Abstract
Objective: To investigate the protective effects and mechanisms of action of dexamethasone and Salvia miltiorrhiza on multiple organs in rats with severe acute pancreatitis (SAP). Methods: The rats were divided into sham-operated, model control, dexamethasone treated, and Salvia miltiorrhiza treated groups. At 3, 6, and 12 h after operation, the mortality rate of different groups, pathological changes, Bcl-2-associated X protein (Bax) and nuclear factor-κB (NF-κB) protein expression levels in multiple organs (the pancreas, liver, kidneys, and lungs), toll-like receptor 4 (TLR-4) protein levels (only in the liver), intercellular adhesion molecule 1 (ICAM-1) protein levels (only in the lung), and terminal deoxynucleotidy transferase mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) staining expression levels, as well as the serum contents of amylase, glutamate-pyruvate transaminase (GPT), glutamic-oxaloacetic transaminase (GOT), blood urea nitrogen (BUN), and creatinine (CREA) were observed. Results: The mortality rate of the dexamethasone treated group was significantly lower than that of the model control group (P<0.05). The pathological changes in multiple organs in the two treated groups were relieved to different degrees (P<0.05 and P<0.01, respectively), the expression levels of Bax and NF-κB proteins, and apoptotic indexes of multiple organs were reduced (P<0.05 and P<0.01, respectively). The contents of amylase, GPT, GOT, BUN, and CREA in the two treated groups were significantly lower than those in model control groups (P<0.05 and P<0.01, respectively). The expression level of ICAM-1 protein in the lungs (at 3 and 12 h) in the dexamethasone treated group was significantly lower than that in the Salvia miltiorrhiza treated group (P<0.05). The serum contents of CREA (at 12 h) and BUN (at 6 h) of the Salvia miltiorrhiza treated group were significantly lower than those in the dexamethasone treated group (P<0.05). Conclusions: Both dexamethasone and Salvia miltiorrhiza can reduce the inflammatory reaction, regulate apoptosis, and thus protect multiple organs of rats with SAP.
Keywords: Dexamethasone, Salvia miltiorrhiza, Pancreatitis, Multiple organs, Rats, Apoptosis, NF-κB, TLR-4, ICAM-1, Tissue microarrays
1. Introduction
When severe acute pancreatitis (SAP) develops, the release of a variety of inflammatory mediators causes a cascade-like reaction. Simultaneous with the massive necrosis of pancreatic tissue, multiple organ failure often results. Inflammatory reaction induced by various inflammatory mediators is one of the major factors contributing to the injury of multiple organs, such as the lungs, kidneys, and liver, often resulting in death (Mizuguchi et al., 2004; Zhang et al., 2007a; 2007b). In SAP, the production of a large number of cytokines and vasoactive substances can, directly or indirectly, give rise to decreased local blood flow and blood flow velocity, leukocyte adhesion, and increased capillary permeability, which can cause microcirculatory disturbances (Strate et al., 2003; von Dobschuetz et al., 2004). Microcirculatory disturbance is one of the trigger factors for the development of acute pancreatitis (AP) (Foitzik et al., 2000; Qu et al., 2003). During the development and progression of SAP, obvious immune dysregulation develops, which manifests mainly as an excessive immune response in the early stages, and immunosuppression in the late stages. Apoptosis is a self-protective strategy employed by the body for removal of destroyed cells by initiating programmed gene expression under certain pathophysiological conditions. There is a kind of substantial distinction between apoptosis and necrosis (Samuilov et al., 2000). The extent of apoptosis is associated with SAP-induced multiple organ injury (Takeyama, 2005; Zhang et al., 2007c). Salvia miltiorrhiza (‘Danshen’ in Chinese) injection and dexamethasone injection are representative traditional Chinese and Western medicines, respectively, most frequently used for the treatment of SAP patients. Salvia miltiorrhiza exerts therapeutic effects on SAP mainly by improving microcirculation, while dexamethasone acts mainly through suppressing inflammatory mediators (Zhang et al., 2007d; 2007e). Salvia miltiorrhiza is a commonly used Chinese herb for promoting blood flow and removing blood stasis. Its main active ingredients are water-soluble materials, including danshensu, salvianolic acid, and protocatechualdehyde (Cui et al., 2007). These compounds can protect endothelial cells, and prevent inflammation, lipid peroxidation, and calcium overload (Kim et al., 2005; Li et al., 2006; Ding and Yuan, 2007; Lam et al., 2007; Zhao et al., 2008). Studies have proved that Salvia miltiorrhiza can mitigate the pathological changes in the liver, lung, kidney, intestine, and stomach of SAP rats, and prevent the occurrence of multiple organ failure (Zhang et al., 2002a; 2002b; 2002c; 2003; 2005). Dexamethasone has anti-inflammatory, anti-toxic, and anti-shock effects. Since it can inhibit the inflammatory response in many diseases, dexamethasone is widely used in the treatment of severe infections (Zhang X.P., et al., 2006; 2008; Zhang X., et al., 2008). Moreover, it can inhibit the expression of multiple inflammatory mediator genes and antagonize the effects of inflammatory mediators by increasing the synthesis of anti-inflammatory proteins, thereby producing therapeutic effects on SAP rats (Sugiyama et al., 2005; Kandil et al., 2006; Muller et al., 2008).
In the present study, we investigated the protective effects and mechanisms of action of dexamethasone and Salvia miltiorrhiza injections on multiple organs of SAP rats by analyzing multiple parameters, and compared the respective advantages of the two drugs.
2. Materials and methods
2.1. Experimental animals
Clean grade male Sprague-Dawley (SD) rats of 250–300 g body weight were obtained from the Experimental Animal Center of the Medical School, Zhejiang University, China.
2.2. Experimental drugs and reagents
Sodium taurocholate was obtained from the Sigma Company (St. Louis, MO 63103, USA). Dexamethasone injection was obtained from Zhejiang Xinchang Pharmaceutical Company, China. Salvia miltiorrhiza injection was obtained from Chiatai Qingchunbao Pharmaceutical Co., Ltd. (Hangzhou, China). Bcl-2-associated X protein (Bax) and nuclear factor-κB (NF-κB) P65 antibodies were purchased from the Santa Cruz Company (California 95060, USA). Intercellular adhesion molecule 1 (ICAM-1) antibody was purchased from Gene Company Limited (Shanghai, China); toll-like receptor 4 (TLR-4) antibody was purchased from Wuhan Boster Biological Technology Co., Ltd. Hubei, China. The main reagent for DNA nick in situ end-labeling (terminal deoxynucleotidy transferase mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL)) staining (TaKaRa in situ apoptosis detection kit) was purchased from the TaKaRa Biotechnology Co., Ltd. (Jindu, Japan). The serum contents of amylase, glutamate-pyruvate transaminase (GPT), glutamic-oxaloacetic transaminase (GOT), blood urea nitrogen (BUN), and creatinine (CREA) were determined using an automatic biochemistry analyzer, and the reagents were purchased from the Sysmex Biological Technology Co. Ltd. (Xi’an, China).
2.3. Experimental methods and animal groupings
A total of 144 rats were randomly divided into sham-operated, model control, dexamethasone treated, and Salvia miltiorrhiza treated groups (n=36 each). These groups were further randomly subdivided into 3, 6, and 12 h groups (n=12 each) according to time points after operation. Fifteen minutes after SAP model was induced, the dexamethasone treated and Salvia miltiorrhiza treated groups were injected via the femoral vein. The sham-operated and model control groups were injected with saline of the same volume at 15 min after the operation. At the corresponding time points after operation, the bloods from heart and tissue specimens of multiple organs (the pancreas, liver, kidneys, and lungs) were collected. The procedures were approved by the ethics committee of Zhejiang Cancer Hospital and Zhejiang University. The following observations were made: (1) after the mortality rates were recorded, the pathological changes in the multiple organs were examined and pathological severity scores were assigned; (2) the amylase contents and hepatic and renal function indexes (GPT, GOT, BUN, and CREA) were determined; (3) tissue microarrays of the multiple organs were prepared (1.5 mm diameter), and then the expression levels of Bax and NF-κB proteins (in the multiple organs), TLR-4 protein (only in the liver), and ICAM-1 protein (only in the lungs), were determined, TUNEL staining (in the multiple organs) was conducted, and apoptotic indexes were recorded.
2.4. Preparation of animal models, therapeutic regimens, and statistical analysis methods
Related research was reviewed in a recent article (Zhang et al., 2010). Briefly, sodium taurocholate (3.5%, 0.1 ml/100 g) was transfused into the bile duct in the direction of the papilla at a flow rate of 0.2 ml/min using a microinjection pump. Fifteen minutes after successful operation, a single dose injection was given, and then the corresponding medication was continued with a micro-infusion pump via the femoral vein. The Kruskal-Wallis test was used for comparisons among the four groups, and the Mann-Whitney test for comparisons within each of the four groups. Differences were considered significant when P<0.05.
3. Results
3.1. Comparison of mortality rate
A related study was reported in a recent article (Zhang et al., 2010). Briefly, at 12 h, the mortality rate was significantly higher in the model control group than in the sham-operated group (P<0.001) or the dexamethasone treated group mortality was significantly lower than that of the model control group (P<0.05).
3.2. Pathological changes in multiple organs
3.2.1. Gross pathological changes in the pancreas
Sham-operated group: the pancreas was intact in structure and yellowish in color. At all time points after operation, no obvious abnormalities were seen in the pancreas or peripancreatic fat and omenta. Model control group: the pathological changes in the pancreas were aggravated with postoperative duration. At 3 h, pancreatic congestion and edema, some of which showed jelly-like hemorrhage or necrosis, were obvious. At 6 and 12 h, the extent and scope of pancreatic congestion and edema were increased; a moderate number of saponified spots were visible in the peripancreatic epiploon; jelly-like changes occurred in the pancreas; pronounced hemorrhage and necrosis were seen. Treated groups: the extent and scope of pancreatic congestion, edema, hemorrhage, and necrosis were comparatively reduced to various degrees. The number of saponified spots was reduced compared to that in the model control group, an effect obvious in both treated groups.
3.2.2. Gross pathological changes in the lungs
Sham-operated group: the color and shape of both lungs were normal. Model control group: congestion and edema were seen in both lungs; the lungs had a pink color; dark red bleeding points on the surface of some areas of pulmonary lobes were seen (3 h). The pathological changes in the lungs were aggravated, and dark red plaques were seen on the surface (6 h, 12 h). Treated groups: the pathological changes in the lungs showed various degrees of mitigation, which was most obvious in the dexamethasone treated group.
3.2.3. Gross pathological changes in the liver
Sham-operated group: no obvious liver swelling was seen, and the liver was normal in color. Model control group: the liver showed mild swelling; some granular plaques with obscure boundaries were seen in some areas of the livers of individual rats (3 h). The liver became pale and turbid or showed congestion; scattered and irregular shaped gray plaques or necrotic spots were seen in some rats, and were especially obvious at the edge of the liver (6 h, 12 h). Treated groups: the pathological changes in the liver showed various degrees of mitigation.
3.2.4. Gross pathological changes in the kidneys
Sham-operated group: no kidney swelling was seen, and the kidneys were normal in morphology. Model control group: no obvious pathological changes in the kidneys were seen (3 h); renal swelling and capsular tension were observed, bleeding points were scattered on the surface of the renal capsule in some rats, and slightly bloody intrapelvic urine was seen in severe cases (6 h, 12 h). Treated groups: the pathological changes in the kidneys showed various degrees of mitigation.
3.2.5. Pathological changes in the pancreas under light microscopy
Sham-operated group: the pancreas was normal in most rats. Focal edema and infiltration of a small number of inflammatory cells were seen in the pancreas of a few rats. Model control group: the pathological changes in the pancreas were aggravated with an increase in postoperative duration. At 3 h, most rats showed interlobular and interacinar edema and acinar necrosis, as well as inflammatory cell infiltration, while a few showed hemorrhage and fat necrosis. At 6 h, all rats showed interlobular and interacinar edema and acinar necrosis, as well as inflammatory cell infiltration, while some showed hemorrhage and fat necrosis. At 12 h, all rats showed interlobular and interacinar edema, acinar necrosis, and inflammatory cell infiltration, as well as hemorrhage, while some rats showed fat necrosis. Salvia miltiorrhiza treated group: at 3 h, some rats showed interlobular and interacinar edema, and interlobular or perivascular inflammatory cell infiltration, as well as acinar necrosis, while some individual rats showed no obvious abnormality. At 6 and 12 h, most rats showed interlobular and interacinar edema, and inflammatory cell infiltration, as well as acinar necrosis. Dexamethasone treated group: at 3 h, most rats showed interlobular and interacinar edema, and interlobular or perivascular inflammatory cell infiltration, as well as occasional acinar necrosis. A small number of rats showed hemorrhage and fat necrosis, and a few showed no obvious abnormality. At 6 h, all rats showed interlobular and interacinar edema, acinar necrosis, and inflammatory cell infiltration, as well as acinar necrosis, while a small number showed mild hemorrhage and fat necrosis. At 12 h, the pancreas of all rats showed interlobular and perivascular inflammatory cell infiltration. Some rats showed interlobular and interacinar edema and others showed acinar necrosis.
3.2.6. Pathological changes in the lungs under light microscopy
Sham-operated group: most rats showed chronic inflammatory cell infiltration in the interstitium, while a few showed no abnormality. Model control group: at 3 h, most rats showed chronic inflammatory cell infiltration in the interstitium, hyperplasia and widening of the alveolar septum, and local deposition of plasma substances into the wall of small arteries, while a few showed only capillary distension and congestion in the alveolar septum, hyperplasia and widening of the alveolar septum, and inflammatory cell infiltration in the interstitium. At 6 h, most rats showed chronic inflammatory cell infiltration in the interstitium, hyperplasia and widening of the alveolar septum, and local deposition of plasma substances into the wall of small arteries, while some showed only capillary distension and congestion in the alveolar septum, hyperplasia and widening of the alveolar septum, and inflammatory cell infiltration in the interstitium. At 12 h, some rats showed chronic inflammatory cell infiltration in the interstitium, capillary distension and congestion in the alveolar wall, and hyperplasia and widening of the alveolar septum while a few showed only inflammatory cell infiltration in the interstitium. Salvia miltiorrhiza treated group: no obvious differences in the pathological changes in the lungs were observed among different time points. Some rats showed inflammatory cell infiltration in the interstitium, and hyperplasia and widening of the alveolar wall. Two rats showed no obvious abnormality in the lungs at 3 and 6 h, respectively (Fig. 1). Dexamethasone treated group: at 3 and 6 h, most rats showed chronic inflammatory cell infiltration in the interstitium, hyperplasia and widening of the alveolar septum, and capillary distension and congestion, while a few showed only chronic inflammatory cell infiltration in the interstitium. Four rats showed no obvious abnormality in the lungs at 3 h (in three cases) and 6 h (in one case) after operation. At 12 h, most rats showed chronic inflammatory cell infiltration in the interstitium, capillary distension and congestion in the alveolar wall, and hyperplasia and widening of the alveolar septum, while a few showed only chronic inflammatory cell infiltration in the interstitium.
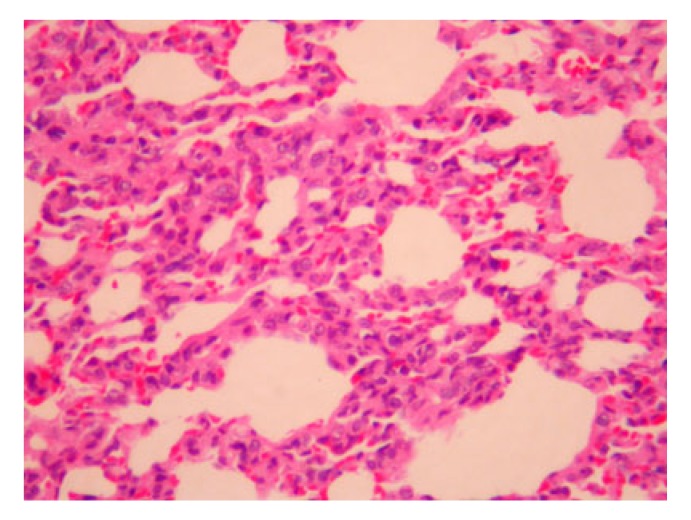
Fig. 1.
Light microscopy of the lungs with Salvia miltiorrhiza treated group at 12 h
Mild thickening of the alveolar septum and exudation of a few inflammatory cells (H&E staining, 200× magnification)
3.2.7. Pathological changes in the liver under light microscopy
Sham-operated group: the structure of the hepatic lobules was intact, inflammatory cell infiltration in the portal area was occasionally seen, and the majority of hepatic cells showed normal morphology. A few rats showed swelling and eosinophilic denaturation of hepatic cells, as well as narrowing of hepatic sinusoids, while some showed hyperplasia of Kupffer cells. Model control group: the pathological changes in the liver were aggravated with an increase in postoperative duration. At 3 h, most rats showed swelling of hepatic cells, narrowing of hepatic sinusoids, neutrophil infiltration and edema in the portal area, eosinophilic denaturation or necrosis of hepatic cells, hyperplasia and hypertrophy of Kupffer cells (Grade I of pathological changes), as well as spotty necrosis, while a few showed patchy necrosis of hepatic cells. At 6 h, most rats showed Grade I pathological changes accompanied by spotty necrosis, while some showed patchy necrosis of hepatic cells. At 12 h, most rats showed Grade I pathological changes accompanied by interconnected spotty necrosis, while some showed patchy necrosis of hepatic cells and hemorrhage. Salvia miltiorrhiza treated group: at all time points, the pathological changes in the liver in the Salvia miltiorrhiza treated group were milder than those in the model control group. At 3 h, most rats showed swelling of hepatic cells, narrowing of hepatic sinusoids, eosinophilic denaturation, and necrosis of hepatic cells, as well as hyperplasia of Kupffer cells. The livers of some rats showed neutrophil infiltration in the portal area. Very few rats showed Grade I pathological changes accompanied by spotty or patchy necrosis. At 6 h, most rats showed swelling of hepatic cells, narrowing of hepatic sinusoids, eosinophilic denaturation of hepatic cells, as well as hyperplasia of Kupffer cells. A few rats showed neutrophil infiltration in the portal area. Very few rats showed Grade I pathological changes accompanied by spotty or patchy necrosis. At 12 h, most rats showed swelling of hepatic cells, narrowing of hepatic sinusoids, eosinophilic denaturation of hepatic cells, and hyperplasia of Kupffer cells, as well as neutrophil infiltration in the portal area. Very few rats showed spotty necrosis or patchy hemorrhage and necrosis. A few showed minor changes in hepatic cells. Dexamethasone treated group: the pathological changes in the liver were mitigated with an increase in postoperative duration. At 3 h, most rats showed Grade I pathological changes while a few showed Grade III pathological changes. At 6 h, most rats showed Grade I pathological changes, while very few showed Grade III pathological changes. One rat showed no obvious abnormality in the liver. At 12 h, some rats showed spotty necrosis, while others showed swelling of hepatic cells, narrowing of hepatic sinusoids, and hyperplasia of Kupffer cells (Fig. 2).
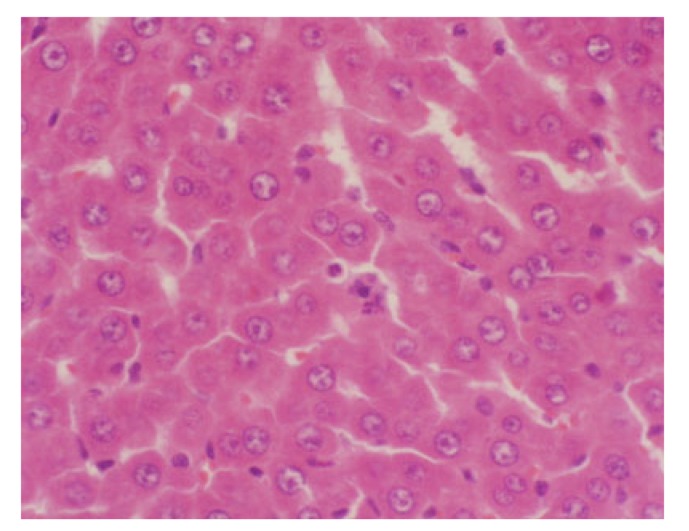
Fig. 2.
Light microscopy of the liver with dexamethasone treated group at 12 h
Swelling of hepatic cells, narrowing of hepatic sinusoids, eosinophilic change of hepatic cells, and spotty necrosis of hepatic cells (H&E staining, 200× magnification)
3.2.8. Pathological changes in the kidneys under light microscopy
Sham-operated group: at all time points, the kidneys were basically normal. A few rats showed swelling of renal tubular epithelium, interstitial edema, and narrowing of renal tubular lumen, while very few showed hyperplasia of glomerular cells. Model control group: the pathological changes in the kidneys were aggravated with an increase in postoperative duration. At 3 h, most rats showed glomerular capillary congestion and scattered necrosis of renal tubular epithelial cells, while some showed interstitial edema and inflammatory cell infiltration, swelling of renal tubular epithelial cells, narrowing of renal tubular lumen and protein casts in the lumen. At 6 h, most rats showed glomerular capillary congestion, swelling of renal tubular epithelial cells, narrowing of renal tubular lumen, protein casts in the lumen, as well as scattered necrosis of renal tubular epithelial cells, while a few showed patchy necrosis of the renal tubular epithelium. At 12 h, most rats showed glomerular capillary congestion, swelling of renal tubular epithelial cells, narrowing or closure of the renal tubular lumen, protein casts in the lumen, as well as patchy necrosis of renal tubular epithelial cells, while a few rats showed scattered patchy necrosis of the renal tubular epithelium (Fig. 3a). Salvia miltiorrhiza treated group: at 3 h, most rats showed glomerular capillary congestion, swelling of renal tubular epithelial cells, protein casts in the lumen, patchy necrosis of renal tubular epithelial cells, interstitial edema and inflammatory cell infiltration in the interstitium, while very few showed scattered patchy necrosis of the renal tubular epithelium. At 6 and 12 h, most rats showed glomerular capillary congestion, swelling of renal tubular epithelial cells, narrowing of the renal tubular lumen, protein casts in the lumen, patchy necrosis of the renal tubular epithelium, as well as interstitial edema (Fig. 3b). Dexamethasone treated group: at 3 h, a few rats had no glomerular abnormality, but showed swelling of renal tubular epithelial cells, narrowing of the renal tubular lumen and protein casts in the lumen. Most rats showed glomerular congestion and scattered necrosis of renal tubular epithelial cells. A few showed patchy necrosis of the renal tubular epithelium. At 6 and 12 h, most rats showed glomerular capillary congestion and scattered necrosis of renal tubular epithelial cells. A few showed patchy necrosis of the renal tubular epithelium and interstitial edema. Some rats had eosinophilic granules in the space between glomerular capillaries, as well as in the glomerulus and the cytoplasm of renal tubular epithelial cells.
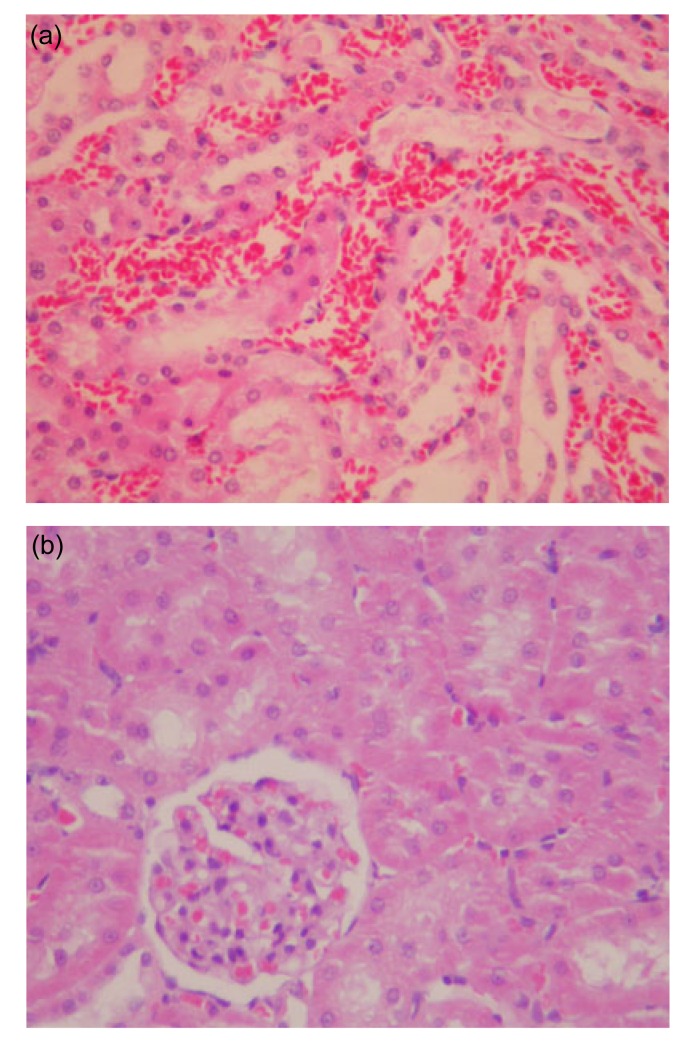
Fig. 3.
Light microscopy of the kidneys with Salvia miltiorrhiza treated and model control groups at 12 h
(a) Model control group at 12 h: renal interstitial congestion and hemorrhage as well as obvious degeneration of renal tubules; (b) Salvia miltiorrhiza treated group at 12 h: obvious turbidity of renal tubular epithelial cells (H&E staining, 200× magnification)
3.3. Comparison of pathological severity scores of multiple organs
The treated groups and the model control group (at all time points) had significantly higher scores than the sham-operated group (P<0.05 or P<0.01). The scores for the pancreas of the treated groups (at 12 h) were significantly lower than that of the model control group (P<0.05). The score for the liver of the Salvia miltiorrhiza treated group (at 6 h) was significantly lower than that of the model control group (P<0.05). The scores for the kidneys of the treated groups (at 6 and 12 h) were significantly lower than that of the model control group (P<0.01) (Table 1).
Table 1.
Comparison of pathological severity scores for multiple organs
| Organ | Time (h) | M (QR) |
|||
| Sham-operated group | Model control group | Dexamethasone treated group | Salvia miltiorrhiza treated group | ||
| Pancreas | 3 | 0.0 (0.0) | 9.0 (3.0)** | 8.0 (4.0)** | 7.0 (3.0)** |
| 6 | 0.0 (0.0) | 9.0 (5.0)** | 7.0 (8.0)** | 7.0 (2.0)** | |
| 12 | 0.0 (0.0) | 12.0 (2.0)** | 9.0 (4.0)** + | 7.0 (3.0)** + | |
| Liver | 3 | 0.0 (1.0) | 2.0 (1.0)** | 1.0 (1.0)** | 1.0 (1.0)** |
| 6 | 0.0 (1.0) | 2.0 (1.0)** | 2.0 (1.0)** | 1.0 (0.0)** + | |
| 12 | 0.0 (1.0) | 2.0 (1.0)** | 1.0 (1.0)** | 1.0 (2.0)** | |
| Kidney | 3 | 0.0 (1.0) | 2.0 (0.0)** | 2.0 (0.0)** | 2.0 (1.0)** |
| 6 | 0.0 (1.0) | 3.0 (0.0)** | 2.0 (1.0)** ++ | 2.0 (0.0)** ++ | |
| 12 | 0.0 (1.0) | 3.0 (0.0)** | 2.0 (0.0)** ++ | 2.0 (0.0)** ++ | |
| Lung | 3 | 0.0 (1.0) | 2.0 (1.0)* | 1.0 (2.0)* | 2.0 (1.0)* |
| 6 | 0.0 (1.0) | 3.0 (2.0)* | 2.0 (1.0)* | 2.0 (2.0)* | |
| 12 | 0.0 (1.0) | 2.0 (2.0)* | 1.0 (1.0)* | 2.0 (2.0)* | |
P<0.05, compared to the sham-operated group
P<0.01, compared to the sham-operated group
P<0.05, compared to the model control group
P<0.01, compared to the model control group
M (QR): median (quartile range)
3.4. Comparison of Bax protein expression level in multiple organs
The expression levels in the pancreas (at 6 h), liver, kidneys, and lungs (at all time points) of the sham-operated group were significantly lower than those of the model control group (P<0.05). The expression levels in the pancreas (at all time points) and liver (at 3 and 6 h) of the sham-operated group were significantly lower than those of the dexamethasone treated group (P<0.05 or P<0.01). The expression levels in the pancreas (at 3 and 12 h) and the liver (at 3 and 6 h) of the sham-operated group were significantly lower than those of the Salvia miltiorrhiza treated group (P<0.05). The expression levels in the pancreas (at 6 h) of the dexamethasone treated group were significantly higher than those of the model control group (P<0.05). The expression levels in the liver (at all time points) and the kidneys (at 6 and 12 h) of the dexamethasone treated group, as well as the liver (at all time points), lungs (at 12 h), and kidneys (at all time points) of the Salvia miltiorrhiza treated group, were significantly lower than those of the model control groups (P<0.05 or P<0.01). The expression levels in the pancreas (at 6 h) of the Salvia miltiorrhiza treated group were significantly lower than those of the dexamethasone treated group (P<0.05) (Table 2).
Table 2.
Comparison of Bax protein levels of multiple organs
| Organ | Time (h) | M (QR) |
|||
| Sham-operated group | Model control group | Dexamethasone treated group | Salvia miltiorrhiza treated group | ||
| Pancreas | 3 | 0.0 (0.0) | 0.0 (1.0) | 1.0 (1.0)* | 1.0 (1.0)* |
| 6 | 0.0 (0.0) | 0.0 (1.0)* | 1.0 (1.0)* + | 0.0 (1.0)a | |
| 12 | 0.0 (0.0) | 0.0 (1.0) | 1.0 (1.0)* | 1.0 (0.0)* | |
| Liver | 3 | 0.0 (0.0) | 1.0 (1.0)* | 1.0 (1.0)+ * | 0.0 (1.0)+ * |
| 6 | 0.0 (0.0) | 2.0 (1.0)* | 0.0 (2.0)+ * | 0.0 (1.0)+ * | |
| 12 | 0.0 (0.0) | 1.0 (2.0)* | 0.0 (0.0)+ | 0.0 (1.0)+ | |
| Kidney | 3 | 0.0 (1.0) | 1.0 (1.0)** | 0.0 (2.0) | 0.0 (0.0)+ |
| 6 | 0.0 (0.0) | 2.0 (2.0)** | 0.0 (2.0)++ | 0.0 (1.0)+ | |
| 12 | 0.0 (0.0) | 2.0 (0.0)** | 0.0 (0.0)++ | 0.0 (1.0)+ | |
| Lung | 3 | 0.0 (0.0) | 1.0 (1.0)* | 1.0 (1.0) | 0.0 (1.0) |
| 6 | 0.0 (0.0) | 1.0 (2.0)* | 1.0 (2.0) | 0.0 (1.0) | |
| 12 | 0.0 (0.0) | 1.0 (1.0)* | 1.0 (1.0) | 0.0 (1.0)+ | |
P<0.05, compared to the sham-operated group
P<0.01, compared to the sham-operated group
P<0.05, compared to the model control group
P<0.01, compared to the model control group
P<0.05, compared to dexamethasone treated group
M (QR): median (quartile range)
3.5. Comparison of NF-κB protein expression levels in multiple organs
The expression levels in the pancreas, lungs, liver (at all time points) and kidneys (at 6 and 12 h) of the sham-operated group were significantly lower than those of the model control group (P<0.05 or P<0.01). The expression levels in the liver (at 3 h) of the sham-operated group were significantly lower than those of the dexamethasone treated group (P<0.05). The expression levels in the pancreas (at 6 and 12 h), lungs (all time points), liver (at 6 and 12 h), and kidneys (at 12 h) of the dexamethasone treated group, as well as the pancreas, lungs (all time points), and liver (at 6 h) were significantly lower than those of the model control group (P<0.05). However, no marked differences were found between the treated groups (P>0.05) (Table 3).
Table 3.
Comparison of NF-κB protein levels of multiple organs
| Organ | Time (h) | M (QR) |
|||
| Sham-operated group | Model control group | Dexamethasone treated group | Salvia miltiorrhiza treated group | ||
| Pancreas | 3 | 0.0 (1.0) | 1.0 (0.0)* | 1.0 (1.0) | 0.0 (1.0)+ |
| 6 | 0.0 (1.0) | 2.0 (1.0)* | 1.0 (1.0)+ | 0.0 (1.0)+ | |
| 12 | 0.0 (1.0) | 2.0 (1.0)* | 0.0 (0.0)+ | 1.0 (1.0)+ | |
| Liver | 3 | 0.0 (0.0) | 1.0 (0.0)* | 1.0 (1.0)* | 1.0 (1.0) |
| 6 | 0.0 (1.0) | 1.0 (1.0)* | 0.0 (0.0)+ | 0.0 (1.0)+ | |
| 12 | 0.0 (1.0) | 1.0 (1.0)* | 0.0 (0.0)+ | 1.0 (1.0) | |
| Kidney | 3 | 0.0 (1.0) | 1.0 (1.0) | 0.0 (1.0) | 0.0 (1.0) |
| 6 | 0.0 (1.0) | 1.0 (1.0)** | 1.0 (2.0) | 1.0 (1.0) | |
| 12 | 0.0 (1.0) | 2.0 (1.0)** | 0.0 (0.0)+ | 1.0 (1.0) | |
| Lung | 3 | 0.0 (1.0) | 2.0 (1.0)* | 1.0 (1.0)+ | 0.0 (1.0)+ |
| 6 | 0.0 (1.0) | 2.0 (1.0)* | 0.0 (1.0)+ | 0.0 (1.0)+ | |
| 12 | 0.0 (1.0) | 1.0 (1.0)* | 0.0 (0.0)+ | 0.0 (1.0)+ | |
P<0.05, compared to the sham-operated group
P<0.01, compared to the sham-operated group
P<0.05, compared to the model control group
M (QR): median (quartile range)
3.6. Comparison of apoptotic index in multiple organs
The indexes of the pancreas (at 12 h), kidneys, and liver (at all time points) of the sham-operated group were significantly lower than those of the model control group (P<0.05 or P<0.01). The indexes of the pancreas (at 6 and 12 h) of the sham-operated group were significantly lower those of the treated groups (P<0.05). The indexes of the liver (at all time points) and the kidneys (at 6 and 12 h) of the dexamethasone treated group, as well as the liver and the kidneys (at all time points) of the Salvia miltiorrhiza treated group, were significantly lower those of the model control groups (P<0.05). No marked differences were noted between the treated groups (P>0.05) (Table 4 and Figs. 4–6).
Table 4.
Comparison of apoptoic indexes of multiple organs
| Organ | Time (h) | M (QR) |
|||
| Sham-operated group | Model control group | Dexamethasone treated group | Salvia miltiorrhiza treated group | ||
| Pancreas | 3 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.005) |
| 6 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.03)* | 0.01 (0.02)* | |
| 12 | 0.0 (0.0) | 0.0 (0.001)* | 0.0 (0.01)* | 0.0 (0.01)* | |
| Liver | 3 | 0.0 (0.0) | 0.005 (0.015)** | 1.0 (0.0)+ | 0.0 (0.0)+ |
| 6 | 0.0 (0.0) | 0.02 (0.065)** | 0.0 (0.0)+ | 0.0 (0.0)+ | |
| 12 | 0.0 (0.0) | 0.02 (0.045)** | 0.0 (0.0)+ | 0.0 (0.0)+ | |
| Kidney | 3 | 0.0 (0.0) | 0.0 (0.01)** | 0.0 (0.0) | 0.0 (0.0)+ |
| 6 | 0.0 (0.0) | 0.01 (0.01)** | 0.0 (0.0)+ | 0.0 (0.0)+ | |
| 12 | 0.0 (0.0) | 0.005 (0.03)** | 0.0 (0.0)+ | 0.0 (0.0)+ | |
P<0.05, compared to the sham-operated group
P<0.01, compared to the sham-operated group
P<0.05, compared to the model control group
M (QR): median (quartile range)
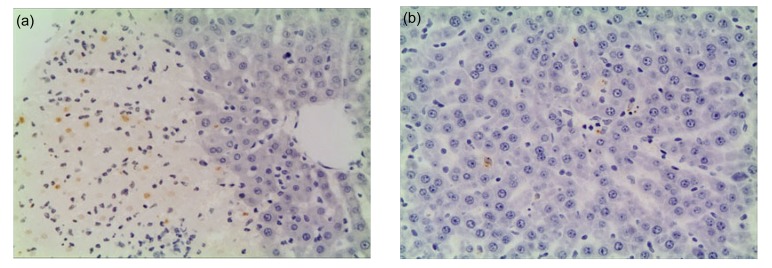
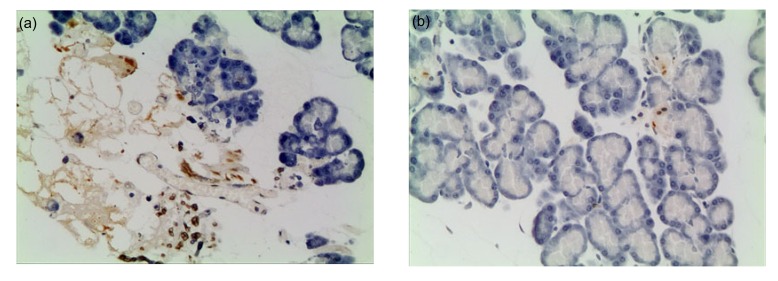
Fig. 4.
Pathological changes in the liver under light microscopy
(a) Model control group at 12 h. The structure of the liver tissue remains, and significant inflammatory cells infiltration and a large number of apoptotic cells can be seen in the necrotic areas of liver. (b) Salvia miltiorrhiza treated group at 12 h. Liver damage is mild, a small amount of inflammatory cells infiltration and a few of apoptotic cells can be seen (TUNEL staining, 200× magnification)
Fig. 6.
Pathological changes in the pancreas under light microscopy
(a) Model control group at 12 h. The structure of the pancreatic tissue remains, and a part of necrosis in the pancreas lobular and a large number of apoptotic cells can be seen. (b) Dexamethasone treated group at 12 h. Pancreatic tissue structure is clear, and there is a small number of apoptotic cells (TUNEL staining, 200× magnification)
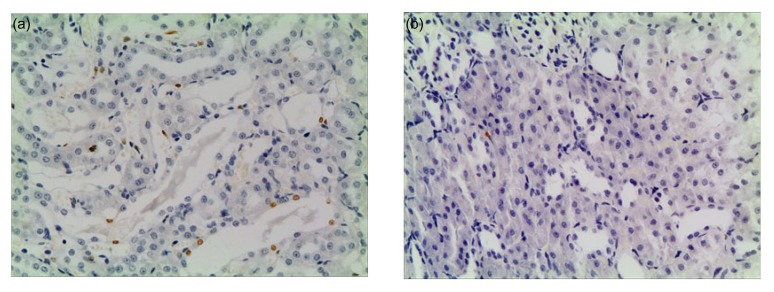
Fig. 5.
Pathological changes in the kidneys under light microscopy
(a) Model control group at 12 h. Renal tissue structure is clear, and renal tubular epithelial cells have many apoptotic cells. (b) Dexamethasone treated group at 12 h. Renal tissue structure is clear and has no significantly damage, and apoptotic cells can be seen occasionally (TUNEL staining, 200× magnification)
3.7. Comparison of TLR-4 protein expression levels in the liver
No marked differences were found among all the groups (P>0.05) (Table 5).
Table 5.
Comparison of TLR-4 and ICAM-1 protein expression levels of multiple organs
| Organ | Time (h) | M (QR) |
|||
| Sham-operated group | Model control group | Dexamethasone treated group | Salvia miltiorrhiza treated group | ||
| TLR-4 | 3 | 0.0 (1.0) | 1.0 (2.0) | 0.0 (1.0) | 0.0 (1.0) |
| 6 | 0.0 (0.0) | 0.0 (2.0) | 0.0 (0.0) | 0.0 (0.0) | |
| 12 | 0.0 (1.0) | 1.0 (2.0) | 0.0 (0.0) | 0.0 (1.0) | |
| ICAM-1 | 3 | 0.0 (1.0) | 0.0 (2.0) | 0.0 (0.0)* + | 0.0 (1.0)a |
| 6 | 0.0 (1.0) | 1.0 (2.0) | 0.0 (1.0) | 0.0 (1.0) | |
| 12 | 0.0 (1.0) | 1.0 (1.0)* | 0.0 (0.0)+ | 0.0 (1.0) | |
P<0.05, compared to the sham-operated group
P<0.05, compared to the model control group
P<0.05, compared to dexamethasone treated group
M (QR): median (quartile range); ICAM-1: intercellular adhesion molecule 1; TLR-4: toll-like receptor 4
3.8. Comparison of ICAM-1 protein expression levels in the lungs
The sham-operated group had significantly lower ICAM-1 levels than the model control group at 12 h (P<0.05). Expression levels in the dexamethasone treated group were significantly lower those of the model control group at 3 and 12 h (P<0.05). The dexamethasone treated group had significantly lower expression levels than the Salvia miltiorrhiza treated group at 3 h (P<0.05) (Table 5).
3.9. Comparison of serum amylase content
The amylase content of the sham-operated group was significantly lower than those of the model control group and the two treated groups at all time points (P<0.01). The two treated groups had significantly lower levels than the model control group at 12 h (P<0.05) (Table 6).
Table 6.
Comparision of different blood indexes
| Index | Time (h) | Amylase (U/L) | GPT (U/L) | GOT (U/L) | BUN (mmol/L) | CREA (μmol/L) |
| Sham-operated group | 3 | 1 510.83±276.27 | 122.25±70.18 | 196.75±52.60 | 7.19±1.81 | 48.17±12.59* |
| 6 | 1 583.92±279.29 | 112.00±60.61 | 221.17±54.06 | 6.86±1.70 | 56.08±18.40* | |
| 12 | 1 721.50±193.47 | 113.33±42.44 | 202.67±64.24 | 7.37±1.74 | 41.00±12.77* | |
| Model control group | 3 | 5 635.36±1 387.94** | 310.09±168.72** | 609.64±287.45* | 15.48±4.30** | 50.25±14.99* |
| 6 | 6 273.43±2 970.86** | 370.71±125.15** | 597.71±313.15* | 17.50±4.15** | 58.50±17.98* | |
| 12 | 9 708.50±3 008.06** | 535.67±213.28** | 734.00±425.84* | 22.49±4.74** | 45.44±16.31* b | |
| Dexamethasone treated group | 3 | 5 186.58±959.18** | 196.50±85.48 | 547.17±210.43* | 11.27±2.84** ++ | 52.83±17.70* |
| 6 | 5 402.09±1 421.92** | 202.73±96.64 | 529.00±201.55* | 15.40±5.59** b | 50.58±17.00* + | |
| 12 | 5 738.44±2 138.37** + | 283.44±145.45* + | 554.78±205.28* | 13.67±3.88** ++ | 49.56±9.00* b | |
| Salvia miltiorrhiza treated group | 3 | 5 623.92±1 684.45** | 259.42±140.71* | 494.08±217.60* | 11.42±2.68* + | 49.08±14.92** |
| 6 | 5 994.92±1 729.72** | 269.00±218.71 | 481.25±196.51* | 11.00±3.81* + | 46.08±16.72** + | |
| 12 | 6 431.71±1 285.00** + | 402.71±294.83* | 544.43±148.82* | 10.95±3.60+ | 27.71±10.11 |
P<0.05, compared to the sham-operated group
P<0.01, compared to the sham-operated group
P<0.05, compared to the model control group
P<0.01, compared to the model control group
P<0.05, compared to the Salvia miltiorrhiza treated group
Data were expressed as mean±standard deviation (SD). GPT: glutamate-pyruvate transaminase; GOT: glutamic-oxaloacetic transaminase; BUN: blood urea nitrogen; CREA: creatinine
3.10. Comparison of serum GPT content
The sham-operated group (at all time points) had a significantly lower GPT content than the model control group (P<0.01), the dexamethasone treated group (at 12 h), and the Salvia miltiorrhiza treated group (at 3 and 12 h). The dexamethasone treated group (at 12 h) had a significantly lower GPT content than the model control group (P<0.05) (Table 6).
3.11. Comparison of serum GOT content
The sham-operated group had a significantly lower GOT content than the model control group and treated groups at all time points (P<0.05) (Table 6).
3.12. Comparison of serum BUN content
The sham-operated group had a significantly lower BUN content than the dexamethasone treated group at all time points (P<0.01) and the Salvia miltiorrhiza treated group at 3 and 6 h (P<0.05). The dexamethasone treated groups had significantly lower BUN contents than the model control group at 3 and 12 h (P<0.01). The Salvia miltiorrhiza treated group had significantly lower BUN contents than the model control groups at all time points (P<0.05) (Table 6).
3.13. Comparison of serum CREA content
The sham-operated group had a significantly lower CREA content than the model control groups and treated groups at all time points (P<0.05), and the Salvia miltiorrhiza treated group at 3 and 6 h (P<0.01). The Salvia miltiorrhiza treated and dexamethasone treated groups had significantly lower CREA contents than the model control group at 6 h (P<0.05). The CREA content of the Salvia miltiorrhiza treated group was significantly lower than that of the dexamethasone treated group at 12 h (P<0.05) (Table 6).
4. Discussion
After treatment with dexamethasone and Salvia miltiorrhiza, the pathological changes in multiple organs of SAP rats showed variable degrees of mitigation. In addition, both drugs could protect the hepatic and renal functions of SAP rats and decrease the contents of amylase and CREA in the blood, suggesting that both drugs can exert protective effects on multiple organs of SAP rats.
Apoptosis is governed by genes. Bax is an apoptosis-inducing gene that belongs to the same family as the bcl-2 gene. Transfection of the Bax gene into cells can increase the apoptosis index (Sha et al., 2008), promoting the formation of Bax homodimers and thus can enhance the occurrence of apoptosis (Gomez et al., 2001). Both dexamethasone and Salvia miltiorrhiza can suppress the expression of Bax protein in multiple organs and mitigate apoptosis-induced multiple organ injury. This result is consistent with the conclusions previously drawn by other researchers (Qu et al., 2003). In this study, we found that dexamethasone can potentially induce apoptosis of pancreatic acinar cells, although its inductive effect is not apparent. Surprisingly, both the up-regulation of Bax protein expression in pancreatic acinar cells and the down-regulation of this protein expression in the liver, kidneys, and lungs could show protective effects on corresponding organs or tissues. To date, there is no convincing explanation for this observation. We speculate that an increase in pancreatic apoptosis may mitigate secondary pancreatic damage since extensive hemorrhage and necrosis are present in the pancreas. In contrast, the pathological changes in the liver, kidneys, and lungs are milder than those in the pancreas. Thus, apoptosis as a mode of cell death will inevitably damage the structure and functions of these organs.
NF-кB is involved in the development of SAP through up-regulating the expressions of ICAM-1 and TNF-α genes. The up-regulation of NF-кB can aggravate SAP-induced multiple organ injury (Meng et al., 2005; Xu et al., 2007; Campo et al., 2008; Song et al., 2008). The results of this study showed that both dexamethasone and Salvia miltiorrhiza can down-regulate the expression of NF-кB protein, reduce the production of inflammatory mediators, and thereby exert protective effects on multiple organs of SAP rats. ICAM-1 can interact with the integrin on the surface of granulocytes and play a key role in the adhesion between leukocytes and vascular endothelial cells. The over-expression of ICAM-1 is associated with SAP-induced multiple organ injury (Martín Alonso et al., 2007). This study proved that dexamethasone was able to reduce the expression level of ICAM-1 protein in the lungs, thus exerting some protective effects.
Our results show that the expression levels of ICAM-1 protein in the lungs of rats in the dexamethasone treated group were significantly lower than those in the Salvia miltiorrhiza treated group, and the contents of CREA and BUN in serum in the Salvia miltiorrhiza treated group were significantly lower than those in the dexamethasone treated group, suggesting that the two drugs have respective advantages and may be used together.
Footnotes
Project (No. 2010382) supported by the Foundation for the Excellent Middle-Aged and Talented Young Persons of Zhejiang Province “151”, China
References
- 1.Campo GM, Avenoso A, Campo S, Nastasi G, Traina P, D′Ascola A, Calatroni A. Chondroitin-4-sulphate reduced oxidative injury in caerulein-induced pancreatitis in mice: the involvement of NF-kappaB translocation and apoptosis activation. Exp Biol Med (Maywood) 2008;233(6):741–752. doi: 10.3181/0711-RM-318. [DOI] [PubMed] [Google Scholar]
- 2.Cui Y, Li Y, Shi W, Yang M, Zhao X, Xia Z. Separation of water-soluble active components in Salvia miltiorrhiza bge. f. alba using capillary zone electrophoresis. Se Pu. 2007;25(5):686–689. (in Chinese) [PubMed] [Google Scholar]
- 3.Ding M, Yuan YJ. Study on the mechanisms of an extract of Salvia miltiorrhiza on the regulation of permeability of endothelial cells exposed to tumor necrosis factor-α. J Pharm Pharmacol. 2007;59(7):1027–1033. doi: 10.1211/jpp.59.7.0016. [DOI] [PubMed] [Google Scholar]
- 4.Foitzik T, Eibl G, Hotz HG, Faulhaber J, Kirchengast M, Buhr HJ. Endothelin receptor blockade in severe acute pancreatitis leads to systemic enhancement of capillary permeability and improved survival rates. Surgery. 2000;128(3):399–407. doi: 10.1067/msy.2000.107104. [DOI] [PubMed] [Google Scholar]
- 5.Gomez G, Lee HM, He Q, Englander EW, Uchida T, Greeley GH., Jr Acute pancreatitis signals activation of apoptosis-associated and survival genes in mice. Exp Biol Med (Maywood) 2001;226(7):692–700. doi: 10.1177/153537020222600716. [DOI] [PubMed] [Google Scholar]
- 6.Kandil E, Lin YY, Bluth MH, Zhang H, Levi G, Zenilman ME. Dexamethasone mediates protection against acute pancreatitis via up-regulation of pancreatitis-associated proteins. World J Gastroenterol. 2006;12(42):6806–6811. doi: 10.3748/wjg.v12.i42.6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JS, Narula AS, Jobin C. Salvia miltiorrhiza water-soluble extract, but not its constituent salvianolic acid B, abrogates LPS-induced NF-kappaB signaling in intestinal epithelial cells. Clin Exp Immunol. 2005;141(2):288–297. doi: 10.1111/j.1365-2249.2005.02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam FF, Yeung JH, Chan KM. Relaxant effects of Salvia miltiorrhiza aqueous extract and its constituent danshensu on rat coronary artery are mediated by inhibition of calcium channels. Vascul Pharmacol. 2007;46(4):271–277. doi: 10.1016/j.vph.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Li D, Xiong SD, Du DB. Inhibitory effects of Salvia miltiorrhiza injection coordinated with dexamethasone on interleukin-13 and eotaxin expression in lung of asthmatic rats. Chin J Int Trad West Med. 2006;26(11):1007–1110. (in Chinese) [PubMed] [Google Scholar]
- 10.Martín Alonso MA, Santamaría A, Saracíbar E, Arranz E, Garrote JA, Almaraz A, Caro-Patón A. Cytokines and other immunological parameters as markers of distant organ involvement in acute pancreatitis. Med Clin (Barc) 2007;128(11):401–406. doi: 10.1157/13100335. [DOI] [PubMed] [Google Scholar]
- 11.Meng Y, Ma QY, Kou XP, Xu J. Effect of resveratrol on activation of nuclear factor kappa-B and inflammatory factors in rat model of acute pancreatitis. World J Gastroenterol. 2005;11(4):525–528. doi: 10.3748/wjg.v11.i4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuguchi T, Mukaiya M, Imaizumi H, Kimura Y, Masuda Y, Katsuramaki T, Asai Y, Hirata K. Successful management of severe acute pancreatitis with multiple organ failure. Pancreas. 2004;28(2):211–213. doi: 10.1097/00006676-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Muller CA, Belyaev O, Appelros S, Buchler M, Uhl W, Borgstrom A. Dexamethasone affects inflammation but not trypsinogen activation in experimental acute pancreatitis. Eur Surg Res. 2008;40(4):317–324. doi: 10.1159/000118027. [DOI] [PubMed] [Google Scholar]
- 14.Qu JG, Zhang JX, Cheng GZ. Effect of urinastatin on microcirculation of extrapancreatic organs in rats with acute necrotizing pancreatitis. Chin J Bases Clin General Surg. 2003;10(3):234–235. (in Chinese) [Google Scholar]
- 15.Samuilov VD, Oleskin AV, Lagunova EM. Programmed cell death. Biochemistry (Mosc) 2000;65(8):873–887. [PubMed] [Google Scholar]
- 16.Sha H, Ma Q, Jha RK, Xu F, Wang L, Wang Z, Zhao Y, Fan F. Resveratrol ameliorates hepatic injury via the mitochondrial pathway in rats with severe acute pancreatitis. Eur J Pharmacol. 2008;601(1-3):136–142. doi: 10.1016/j.ejphar.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Song JM, Liu HX, Li Y, Zeng YJ, Zhou ZG, Liu HY. Extracellular heat-shock protein 70 aggravates cerulein-induced pancreatitis through toll-like receptor-4 in mice. Chin Med J (Engl) 2008;121(15):1420–1425. [PubMed] [Google Scholar]
- 18.Strate T, Mann O, Kleinhans H, Schneider C, Knoefel WT, Yekebas E, Standl T, Bloechle C, Izbicki JR. Systemic intravenous infusion of bovine hemoglobin significantly reduces microcirculatory dysfunction in experimentally induced pancreatitis in the rat. Ann Surg. 2003;238(5):765–771. doi: 10.1097/01.sla.0000094442.12395.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama Y, Kato S, Abe M, Mitsufuji S, Takeuchi K. Different effects of dexamethasone and the nitric oxide synthase inhibitor L-NAME on caerulein-induced rat acute pancreatitis, depending on the severity. Inflammopharmacology. 2005;13(1-3):291–301. doi: 10.1163/156856005774423728. [DOI] [PubMed] [Google Scholar]
- 20.Takeyama Y. Significance of apoptotic cell death in systemic complications with severe acute pancreatitis. J Gastroenterol. 2005;40(1):1–10. doi: 10.1007/s00535-004-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Dobschuetz E, Pahernik S, Hoffmann T, Kiefmann R, Heckel K, Messmer K, Mueller-Hoecker J, Dellian M. Dynamic intravital fluorescence microscopy—a novel method for the assessment of microvascular permeability in acute pancreatitis. Microvasc Res. 2004;67(1):55–63. doi: 10.1016/j.mvr.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Xu P, Zhou XJ, Chen LQ, Chen J, Xie Y, Lv LH, Hou XH. Pioglitazone attenuates the severity of sodium taurocholate-induced severe acute pancreatitis. World J Gastroenterol. 2007;13(13):1983–1988. doi: 10.3748/wjg.v13.i13.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang JX, Cheng GZ, Li L, Qu JG. The effect of labiatae on changes of gastric mucosal microcirculation in rats with acute necrotizing pancreatitis. Jiangsu Monthly J. 2002;28(7):496–498. (in Chinese) [Google Scholar]
- 24.Zhang JX, Cheng GZ, Li L, Qu JG. The effect and protection of labiatae on intestine microcirculation in rats with acute pancreatitis. Chin J Hepatobiliary Surg. 2002;8(12):753–754. (in Chinese) [Google Scholar]
- 25.Zhang JX, Cheng GZ, Li L, Cheng JX, Zhang Q. The effect of labiatae on acute necrotizing pancreatitis in rats with lung injury. Chin J Emerg Med. 2002;11(2):101–104. (in Chinese) [Google Scholar]
- 26.Zhang JX, Qu JG, Li L, Xie R, Cheng GZ. The effect of labiatae on renal injury in rats with acute necrotizing pancreatitis. Chin J Emerg Med. 2003;12(2):97–102. (in Chinese) [Google Scholar]
- 27.Zhang X, Chen L, Luo L, Tian H, Feng G, Cai Y, Xu R, Wang K, Wang Z. Study of the protective effects of dexamethasone on ileum mucosa injury in rats with severe acute pancreatitis. Pancreas. 2008;37(3):e74–e82. doi: 10.1097/MPA.0b013e3181800d11. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XP, Li ZJ, Liu DR. Progress in research into the mechanism of radix Salviae miltiorrhizae in treatment of acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2006;5(4):501–504. [PubMed] [Google Scholar]
- 29.Zhang XP, Lin Q, Zhou YF. Progress of study on the relationship between mediators of inflammation and apoptosis in acute pancreatitis. Dig Dis Sci. 2007;52(5):1199–1205. doi: 10.1007/s10620-006-9388-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XP, Ye Q, Jiang XG, Ma ML, Zhu FB, Zhang RP, Cheng QH. Preparation method of an ideal model of multiple organ injury of rat with severe acute pancreatitis. World J Gastroenterol. 2007;13(34):4566–4573. doi: 10.3748/wjg.v13.i34.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang XP, Zhang L, Wang Y, Cheng QH, Wang JM, Cai W, Shen HP, Cai J. Study of the protective effects of dexamethasone on multiple organ injury in rats with severe acute pancreatitis. JOP. 2007;8(4):400–412. [PubMed] [Google Scholar]
- 32.Zhang XP, Zhang L, Chen LJ, Cheng QH, Wang JM, Cai W, Shen HP, Cai J. Influence of dexamethasone on inflammatory mediators and NF-kappaB expression in multiple organs of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13(47):6385–6395. doi: 10.3748/wjg.13.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang XP, Chen L, Hu QF, Tian H, Xu RJ, Wang ZW, Wang KY, Cheng QH, Yan W, Li Y, et al. Effects of large dose of dexamethasone on inflammatory mediators and pancreatic cell apoptosis of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13(41):5506–5511. doi: 10.3748/wjg.v13.i41.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang XP, Xu HM, Jiang YY, Yu S, Cai Y, Lu B, Xie Q, Ju TF. Influence of dexamethasone on mesenteric lymph node of rats with severe acute pancreatitis. World J Gastroenterol. 2008;14(22):3511–3517. doi: 10.3748/wjg.14.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang XP, Pan Y, Huang XM, Feng GH, Ma ML, Ni J, Zhang FJ. Effects of dexamethasone and Salvia miltiorrhiza on the small intestine and immune organs of rats with severe acute pancreatitis. Inflammation. 2010;33(4):259–266. doi: 10.1007/s10753-010-9180-9. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Shi CX, Li YX. Effect of Salvia miltiorrhiza on mRNA expression of inducible nitric oxide synthase and organ injury in rats with severe acute pancreatitis. Chin J Int Trad West Med. 2005;25(11):1012–1015. (in Chinese) [PubMed] [Google Scholar]
- 37.Zhao GR, Zhang HM, Ye TX. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem Toxicol. 2008;46(1):73–81. doi: 10.1016/j.fct.2007.06.034. [DOI] [PubMed] [Google Scholar]