Abstract
Previously, both primary and secondary anti-D alloimmunizations induced by “Asian type” DEL (RHD1227A allele) were observed in two incidents. We investigated how often these alloimmunization events occur. The transfusions of any D-negative patients were investigated in the First Affiliated Hospital of Xi’an Jiaotong University Medical College, China, during the entire 2009. The antigens of D, C, c, E, and e were routinely serotyped. The “Asian type” DEL variant was genotyped and the RHD heterozygote was determined through two published methods. The changes in anti-D levels were monitored by the indirect antiglobulin test (IAT) and flow cytometry. Thirty D-negative transfused patients were included in the study. We focused on 11 recipients who were transfused with packed red blood cells (RBCs) from DEL donors at least one time. Of those 11 recipients, seven were anti-D negative before transfusion and four were anti-D positive (one patient with an autoantibody). One of the seven pre-transfusion anti-D negative patients produced a primary-response anti-D after being transfused with 400 ml of DEL blood twice. All four pre-transfusion antibody positive patients were not observed hemoglobin (Hb) levels increased, as expected after transfusions. Two patients had an increase in anti-D from 1:8 to 1:64 by IAT, which was also shown by flow cytometry. None of the patients experienced an acute hemolytic episode. Our data indicated that the primary anti-D induced by DEL transfusion or the secondary anti-D elevated by DEL in a truly D-negative patient might not be unusual. We suggest that a truly D-negative childbearing-aged woman should avoid DEL transfusion to protect her from primary anti-D allosensitization. In addition, anti-D positive recipients should also avoid DEL red cell transfusion due to the delayed hemolytic transfusion reaction (DHTR).
Keywords: Rh blood group, DEL, Allo-anti-D, Transfusion, Pregnancy, Delayed hemolytic transfusion reaction
1. Introduction
The Rhesus (Rh) blood group is of clinical importance because the D antigen presents a strong immunogenicity in transfusion and mother-fetus alloimmunization. Commonly D, encoded by either RH genes or RHD, is divided into D-positive and D-negative phenotypes. It has been observed that D is weakly expressed in many D variants, such as DEL (or Del) (Okubo et al., 1984). The D antigen on a DEL red blood cell (RBC) is so weak that it is serotyped as D-negative by a conventional indirect antiglobulin test (IAT), and needs to be confirmed by an absorption-elution test. Currently neither patients in clinics nor donors in blood banks are screened for DEL, which means we deem DEL as a D-negative donor and a D-negative recipient in transfusion.
DEL alleles were first described by Chang et al. (1998). Although most of them are observed in individuals of European descent, the DEL variant is quite rare in these populations (Wagner et al., 2001), and is absent in individuals of African descent. In East Asia, however, the DEL variant is presented at a rate of 28% in Japanese (Fukumori et al., 1997) and 17% in Koreans among apparent D-negative persons (Kim et al., 2005). We reviewed a number of published papers on DEL that were conducted in Hong Kong, Taiwan, and mainland China (including Chinese papers that weren’t shown in the references) (Mak et al., 1993; Sun et al., 1998; Shao et al., 2002; Chen et al., 2004; Wang et al., 2005; Xu et al., 2005; Sun et al., 2008; Li et al., 2009). There were 1 869 (approximately 27.1%) DELs found by elution or DNA detection among 6 906 apparent D-negative cases. The DELs were genotyped, and it was found that more than 98% (667/678) were carrying the RHD1227A allele, which predominates in East Asians, and is thus called the “Asian type” (Körmöczi et al., 2005; Shao, 2010; Shao et al., 2010).
Although DEL is nearly D antigen negative, some truly D-negative patients were still observed being induced allo-anti-D by DEL red cells in transfusions in Europeans and Asians (Körmöczi et al., 2005; Wagner et al., 2005; Yasuda et al., 2005; Kim et al., 2009). In a case report, Yasuda et al. (2005) reported a secondary anti-D alloimmunization in a woman who received a RHD1227A DEL blood transfusion in Japan, and more recently, Kim et al. (2009) observed a primary allo-anti-D immunization in a 68-year-old Korean man who was transfused with “Asian type” DEL red cells. These studies, along with the high rate of DEL in apparent D-negative individuals, suggest that further clinical investigation is needed to determine whether DEL should be identified in clinics in East Asian countries.
2. Materials and methods
2.1. Design
This study started in January 2009 and lasted throughout the whole year. We routinely observed every D-negative transfusion in the First Affiliated Hospital of Xi’an Jiaotong University Medical College, Shanxi Province, central China. The D antigen was detected for blood recipients in saline, then D-negative donor blood was applied for transfusion. The weak D and partial D phenotypes are rare in Chinese populations. The DEL variant is not currently tested and distinguished from apparent D-negative blood (determined by IAT) within the blood supply institutions of China. For this study, after pre-transfusion tests, both recipient and donor samples of any D-negative transfusions were collected and sent to the Blood Group Reference Laboratory, Shaanxi Blood Center in Xi’an for further serological and genetic analyses. The following information was recorded: recipients, alloimmunization histories, blood transfusions, transfusion reactions, and the results of anti-D, hemoglobin (Hb), indirect bilirubin and haptoglobin tests.
2.2. Serological tests
The routine blood tests for Hb and hematocrit (HCT) or packed cell volume were performed by using a blood cell counter (Hematology Analyzer MEK 6400, Nihon Kohde, Tokyo, Japan) up to 24 h prior to transfusion and then again 24 h post-transfusion. When necessary, the tests were repeated at other points in time. Serum haptoglobin and bilirubin levels were also tested. The pre-transfusion tests, including irregular serum antibody screening and cross-matching tests, were performed using a commercial gel card method (Diana, Branchville, NJ, USA). When irregular antibodies were detected, the serum sample would be re-screened and identified through conventional IAT using erythrocyte screening cells (Immucor Inc., GA, USA, or Sanquin Reagents, Amsterdam, the Netherlands, or Shanghai Blood Center, China), and an erythrocyte panel for antibody specificity identification (Immucor Inc., GA, USA, or Sanquin Reagents, Amsterdam, the Netherlands). Autoantibody was tested too, but the procedure was not completed systematically. The post-transfusion anti-D was tested during the 4th week after the first transfusion. Occasionally, patients were unavailable for additional testing. For Rh blood group typing, the C, c, E, and e antigens were tested in saline (anti-C: MS24; anti-E: MS12; anti-c: MS33; and anti-e: MS16. Immucor Diagnostik GmbH, Roeder-mark, Germany), and the D was typed by IAT (anti-D, IgM+IgG, Clone 175-2 and Clone 415 1E4. Dominion Biological Limited, Nova Scotia, Canada, or Immucor Inc., Norcross, GA, USA).
2.3. Genotyping
Genomic DNA was isolated from patient and donor whole blood samples (Promega wizard genomic DNA extraction kit, Promega Corporation, Madison, WI, USA). DNA was also extracted from the patient’s husband if her serum anti-D detected positive. The RHD was first genotyped through a sequence-specific polymerase chain reaction method (PCR-SSP) by using a commercial kit for partial D detection (BAG Health Care GmbH, Lich, Germany). This method detects all ten exons of RHD and typed C, c, E, and e of the RHCE gene according to the manufacture’s procedures. If a sample was RHD positive for any of the exons, it would be sent to Shenzhen Blood Center for detection of the RHD1227A allele (“Asian type” DEL) and the RHD zygosities using methods detailed elsewhere (Shao et al., 2002; Perco et al., 2003).
2.4. Flow cytometry
To remove the ambiguities inherent to the IAT method, the changes of serum anti-D titer were also confirmed by flow cytometry. The serum samples were mixed 4:1 with 4% RBC suspension in phosphate buffer saline (PBS). The D-positive red cells were pooled from three previously typed DCCee (DCe/DCe) donors (Shao et al., 2002). The D-negative control cells were pooled from three ddccee samples (Shao, 2010). The negative control serum sample came from a D-positive, AB donor. The tubes were incubated at 37 °C for 45 min, and shaken every 15 min. The RBCs were washed with 0.9% (9 g/L) saline solution 5 times, and the supernatant was removed. A total of 15 μl of goat anti-human IgG antigen-binding fragments (Fab) antibody labeled with fluorescein isothiocyanate (FITC) was added (Jackson Immuno Researc, AP, USA), and the mixture was kept in a light-proof container at room temperature for 24 min, and shaken by hand every 5 min. The RBCs were then washed five times with normal saline solution. On the last wash, the supernatant was removed and 2 ml of normal saline was added and mixed for cytometry (FACSCanto II, BD Biosciences, MA, USA). A total of 30 000 to 50 000 events were collected. The median fluorescence intensities were compared across the samples.
3. Results
In 2009 at the Department of Transfusion, Hospital of Xi’an Jiaotong University Medical College, there were 30 D-negative (in IAT) transfusion inpatients. Among them, 11 were transfused with packed red blood cells (PRBCs) or washed red blood cells (WRBCs) from “Asian type” DEL donors at least once. Two patients were found to be heterozygous for the RHD1227A allele (Del/dCcee, 18.2%) and nine patients were dd (81.8%, Table 1, patient No. 15 has nonfunctional RHD allele, RHD-RHCE(2-9)-D). We found that four of the 11 patients had anti-D antibodies before transfusion (one had autoantibody). After two units of PRCBs transfused (one unit is derived from 200 ml of whole blood), two patients tested anti-D titers jumped from 1:8 to 1:64 (50.0%). In the seven anti-D negative patients (two are DEL variants), one of them developed anti-D (1:2) after being transfused twice with two units of DEL PRBCs (14.3%). It was not surprising that the “Asian type” DEL elicits or enhances anti-D to a truly D-negative blood recipient, although primary anti-D is difficult to test. There was no clinical evidence of acute hemolysis or rise in serum haptoglobin in any of the patients.
Table 1.
Truly D-negative patients transfused with DEL RBCs
| Patient No. | Sex | Age (year) | Diagnosis | Rh phenotype | Immune history | Pre-transfusion Abs | Transfusion (Rh/Vol (ml)) |
Post-transfusion anti-D | Reaction | ||
| 1 | 2 | 3 | |||||||||
| 2 | M | 52 | Fracture | ddccee | None | None | DEL/400 | None | None | ||
| 3 | F | 33 | DUB | ddccee | G2P1 with D+ child | Anti-D 1:8 | DEL/400 | 1:64 | Hb: 60→55 g/L | ||
| 7 | M | 45 | Colon cancer | ddCcee | Probably D+ transfusion | Anti-D 1:8 | DEL/400 | dd/400 | 1:64 | Hb: 55→57→60 g/L | |
| 8 | M | 42 | Hemolytic anemia | ddccEE | Apparent D− transfusion | Auto-Ab+ | dd/400 | DEL/400 | dd/200 | Auto-Ab+ | Hb: 38→40→40→46 g/L |
| 11 | F | 28 | Caesarean birth | ddccee | G3P1 with D+ child, probably D+ transfusion | Anti-D 1:512 | DEL/400 | 1:512 | Hb: 46→48 g/L | ||
| 15 | M | 68 | Gastric carcinoma | ddCcee | None | None | DEL/400 | DEL/400 | 1:2 | Hb: 57→62→68 g/L | |
| 17 | F | 31 | Trauma | ddccEE | G1P1 with D+ child | None | DEL/400 | None | None | ||
| 20 | M | 27 | MDS | ddCcee | None | None | DEL/400 | None | Rash | ||
| 25 | F | 12 | AML | ddCcee | None | None | DEL/200 | None | None | ||
DUB: dysfunctional uterine bleeding; MDS: myelodysplastic syndromes; AML: acute myeloid leukaemia; Vol: volume; Ab: antibody
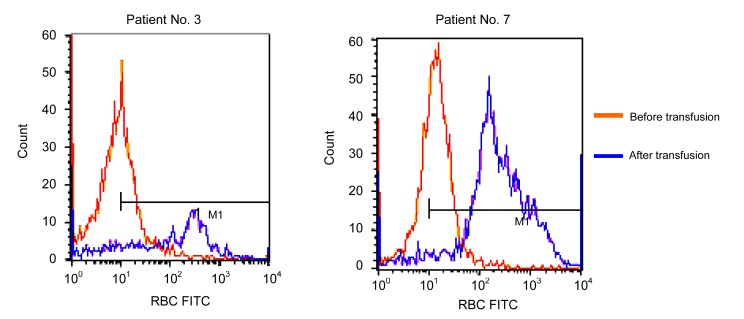
Patient No. 3 (Table 1) was a 33-year-old woman, who was diagnosed with dysfunctional uterine bleeding (DUB). She had two gestations and one parturition (G2P1). She denied any history of transfusion, and denied ever receiving Rh immune globulin prophylaxis. Her husband was a D-positive phenotype with D/D genotype. The day before transfusion, a routine blood test showed whole blood Hb of 60 g/L, RBC 1.80×1012 L−1, platelet count (PC) 80×109 L−1, and HCT of 0.22. In pre-transfusion tests, serum antibody screening and identification showed only anti-D positive at a titer of 8-fold. Two units of cross-match-compatible group B and D-negative PRBCs were transfused simultaneously. No acute transfusion reactions were observed; however, the routine blood test on the third day showed Hb 55 g/L, RBC 1.68×1012 L−1, PC 70×109 L−1, and HCT 0.17, and her serum indirect bilirubin rose to 18.5 μmol/L, which indicated a delayed hemolytic transfusion reaction (DHTR) had occurred. No further transfusions were prescribed. Upon recover, the patient was discharged from the hospital two weeks later. Twenty-five days post transfusion, a blood sample was drawn and an IAT detected anti-D at a titer of 1:64. The titers of pre-transfusion anti-D and post-transfusion anti-D were also compared by flow cytometry (Fig. 1).
Fig. 1.
Flow cytometry analysis of anti-D before and after transfusion
The fluorescein isothiocyanate (FITC) intensities of pre-transfusion and post-transfusion anti-D to D-positive red cells were compared. In patient No. 3, the mean fluorescence intensity and the peak fluorescence intensity before transfusion were 10.22 and 14.28, respectively. Both values after transfusion were 224.74 and 317.54. In patient No. 7, the values were 11.56 and 19.38 pre-transfusion, and 315.35 and 233.26 post-transfusion, respectively
Patient No. 7 was a 45-year-old man with colon carcinoma. His family members declared that he had a transfusion of whole blood 20 years earlier during an appendicitis operation in a county hospital. The number of blood units transfused was unknown; however, Rh(D) blood group testing was not required in China at that time. After being transfused with two units of PRBCs from a DEL donor, his anemia had not improved significantly (Hb 55 to 57 g/L). A second bag of red cells (two units) was applied on the third day, and his Hb increased to 60 g/L. The patient’s serum indirect bilirubin remained in the normal range. Four weeks after transfusion, IAT detected a 4-fold increase in his anti-D (1:8 to 1:64), which was confirmed by flow cytometry (Fig. 1).
Patient No. 11 was a woman at our center who needed a caesarean section due to hemolytic disease of the fetus and the newborn (HDFN). Her anti-D titer was 1:512. She had a history of transfusion due to a childhood surgery and had two miscarriages. She received two units of DEL PRBCs after her operation. Her child recovered with a treatment of exchanged blood. Her anti-D titer remained unchanged by the 4th week of testing, but her Hb dropped and her serum indirect bilirubin rose by the fifth day (26.1 μmol/L) indicating a DHTR.
Seven other patients, who tested negative for anti-D at admission, had no history of D-positive blood transfusions. Patient No. 4 was transfused with apparent D-negative red cells many times. Patient No. 17 was recorded G1P1 (her husband was tested D/D). After DEL transfusions, six of them had no detectable anti-D (two patients, Nos. 4 and 13, were typed as DEL and would not be expected to develop anti-D from DEL transfusions). However, one male patient (No. 15), with no transfusion history, received DEL PRBCs twice, and 22 d after his first transfusion he was found to have a 1:2 titer of anti-D. This appears to be characteristic of primary anti-D alloimmunization.
4. Discussion
Although the D-negative phenotype is rare in East Asian populations (0.3%–0.5%) compared to Caucasians (about 15%), the DEL variant is common among the apparent D-negative individuals. The highest rate reported is approximately one-third (Mak et al., 1993). In this study, among all 30 apparent D-negative patients, six were genotyped DEL (20%), and among the donors to those patients, 12 of 51 are DEL (29%) (data not shown). This indicates that a truly D-negative blood recipient frequently receives DEL blood. It is, therefore, important to explore the effects of the DEL phenotype in clinical blood transfusions or for mother-fetus alloimmunization prophylaxis in East Asia countries.
Commonly we see a donor with a weak D phenotype as a D-positive in transfusion. Although D antigen can be detected on DEL red cells, we deem a DEL as a D-negative donor as there are fewer than 22 RhD antigens per “Asian type” DEL red cell (Körmöczi et al., 2005), and a DEL is routinely typed as D-negative by IAT. According to this study, we should reconsider our screening policy for DEL in donors and recipients. Although the cases reporting primary and secondary anti-D immunizations by DEL (Yasuda et al., 2005; Kim et al., 2009) were once considered a rare event, our primary data show that 50% of truly D-negative patients with anti-D developed DHTR after DEL RBCs transfusion, and 14.3% of apparent D-negative patients with no detected anti-D developed primary allosensitization. If we excluded two DEL recipients (Shao, 2010; Shao et al., 2010), the latter number would be 20.0%. This indicates that the ineffective transfusions elicited by the “Asian type” DELs are a frequent occurrence. Therefore, a truly D-negative woman of childbearing age, should be protected from primary anti-D allosensitization by transfusing her with DEL red cells. In addition, for an anti-D positive recipient, the use of DEL blood, for an effective red cell transfusion, should be avoided. The first step, however, should include screening all donors and recipients for the DEL phenotype.
Another important consideration to the Rh(D) blood group is prophylaxis of mother-fetus alloimmunization, the hemolytic disease of the fetus and the newborn. When a mother is a DEL variant, it is safe to give birth to a D-positive child (Shao, 2010; Shao et al., 2010). However, when a mother is truly D-negative and the fetus or newborn is a DEL variant, according to our study it is important to consider the possible occurrence of allosensitization or a secondary alloimmunization. To our knowledge, an HDFN case due to a DEL variant newborn as never been reported. Maybe a mild DEL HDFN is easily ignored as physiological jaundice. Further research is needed in order to implement a prophylaxis policy in East Asian countries.
It is well-known that the DEL variant occurs in approximately 1/4–1/3 of apparent D-negative East Asian individuals. Although more investigation is needed, our primary data indicates that it is not unusual that the “Asian type” DEL RBCs induce a DHTR in a truly D-negative blood recipient. Therefore it is suggested that blood centers and blood banks need to identify the presence of the DEL variant in apparent D-negative donors and recipients. A truly D-negative blood recipient should not receive DEL transfusion in order to protect him or her from primary anti-D allosensitization or severe DHTR.
Acknowledgments
We appreciated Mr. Andrew DYER, Shenzhen Blood Center, China, for his assistance in editing this paper.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30670893) and the Foundation of Science and Technology Development Scheme of Shaanxi Province (No. 2010K16-01-12), China
References
- 1.Chang JG, Wang JC, Yang TY, Tsan KW, Shih MC, Peng CT, Tsai CH. Human RhD is caused by a deletion of 1 013 bp between introns 8 and 9 including exon 9 of RHD gene. Blood. 1998;92(7):2602–2604. [PubMed] [Google Scholar]
- 2.Chen JC, Lin TM, Chen YL. RHD 1227A is an important genetic marker for RhDel individuals. Am J Clin Pathol. 2004;122(2):193–198. doi: 10.1309/3XMF2NV5707TJE7X. [DOI] [PubMed] [Google Scholar]
- 3.Fukumori Y, Hori Y, Ohnoki S, Nagao N, Shibata H, Okubo Y, Yamaguchi H. Further analysis of Del (RHD-elute) using polymerase chain reaction (PCR) with RHD gene-specific primers. Transfus Med. 1997;7(3):227–231. doi: 10.1046/j.1365-3148.1997.d01-31.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim JY, Kim SY, Kim CA, Yon GS, Park SS. Molecular characterization of D-Korean persons: development of a diagnostic strategy. Transfusion. 2005;45(3):345–352. doi: 10.1111/j.1537-2995.2005.04311.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim KH, Kim KE, Woo KS, Han JY, Kim JM, Park KU. Primary anti-D immunization by DEL red blood cells. Korean J Lab Med. 2009;29(4):361–365. doi: 10.3343/kjlm.2009.29.4.361. [DOI] [PubMed] [Google Scholar]
- 6.Körmöczi GF, Gassner C, Shao CP, Uchikawa M, Legler TJ. A comprehensive analysis of DEL types: partial DEL individuals are prone to anti-D alloimmunization. Transfusion. 2005;45(10):1561–1567. doi: 10.1111/j.1537-2995.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Hou L, Guo ZH, Ye LY, Yue DQ, Zhu ZY. Molecular basis of the RHD gene in blood donors with DEL phenotypes in Shanghai. Vox Sang. 2009;97(2):139–146. doi: 10.1111/j.1423-0410.2009.01181.x. [DOI] [PubMed] [Google Scholar]
- 8.Mak KH, Yan KF, Cheng SS, Yuen MY. Rh phenotypes of Chinese blood donors in Hong Kong, with special reference to weak D antigens. Transfusion. 1993;33(4):348–351. doi: 10.1046/j.1537-2995.1993.33493242645.x. [DOI] [PubMed] [Google Scholar]
- 9.Okubo Y, Yamaguchi H, Tomita T, Nagao N. A D variant, Del? Transfusion. 1984;24(6):542. doi: 10.1046/j.1537-2995.1984.24685066827.x. [DOI] [PubMed] [Google Scholar]
- 10.Perco P, Shao CP, Mayr WR, Panzer S, Legler TJ. Testing for the RHD zygosity with three different methods revealed altered Rhesus boxes and a new weak D type. Transfusion. 2003;43(3):335–339. doi: 10.1046/j.1537-2995.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 11.Shao CP. Transfusion of RhD-positive blood in “Asian type” DEL recipients. N Engl J Med. 2010;362(5):472–473. doi: 10.1056/NEJMc0909552. [DOI] [PubMed] [Google Scholar]
- 12.Shao CP, Maas JH, Su YQ, Köhler M, Legler TJ. Molecular background of Rh D-positive, D-negative, Del and weak D phenotypes in Chinese. Vox Sang. 2002;83(2):156–161. doi: 10.1046/j.1423-0410.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- 13.Shao CP, Xu H, Xu Q, Sun GD, Li JP, Zhang BW, Liang XH, Liu Z, Zhou Y, Li D, Zhuang NB. Antenatal Rh prophylaxis is unnecessary for “Asia type” DEL women. Transfusion Clin Biol. 2010;17(4):260–264. doi: 10.1016/j.tracli.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Sun CF, Liu JP, Chen DP. Use of real time PCR for rapid detection of Del phenotype in Taiwan. Ann Clin Lab Sci. 2008;38(3):258–263. [PubMed] [Google Scholar]
- 15.Sun CF, Chou CS, Lai NC, Wang WT. RHD gene polymorphisms among RhD-negative Chinese in Taiwan. Vox Sang. 1998;75(1):52–57. doi: 10.1046/j.1423-0410.1998.7510052.x. [DOI] [PubMed] [Google Scholar]
- 16.Wagner FF, Frohmajer A, Flegel WA. RHD positive haplotypes in D negative Europeans. BMC Genet. 2001;2:10. doi: 10.1186/1471-2156-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner T, Körmöczi GF, Buchta C, Vadon M, Lanzer G, Mayr WR, Legler TJ. Anti-D immunization by DEL red blood cells. Transfusion. 2005;45(4):520–526. doi: 10.1111/j.0041-1132.2005.04256.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang YH, Chen JC, Lin KT, Lee YJ, Yang YF, Lin TM. Detection of RhDel in Rh D-negative persons in clinical laboratory. J Lab Clin Med. 2005;146(6):321–325. doi: 10.1016/j.lab.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Xu Q, Grootkerk-Tax MG, Maaskant-van Wijk PA, van der Schoot CE. Systemic analysis and zygosity determination of the RHD gene in a D-negative Chinese Han population reveals a novel D-negative RHD gene. Vox Sang. 2005;88(1):35–40. doi: 10.1111/j.1423-0410.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda H, Ohto H, Sakuma S, Ishikawa Y. Secondary anti-D immunization by Del red blood cells. Transfusion. 2005;45(10):1581–1584. doi: 10.1111/j.1537-2995.2005.00579.x. [DOI] [PubMed] [Google Scholar]