Abstract
Objective: This study was aimed at assessing the dynamics of vitronectin (VN), laminin (LN), and heparan sulfate/heparin (HS/HP) content changes during experimental burn healing. Methods: VN, LN, and HS/HP were isolated and purified from normal and injured skin of domestic pigs, on the 3rd, 5th, 10th, 15th, and 21st days following thermal damage. The wounds were treated with apitherapeutic agent (propolis), silver sulfadiazine (SSD), physiological salt solution, and propolis vehicle. VN and LN were quantified using an immunoenzymatic assay and HS/HP was estimated by densitometric analysis. Results: Propolis treatment stimulated significant increases in VN, LN, and HS/HP contents during the initial phase of study, followed by a reduction in the estimated extracellular matrix molecules. Similar patterns, although less extreme, were observed after treatment with SSD. Conclusions: The beneficial effects of propolis on experimental wounds make it a potential apitherapeutic agent in topical burn management.
Keywords: Apitherapeutic agent, Silver sulfadiazine, Laminin, Vitronectin, Heparan sulfate/heparin, Wound healing
1. Introduction
Propolis possesses a plethora of biological and therapeutic mechanisms, such as immunomodulatory, antitumor, antiinflammatory, antioxidant, antibacterial, antiviral, antifungal, and antiparasitic actions and can accelerate wound healing and reepithelization (McLennan et al., 2008; Sforcin and Bankova, 2011). Particular propolis compounds have been extensively analyzed; however, little experimental research of its effects on burn wound healing has been carried out (Sforcin and Bankova, 2011). Skin repair after injury proceeds via a finely-tuned pattern of integrated phases: hemostasis, inflammation, proliferation, and remodeling, which all involve a number of cellular and molecular processes (Tong et al., 2008; Goh et al., 2010; Liu et al., 2010; Groah et al., 2011). These include migration and proliferation of epidermal cells and keratinocytes, fibroblast adherence, and extracellular matrix (ECM) contraction (Chen et al., 2005; Goh et al., 2010; Kikkawa et al., 2010). These mechanisms require deposition not only of multidomain adhesive glycoproteins such as laminin (LN) and vitronectin (VN) but also of glycosaminoglycans (GAGs) such as heparan sulfate/heparin (HS/HP) (Schultz and Wysocki, 2009). LN is essential for cell-cell recognition, differentiation, cell survival, and force transmission (Voermans et al., 2008). This omnipresent glycoprotein of all basement membranes is composed of three distinct subunits (α-, β-, and γ-chains), which oligomerize to form a cruciform or T-shaped heterotrimer (Voermans et al., 2008; Durbeej, 2010; Roediger et al., 2010). In vertebrates, five α, three β, and three γ chains, have been identified to create 18 different LN isoforms (Durbeej, 2010). Each LN chain contains specific domains capable of interacting with cellular receptors such as integrins and extracellular ligands including heparan sulfate proteoglycans (HSPGs) (Tzu and Marinkovich, 2008; Durbeej, 2010). Binding of LN to cell surface receptors facilitates its multimerization (Ragbow et al., 2006). Another multiadhesive glycoprotein, VN, present in plasma as a single-chain (75 kDa) or two-chain (10 and 65 kDa) form, undergoes a multimerization process in the ECM (Ekmekçi and Ekmekçi, 2006; Sano et al., 2007). Human VN, also known as a serum-spreading factor or complement S-protein, consists of four structurally different domains: somatomedin B (SMB), hemopexin 1 (N-glycosylated), hemopexin 2, and a connecting region linking the somatomedin and hemopexin 1 domains (Ekmekçi and Ekmekçi, 2006; Piccard et al., 2007). Due to the Arg-Gly-Asp (RGD) amino acid sequence occurring in the SMB domain, VN binds integrins and serves as a cell attachment site promoting cellular adhesion and spreading (Ekmekçi and Ekmekçi, 2006). VN and LN interact with HS/HP proteoglycans (Beauvais et al., 2009; Durbeej, 2010). HS/HP is attached by covalent linkage to the core protein, forming in vivo HSPG (Wegrowski et al., 2006). HS/HP is a linear polymer consisting of repeating disaccharide subunits composed of α(1→4) linked uronic acid (either D-glucuronic acid (GlcA) or L-iduronic acid (IdoA)) and D-glucosamine (Wang et al., 2010; Malavaki et al., 2011). HS is less sulfated with lower IdoA content compared with HP—the highest negatively charged GAG (Malavaki et al., 2011). HS/HP is recognized as a pivotal player in angiogenesis, cell growth, migration, and differentiation (Wegrowski et al., 2006; Malavaki et al., 2011). The expressions of VN, LN, and HS/HP can be changed over the course of different physiological and pathological conditions, resulting in a modified cellular and molecular course of action occurring in the tissue regenerating process. Hence, the aim of this paper was to investigate the influence of the apitherapeutic agent on LN, VN, and HS/HP content changes during experimental burn healing.
2. Materials and methods
2.1. Therapeutic agents
Propolis formulation (apitherapeutic ointment) accepted by the National Institute of Hygiene (certificate number: HŻ/06107/00; date: Nov. 4, 2000). Dermazin—1% (0.01 g/ml) silver sulfadiazine (SSD) cream, Sandoz/Lek, Poland.
2.2. Tissue materials
The study protocol was approved by the Ethics Committee of the Medical University of Silesia, Poland. Four 16-week-old domesticated pigs were chosen for the evaluation of wound repair because of the many similarities of pig skin to human skin. Seventy-two contact burn wounds were inflicted according to the methods proposed by Hoekstra et al. (1993) and Brans et al. (1994). Pigs were housed according to the Good Laboratory Practice (GLP) Standards of Polish Veterinary Law. Animals were divided into control (n=2) and experimental (n=2) groups. In the control group wounds were treated with physiologic saline (NaCl) to observe the healing process occurring without management (one animal) or with a propolis vehicle in order to exclude its possible effect on the propolis properties (another animal), twice a day, throughout 21 d. In the experimental group, burns were treated with propolis (one animal) or SSD (another animal), twice a day, for 21 d. Biopsies, in three replications, were taken from healthy skin at Day 0 and from the wound bed on post-burn Days 3, 5, 10, 15, and 21.
After burn infliction, thermally damaged tissues were rinsed with an antiseptic agent and then treated with propolis, SSD, propolis vehicle, and NaCl, respectively. In the case of burn wounds treated with the propolis, SSD, and propolis vehicle, the wound surface was covered with 0.50–0.75 cm layer of topically applied experimental agent. The wounds were then covered with a woven cotton material. The wounds left by the biopsy were covered with collagen dressing.
2.3. Extraction and assay of tissue VN and LN
Tissue samples, after homogenization with acetone (30 000 r/min, 4 °C for 30 min) and weighing, were treated with 2 mol/L urea solution in 0.05 mol/L Tris-HCl buffer (pH=7.2) containing 0.2 mol/L NaCl, 0.005 mol/L ethylenediaminetetraacetic acid (EDTA), 0.05 mol/L ε-amino caproic acid, and 0.001 mol/L phenylmethylsulfonyl fluoride. Extraction was carried out using constant shaking (4 °C for 24 h). Samples were then centrifuged (19 000×g for 1 h). Tissue pellets were repeatedly extracted as described above. Obtained supernatants were combined and treated with 100% (1 g/ml) trichloroacetic acid (TCA) to achieve 10% acid solution. Precipitation was carried out at 4 °C for 12 h. Protein precipitates were centrifuged (19 000×g for 1 h) and then washed twice with ethanol to remove the TCA. The first wash was conducted with 80% ethanol solution at 20 °C for 2 h with gentle shaking. The second wash was conducted with anhydrous ethanol (Takasaki et al., 1991). Dried protein pellets were stored at −80 °C until quantified. The estimation of LN and VN contents in the protein pellets was obtained using the direct immunoenzymatic method. The following procedure was applied: proteins extracted from samples (0.1 g of dry tissue) of healthy and burned pig skin were dissolved in phosphate buffered saline (PBS) buffer (pH 7.4), and added to microtiter plate wells (Immulon 2HB, Thermo Labsystems, USA) and allowed to adsorb. Well coating was conducted overnight at 4 °C. Upon coating, wells were washed three times with 250 μl of PBS containing 0.05% Tween 20 (i.e., washing buffer) and incubated for 1 h in the washing buffer with 1% (0.01 g/ml) bovine serum albumin (BSA) to prevent nonspecific binding. After extensive rinsing with the washing buffer, coated proteins were exposed for 1 h at room temperature to mouse monoclonal anti-porcine LN antibody (Sigma, L8271) or rabbit anti-porcine VN antibody (CosmoBio, LSL-LB-2096). Subsequently, after rinsing with washing buffer, the appropriate secondary antibodies (i.e., goat anti-mouse and goat anti-rabbit IgG, respectively) conjugated to peroxidase were applied for 1 h at room temperature. Both sera were used at dilution of 1:50 000. After exhaustive rinsing, the colorimetric reaction was initiated by addition of 100 μl of peroxidase substrate 3,3′,5,5′-tetramethylbenzidine, and stopped after 30 min with 100 μl of 1 mol/L HCl. Absorbance in particular wells was measured at 450 nm using an enzyme-linked immunosorbent assay (ELISA) microplate reader TECAN infinite M200. The following antibodies were used: an anti-mouse IgG (Fab specific) peroxidase conjugate antibody developed in goat (Sigma, A2304) and an anti-rabbit IgG (whole molecule) peroxidase conjugate antibody developed in goat (Sigma, A9169).
2.4. Extraction and assay of tissue HS/HP
GAG isolation was carried out according to methods described by Scott (1960) and van Amerongen et al. (1990). Briefly, tissue samples, after homogenization with acetone (30 000 r/min, 4 °C for 30 min), were exhaustively digested with papain (25 mg added to 1 g of dry tissue) in 0.1 mol/L PBS (pH 6.5) for 48 h at 65 °C, to release GAG chains from proteoglycan (PG) core proteins. Peptides generated by papain action, as well as proteins resistant to the enzyme, were removed by precipitation with 100% (1 g/ml) TCA, for 24 h at 4 °C. The mixture was then centrifuged (14 000×g for 20 min at 4 °C). Supernatants (I) containing GAG were restored, while protein pellets were washed with 7% (0.07 g/ml) TCA and centrifuged (14 000×g for 20 min at 4 °C). Both supernatants were combined and subsequently dialyzed against distilled water at exclusion 8–15 kDa (Visking Serva) for 20 h at 4 °C. GAGs were precipitated with ethanol, dissolved in 0.5 mol/L potassium acetate, and reprecipitated with 3 volumes 96% ethanol. After centrifugation (15 500×g for 25 min at 4 °C), the supernatant was discarded, and GAG pellets were dissolved in deionized water and stored at −75 °C until used for biochemical analysis. The total amounts of GAG were quantified by the hexuronic acid assay according to Blumenkrantz and Asboe-Hansen (1973) as modified by Slim et al. (1994). Samples of isolated GAG were electrophoresed on cellulose acetate, before and after the use of enzymes specifically eliminating particular GAG types. The following GAG digestion factors were used: chondroitinase ABC (pH 6.0), chondroitinase ABC (pH 8.0), and chondroitinase B (pH 7.5) (Sigma Aldrich, Poland). Electrophoretic fractionation of GAG was performed as described (Komosińska-Vassev et al., 2008). Obtained electrophoregrams were analyzed by gel documentation system (G:BOX BioImaging Systems).
2.5. Statistical analysis
Repeated measures analysis of variances (ANOVA) was applied to test significance of univariate measures of factors with more than two levels (in our research, six levels: Day 0 and post-burn Days 3, 5, 10, 15, 21) followed by Tukey’s post-hoc tests. Sphericity assumptions were verified and eventually, Greenhouse and Geisser (1959) correction and Huynh and Feldt (1970) adjustments were used. Pearson’s coefficient was used to estimate the association between two of all analyzed variables because of the normal distribution found in our results. P<0.05 was considered to indicate statistical significance (StatSoft, Statistica Version 10).
3. Results
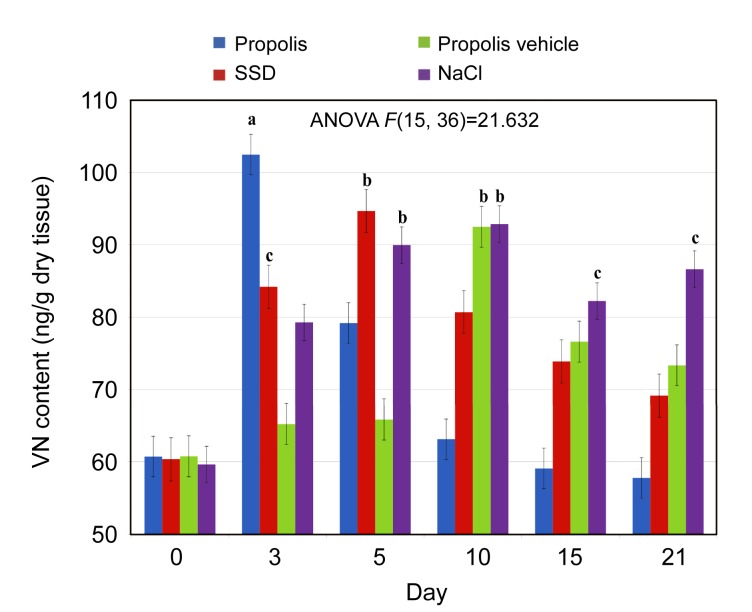
The experimental analysis allowed us to identify two multiadhesive glycoproteins VN and LN as well as GAGs such as HS/HP. As shown in Fig. 1, an increase in VN content in the course of burn repair process, particularly visible after propolis application, was followed by the reduction in VN amount after the 3rd day of the experiment. When SSD was applied, the VN content increased until Day 5 and then began to decrease over the next few days. In contrast, NaCl and propolis vehicle weakly stimulated VN deposition, which peaked at Day 10. The differences in VN content between the first and the last days of the experiment were statistically significant in the case of NaCl-treated wounds (P<0.05). On the other hand, at the final phase of burn healing, propolis (as compared to SSD and propolis vehicle) stimulated the greatest degree of VN level reduction down to the levels resembling those in healthy skin.
Fig. 1.
Dynamics of vitronectin (VN) content alterations in normal skin and skin samples taken from the wound burns treated with propolis, SSD, NaCl, and propolis vehicle
Results are expressed as mean±standard error of the mean (SEM) of the assays performed in triplicate. a P<0.001, b P<0.01, c P<0.05 compared with values determined on Day 0. The different colors represent different treatments (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
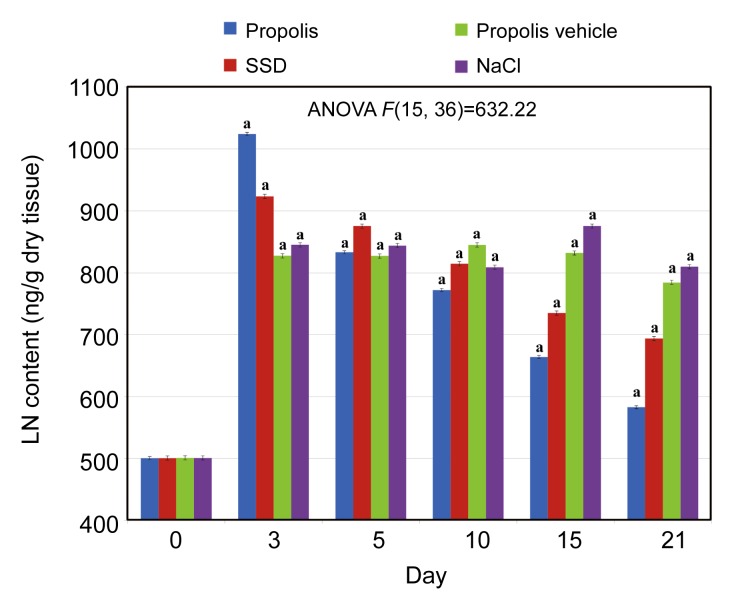
It was also found that during the healing process, the LN amount derived from burned tissue varied depending on the applied agent. The most marked increase in LN content (Days 0–3), followed by a reduction (both alterations statistically significant), was observed in the burned tissue after propolis treatment. Similar changes were found when SSD was applied. A statistically significant trend in LN content was observed in tissues treated with NaCl and propolis vehicle until the 3rd day of the study. Over the next few days, the LN content changed marginally as compared to effects of propolis and SSD treatments. The differences in LN content between the first and the last days of the experiment were statistically significant in all studied tissue samples. The results obtained are presented in Fig. 2.
Fig. 2.
Dynamics of laminin (LN) content alterations in normal skin and skin samples taken from the wound burns treated with propolis, SSD, NaCl, and propolis vehicle
Results are expressed as mean±SEM of the assays performed in triplicate. a P<0.001 compared with values determined on Day 0. The definition of different colors is the same to Fig. 1
The electrophoretic analysis of tissue GAGs confirmed the presence of chondroitin sulfate (CS), dermatan sulfate (DS), hyaluronic acid (HA), and HS/HP (Fig. 3).
Fig. 3.
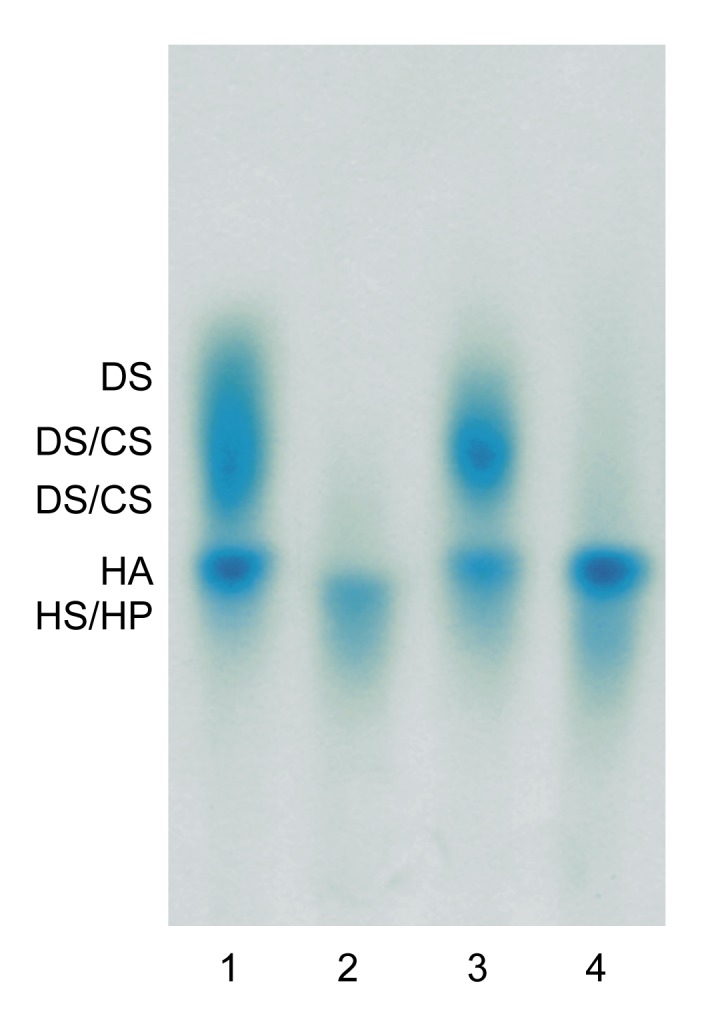
Typical electrophoregram of glycosaminoglycans (GAGs) isolated from burned tissue treated with propolis
Lane 1: sample containing GAGs submitted to electrophoresis without any previous treatment (presence of chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate/heparin (HS/HP), hyaluronic acid (HA)); Lane 2: material resistant to the chondroitinase ABC (pH 6.0) action (presence of HS/HP); Lane 3: material resistant to chondroitinase B action (presence of CS, HS/HP, and HA); Lane 4: product of chondroitinase ABC (pH 8.0) depolymerization (presence of HS/HP and HA)
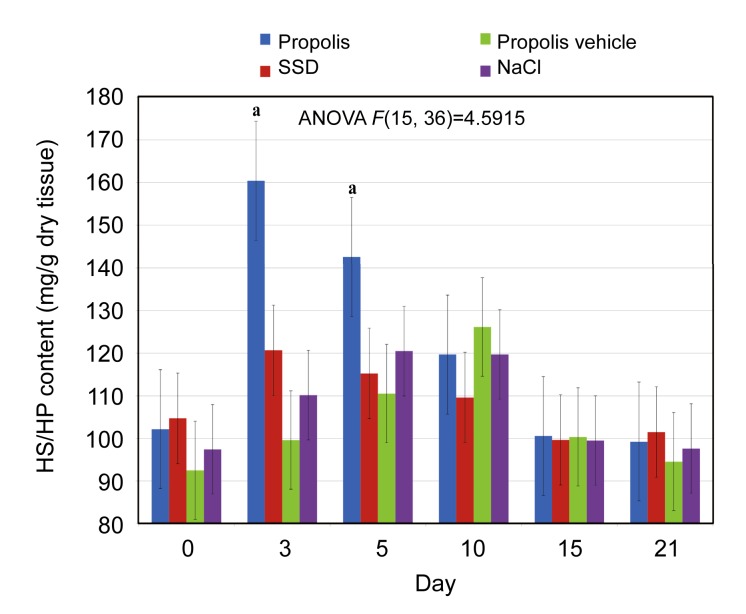
Short-term elevation in wound HS/HP amount was observed only in the case of propolis treatment. It was visible in the first three post-burn days. In the case of skin treated with SSD, propolis vehicle, and NaCl, changes in HS/HP amount were not found. The results obtained are presented in Fig. 4.
Fig. 4.
Dynamics of the heparan sulfate/heparin (HS/HP) content alterations in normal skin and skin samples taken from the wound burns treated with propolis, SSD, NaCl, and propolis vehicle
Results are expressed as mean±SEM of the assays performed in triplicate. a P<0.001 compared with values determined on Day 0. The definition of different colors is the same to Fig. 1
As can be seen from Table 1, during experimental tissue healing positive correlations were observed between: LN and VN isolated from tissues treated with propolis; LN extracted from tissues treated with propolis and VN of SSD-supplied tissues; LN and VN of SSD-treated tissues; LN and VN of NaCl-treated tissues; LN extracted from tissues treated with propolis vehicle and VN of NaCl-treated tissues.
Table 1.
Correlation between laminin (LN) and vitronectin (VN) levels in porcine tissue samples treated with propolis, SSD, NaCl, and propolis vehicle
| Treatment | VN propolis | VN SSD | VN NaCl | VN propolis vehicle |
| LN propolis | r=0.90 (P<0.05) | r=0.82 (P<0.05) | NS | NS |
| LN SSD | NS | r=0.91 (P<0.05) | NS | NS |
| LN NaCl | NS | NS | r=0.85 (P<0.05) | NS |
| LN propolis vehicle | NS | NS | r=0.91 (P<0.05) | NS |
Pearson’s correlation coefficient (r) was used to estimate the association between two of all analyzed variables. NS: non-significant
Moreover, positive correlations were observed between: HS/HP and VN of propolis-supplied tissues; HS/HP extracted from tissues treated with SSD and VN of propolis-supplied tissues (Table 2).
Table 2.
Correlation between heparan sulfate/heparin (HS/HP) and vitronectin (VN) levels in porcine tissue samples treated with propolis, SSD, NaCl, and propolis vehicle
| Treatment | VN propolis | VN SSD | VN NaCl | VN propolis vehicle |
| HS/HP propolis | r=0.94 (P<0.05) | NS | NS | NS |
| HS/HP SSD | r=0.88 (P<0.05) | NS | NS | NS |
| HS/HP NaCl | NS | NS | NS | NS |
| HS/HP propolis vehicle | NS | NS | NS | NS |
Pearson’s correlation coefficient (r) was used to estimate the association between two of all analyzed variables. NS: non-significant
Furthermore, as can be seen from Table 3, during experimental tissue healing, positive correlations were observed between: HS/HP and LN of propolis-supplied tissues; HS/HP extracted from tissues treated with SSD and LN of propolis-supplied tissues; HS/HP extracted from tissues treated with propolis vehicle and LN of SSD-treated tissues.
Table 3.
Correlation between heparan sulfate/heparin (HS/HP) and laminin (LN) levels in porcine tissue samples treated with propolis, SSD, NaCl, and propolis vehicle
| Treatment | LN propolis | LN SSD | LN NaCl | LN propolis vehicle |
| HS/HP propolis | r=0.96 (P<0.05) | NS | NS | NS |
| HS/HP SSD | r=0.93 (P<0.05) | NS | NS | NS |
| HS/HP NaCl | NS | NS | NS | NS |
| HS/HP propolis vehicle | NS | r=0.86 (P<0.05) | NS | NS |
Pearson’s correlation coefficient (r) was used to estimate the association between two of all analyzed variables. NS: non-significant
4. Discussion
Wound healing occurs in multiple stages over several days, and involves increased cell migration and proliferation as well as de novo synthesis of connective tissue (Chen et al., 2005; El Kahi et al., 2009). It is generally accepted that the fibroblasts, keratinocytes, and epidermal cells are pivotal cell “players” throughout the wound healing process (El Kahi et al., 2009; Räsänen and Vaheri, 2010). During tissue regeneration, interaction of keratinocytes and fibroblasts determines conversion of the wound microenvironment from an inflammatory to a synthesis-driven granulation tissue (Werner et al., 2007). Epidermal cells exhibit immunoregulatory functions when skin homeostasis is disrupted by damage or inflammation (Havran and Jameson, 2010). However, fibroblasts, keratinocytes, and epidermal cells are unable to fulfill their special roles during tissue regeneration without the simultaneous accumulation of multiadhesive glycoproteins such as VN and LN or GAGs such as HS/HP in the wound bed. VN, in the presence of growth factors, stimulates keratinocyte migration, proliferation, as well as protein synthesis (Hyde et al., 2004). LN is necessary for migration and proliferation of epidermal cells (Kikkawa et al., 2010). HS/HP is indispensable for adhesive and contractile signaling resulting in myofibroblast formation and wound closure (Chen et al., 2005). The present paper demonstrates the influences of apitherapeutic agents, propolis, and SSD on VN, LN, and HS/HP expression in matrix of experimental burn wounds. Propolis is well known for its antimicrobial, anti-inflammatory, and healing time-reducing properties (Olczyk et al., 2010). SSD is the agent of choice for topical burn therapy, effective in controlling the infection of damaged skin (Costagliola and Agrosi, 2005). Unfortunately, SSD may lead to prolongation of the wound reepithelization process as well as decrease mechanical strength of the dermal tissue (Costagliola and Agrosi, 2005). For the effective comparison of propolis and SSD influence on VN, LN, and HS/HP deposition in the wound bed matrix, the animal model was implemented. Animal wound models constitute the gold standard for understanding reepithelization, angiogenesis, inflammation, and scar formation (Geer et al., 2004).
It was found that propolis stimulated the increase in VN content until the 3rd day of the experiment. It is conceivable that this biological effect of propolis is associated with its ability to stimulate the expression of transforming growth factor-β (TGF-β) (Ansorge et al., 2003; Moura et al., 2011), which in turn induces VN synthesis. TGF-β is responsible for the enhanced deposition of VN which participates in the early phases of wound repair such as hemostasis and inflammation (Koli et al., 1991; Ekmekçi and Ekmekçi, 2006; Bernards and Jiang, 2008). During the following days of the experiment, propolis-treated tissues experienced a reduction in VN amounts down to the level characteristic for healthy skin. This phenomenon is crucial in the later stages of the healing process, because VN stabilizes plasminogen activator inhibitor-1 (PAI-1) both in the plasma and ECM. PAI-1 may cause excessive accumulation of newly synthesized collagen by keloid fibroblasts, representing a novel mechanism participating in keloid pathogenesis (Tuan et al., 2008). The slower and later occurring growth of VN amount followed by its subsequent decrease was found in the case of wounds treated with SSD.
We have also found that propolis contributed to increases in LN levels in the matrix of the burn wound at the beginning of the study, followed by a decrease later in the study. We propose that the significant initial growth of LN content is related to the apitherapeutic agent induced TGF-β expression (Ansorge et al., 2003; Moura et al., 2011). Our results are in consistent with those of Natarajan et al. (2006). Early LN expression is of particular importance in the course of the tissue regeneration process due to the properties of LN, i.e., promoting and supporting cell migration, matrix assembly, and signaling (Köhling et al., 2006; Sugawara et al., 2008). It is noteworthy that subsequent reduction of LN level in propolis-treated burns may also be an event stimulating wound repair. Over the course of tissue regeneration, LN can be depolymerized by matrix metalloproteinases (MMPs), which lead to formation of matrikines, peptides liberated by partial proteolysis of extracellular macromolecules. The epidermal growth factor-like repeats and angiogenic LN-derived peptides are examples of matrikines (Malinda et al., 2008). The same patterns were also observed in the case of SSD implementation; however, the changes were expressed to a lesser extent.
Due to the necessity to quantify the differences in LN metabolism between variously treated contact post-burn wounds of porcine skin, we used LAM-89 monoclonal antibody (Sigma). The antibody, as the only commercially accessible one, makes possible quantitative evaluation of porcine LN by ELISA. However, detailed specificity of LAM-89 toward various porcine LN isoforms is unknown. On the other hand, Geberhiwot et al. (2000) reported that LAM-89, which initially was intended for human LN detection, shows reactivity with its β1 chain. In addition, the clone LAM-89 reacts both with human LN localized in the basement membrane of the dermal-epidermal junction and LN associated with dermal vasculature (El-Hadidy and El-Hadidy, 2003). Thus, taking this into account, along with structural and functional homology between human and pig LNs, and the observed cross-reactivity of LAM-89 with LN from healing wounds of porcine skin, we suppose that porcine isoform containing β1 chain, most probably 511 one, was detected.
Besides LN, HS/HP actively influences dermal-epidermal junction and takes part in the wound healing process (Sugawara et al., 2008; Tong et al., 2008; Iriyama et al., 2011). We observed a short-term increase in HS/HP content in propolis-treated burn wounds. Initial growth of HS/HP amount is pivotal during early stages of tissue repair. It is known that HS/HP plays a key role in chemical signaling between cells through binding and regulating the activities of heparin-binding growth factors, proteolytic enzymes, and protease inhibitors (Tong et al., 2008). However, after injury, HSPGs are degraded by locally secreted factors such as heparanases (Tong et al., 2008). We propose that initially enhanced HS/HP deposition resulted from the antioxidative property of the propolis compound, caffeic acid phenethyl ester, which stimulates superoxide dismutase (SOD) activity (Yildiz et al., 2009). SOD attenuates heparanase expression (Teoh et al., 2009). However, the reduction in HS/HP content during the following days of the experiment is closely related to the later stages of repair process. Elevated HSPG expression during final stages of wound healing may correlate with enhanced fibroblast activation leading to the development of unabated wound repair, resulting in excessive ECM deposition and contraction (Chen et al., 2005).
Moreover, a close linear correlation found between LN and both VN and HS/HP of propolis-treated burns, in contrast to the correlation observed between LN and VN of SSD-supplied wounds, may suggest that the apitherapeutic agent is an effective factor in controlling ECM remodeling during the healing process.
The results obtained demonstrate that propolis modulates VN, LN, and HS/HP metabolism, leading to the better regulation of the primary wound healing cellular events including epidermal cell and keratinocyte migration and proliferation as well as fibroblast activation providing reepithelization and wound closure (Chen et al., 2005; McLennan et al., 2008; Goh et al., 2010; Kikkawa et al., 2010).
It should be emphasized that presented results correspond to histological data described previously (Olczyk et al., 2008). We have found that propolis stimulated faster healing and diminished inflammation as compared with SSD, propolis vehicle, and NaCl action. However, at the beginning of the study (3 d post-burn), coagulative necrosis, inflammatory infiltration, and the lack of stratified squamous epithelium were found in the case of wounds treated with all agents. Only after the 5th day of the experiment did differences in the intensity of healing process vary depending on the applied agent. Only after propolis application did the initial symptoms of granulation (5 d post-burn), followed by enhanced granulation and the first reepithelization signs (10 d post-burn), occur. On Day 15 the extensive reepithelization was visible both on the border as well as in the central part of tissue damage and was accompanied by initial collagen accumulation. During the final days of the study (21 d post-burn), the new epithelium covered the propolis-treated wounds with simultaneous abundant collagen deposition in the burned tissue. The healing process was significantly delayed in the case of the other applied agents, particularly with the propolis vehicle and NaCl.
The beneficial effects of propolis treatment in burn healing found in our study, may suggest a potential application of this apitherapeutic agent in topical burn management.
Footnotes
Project (KNW-2-138/09) supported by the Medical University of Silesia, Poland
References
- 1.Ansorge S, Reinhold D, Lendeckel U. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-β1 production of human immune cells. Z Naturforsch C. 2003;58(7-8):580–589. doi: 10.1515/znc-2003-7-823. [DOI] [PubMed] [Google Scholar]
- 2.Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates αvβ3 and αvβ5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206(3):691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernards MT, Jiang S. pH-induced conformation changes of adsorbed vitronectin maximize its bovine aortic endothelial cell binding ability. J Biomed Mater Res A. 2008;87(2):505–514. doi: 10.1002/jbm.a.31778. [DOI] [PubMed] [Google Scholar]
- 4.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 5.Brans TA, Dutrieux RP, Hoekstra MJ, Kreis RW, du Pont JS. Histopathological evaluation of scalds and contact burns in the pig model. Burns. 1994;20:S48–S51. doi: 10.1016/0305-4179(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Shi-Wen X, van Beek J, Kennedy L, McLeod M, Renzoni EA, Bou-Gharios G, Wilcox-Adelman S, Goetinck PF, Eastwood M, et al. Matrix contraction by dermal fibroblasts requires transforming growth factor-β/activin-linked kinase 5, heparan sulfate-containing proteoglycans, and MEK/ERK: insights into pathological scarring in chronic fibrotic disease. Am J Pathol. 2005;167(6):1699–1711. doi: 10.1016/S0002-9440(10)61252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costagliola M, Agrosi M. Second-degree burns: a comparative, multicenter, randomized trial of hyaluronic acid plus silver sulfadiazine vs. silver sulfadiazine alone. Curr Med Res Opin. 2005;21(8):1235–1240. doi: 10.1185/030079905X56510. [DOI] [PubMed] [Google Scholar]
- 8.Durbeej M. Laminins. Cell Tissue Res. 2010;339(1):259–268. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 9.Ekmekçi OB, Ekmekçi H. Vitronectin in atherosclerotic disease. Clin Chim Acta. 2006;368(1-2):77–83. doi: 10.1016/j.cca.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 10.El-Hadidy MR, El-Hadidy AR. Clinical and histological evaluation of acellular allograft dermal matrix in full-thickness burns. Egypt J Plast Reconstr Surg. 2003;27(1):61–72. [Google Scholar]
- 11.El Kahi CG, Atiyeh BS, Abdallah Hajj Hussein I, Jurjus R, Dibo SA, Jurjus A, Jurjus A. Modulation of wound contracture α-smooth muscle actin and multispecific vitronectin receptor integrin αvβ3 in the rabbit’s experimental model. Int Wound J. 2009;6(3):214–224. doi: 10.1111/j.1742-481X.2009.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geberhiwot T, Wondimu Z, Salob S, Pikkarainenc T, Kortesmaac J, Tryggvasonc K, Virtanend I, Patarroyoa M. Chain specificity assignment of monoclonal antibodies to human laminins by using recombinant laminin β1 and γ1 chains. Matrix Biol. 2000;19(2):163–167. doi: 10.1016/S0945-053X(00)00056-1. [DOI] [PubMed] [Google Scholar]
- 13.Geer DJ, Swartz DD, Andreadis ST. In vivo model of wound healing based on transplanted tissue-engineered skin. Tissue Eng. 2004;10(7):1006–1017. doi: 10.1089/1076327041887727. [DOI] [PubMed] [Google Scholar]
- 14.Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, Tan CK, Huang RL, Sze SK, Tang MB, et al. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J Biol Chem. 2010;285(43):32999–33009. doi: 10.1074/jbc.M110.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24(2):95–112. doi: 10.1007/BF02289823. [DOI] [Google Scholar]
- 16.Groah SL, Libin A, Spungen M, Nguyen KL, Woods E, Nabili M, Ramella-Roman J, Barritault D. Regenerating matrix-based therapy for chronic wound healing: a prospective within-subject pilot study. Int Wound J. 2011;8(1):85–95. doi: 10.1111/j.1742-481X.2010.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol. 2010;184(10):5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoekstra MJ, Hupkens P, Dutrieux RP, Bosch MMC, Brans TA, Kreis RW. A comparative burn wound model in the New Yorkshire pig for the histopathological evaluation of local therapeutic regimens: silver sulfadiazine cream as a standard. Br J Plast Surg. 1993;46(7):585–589. doi: 10.1016/0007-1226(93)90111-N. [DOI] [PubMed] [Google Scholar]
- 19.Huynh H, Feldt LS. Conditions under which mean square ratios in repeated measurements designs have exact F-distributions. J Amer Statist Assoc. 1970;65(332):1582–1589. doi: 10.1080/01621459.1970.10481187. [DOI] [Google Scholar]
- 20.Hyde C, Hollier B, Anderson A, Harkin D, Upton Z. Insulin-like growth factors (IGF) and IGF-binding proteins bound to vitronectin enhance keratinocyte protein synthesis and migration. J Invest Dermatol. 2004;122(5):1198–1206. doi: 10.1111/j.0022-202X.2004.22527.x. [DOI] [PubMed] [Google Scholar]
- 21.Iriyama S, Hiruma T, Tsunenaga M, Amano S. Influence of heparan sulfate chains in proteoglycan at the dermal-epidermal junction on epidermal homeostasis. Exp Dermatol. 2011;20(10):810–814. doi: 10.1111/j.1600-0625.2011.01330.x. [DOI] [PubMed] [Google Scholar]
- 22.Kikkawa Y, Takaki S, Matsuda Y, Okabe K, Taniguchi M, Oomachi K, Samejima T, Katagiri F, Hozumi K, Nomizu M. The influence of tribenoside on expression and deposition of epidermal laminins in HaCaT cells. Biol Pharm Bull. 2010;33(2):307–310. doi: 10.1248/bpb.33.307. [DOI] [PubMed] [Google Scholar]
- 23.Koli K, Lohi J, Hautanen A, Keski-Oja J. Enhancement of vitronectin expression in human HepG2 hepatoma cells by transforming growth factor-β1. Eur J Biochem. 1991;199(2):337–345. doi: 10.1111/j.1432-1033.1991.tb16129.x. [DOI] [PubMed] [Google Scholar]
- 24.Komosińska-Vassev K, Winsz-Szczotka K, Kuznik-Trocha K, Olczyk P, Olczyk K. Age-related changes of plasma glycosaminoglycans. Clin Chem Lab Med. 2008;46(2):219–224. doi: 10.1515/CCLM.2008.048. [DOI] [PubMed] [Google Scholar]
- 25.Köhling R, Nischt R, Vasudevan A, Ho M, Weiergräber M, Schneider T, Smyth N. Nidogen and nidogen-associated basement membrane proteins and neuronal plasticity. Neurodegener Dis. 2006;3(1-2):56–61. doi: 10.1159/000092094. [DOI] [PubMed] [Google Scholar]
- 26.Liu TL, Miao JC, Sheng WH, Xie YF, Huang Q, Shan YB, Yang JC. Cytocompatibility of regenerated silk fibroin film: a medical biomaterial applicable to wound healing. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2010;11(1):10–16. doi: 10.1631/jzus.B0900163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malavaki CJ, Theocharis AD, Lamari FN, Kanakis I, Tsegenidis T, Tzanakakis GN, Karamanos NK. Heparan sulfate: biological significance, tools for biochemical analysis and structural characterization. Biomed Chromatogr. 2011;25(1-2):11–20. doi: 10.1002/bmc.1536. [DOI] [PubMed] [Google Scholar]
- 28.Malinda KM, Wysocki AB, Koblinski JE, Kleinman HK, Ponce ML. Angiogenic laminin-derived peptides stimulate wound healing. Int J Biochem Cell Biol. 2008;40(12):2771–2780. doi: 10.1016/j.biocel.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLennan SV, Bonner J, Milne S, Lo L, Charlton A, Kurup S, Jia J, Yue DK, Twigg SM. The anti-inflammatory agent propolis improves wound healing in a rodent model of experimental diabetes. Wound Rep Reg. 2008;16(5):706–713. doi: 10.1111/j.1524-475X.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- 30.Moura SA, Ferreira MA, Andrade SP, Reis ML, Noviello MD, Cara DC. Brazilian green propolis inhibits inflammatory angiogenesis in a murine sponge model. Evid Based Complement Alternat Med. 2011;2011:182703. doi: 10.1093/ecam/nep197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natarajan E, Omobono JD, 2nd, Guo Z, Hopkinson S, Lazar AJ, Brenn T, Jones JC, Rheinwald JG. A keratinocyte hypermotility/growth-arrest response involving laminin 5 and p16INK4A activated in wound healing and senescence. Am J Pathol. 2006;168(6):1821–1837. doi: 10.2353/ajpath.2006.051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olczyk P, Wróblewska-Adamek I, Stojko J, Komosińska-Vassev K, Olczyk K. Histopathological evaluation of Propol-T and silver sulfadiazine therapeutic efficacy in burn healing. Farm Pol. 2008;63(24):1108–1116. (in Polish) [Google Scholar]
- 33.Olczyk P, Komosińska-Vassev K, Koźma EM, Winsz-Szczotka K, Stojko J, Klimek K, Olczyk K. Assessment of glucuronosyl epimerization of dermatan sulfate chains in the course of burned wound healing. Bull Vet Inst Pulawy. 2010;54:625–629. [Google Scholar]
- 34.Piccard H, van den Steen PE, Opdenakker G. Hemopexin domains as multifunctional liganding modules in matrix metalloproteinases and other proteins. J Leukoc Biol. 2007;81(4):870–892. doi: 10.1189/jlb.1006629. [DOI] [PubMed] [Google Scholar]
- 35.Ragbow R, Seyer J, Kang A. Connective Tissue of the Subendothelium. In: Creager MA, Loscalzo J, Dzau VJ, editors. Vascular Medicine: A Companion to Braunwald’s Heart Disease. 7th Ed. Philadelphia: Saunders Elsevier; 2006. pp. 31–60. [Google Scholar]
- 36.Räsänen K, Vaheri A. Proliferation and motility of HaCaT keratinocyte derivatives is enhanced by fibroblast nemosis. Exp Cell Res. 2010;316(10):1739–1747. doi: 10.1016/j.yexcr.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Roediger M, Miosge N, Gersdorff N. Tissue distribution of the laminin β1 and β2 chain during embryonic and fetal human development. J Mol Histol. 2010;41(2-3):177–184. doi: 10.1007/s10735-010-9275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sano K, Asanuma-Date K, Arisaka F, Hattori S, Ogawa H. Changes in glycosylation of vitronectin modulate multimerization and collagen binding during liver regeneration. Glycobiology. 2007;17(7):784–794. doi: 10.1093/glycob/cwm031. [DOI] [PubMed] [Google Scholar]
- 39.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Rep Reg. 2009;17(2):153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 40.Scott JE. Aliphatic Ammonium Salts in the Assay of Acidic Polysaccharides from Tissues. In: Glick D, editor. Methods of Biochemical Analysis. New York: Wiley; 1960. pp. 145–197. [DOI] [PubMed] [Google Scholar]
- 41.Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011;133(2):253–260. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 42.Slim GC, Furneaux RH, Yorke SC. A procedure for the analysis of glycosaminoglycan mixtures based on digestion by specific enzymes. Carbohydr Res. 1994;255:285–293. doi: 10.1016/S0008-6215(00)90985-6. [DOI] [PubMed] [Google Scholar]
- 43.Sugawara K, Tsuruta D, Ishii M, Jones JC, Kobayashi H. Laminin-332 and -511 in skin. Exp Dermatol. 2008;17(6):473–480. doi: 10.1111/j.1600-0625.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- 44.Takasaki I, Chobanian AV, Brecher P. Biosynthesis of fibronectin by rabbit aorta. J Biol Chem. 1991;266(26):17686–17694. [PubMed] [Google Scholar]
- 45.Teoh ML, Fitzgerald MP, Oberley LW, Domann FE. Overexpression of extracellular superoxide dismutase attenuates heparanase expression and inhibits breast carcinoma cell growth and invasion. Cancer Res. 2009;69(15):6355–6363. doi: 10.1158/0008-5472.CAN-09-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong M, Zbinden MM, Hekking IJ, Vermeij M, Barritault D, van Neck JW. RGTA OTR 4120, a heparan sulfate proteoglycan mimetic, increases wound breaking strength and vasodilatory capability in healing rat full-thickness excisional wounds. Wound Rep Reg. 2008;16(2):294–299. doi: 10.1111/j.1524-475X.2008.00368.x. [DOI] [PubMed] [Google Scholar]
- 47.Tuan TL, Hwu P, Ho W, Yiu P, Chang R, Wysocki A, Benya PD. Adenoviral overexpression and small interfering RNA suppression demonstrate that plasminogen activator inhibitor-1 produces elevated collagen accumulation in normal and keloid fibroblasts. Am J Pathol. 2008;173(5):1311–1325. doi: 10.2353/ajpath.2008.080272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tzu J, Marinkovich MP. Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol. 2008;40(2):199–214. doi: 10.1016/j.biocel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Amerongen JP, Lemmens AG, Tonino GJM. Glycosaminoglycans in Dental Pulp. In: Olgart IK, editor. Dynamic Aspects of Dental Pulp: Molecular Biology, Pharmacology and Pathophysiology. London: Chapman and Hall; 1990. pp. 259–276. [DOI] [Google Scholar]
- 50.Voermans NC, Bönnemann CG, Huijing PA, Hamel BC, van Kuppevelt TH, de Haan A, Schalkwijk J, van Engelen BG, Jenniskens GJ. Interactions between extracellular matrix and growth factors in wound healing. Neuromuscul Disord. 2008;18(11):843–856. doi: 10.1016/j.nmd.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Xu Y, Yang B, Tiruchinapally G, Sun B, Liu R, Dulaney S, Liu J, Huang X. Preactivation-based, one-pot combinatorial synthesis of heparin-like hexasaccharides for the analysis of heparin-protein interactions. Chem Eur J. 2010;16(28):8365–8375. doi: 10.1002/chem.201000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wegrowski Y, Milard AL, Kotlarz G, Toulmonde E, Maquart FX, Bernard J. Cell surface proteoglycan expression during maturation of human monocytes-derived dendritic cells and macrophages. Clin Exp Immunol. 2006;144(3):485–493. doi: 10.1111/j.1365-2249.2006.03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 54.Yildiz Y, Serter M, Ek RO, Ergin K, Cecen S, Demir EM, Yenisey C. Protective effects of caffeic acid phenethyl ester on intestinal ischemia-reperfusion injury. Dig Dis Sci. 2009;54(4):738–744. doi: 10.1007/s10620-008-0405-9. [DOI] [PubMed] [Google Scholar]