Abstract
This study was aimed to compare the effects of acute and chronic psychological stress on metabolic factors. Forty-two male Wistar rats were divided into control and stressed groups. Stress was applied by a communication box acutely (1 d) and chronically (15 and 30 d). Blood sampling was carried out by retro-orbital-puncture method. The plasma levels of glucose, cholesterol, triglyceride, insulin, and corticosterone were measured. In addition, feed and water intake, latency to eat and drink, adrenal and body weights were determined. Acute and chronic psychological stress did not significantly change basal plasma corticosterone levels. However, immediately (1 min) after acute exposure to stress, plasma corticosterone level increased compared to that before stress exposure. Acute stress increased plasma insulin levels significantly. Fifteen days of stress exposure resulted in plasma glucose increase. Chronic stress significantly increased feed intake, latency to eat, and adrenal weight compared to acute stress. The body weights of both control and stressed groups increased markedly during the experiment. Homeostasis model assessment of insulin resistance (HOMA-IR) index did not change significantly in the stressed group. In conclusion, application of acute and chronic psychological stress leads to different metabolic and/or behavioral changes but the metabolic changes resulting from acute exposure to stress seem to be more pronounced.
Keywords: Psychological stress, Corticosterone, Insulin, Glucose, Cholesterol, Triglyceride
1. Introduction
As Hans Selye believed, “stress is a general adaptation syndrome”, i.e., a single stereotypic response elicited by any demand upon the body (Rosmond, 2005). The adaptation response to stress would be different according to the type, duration, intensity, and history of the stress (Jean Kant et al., 1985; Pitman et al., 1990; Ishikawa et al., 1992; Ricart-Jane et al., 2002; Rai et al., 2003). Psychological stress as a more frequent type of stress in humans evokes adaptive responses such as changes in plasma levels of some of the blood parameters and hormones and subsequent behavioral and metabolic alterations to survive. Acute restraint, shaking, and restraint plus shaking for 2 h each increased plasma corticosterone level significantly in male rats (Dhabhar and McEwen, 1997). In addition, acute water immersion stress (as a psychological stress) increased plasma noradrenaline, adrenaline, corticosterone, and glucose levels in male rats (de Boer et al., 1990). Moreover, acute noise stress (another example of psychological stress) decreased serum glucose and insulin concentrations (Armario et al., 1985) but enhanced plasma corticosterone levels (Armario et al., 1984). Acute immobilization stress that has a psychological component (van de Kar and Blair, 1999), increased plasma glucose and corticosterone levels during the stress session, whereas plasma insulin concentration was increased at the beginning of the stress session and returned to its normal value at the end (Yamada et al., 1993). On the other hand, the response to chronic stress is completely different from that to acute short-term stress as mentioned above. In this regard, chronic exposure to restraint, shaking and restraint plus shaking for 6 h (during 6 h the stressors were changed every hour) resulted in a marked reduction of the stress-induced increase in plasma corticosterone level (Dhabhar and McEwen, 1997). Moreover, chronic water immersion caused a smaller increase of plasma noradrenaline, adrenaline, corticosterone, and glucose levels in the rats as compared to acute stress exposure (de Boer et al., 1990). The chronic noise exposure caused no changes in the serum levels of insulin, glucose (Armario et al., 1985) and also basal plasma level of corticosterone (Armario et al., 1984). In addition, repeated immobilization increased plasma corticosterone, decreased plasma insulin, and had no effect on plasma glucose concentrations (Makino et al., 1999).
In human societies, psychological stress in acute and chronic forms is much too prevalent and by changing some metabolic and hormonal parameters, may be the basis of many disorders (Rosmond, 2005; Dong et al., 2011), but whether acute or chronic psychological stress induces more profound metabolic defects is still an unanswered question. In this regard, the first step is to choose animal models of psychological stress which are very close to human psychological stress, followed by the evaluation of the metabolic consequences of acute and chronic application of the stressors. Therefore, this study aimed to use a communication box (com-box) to induce a psychological stress without a physical component in rats, and to compare some metabolic (plasma glucose, triglyceride, and cholesterol), hormonal (insulin and corticosterone), and behavioral (feed and water intake, latency to eat and drink) parameters following acute and chronic exposure to the stress.
2. Materials and methods
2.1. Animals
Forty-two male Wistar rats (Pasteur Institute, Tehran, Iran) with an initial body weight of 170–250 g were used. The animals were housed three per plastic cage at a constant temperature ((22±2) °C) and a 12-h light cycle with free access to standard feed (standard pellets, pars production and distribution of animal feed company, Iran) and tap water. For fasting conditions, feed was removed for 16 h before the beginning of the trial. Rats were adjusted to the environment for one week prior to the experiments. Animals’ body weight was measured on the first and last days of the experiments by a digital scale (FEW, Japan, sensitivity 0.1 g). All procedures were approved by the Animal Care and Use Committee of the Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Iran.
2.2. Stress procedure
A com-box (Borje Sanat, Iran) was used as a psychological stress stimulus devise. This device (48 cm×48 cm×50 cm) is divided into nine compartments (16 cm×16 cm×50 cm) by transparent plastic sheets. In each session, five rats were exposed to the electrical foot-shock (1 mA, 1 Hz) for a 10-s duration every 60 s (1 h/d) through the stainless steel grids. Four remaining animals in the other compartments were exposed to psychological stress by observing the animals under foot-shock stress (Endo et al., 2001). In two sessions, eight animals were psychologically stressed, seven of which were used in this study.
The animals of the stressed group were subdivided into acutely stressed (i.e., 1 d exposure to stress) and chronically stressed (i.e., 15 and 30 d exposure to stress) groups. The stressed groups were exposed to psychological stress (1 h/d) for 10 to 14 h. The animals of the control group had the same subgroups and served as the control Days 1, 15, and 30. These animals were placed in the box (1 h/d) without receiving any stress.
2.3. Blood sampling
Blood samples were obtained by orbital sinus puncture method (Hoff, 2000) under light isoflurane anesthesia. The samples were collected in an Eppendorf tube containing 0.5% heparin and centrifuged at 3 000×g for 5 min (Toleikis and Godin, 1995). The plasma was separated and kept at −80 °C until used.
2.4. Assessment of plasma glucose, insulin, triglyceride, cholesterol, and corticosterone levels
To evaluate plasma glucose, insulin, triglyceride, and cholesterol levels in both the control and stressed groups, blood sampling was performed 1 d after the last session of the trial (i.e., on Days 2, 16, and 31 of the experiment) at 8:00 to 8:30 AM.
To determine the basal plasma corticosterone concentration, blood samples were obtained before placing the animals in the com-box for the first time (basal-before, B-B) and 1 d after the last session of the trial (basal-after, B-A) at 8:00 to 8:30 AM. Moreover, blood sampling was performed on Days 1, 15, and 30 of the experiment immediately after removing the animals from the com-box (with or without stress).
Plasma samples were analyzed for insulin and corticosterone concentrations by a rat insulin enzyme-linked immunosorbent assay (ELISA) kit (Mercodia, Sweden) and corticosterone ELISA kit (DRG, Germany), respectively. Plasma glucose concentration was determined using the glucose oxidase method (Pars Azmoon, Iran). Plasma triglyceride and cholesterol levels were measured by colorimetric method (Pars Azmoon, Iran). The intra- and inter-assay coefficients of variations for insulin were 3.40% and 2.20%, for corticosterone 4.08% and 6.35%, for glucose 1.74% and 1.19%, for triglyceride 1.82% and 1.60%, and for cholesterol 1.62% and 1.22%.
2.5. Homeostasis model assessment of insulin resistance (HOMA-IR) index
Fasting plasma glucose and insulin were measured to determine HOMA-IR index, the formula is: HOMA-IR=(c i×c g)/22.5, where c i is fasting insulin level (μU/ml) and c g is fasting glucose level (mmol/L) (Farahani et al., 2010).
2.6. Weight of adrenal glands
Rats were anesthetized with isoflurane and decapitated, and the abdomen was opened. The adrenal glands were carefully isolated from adhering adipose tissue and weighed immediately (Hoeflich et al., 2002) by a digital scale (Sartorius, Germany, resolution 0.1 mg).
2.7. Feed and water intake and latency to eat and drink
Feed and water intakes were measured once a week throughout the experiment by measuring the difference between the amount of feed or the volume of water put in the cage and the remaining amount after 24 h. Moreover, after each session of an animal being in the com-box, the time lag between the removal and starting to consume feed and water was recorded and the mean values of Days 7 to 30 were compared with the value of Day 1 (Macht et al., 2001).
2.8. Statistical analysis
Results are presented as mean±standard error of the mean (SEM). A mixed analysis of variance (ANOVA) with repeated measures within the stressed and control groups (day was considered as a repeated factor) and independent measures between the two groups (stress was considered as an independent factor) was performed by SPSS Version 9.0 program package. P<0.05 was considered statistically significant.
3. Results
3.1. Effects of acute and chronic stress on plasma corticosterone concentration and adrenal gland weight
Acute (1 d) and chronic (15 and 30 d) exposure to psychological stress had no significant effects on basal (both B-B and B-A) plasma corticosterone concentrations. However, immediately after acute (Day 1) exposure to stress, plasma corticosterone concentration was increased as compared to that before stress exposure (P<0.001). The chronically stressed animals showed a reduction in plasma corticosterone level immediately after stress exposure on Days 15 and 30 as compared to the same group on Day 1; however, only on Day 15 the reduction was significant (P<0.01) (Table 1).
Table 1.
Plasma corticosterone concentrations and adrenal gland weights of control and stressed rats
| Group | Corticosterone concentration (nmol/ml) |
Adrenal gland weight (mg) | ||
| B-B1 | B-A2 | After3 | ||
| Control | ||||
| Day 1 | 0.32±0.07 | 0.33±0.05 | 0.36±0.06 | 29.30±1.82 |
| Day 15 | 0.31±0.03 | 0.31±0.07 | 0.32±0.04 | 32.39±1.84 |
| Day 30 | 0.31±0.04 | 0.24±0.05 | 0.29±0.05 | 36.20±2.89 |
| Stressed | ||||
| Day 1 | 0.29±0.05 | 0.40±0.06 | 0.55±0.03m | 30.91±0.97 |
| Day 15 | 0.34±0.06 | 0.31±0.04 | 0.30±0.08c | 35.07±1.98 |
| Day 30 | 0.30±0.04 | 0.27±0.05 | 0.36±0.07 | 42.61±1.79a b d e |
Basal-before stress exposure
Basal-after stress exposure
Immediately after removing from com-box
P<0.001 significant difference versus Day 1 of the control group
P<0.001 significant difference versus Day 1 in the same group
P<0.01 significant difference versus Day 1 in the same group
P<0.05 significant difference versus Day 15 in the same group
P<0.01 significant difference versus Day 15 in the control group
P<0.001 significant difference versus B-B in the same group
Values are expressed as mean±SEM of 7 rats. Letters a to e show differences in column and letter m shows difference in row
Acute stress had no significant effect on the adrenal gland weight, whereas in the stressed group, chronic stress (Day 30) caused a significant increase in the weight of adrenal glands as compared to Day 1 of the control and stressed groups (P<0.001) and Day 15 of the control (P<0.01) and stressed (P<0.05) groups (Table 1).
3.2. Effects of acute and chronic stress on plasma glucose and insulin concentrations and HOMA-IR index
Acute (1 d) exposure to psychological stress had no significant effects on plasma glucose concentration, whereas chronic stress (15 d) significantly increased plasma level of glucose as compared to Day 1 (P<0.01). However, 30 d exposure to stress had no significant effect on plasma glucose concentration (Table 2).
Table 2.
Effects of acute and chronic psychological stress on plasma insulin, glucose, triglyceride, and cholesterol concentrations and HOMA-IR index
| Group | Insulin (μg/L) | Glucose (mg/dl) | Triglyceride (mg/dl) | Cholesterol (mg/dl) | Fasting glucose (mg/dl) | Fasting insulin (μg/L) | HOMA-IR index |
| Control | |||||||
| Day 1 | 0.77±0.10 | 116.84±6.91 | 179.10±6.47 | 107.37±4.16 | 98.39±5.40 | 0.76±0.15 | 4.47±0.82 |
| Day 15 | 1.10±0.14 | 124.06±3.64 | 180.54±5.38 | 118.03±5.79 | 104.39±5.02 | 0.62±0.18 | 4.13±1.29 |
| Day 30 | 1.17±0.13 | 120.68±3.85 | 191.04±6.53 | 108.68±7.60 | 114.29±4.87 | 0.99±0.43 | 4.07±1.14 |
| Stressed | |||||||
| Day 1 | 1.61±0.24** | 110.07±3.48 | 184.13±7.42 | 113.61±5.10 | 87.82±1.18 | 0.71±0.06 | 3.86±0.29 |
| Day 15 | 1.05±0.14 | 124.53±4.71## | 194.07±12.37 | 108.84±3.06 | 104.18±3.85 | 0.99±0.37 | 3.73±1.71 |
| Day 30 | 1.43±0.28 | 115.39±3.40 | 175.49±3.08 | 122.88±2.96 | 103.19±4.88 | 0.69±0.17 | 4.49±1.16 |
Values are expressed as mean±SEM of 7 rats
P<0.01 significant difference versus the same day of the control group
P<0.01 significant difference versus Day 1 in the same group
Psychological stress caused significant elevation of plasma insulin levels on the first day of the experiment as compared to the respective control group (P<0.01). Acute and chronic psychological stress did not change fasting plasma glucose and insulin levels (Table 2).
The HOMA-IR index was not significantly different between the control and stressed groups (Table 2).
3.3. Plasma cholesterol and triglyceride changes
No significant difference was observed between plasma cholesterol levels of the control and stressed groups throughout the trial (Table 2).
Acute and chronic psychological stress caused no significant changes in the plasma triglyceride concentration. However, a non-significant reduction of plasma triglyceride was observed following 30 d stress exposure compared to the control Day 30 (Table 2).
3.4. Feed and water intake and latency to eat and drink
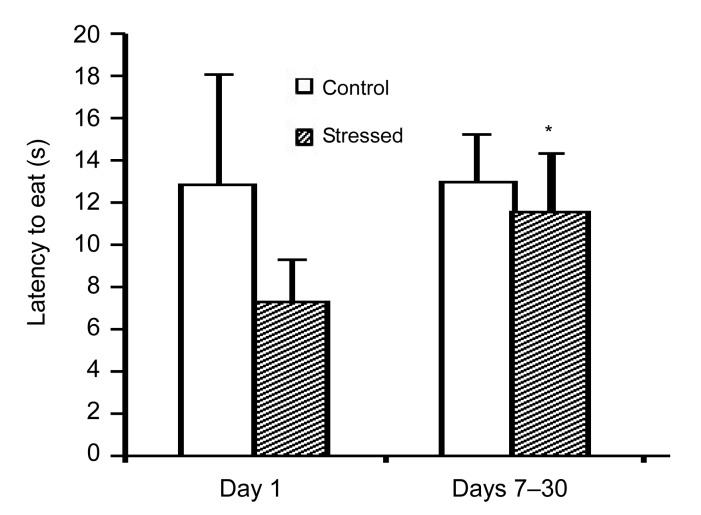
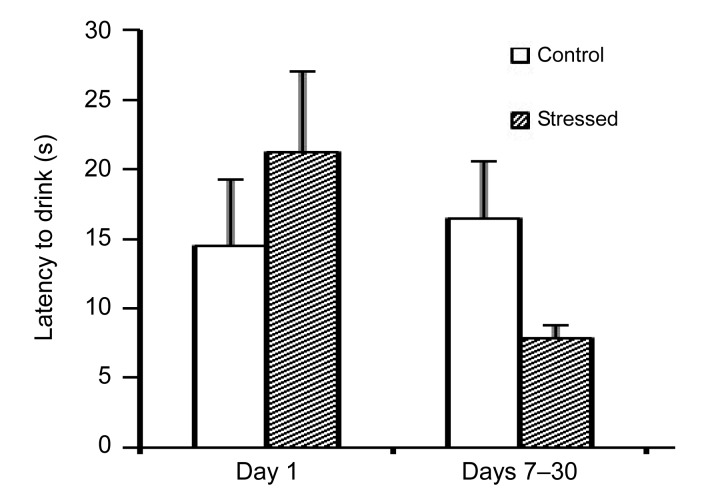
The animals which were chronically exposed to stress showed a significant increase in feed intake on Day 21 of the experiment as compared to Day 1 (P<0.01) and on Day 30 of the experiment as compared to Day 1 and the control animals on Day 30 (P<0.01), as shown in Table 3. Furthermore, the latency to eat following chronic stress exposure (Days 7–30) was increased compared to acute exposure to stress (Day 1), as shown in Fig. 1. Acute and chronic psychological stress had no significant effects on water intake (Table 3) or on the latency to drink (Fig. 2).
Table 3.
Effects of acute and chronic psychological stress on feed and water intake
| Group | Feed intake each rat (g/d) | Water intake each rat (ml/d) |
| Control | ||
| Day 1 | 18.93±2.15 | 28.43±3.10 |
| Day 7 | 17.22±0.82 | 33.71±3.47 |
| Day 15 | 18.07±0.33 | 42.71±6.59 |
| Day 21 | 21.20±1.27 | 44.40±3.92 |
| Day 30 | 17.87±0.89 | 41.73±5.55 |
| Stressed | ||
| Day 1 | 16.54±1.42 | 33.00±1.89 |
| Day 7 | 18.80±0.61 | 29.74±1.45 |
| Day 15 | 20.21±1.21 | 37.14±1.82 |
| Day 21 | 23.69±2.88## | 36.86±3.69 |
| Day 30 | 24.24±1.98** ## | 37.71±1.89 |
Values are expressed as mean±SEM of 7 rats
P<0.01 significant difference versus the same day of the control group
P<0.01 significant difference versus Day 1 of the same group
Fig. 1.
Effects of acute and chronic psychological stress on latency to eat
The latency to eat following chronic stress exposure (Days 7–30) was increased compared to acute stress exposure. Each column represents mean±SEM of 7 rats. * P<0.05 significant difference versus Day 1 of the same group
Fig. 2.
Effects of acute and chronic psychological stress on latency to drink
Acute and chronic psychological stress had no significant effects on latency to drink. Each column represents mean±SEM of 7 rats
3.5. Body weight variations
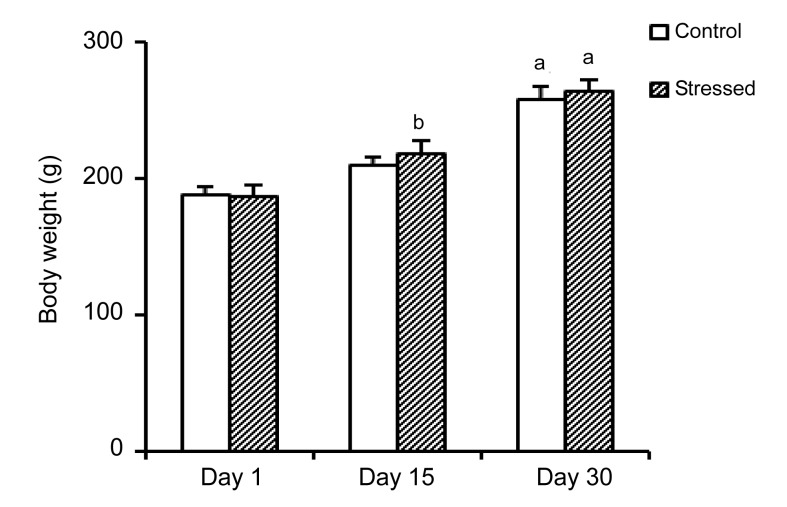
The body weights of both control and stressed rats had a rising trend. The weights of control and stressed rats showed a significant (P<0.001) increase on Day 30 as compared to Days 1 and 15. In addition, the weight of stressed rats on Day 15 had a significant increase as compared to Day 1 (P<0.01) (Fig. 3).
Fig. 3.
Body weights of the control and stressed groups
Each column represents mean±SEM of 7 rats. a P<0.001 significant difference versus Days 1 and 15, b P<0.01 significant difference versus Day 1 of the same group
4. Discussion
In the present study, acute stress elevated plasma insulin concentration but did not change plasma glucose level. The plasma concentrations of glucose and insulin remained unchanged following chronic (30 d) stress exposure. In addition, exposure to stress for the first time (Day 1) significantly increased plasma corticosterone concentration immediately after exposure, whereas by chronic exposure to stress plasma corticosterone level decreased while the adrenal weight increased. Moreover, chronic exposure to stress significantly increased feed intake and latency to eat compared to acute stress. As a whole, the results of the present study showed a relatively typical metabolic response to acute and chronic stress.
Activation of the sympathoadrenal system leads to catecholamine release from nerves and the adrenal medulla. Activation of hypothalamic-pituitary-adrenal (HPA) axis in turn results in corticotrophin secretion from adenohypophysis and finally corticosterone, as the main glucocorticoid in rodents, is released from the adrenal cortex (Teague et al., 2007). In this regard, in the present study the up-regulation of gluconeogenesis, glycogenolysis, and glucose transport probably induced by the short-term increase of plasma corticosterone level immediately after the first stress exposure (acute stress) may keep the plasma glucose level to be unchanged (Andrews and Walker, 1999). In some other studies, acute exposure to immobilization (Ricart-Jane et al., 2002; Rai et al., 2003) increased plasma glucose and insulin concentrations. Since stress can increase plasma catecholamine beside corticosterone levels and the secretion of the catecholamines is a part of the “fight or flight” response that stimulates glycogenolysis and increases the basal metabolic rates and productions of glucose and insulin as well (Teague et al., 2007), the different observed results may reflect different stress level threshold responses, differential rates of glucose and insulin production, or other intrinsic metabolic differences between animals used in the various studies.
In the chronically stressed rats of this study, an increase of plasma glucose concentration was observed on Day 15, but not on Day 30 as compared to Day 1, while plasma corticosterone showed no significant change. This increase of plasma glucose may be related to the possible increase of plasma adrenaline and noradrenaline (not measured in this study) following stress exposure. However, by increasing the days of exposure to stress the catecholamines’ response may be adapted and therefore may cause the plasma glucose level on Day 30 not to change significantly (de Boer et al., 1990). It is noteworthy that the patterns of corticosterone and catecholamine of sympathoadrenal response and adaptation to intermittent stress are different (de Boer et al., 1989).
According to the results of the present study, HOMA-IR index, which is a method to quantify insulin resistance, also did not change significantly following acute and chronic stress. Thus it seems that this kind of stress in acute or chronic form did not have a significant effect on insulin resistance. In agreement with our results, it was shown that inducing a moderate hypercortisolism by hydrocortisone infusion (for 3 h) in human subjects, which is equivalent to that observed in response to a mild stress, did not change HOMA-IR index (Darmon et al., 2006). On the other hand, chronic exposure of normal chow fed male Wistar rats to electric foot-shock assisted with noise for 10 weeks (Fu et al., 2009) increased HOMA-IR index. The differences between results may be due to the variation in the type, duration, and intensity of the applied stressor.
Consistent with the present study, previous experiments have shown that different types of stress including immobilization (30 min) (Ricart-Jane et al., 2002), restraint, shaking, and restraint plus shaking (2 h) (Dhabhar and McEwen, 1997) in rats significantly increased plasma corticosterone level when applied acutely, whereas chronic ethanol injection (Spencer and McEwen, 1990), noise (Armario et al., 1984), and electroconvulsive shock (Thiagarajanet al., 1989) in rats decreased plasma corticosterone concentration compared to the first stress exposure. Moreover, in the chronically stressed groups of the present study the plasma corticosterone levels immediately or one day after the last stress exposure decreased to the levels of the control groups, even though the weight of the adrenal glands was increased in those animals. In general, the plasma corticosterone level decreased as the animal adapted to the stressor (Teague et al., 2007). Thus the increased adrenal weight together with a return of corticosterone to control values in the chronically stressed rats could suggest a possible late adaptation following initial mass increases in early coping attempts or exhaustion of the adrenal gland, although the involvement of the negative feedback mechanism cannot be ruled out (Teague et al., 2007). It is also possible that a certain adaptation at the cellular level may be contributing, though this remains to be elucidated. In this regard, several studies showed that following chronic exposure to some kinds of stressors (with lower intensity or having psychological aspect) the adaptation may occur (Zelena et al., 2003). Moreover, in previous studies, it has also been shown that despite adaptation to stress, the weight of the adrenal glands was increased, which in turn may indicate the demand on the ability of the adrenals to secrete even higher amounts of corticosterone.
In the present experiment plasma triglyceride concentration showed a non-significant reduction following chronic stress exposure whereas plasma cholesterol remained unchanged. Chronic immobilization for 2 periods of 5 and 4 consecutive days, separated by 2 d without stress in rats and chronic stress combining acoustic and restraint stress in mice also decreased plasma triglyceride but increased plasma cholesterol concentrations (Ricart-Jane et al., 2002; Depke et al., 2008). Acute stress combining acoustic and restraint stress in mice had no significant effect on any of the mentioned parameters (Depke et al., 2008). Acute foot-shock (Ghalami et al., 2011) and immobilization stress in 24-h fasted rats (with chow diet) (Hershock and Vogel, 1989) decreased plasma triglyceride but did not change plasma cholesterol. The variations in the results may be attributed to the differences in type, intensity, and duration of stress and also feeding state. The reduction in the plasma triglyceride may be due to enhanced activity of lipoprotein lipase (Starzec et al., 1981; Ricart-Jane et al., 2002) or decrease in endogenous triglyceride production (Robertson and Smith, 1976).
Chronic exposure to psychological stress, in this study, did not change water intake but increased feed intake as well as body weight compared to the first day. This result may indicate that a balance between feed intake and energy consumption was maintained by the possible activation of the sympathetic nervous system following stress exposure, which stimulates thermogenesis and metabolism (Armario et al., 1986; Dorfman et al., 2009). However, no significant difference was observed compared to the control group. According to the previous studies, chronic exposure to stress led to different results in relation with the feed intake and weight gain. For instance, repeated immobilization in male Wistar rats (2 h daily, for 2 periods of 5 and 4 consecutive days, separated by 2 d of rest) (Ricart-Jane et al., 2002) and in male Sprague-Dawley rats (2 h/d, for 15 d) (Martí et al., 1993) decreased feed intake and weight gain, whereas intermittent shaker stress for 2-min periods (150 cycles/min), 45 times/d for 7 d in male C57BL6 mice increased water intake, induced no change in feed intake, and significantly decreased body weight (Bernatova et al., 2002). Repeated social stress (for 6 d) increased feed intake during the light period but decreased weight gain in male Sprague-Dawley rats (Bhatnagar et al., 2006). At last, a chronic social defeat increased both feed intake and body weight in male Syrian hamsters as compared to the controls (Foster et al., 2006).
In this experiment, latency to eat in the chronic stressed animals (Days 7–30) increased significantly as compared to Day 1 (acute stress), whereas latency to drink in the animals remained unchanged. Acute restraint stress (30 min in a plexiglass tube) increased latency to eat and decreased feed intake (Tabarin et al., 2007). Twenty-one days of chronic unpredictable stress in mice (Koo et al., 2010) and also social defeat stress in Wistar rats (Vidal et al., 2011) increased latency to drink. In a study on Sprague-Dawley rats, noise stress (95 dB white noise) increased latency to eat and also feed intake in hypophagic rats, but in normophagic rats the feed intake was not changed (Macht et al., 2001). The reasons for the stress-induced alterations in latency to eat and drink are not fully understood. Overall, the discrepancy in the results may be related to the differences in race, type, intensity, and duration of stress. Therefore, further investigation is necessary to be carried out. The combination of increased latency to eat and feed intake observed in this study is a somewhat different result compared to human studies. In general, from the studies on human subjects it seems that high arousal or intense emotions (such as fear, anger, or negative mood) suppress feed-intake and motivation to eat, whereas low to moderate emotions may increase feed-intake and motivation to eat (Macht, 2008).
On the basis of the “stress spectrum hypothesis” introduced by Dhabhar and McEwen (1997), the present study can propose that acute psychological stress used in this research might lead to an immediate physiological stress response which was stopped rapidly according to basal B-A value of the plasma corticosterone level (Table 1). The short-term increase of plasma corticosterone level immediately after stress exposure was beneficial for the stressed rats to promote adaptation and survival (Teague et al., 2007) which are the characteristics of a “eustress spectrum”. By progression to chronic stress (Days 15 and 30), it seems that the animals’ response to stress arrived into the middle part of the spectrum (i.e., “resilience”), which helps survival of the animals for longer periods under increasingly demanding conditions. If the stress continued for a longer duration it would be possible that the stress response of the animals get into the other end of the stress spectrum (i.e., “distress”) in which apparent precipitation or exacerbation of metabolic impairments may occur (Dhabhar and McEwen, 1997).
From the results of this experiment it can be concluded that acute exposure to psychological stress may lead to more profound metabolic changes than chronic stress. This profound effect of acute stress should be considered in any intervention of stress managements.
Footnotes
Project (No. 919) supported by the Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
References
- 1.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond) 1999;96(5):513–523. doi: 10.1042/CS19980388. [DOI] [PubMed] [Google Scholar]
- 2.Armario A, Castellanos JM, Balasch J. Adaptation of anterior pituitary hormones to chronic noise stress in male rats. Behav Neural Biol. 1984;41(1):71–76. doi: 10.1016/S0163-1047(84)90745-3. [DOI] [PubMed] [Google Scholar]
- 3.Armario A, Castellanos JM, Balasch J. Chronic noise stress and insulin secretion in male rat. Physiol Behav. 1985;34(3):359–361. doi: 10.1016/0031-9384(85)90196-9. [DOI] [PubMed] [Google Scholar]
- 4.Armario A, Lopez-Calderon A, Jolin T, Balasch J. Response of anterior pituitary hormones to chronic stress. The specificity of adaptation. Neurosci Biobehav Rev. 1986;10(3):245–250. doi: 10.1016/0149-7634(86)90011-4. [DOI] [PubMed] [Google Scholar]
- 5.Bernatova I, Key MP, Lucot JB, Morris M. Circadian differences in stress-induced pressor reactivity in mice. Hypertension. 2002;40(5):768–773. doi: 10.1161/01.HYP.0000036405.27562.02. [DOI] [PubMed] [Google Scholar]
- 6.Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18(1):13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- 7.Darmon P, Dadoun F, Boullu-Ciocca S, Grino M, Alessi MC, Dutour A. Insulin resistance induced by hydrocortisone is increased in patients with abdominal obesity. Am J Physiol Endocrinol Metab. 2006;291(5):995–1002. doi: 10.1152/ajpendo.00654.2005. [DOI] [PubMed] [Google Scholar]
- 8.de Boer SF, van der Gugten J, Slangen JL. Plasma catecholamine and corticosterone responses to predictable and unpredictable noise stress in rats. Physiol Behav. 1989;45(4):789–795. doi: 10.1016/0031-9384(89)90296-5. [DOI] [PubMed] [Google Scholar]
- 9.de Boer SF, Koopmans SJ, Slangen JL, van der Gugten J. Plasma catecholamine, corticosterone and glucose responses to repeated stress in rats: effect of inter stressor interval length. Physiol Behav. 1990;47(6):1117–1124. doi: 10.1016/0031-9384(90)90361-7. [DOI] [PubMed] [Google Scholar]
- 10.Depke M, Fusch G, Domanska G, Geffers R, Vӧlker U, Christine Schuett C, Kiank C. Hypermetabolic syndrome as a consequence of repeated psychological stress in mice. Endocrinology. 2008;149(6):2714–2723. doi: 10.1210/en.2008-0038. [DOI] [PubMed] [Google Scholar]
- 11.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 12.Dong JJ, Lou NJ, Zhao JJ, Zhang ZW, Qiu LL, Zhou Y, Liao L. Evaluation of a risk factor scoring model in screening for undiagnosed diabetes in China population. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(10):846–852. doi: 10.1631/jzus.B1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorfman M, Ramirez VD, Stener-Victorin E, Lara HE. Chronic-intermittent cold stress in rats induces selective ovarian insulin resistance. Biol Reprod. 2009;80(2):264–271. doi: 10.1095/biolreprod.108.070904. [DOI] [PubMed] [Google Scholar]
- 14.Endo Y, Yamauchi K, Fueta Y, Lrie M. Changes of body temperature and plasma corticosterone level in rats during psychological stress induced by the communication box. Med Sci Monitor. 2001;7(6):1161–1165. [PubMed] [Google Scholar]
- 15.Farahani H, Ghasemi A, Roghani M, Zahediasl S. The effect of maternal hypothyroidism on the carbohydrate metabolism and insulin secretion of isolated islets in adult male offspring of rats. Horm Metab Res. 2010;42(11):792–797. doi: 10.1055/s-0030-1262826. [DOI] [PubMed] [Google Scholar]
- 16.Foster MT, Solomon MB, Huhman KL, Bartness TJ. Socialdefeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1284–R1293. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- 17.Fu JH, Xie SR, Kong SJ, Wang Y, Wei W, Shan Y, Luo YM. The combination of a high-fat diet and chronic stress aggravates insulin resistance in Wistar male rats. Exp Clin Endocrinol Diabetes. 2009;117(7):354–360. doi: 10.1055/s-0028-1119406. [DOI] [PubMed] [Google Scholar]
- 18.Ghalami J, Zardooz H, Rostamkhani F, Farrokhi B, Hedayati M. High-fat diet did not change metabolic response to acute stress in rats. EXCLI J. 2011;10:205–217. [PMC free article] [PubMed] [Google Scholar]
- 19.Hershock D, Vogel WH. The effects of immobilization stress on serum triglycerides, nonesterified fatty acids, and total cholesterol in male rats after dietary modifications. Life Sci. 1989;45(2):157–165. doi: 10.1016/0024-3205(89)90290-7. [DOI] [PubMed] [Google Scholar]
- 20.Hoeflich A, Weber MM, Fisch T, Nedbal S, Fottner C, Elmlinger MW, Wanke R, Wolf E. Insulin-like growth factor binding protein 2 (IGFBP-2) separates hypertrophic and hyperplastic effects of growth hormone (GH)/IGF-I excess on adrenocortical cells in vivo. FASEB J. 2002;16(13):1721–1731. doi: 10.1096/fj.02-0349com. [DOI] [PubMed] [Google Scholar]
- 21.Hoff J. Methods of blood collection in the mouse. Lab Animal. 2000;29(10):47–53. [Google Scholar]
- 22.Ishikawa M, Hara C, Ohdo S, Ogawa N. Plasma corticosterone response of rats with sociopsychological stress in the communication box. Physiol Behav. 1992;52(3):475–480. doi: 10.1016/0031-9384(92)90333-W. [DOI] [PubMed] [Google Scholar]
- 23.Jean Kant G, Eggleston T, Landman-Roberts L, Kenion CC, Driver GC, Meyerhoff JL. Habituation to repeated stress is stressor specific. Pharmacol Biochem Behav. 1985;22(4):631–634. doi: 10.1016/0091-3057(85)90286-2. [DOI] [PubMed] [Google Scholar]
- 24.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-κB is a critical mediator of stress impaired neurogenesis and depressive behavior. PNAS. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macht M. How emotions affect eating: a five-way model. Appetite. 2008;50(1):1–11. doi: 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Macht M, Krebs H, Weyers P, Janke W. Effect of stress on feeding behavior in rats: individual differences. Pers Indiv Differ. 2001;30(3):463–469. doi: 10.1016/S0191-8869(00)00037-4. [DOI] [Google Scholar]
- 27.Makino S, Asaba K, Nishiyama M, Hashimoto K. Decreased type 2 corticotropin-releasing receptor mRNA expression in the ventromedial hypothalamus during repeated immobilization stress. Neuroendocrinology. 1999;70(3):160–167. doi: 10.1159/000054472. [DOI] [PubMed] [Google Scholar]
- 28.Martí O, Gavaldà A, Jolín T, Armario A. Effects of regulatory of exposure to chronic immobilization stress on the circadian pattern of pituitary adrenal hormones, growth hormone, and thyroid stimulating hormone in the adult male rat. Psychoneuroendocrinology. 1993;18(1):67–77. doi: 10.1016/0306-4530(93)90056-Q. [DOI] [PubMed] [Google Scholar]
- 29.Pitman DL, Ottenweller JE, Natelson BH. Effect of stressor intensity on habituation and sensitization of glucocorticoid responses in rats. Behav Neurosci. 1990;104(1):28–36. doi: 10.1037/0735-7044.104.1.28. [DOI] [PubMed] [Google Scholar]
- 30.Rai D, Bhatia G, Sen T, Palit G. Comparative study of perturbations of peripheral markers in different stressors in rats. Can J Physiol Pharmacol. 2003;81(12):1139–1146. doi: 10.1139/y03-117. [DOI] [PubMed] [Google Scholar]
- 31.Ricart-Jane D, Rodriguez-Sureda V, Benavides A, Peinado-Onsurbe J, Lopez-Tejero MD, Liobera M. Immobilization stress alters intermediate metabolism and circulating lipoproteins in the rat. Metabolism. 2002;51(7):925–931. doi: 10.1053/meta.2002.33353. [DOI] [PubMed] [Google Scholar]
- 32.Robertson RP, Smith PH. Stress-induced inhibition of triglyceride secretion in vivo in sand rats. Metabolism. 1976;25(12):1583–1590. doi: 10.1016/0026-0495(76)90111-6. [DOI] [PubMed] [Google Scholar]
- 33.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30(1):1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Spencer RL, McEwen BS. Adaptation of the hypothalamic-pituitary-adrenal axis to chronic ethanol stress. Neuroendocrinology. 1990;52(5):481–489. doi: 10.1159/000125632. [DOI] [PubMed] [Google Scholar]
- 35.Starzec JJ, Berger DF, Mason EB, Devito W, Corso C. The effects of differential psychological stress and infantile handling on plasma triglyceride and aortic cholesterol levels in rats. Psychosom Med. 1981;43(6):509–518. doi: 10.1097/00006842-198112000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Tabarin A, Diz-Chaves Y, Consoli D, Monsaingeon M, Bale TL, Culler MD, Datta R, Drago F, Vale WW, Koob GF, et al. Role of the corticotrophin-releasing factor receptor type 2 in the control of food intake in mice: a meal pattern analysis. Eur J Neurosci. 2007;26(8):2303–2314. doi: 10.1111/j.1460-9568.2007.05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teague CR, Dhabhar FS, Barton RH, Beckwith-Hall B, Powell J, Cobain M, Singer B, McEwen BS, Lindon JC, Nicholson JK, et al. Metabonomic studies on the physiological effects of acute and chronic psychological stress in Sprague-Dawley rats. J Proteome Res. 2007;6(6):2080–2093. doi: 10.1021/pr060412s. [DOI] [PubMed] [Google Scholar]
- 38.Thiagarajan AB, Gleiter CH, Mefford IN, Eskay RL, Nutt DJ. Effect of single and repeated electroconvulsive shock on the hypothalamic-pituitary-adrenal axis and plasma catecholamines in rats. Psychopharmacology (Berl) 1989;97(4):548–552. doi: 10.1007/BF00439562. [DOI] [PubMed] [Google Scholar]
- 39.Toleikis PM, Godin DV. Alteration of antioxidant status in diabetic rats by chronic exposure to restraint stressors. Pharmacol Biochem Behav. 1995;52(2):355–366. doi: 10.1016/0091-3057(95)00117-F. [DOI] [PubMed] [Google Scholar]
- 40.van de Kar LD, Blair ML. Forebrain pathways mediating stress induced hormone secretion. Front Neuroendocrinol. 1999;20(1):1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- 41.Vidal J, Buwalda B, Koolhaas JM. Differential long-term effects of social stress during adolescence on anxiety in Wistar and wild-type rats. Behav Process. 2011;87(2):176–182. doi: 10.1016/j.beproc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Yamada F, Inoue S, Saitoh T, Tanaka K, Satoh S, Takamura Y. Glucoregulatory hormones in the immobilization stress induced increase of plasma glucose in fasted and fed rats. Endocrinology. 1993;132(5):2199–2205. doi: 10.1210/en.132.5.2199. [DOI] [PubMed] [Google Scholar]
- 43.Zelena D, Mergl Z, Foldes A, Kovács KJ, Tóth Z, Makara GB. Role of hypothalamic inputs in maintaining pituitary-adrenal responsiveness in repeated restraint. Am J Physiol Endocrinol Metab. 2003;285(5):E1110–E1117. doi: 10.1152/ajpendo.00219.2003. [DOI] [PubMed] [Google Scholar]