triplicate. Human β-actin was amplified as an endogenous control. The relative gene expression levels were calculated using the comparative threshold cycle C t (ΔΔC t) method (according to Applied Biosystems), where the relative expression is calculated as 2−ΔΔCt.
2.3. Establishment of stable ST13 knockdown SW620 cell clones
Small hairpin RNA (shRNA) lentiviral particles used for ST13 knockdown (sc-40684-v) and Mock knockdown (sc-108080) were purchased from Santa Cruz, CA, USA. SW620 cells were infected with shRNA over 48 h in the presence of polybrene (4 μg/ml) in a 6-well plate as described previously (Shi et al., 2012). The shRNA-Mock group was transfected by control shRNA. Stable colonies were selected and isolated in the presence of puromycin at the concentration of 3 μg/ml and evaluated for ST13 mRNA expression by quantitative RT-PCR (qRT-PCR).
2.4. Lentiviral vector construction, virus production and transduction
pcDNA3.1-ST13 was constructed in previous experiments by Prof. Shu ZHENG’s group (Yu et al., 2009). All constructs were made by standard DNA recombination techniques. Briefly, ST13 insert was isolated by PCR amplification from pcDNA3.1-ST13 with two pairs of restriction primers. PCR products were sequenced (ABI Prism 3100 DNA Sequencer, Applied Biosystems, Foster City, CA) and confirmed to contain the entire ST13 coding sequence. The insert was then cloned into the pLenti6.3-MCS-IRES2-EGFP plasmid (Invitrogen), which was co-transfected with Packaging plasmids (Invitrogen) into HEK293T cells. The viral supernatant was harvested, filtered, and concentrated by centrifugation. Lenti-Mock constructs were constructed similarly, only without the ST13 insert. The viral concentrate was diluted in polybrene to infect SW620 cells. A successful transduction was confirmed by visualizing enhanced green fluorescent protein (EGFP; included in the pLenti6.3-IRES2-EGFP vector) and sustained ST13 expression was confirmed at least every two weeks by qRT-PCR. Virus-infected cells were selected with 8 μg/ml blasticidin (Invitrogen). The antibiotic-resistant clones were pooled and used for subsequent assay.
2.5. Cell proliferation assay
Cells were cultured at a density of 5×103 cells/well in triplicate in 96-well plates with 10% fetal bovine serum (FBS) L-15 at 37 °C and 5% CO2 for varying periods and exposed to fresh media every other day. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay was performed every day for up to 5 d. Briefly, 20 μl of 5 mg/ml MTT (Sigma, St. Louis, MO, USA) was added to each well; plates were incubated at 37 °C for 4 h. The generated formazan was dissolved in 150 μl dimethyl sulfoxide (DMSO) and measured with a microplate reader (BioRad, Hercules, CA) at optical density at 570 nm (OD570) for determining the cell viability.
2.6. Plate colony formation assay
Cell colony formation rate was measured by plate colony formation assay. About 200 cells were added to each well of a 6-well plate. Plates were incubated at 37 °C for 14 d, and then were gently washed and stained with crystal violet. Viable colonies containing at least 50 cells were counted.
2.7. Cell cycling analysis
Cells were washed with ice-cold phosphate buffered saline (PBS) twice and fixed with 70% ethanol overnight at 4 °C. The cells were digested with 50 μg/ml RNase A in 100 μl of PBS for 30 min at room temperature and stained with 20 μg/ml propidium iodide (PI; 300 μl) for 20 min. The cell cycling was analyzed on an FACScanner (Coulter Biosciences). All assays were carried out in triplicate (Li et al., 2009).
2.8. Mouse xenograft study
Our animal protocol was approved and performed strictly in accordance with the related ethics regulations of Zhejiang Chinese Medical University. SW620 Mock-knockdown cells (shRNA-Mock) and SW620 ST13-knockdown cells (shRNA-ST13) were cultured until 80%‒90% confluence before harvesting. Cells were trypsinized, washed with PBS twice, and resuspended in serum-free L-15 (Leibovitz’s) medium to a concentration of 5×106 cells per 200 μl. Then, 200 μl cells were injected subcutaneously into the dorsal flanks of 5-week-old female nude mice. Tumor sizes were measured in two dimensions with calipers twice a week and tumor volumes (V, mm3) were calculated as V=L×W 2/2, where L is tumor length and W is tumor width.
2.9. Protein extraction and Western blotting
Cells were harvested in the exponential phase of growth and whole-cell lysates were prepared using the Mammalian Protein Extraction Reagent (Merck, Germany) in accordance with the manufacturer’s instructions. Protein concentrations of samples were determined by the bicinchoninic acid (BCA) protein assay (Pierce, USA). Protein samples (40 μg of each protein) were boiled for 5 min, and Western blot analysis was performed as described previously (Ge et al., 2006). Three independent Western blot assays were performed for all samples. The primary antibodies used were polyclonal antibodies against ST13 (Cell Signaling Technology, USA) and β-actin (Sigma-Aldrich, USA) (Ye et al., 2011).
2.10. Migration assays
Unless specified otherwise, cells (1×105 cells/well) were suspended in 150 μl FBS-free L-15 and dispensed into the upper chambers of transwells (8 μm pore size), with 800 μl of 10% FBS-containing L-15 in the lower chamber. After 48 h, cells on the upper surface were removed with a cotton swab, and cells migrating to the lower membrane surface were fixed with 90% alcohol, stained with crystal violet, and examined under a microscope. A total of six random high-power microscopic fields (400×) per filter were photographed and the numbers of cells were directly counted.
2.11. Statistical analysis
For continuous variables, data were expressed as mean±standard error of the mean (SEM). The difference among groups was determined by analysis of variance (ANOVA) analysis and comparison between two groups was analyzed by the Student’s t-test using the GraphPad Prism software version 4.0 (GraphPad Software Inc., San Diego, CA).
3. Results
3.1. ST13 expression in colorectal cancer cell lines
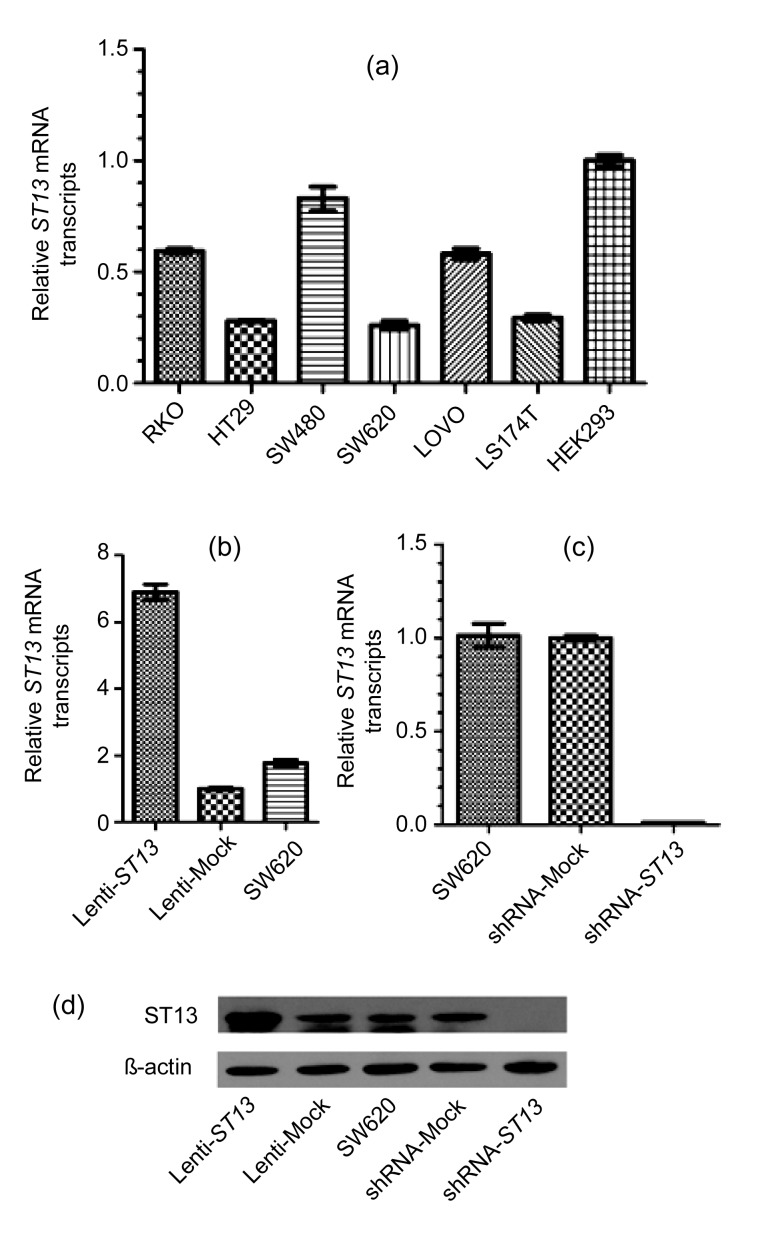
The expression of ST13 mRNA was quantified by qRT-PCR in six human colorectal cancer cells and human embryonic kidney cells (HEK293 cells), and as Fig. 1a indicates, utilizing ΔΔC t method, ST13 expression in mRNA levels was lower in SW620 cells as compared to other five human colorectal cancer cells (RKO, HT29, SW480, LOVO, and LS174T). SW620 easily forms tumors in xenograft mouse. Therefore, SW620 cells were chosen to do a series of function experiments.
Fig. 1.
ST13 expression in colorectal cancer cell lines
(a) qRT-PCR shows relative transcript levels of ST13 in six colorectal cancer cell lines, including RKO, HT29, SW480, SW620, LOVO, and LS174T, and HEK293. The relative quantity was normalized to HEK293 cells, and β-actin was used as an internal control. (b) qRT-PCR shows relative transcript levels of ST13 in ST13-lentivirus-infected Lenti-ST13, control lentivirus-infected Lenti-Mock, and SW620. The relative quantity was normalized to Lenti-Mock cells, and β-actin was used as an internal control. (c) qRT-PCR shows relative transcript levels of ST13 in SW620, control-shRNA-lentivirus-infected shRNA-Mock, and ST13-shRNA-lentivirus-infected shRNA-ST13. The relative quantity was normalized to shRNA-Mock cells, and β-actin was used as an internal control. (d) Western blot results show that ST13 was expressed in SW620 cells with different groups, including Lenti-ST13, Lenti-Mock, SW620, shRNA-Mock, and shRNA-ST13. β-actin was served as loading control. Error bars indicate SEM (n=3 experiments)
As shown in Figs. 1b and 1c, ST13 mRNA expression in the Lenti-ST13 group remarkably increased compared to that in the Lenti-Mock group and wild-type SW620 group, while ST13 mRNA expression in the shRNA-ST13 group remarkably decreased by about 90% compared to that in the shRNA-Mock group and wild-type SW620 group. Furthermore, we performed Western blot to verify the level of ST13 protein in the Lenti-ST13, Lenti-Mock, SW620, shRNA-ST13, and shRNA-Mock groups. The levels of ST13 protein in the Lenti-Mock, wild-type SW620, and shRNA-Mock groups were almost identical, while the level in the Lenti-ST13 group was much higher and the level in the shRNA-ST13 group was almost none (Fig. 1d). These Western blot results were consistent with the results of qRT-PCR.
3.2. Effects of ST13 expression on proliferation ability of SW620 cells in vitro
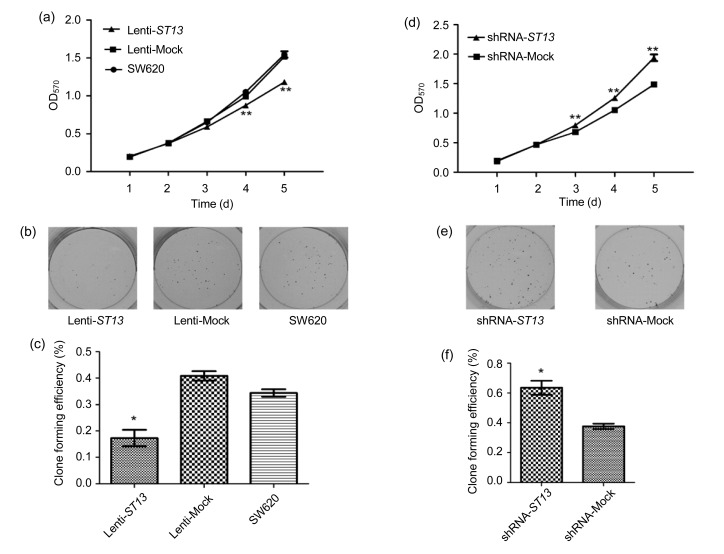
As seen in Fig. 2a, overexpression of ST13 caused a dramatic reduction in the proliferation of the Lenti-ST13 group when compared with that in the Lenti-Mock or SW620 group, especially from the 4th to 5th day after MTT detection (P<0.01, one way ANOVA). As expected, the Lenti-Mock group had the similar growth ability as the SW620 group.
Fig. 2.
Effect of ST13 expression on SW620 cell growth in vitro
(a) Effect of ST13 overexpression on SW620 cell proliferation determined by MTT assay. Error bars indicate SEM (n=3 experiments). ** P<0.01, Lenti-ST13 vs. Lenti-Mock and SW620 (one way ANOVA and Dunnett’s test). (b) Colony formations of Lenti-ST13, Lenti-Mock, and SW620 cells in vitro. (c) Quantitative analyses of colony formations of Lenti-ST13, Lenti-Mock, and SW620 groups. Error bars indicate SEM (n=3 experiments). * P<0.05, Lenti-ST13 vs. Lenti-Mock and SW620 (one way ANOVA and Dunnett’s test). (d) Effect of ST13 knockdown on SW620 cell proliferation determined by MTT assay. Error bars indicate SEM (n=3 experiments). ** P<0.01, shRNA-ST13 vs. shRNA-Mock (Student’s t-test). (e) Colony formations of shRNA-ST13 and shRNA-Mock cells. (f) Quantitative analyses of colony formations of shRNA-ST13 and shRNA-Mock groups. Error bars indicate SEM (n=3 experiments). * P<0.05 shRNA-ST13 vs. shRNA-Mock (Student’s t-test)
Moreover, a significant decrease was observed in the colony formation of the Lenti-ST13 group when compared to the Lenti-Mock or SW620 group (P<0.05, one way ANOVA) (Figs. 2b and 2c).
Knockdown of ST13 remarkably increased the proliferation ability of the shRNA-ST13 group when compared with that of the shRNA-Mock group, especially from the 3rd to 5th day after MTT detection (P<0.01, Student’s t-test) (Fig. 2d). Moreover, a significant increase was observed in the colony formation of the shRNA-ST13 group when compared to the shRNA-Mock group (P<0.05, Student’s t-test) (Figs. 2e and 2f).
3.3. Effects of ST13 expression on the distribution of cell cycle in SW620 cells
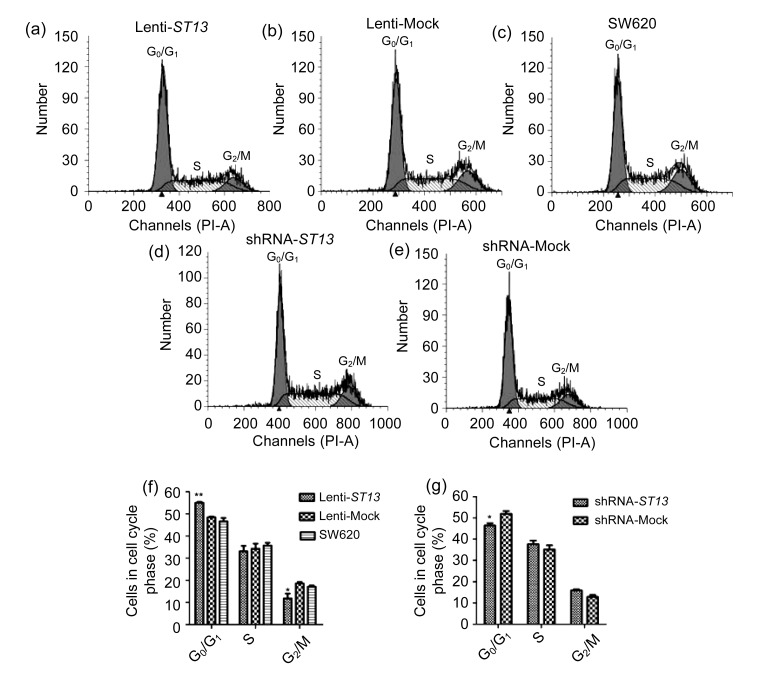
To explore the potential mechanism(s) underlying the action of ST13 in the growth of SW620 cells, cell cyclings of the Lenti-ST13, Lenti-Mock, SW620, shRNA-ST13 and shRNA-Mock groups were characterized by fluorescence-activated cell sorting (FACS) analysis.
As shown in Figs. 3a–3c and 3f, there was no significant difference between the Lenti-Mock and SW620 (P>0.05, one way ANOVA). However, the frequency of the Lenti-ST13 group cells at the G0/G1 phase was significantly higher compared with that in the controls (P<0.01, one way ANOVA) while that in G2/M stage remarkably decreased as compared to the controls (P<0.05, one way ANOVA). The data indicate that up-regulation of ST13 expression arrested Lenti-ST13 cell cycling at the G0/G1 phase, which might inhibit the growth of SW620 cells.
Fig. 3.
Effect of ST13 expression on SW620 cell cycling
(a–e) Cell cycle progressions of Lenti-ST13, Lenti-Mock, SW620 cells, shRNA-ST13, and shRNA-Mock, respectively. (f) The relative frequency of different phases of Lenti-ST13, Lenti-Mock, and SW620 cells. Error bars indicate SEM (n=3 experiments). * P<0.05, Lenti-ST13 vs. Lenti-Mock and SW620; ** P<0.01, Lenti-ST13 vs. Lenti-Mock and SW620 (one way ANOVA and Dunnett’s test). (g) The relative frequency of different phases of shRNA-ST13 and shRNA-Mock cells. Error bars indicate SEM (n=3 experiments). * P<0.05, shRNA-ST13 vs. shRNA-Mock (Student’s t-test)
In Figs. 3d–3e and 3g, the frequency of the shRNA-ST13 group cells at the G0/G1 phase was significantly lower compared with that in the shRNA-Mock group (P<0.05, Student’s t-test). The frequency of the shRNA-ST13 group cells at the S and G2/M phases increased compared to that of the shRNA-Mock group, although the difference was not statistically significant. These data indicate that down-regulation of ST13 expression promoted shRNA-ST13 cell cycling, which might promote the growth of SW620 cells in vitro.
3.4. Effect of ST13 expression on SW620 cell migration in vitro
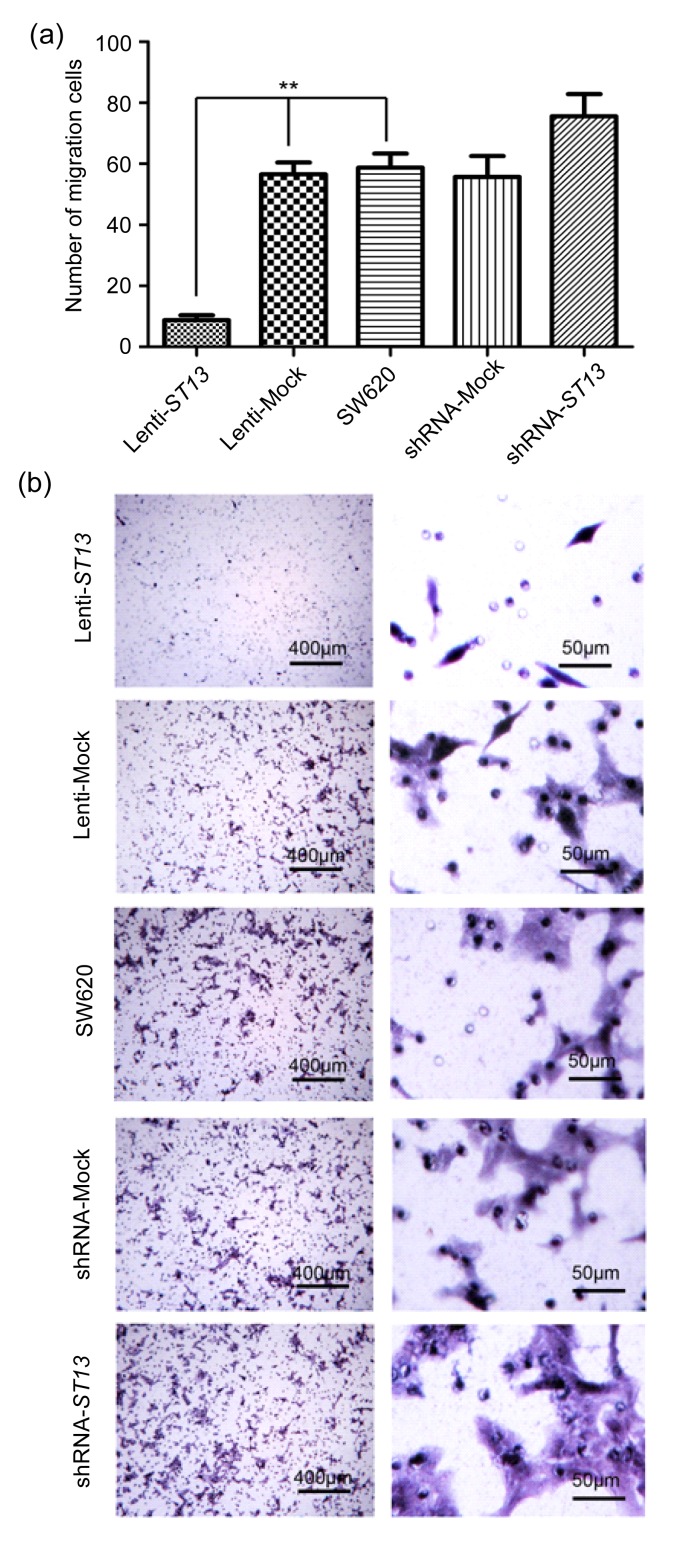
We examined the impact of ST13 expression on the migration of SW620 cells by transwell migration assay (Fig. 4). While an average of 56.5±3.9 Lenti-Mock and 58.7±4.7 SW620 cells per high power field had migrated onto the filter surface, only 8.7±1.6 Lenti-ST13 cells reached the filter (P<0.01, one way ANOVA).
Fig. 4.
Effect of ST13 expression on SW620 cells migration in vitro
Lenti-ST13, Lenti-Mock, SW620, shRNA-Mock, and shRNA-ST13 cells were cultured in the top well of a transwell system and migration of SW620 cells was measured by counting the number of SW620 cells migrating to the filter surface. (a) Quantitative measurement of invaded cells. Data are representatives of each group and expressed as mean±SEM of cells per six high power fields from three separated experiments. ** P<0.01, Lenti-ST13 vs. Lenti-Mock and SW620 (one way ANOVA and Dunnett’s test). (b) Images of Lenti-ST13, Lenti-Mock, SW620, shRNA-Mock, and shRNA-ST13 cells on the filter surface
In contrast, an average of 55.7±6.8 shRNA-Mock and 58.7±4.7 SW620 cells per high power field had migrated onto the filter surface, while an average of 75.6±7.3 shRNA-ST13 cells reached the filter; however, this difference was not significant (P=0.077, one way ANOVA).
3.5. Impact of reduced ST13 expression on the tumor development of inoculated SW620 cells in vivo
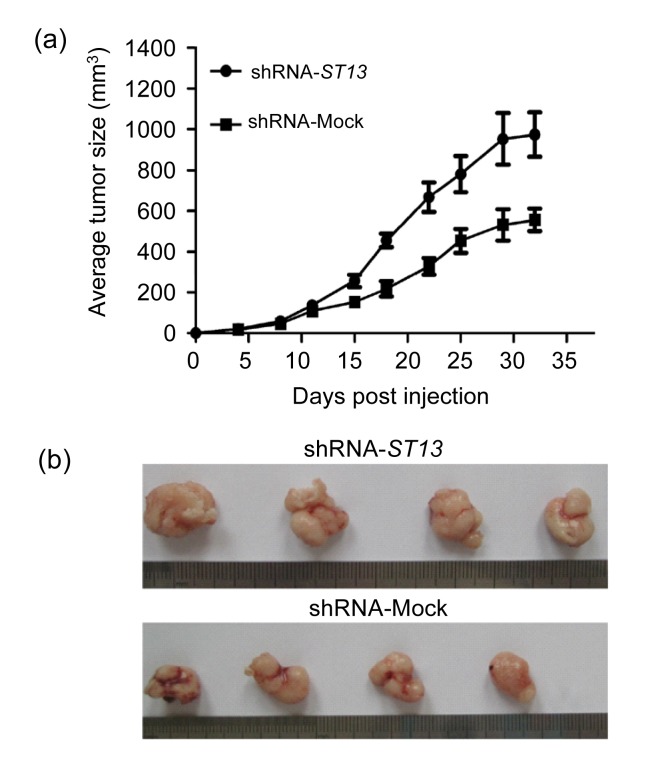
SW620 cells have high tumorigenicity and following inoculation they develop solid tumors in immunocompromised nude mice. To determine the role of ST13 in the tumorigenicity of SW620 cells and development of solid SW620 tumors, shRNA-ST13 or shRNA-Mock cells were injected subcutaneously into nude mice. The development of solid SW620 tumors was monitored for 32 d. As shown in Fig. 5a, the solid tumors were first visible at about 8 d post inoculation and rapidly grew later in the shRNA-ST13 groups. In contrast, the development of shRNA-Mock solid tumors grew slowly and the mean volume of solid SW620 tumors in shRNA-ST13 group increased by about 75%, as compared with that in control groups (Fig. 5b). Collectively, these data indicate that down-regulation of ST13 expression increased the tumorigenicity of SW620 cells in vivo.
Fig. 5.
Effect of ST13 expression on the development and growth of inoculated SW620 cells
BALB/c nude mice (n=4 per group) were inoculated with 5×106 shRNA-ST13 or shRNA-Mock cells and the development of solid SW620 tumors was monitored every 3‒4 d. The mice were sacrificed 32 d post-inoculation and the tumors were taken at the same time. (a) The dynamics of shRNA-ST13 and shRNA-Mock tumor growth. Data are expressed as mean±SEM for each group. (b) The images of individual tumors
4. Discussion
ST13 is the gene encoding the HIP (Höhfeld et al., 1995). In our previous study, ST13 expression was lower in colorectal cancer tissues compared with the tumor-adjacent normal mucosa specimens (Wang et al., 2005). The previous study showed that ST13 re-expression mediated by oncolytic adenovirus within colorectal cancer cells can induce cell apoptosis and exert potent antitumour efficacy in colorectal cancer xenografts in nude mice. Adenovirus-ST13 regulated apoptosis by interacting with ASK1 and inducing JNK activation (Yang et al., 2008; 2010; Yu et al., 2009).
In order to better understand the roles of ST13 in colorectal cancer, we performed lentivirus-mediated overexpression of ST13 and lentivirus-mediated shRNA down-regulation of ST13 expression to make stable transfection cell lines. High expression of ST13 decreased proliferation, while knockdown of ST13 enhanced proliferation ability of SW620 colorectal cancer cells in vitro. We also proved that down-regulation of ST13 expression increased the tumorigenicity of SW620 cells in vivo. Our findings were consistent with previous research that ST13 re-expression mediated by oncolytic adenovirus inhibited tumor cells growth both in vitro and in vivo (Yang et al., 2008; Yu et al., 2009). To explore the potential mechanism(s) underlying the action of ST13 in the growth of SW620 cells with stable transfection, cell cycling was characterized by FACS analysis. The data indicates that up-regulation of ST13 expression arrests Lenti-ST13 cell cycling at the G0/G1 phase and down-regulation of ST13 expression activates shRNA-ST13 cell cycling. This supports that ST13 inhibited SW620 proliferation in the stable transfection situation through cell cycle regulation.
Colorectal cancer is a highly metastatic malignancy. However, little has been reported on the impact of ST13 in migration. We examined the impact of ST13 expression on the migration of SW620 cells by transwell migration assay (Fig. 4). While many control Lenti-Mock and SW620 cells migrated to the filter surface, the numbers of Lenti-ST13 cells on the filter surface decreased by 83%. Therefore, overexpression of ST13 in SW620 cells remarkably reduced the migration capacity of SW620 cells in vitro. We also showed that in the shRNA-ST13 group there were more cells per high power field that had migrated onto the filter surface compared to the shRNA-Mock and SW620 groups, although this difference was not significant (P=0.077, one way ANOVA). SW620 is a very high migration cell line, and knocked-down ST13 did not change the migration of SW620 too much. If another low migration cell line was used, there might have been significant differences of migration between the knocked-down ST13 and Mock groups.
Transforming growth factor-β (TGF-β) is the prototype of a large family of secreted polypeptide growth factors that regulate a multitude of cellular processes affecting proliferation, differentiation, apoptosis, and epithelial-mesenchymal transition (EMT) (Roberts and Sporn, 1993; Roberts, 1998). The tumor suppression of TGF-β is caused by potent inhibition of cell proliferation due to cell cycle arrest in the G1 phase. Such anti-proliferative responses are mediated by a signaling system that includes two types of cell surface receptors and intracellular signal transducers, the SMAD proteins, including SMAD2 (Itoh et al., 2000; Schiffer et al., 2000). Many carcinoma cells are no longer sensitive to growth inhibition by TGF-β. These carcinoma cells at this stage retain the TbR-I/TbR-II/SMAD2/3/4 signaling cascade, oversecrete TGF-β, exhibit EMT (induced by autocrine TGF-β), and become more metastatic and invasive (Derynck et al., 2001; Piek and Roberts, 2001). In previous studies, we used CO-IP to find that ST13 can pull down the HSP70 (data not shown). Yang et al. (2008) also confirmed that transient transfection of ST13 can increase the expression of HSP70. Recent data have shown that HSP70 prevents receptor-dependent phosphorylation and nuclear translocation of SMAD2, and blocks TGF-β-induced EMT (Li et al., 2011). So we speculated that the effects of ST13 in migration might, through increasing HSP70, prevent SMAD2 phosphorylation, block TGF-β-induced EMT and inhibit SW620 migration. Conceivably, overexpression of ST13 may be used as a new strategy for the inhibition of colorectal cancer metastasis.
5. Conclusions
We provide a report assessing the role of ST13 in the tumorigenesis and progression of colorectal cancer. Our findings demonstrate that high expression of ST13 decreased proliferation ability, arrested cell cycle, and inhibited migration while cells knockdown of ST13 expression led to enhanced proliferation ability and activated cell cycle in SW620 colorectal cancer cells. Although the mechanism in which ST13 interacts with other proliferation and migration regulators is poorly understood, ST13 might serve as a novel diagnosis and prognosis biomarker as well as a potential therapeutic target in colorectal cancer.
Acknowledgments
We thank Jun YE, Hai LIU, Zhi-xuan FU, and Zhi-gang CHEN (Cancer Institute, the Second Affiliated Hospital, School of Medicine, Zhejiang University, China) for their technical assistance and the entire laboratory for fruitful discussions.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30973382 and 81101477), the National High-Tech R&D Program (863) of China (No. 2012AA02A506), and the Zhejiang Provincial International Scientific Technology Collaboration Key Project (No. 2009C14010), China
References
- 1.Castells A, Gusella JF, Ramesh V, Rustgi AK. A region of deletion on chromosome 22q13 is common to human breast and colorectal cancers. Cancer Res. 2000;60(11):2836–2839. [PubMed] [Google Scholar]
- 2.Chant ID, Rose PE, Morris AG. Analysis of heat-shock protein expression in myeloid leukaemia cells by flow cytometry. Br J Haematol. 1995;90(1):163–168. doi: 10.1111/j.1365-2141.1995.tb03395.x. [DOI] [PubMed] [Google Scholar]
- 3.Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993;85(7):570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- 4.Csermely P. Proteins, RNAs and chaperones in enzyme evolution: a folding perspective. Trends Biochem Sci. 1997;22(5):147–149. doi: 10.1016/S0968-0004(97)01026-8. [DOI] [PubMed] [Google Scholar]
- 5.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 6.Dong QH, Zheng S, Hu Y, Chen GX, Ding JY. Evaluation of ST13 gene expression in colorectal cancer patients. J Zhejiang Univ-Sci B. 2005;6(12):1170–1175. doi: 10.1631/jzus.2005.B1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge W, Hu H, Ding K, Sun L, Zheng S. Protein interaction analysis of ST14 domains and their point and deletion mutants. J Biol Chem. 2006;281(11):7406–7412. doi: 10.1074/jbc.M510687200. [DOI] [PubMed] [Google Scholar]
- 8.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 9.Höhfeld J, Jentsch S. GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 1997;16(20):6209–6216. doi: 10.1093/emboj/16.20.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Höhfeld J, Minami Y, Hartl FU. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83(4):589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 11.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 12.Irmer H, Höhfeld J. Characterization of functional domains of the eukaryotic co-chaperone Hip. J Biol Chem. 1997;272(4):2230–2235. doi: 10.1074/jbc.272.4.2230. [DOI] [PubMed] [Google Scholar]
- 13.Itoh S, Itoh F, Goumans MJ, Ten Dijke P. Signaling of transforming growth factor-β family members through Smad proteins. Eur J Biochem. 2000;267(24):6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- 14.Kimura E, Enns RE, Alcaraz JE, Arboleda J, Slamon DJ, Howell SB. Correlation of the survival of ovarian cancer patients with mRNA expression of the 60-kD heat-shock protein HSP-60. J Clin Oncol. 1993;11(5):891–898. doi: 10.1200/JCO.1993.11.5.891. [DOI] [PubMed] [Google Scholar]
- 15.Lane DP, Midgley C, Hupp T. Tumour suppressor genes and molecular chaperones. Philos Trans R Soc Lond B Biol Sci. 1993;339(1289):369–372. doi: 10.1098/rstb.1993.0036. discussion 372-373. [DOI] [PubMed] [Google Scholar]
- 16.Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12(3):743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Chai Z, Li Y, Liu D, Bai Z, Li Y, Li Y, Situ Z. BZW1, a novel proliferation regulator that promotes growth of salivary muocepodermoid carcinoma. Cancer Lett. 2009;284(1):86–94. doi: 10.1016/j.canlet.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Kang X, Wang Q. HSP70 decreases receptor-dependent phosphorylation of Smad2 and blocks TGF-beta-induced epithelial-mesenchymal transition. J Genet Genomics. 2011;38(3):111–116. doi: 10.1016/j.jgg.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Mahalingam D, Swords R, Carew JS, Nawrocki ST, Bhalla K, Giles FJ. Targeting HSP90 for cancer therapy. Br J Cancer. 2009;100(10):1523–1529. doi: 10.1038/sj.bjc.6605066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mo Y, Zheng S, Shen D. Differential expression of HSU17714 gene in colorectal cancer and normal colonic mucosa. Chin J Oncol. 1996;18(4):241–243. (in Chinese) [PubMed] [Google Scholar]
- 21.Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32(3):517–530. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 22.Piek E, Roberts AB. Suppressor and oncogenic roles of transforming growth factor-β and its signaling pathways in tumorigenesis. Adv Cancer Res. 2001;83:1–54. doi: 10.1016/S0065-230X(01)83001-3. [DOI] [PubMed] [Google Scholar]
- 23.Powers MV, Workman P. Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett. 2007;581(19):3758–3769. doi: 10.1016/j.febslet.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Prapapanich V, Chen S, Nair SC, Rimerman RA, Smith DF. Molecular cloning of human p48, a transient component of progesterone receptor complexes and an Hsp70-binding protein. Mol Endocrinol. 1996;10(4):420–431. doi: 10.1210/me.10.4.420. [DOI] [PubMed] [Google Scholar]
- 25.Prapapanich V, Chen S, Toran EJ, Rimerman RA, Smith DF. Mutational analysis of the hsp70-interacting protein Hip. Mol Cell Biol. 1996;16(11):6200–6207. doi: 10.1128/mcb.16.11.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prapapanich V, Chen S, Smith DF. Mutation of Hip’s carboxy-terminal region inhibits a transitional stage of progesterone receptor assembly. Mol Cell Biol. 1998;18(2):944–952. doi: 10.1128/mcb.18.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ralhan R, Kaur J. Differential expression of Mr 70000 heat shock protein in normal, premalignant, and malignant human uterine cervix. Clin Cancer Res. 1995;1(10):1217–1222. [PubMed] [Google Scholar]
- 28.Roberts AB. Molecular and cell biology of TGF-β. Miner Electrolyte Metab. 1998;24(2-3):111–119. doi: 10.1159/000057358. [DOI] [PubMed] [Google Scholar]
- 29.Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-β (TGF-β) Growth Factors. 1993;8(1):1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- 30.Schiffer M, von Gersdorff G, Bitzer M, Susztak K, Böttinger EP. Smad proteins and transforming growth factor-β signaling. Kidney Int. 2000;58(S77):45–52. doi: 10.1046/j.1523-1755.2000.07708.x. [DOI] [PubMed] [Google Scholar]
- 31.Shi Z, Bai R, Fu ZX, Zhu YL, Wang RF, Zheng S. Induced pluripotent stem cell-related genes influence biological behavior and 5-fluorouracil sensitivity of colorectal cancer cells. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2012;13(1):11–19. doi: 10.1631/jzus.B1100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi ZZ, Zhang JW, Zheng S. What we know about ST13, a co-factor of heat shock protein, or a tumor suppressor? J Zhejiang Univ-Sci B. 2007;8(3):170–176. doi: 10.1631/jzus.2007.B0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soo ET, Yip GW, Lwin ZM, Kumar SD, Bay BH. Heat shock proteins as novel therapeutic targets in cancer. In Vivo. 2008;22(3):311–315. [PubMed] [Google Scholar]
- 34.Söti C, Csermely P. Chaperones and aging: role in neurodegeneration and in other civilizational diseases. Neurochem Int. 2002;41(6):383–389. doi: 10.1016/S0197-0186(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 35.Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003;22(56):9041–9047. doi: 10.1038/sj.onc.1207114. [DOI] [PubMed] [Google Scholar]
- 36.Wang LB, Zheng S, Zhang SZ, Peng JP, Ye F, Fang SC, Wu JM. Expression of ST13 in colorectal cancer and adjacent normal tissues. World J Gastroenterol. 2005;11(3):336–339. doi: 10.3748/wjg.v11.i3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 38.Yang M, Cao X, Yu MC, Gu JF, Shen ZH, Ding M, Yu DB, Zheng S, Liu XY. Potent antitumor efficacy of ST13 for colorectal cancer mediated by oncolytic adenovirus via mitochondrial apoptotic cell death. Hum Gene Ther. 2008;19(4):343–353. doi: 10.1089/hum.2007.0137. [DOI] [PubMed] [Google Scholar]
- 39.Yang M, Yu M, Guan D, Gu J, Cao X, Wang W, Zheng S, Xu Y, Shen Z, Liu X. ASK1-JNK signaling cascade mediates Ad-ST13-induced apoptosis in colorectal HCT116 cells. J Cell Biochem. 2010;110(3):581–588. doi: 10.1002/jcb.22551. [DOI] [PubMed] [Google Scholar]
- 40.Ye YW, Wu JH, Wang CM, Zhou Y, Du CY, Zheng BQ, Cao X, Zhou XY, Sun MH, Shi YQ. Sox17 regulates proliferation and cell cycle during gastric cancer progression. Cancer Lett. 2011;307(2):124–131. doi: 10.1016/j.canlet.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 41.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5(10):781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 42.Yu DB, Zhong SY, Yang M, Wang YG, Qian QJ, Zheng S, Liu XY. Potent antitumor activity of double-regulated oncolytic adenovirus-mediated ST13 for colorectal cancer. Cancer Sci. 2009;100(4):678–683. doi: 10.1111/j.1349-7006.2009.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Cai X, Schlegelberger B, Zheng S. Assignment of human putative tumor suppressor genes ST13 (alias SNC6) and ST14 (alias SNC19) to human chromosome bands 22q13 and 11q24→q25 by in situ hybridization. Cytogenet Cell Genet. 1998;83(1-2):56–57. doi: 10.1159/000015125. [DOI] [PubMed] [Google Scholar]
- 44.Zheng S, Cai X, Cao J. Application of subtractive hybridization in screening for colorectal cancer negatively related genes. Natl Med J China. 1997;77(4):256–259. (in Chinese) [PubMed] [Google Scholar]