Abstract
Background
: Evidence for the effectiveness of treatments for subjects at ultrahigh risk (UHR) for developing psychosis remains inconclusive. Objective : A new cognitive behavioral intervention specifically targeted at cognitive biases (ie, Cognitive Behavioral Therapy [CBT] for UHR patients plus treatment as usual [TAU] called CBTuhr) is compared with TAU in a group of young help-seeking UHR subjects. Methods : A total of 201 patients were recruited at 4 sites and randomized. In most cases, CBTuhr was an add-on therapy because most people were seeking help for a comorbid disorder. The CBT was provided for 6 months, and the follow-up period was 18 months. Results : In the CBTuhr condition, 10 patients transitioned to psychosis compared with 22 in the TAU condition (χ 2 (1) = 5.575, P = .03). The number needed to treat (NNT) was 9 (95% confidence interval [CI]: 4.7–89.9). At 18-month follow-up the CBTuhr group was significantly more often remitted from an at-risk mental state, with a NNT of 7 (95% CI: 3.7–71.2). Intention-to-treat analysis, including 5 violations against exclusion criteria, showed a statistical tendency (χ 2 (1) = 3.338, P = .06). Conclusions : Compared with TAU, this new CBT (focusing on normalization and awareness of cognitive biases) showed a favorable effect on the transition to psychosis and reduction of subclinical psychotic symptoms in subjects at UHR to develop psychosis.
Key words: cognitive behavioral therapy, ultrahigh risk, cognitive biases, prevention, psychosis, schizophrenia
Introduction
Early intervention programs have led to the detection of people with subclinical psychotic symptoms. Australian researchers were the first to develop the criteria for an “At-Risk Mental State” (ARMS).1,2 These criteria cover young people (aged 14–35 years), in a social decline, with a genetic risk, attenuated psychotic symptoms, or a brief limited period of psychotic symptoms in the past year. These criteria have been tested over the last 15 years and were found to predict the onset of a first episode of psychosis at rates several hundred-fold above the incidence of psychosis in the general population.3,4
The early ultrahigh risk (UHR) studies were mostly performed by tertiary specialized clinics. Only recently, early detection has entered the field of routine psychiatric care. The goals of early detection are the following:
1. postponement or prevention of the transition to frank psychosis
2. reduction of the duration of untreated psychosis to a minimum in patients who develop florid psychosis
3. prevention of mental health services delay.
The first mentioned goal is the main goal: Early detection is of little use without an effective intervention. Several pioneering studies have been performed with the aim of preventing transition to psychosis. The interventions were antipsychotic medication, cognitive behavioral therapy (CBT), omega-3 fish oil, or a combination of these therapies. Although the efficacy of these interventions is encouraging, the results remain inconclusive, thus, warranting more randomized clinical trials.5–7
A characteristic of the current trial is that patients in both the experimental and the control condition have been treated with state-of-the-art treatment for the disorder for which the subject was seeking help. Because the disorder was always treated in both conditions, the experimental add-on intervention targeted the subclinical symptoms and cognitive biases that play a role in the development of psychosis. The UHR subject is characterized by dopamine supersensitivity. The dopamine-2 receptor can exist in a state of high affinity or in a state of low affinity. All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2 receptors.8 In animal models, an increase in sensitivity can be caused by caffeine, cocaine, metamphetamine, ethanol withdrawal (2- to 3-fold), social isolation, amphetamine, or hippocampus lesion (3- to 4-fold), or by gene deletions in pathways for neurotransmitters such as glutamate, dopamine, gamma amino butyric acid, acetylcholine, and norepinephrine. Supersensitivity results in subclinical psychotic symptoms and also exaggerates biases in cognitive processes, such as selective attention for irrelevant cues,9 confirmation bias,10 and data-gathering bias11 and affects high-level cognitive processes.12 Moritz and Woodward described the role of metacognitive processes and biases in the formation and maintenance of delusions and also developed a training program to make patients aware of the biases.13 The jumping-to-conclusions bias is present in prodromal, symptomatic, and remitted patients and also in first-degree relatives. Freeman and colleagues found that despite jumping to conclusions, patients viewed themselves as rather hesitant decision makers who are open to other views and sufficiently weigh the pros and cons of different viewpoints.14 Teaching patients to be aware of their jumping-to-conclusions reasoning style, can consequently be followed by teaching a strategy to trump this bias (eg, whenever the patient notices suspiciousness, the patient is instructed not to trust his first appraisal). Before acting on these impulses, the patient is asked to think of other possible explanations and discuss these with others before acting on his suspicion. The attribution of perceptual aberrations to dopamine supersensitivity can normalize these experiences as quite common with a known cause leading to reduced distress because dysfunctional appraisals are no longer adhered.
This study evaluates an add-on intervention based on CBT, with additional education on the effects of dopamine supersensitivity on perception and reasoning, and exercises to become aware of and correct the effects of cognitive biases. The hypothesis is that the targeted intervention during 6 months will reduce the number of transitions to psychosis and increase the number of subjects who will be relieved from subclinical psychotic symptoms at 6-, 12-, and 18-month follow-up. In addition, we hypothesize that secondary outcomes (such as depression and anxiety) will improve in CBT for ultrahigh risk patients (CBTuhr) compared with treatment as usual (TAU).
Methods
Trial Design
The trial is a randomized controlled trial, in which CBTuhr as an add-on to TAU is compared with TAU, in a group of help-seeking people in mental health services. The study is known as the Dutch Early Detection and Intervention Evaluation (EDIE-NL). The design of this study is approved by the Dutch Union of Medical-Ethics Trial Committees for mental health organizations. The trial was conducted in compliance with the Declaration of Helsinki (amendment of Edinburgh, 2000) and is registered at Current Controlled Trials as trial number ISRCTN21353122 (http://controlled-trials.com/ISRCTN21353122/gaag). Details of the study have been published elsewhere.15
Instruments
1. Prodromal Questionnaire (PQ).16 This is a screening list of 92 statements, of which 45 items refer to subclinical positive symptoms. A cutoff score of ≥18 was used to decide for a diagnostic interview.
2. Comprehensive Assessment of At-Risk Mental State (CAARMS).2 The CAARMS is a semistructured interview that assesses ARMS symptoms. The CAARMS consists of 7 subscales that include 4 positive symptom items (unusual thought content, nonbizarre ideas, perceptual abnormalities, and disorganized speech), 2 cognitive and 3 emotional disturbance items, 3 negative symptom items, 4 behavioral change items, 4 motor/physical changes items, and 8 general psychopathology items. Intensity and frequency of the symptoms is scored on a 7-point Likert scale and distress caused by the symptom on a 0–100 scale. Symptomatic criteria for ARMS are based exclusively on positive symptom items. The EDIE-NL investigators received 2 days of training by Professor A. Yung, who developed the CAARMS criteria. Reliability checks of the Dutch version of the CAARMS were performed approximately every 3 months during the study. The preliminary pairwise interrater concordance of the intensity subscales of the CAARMS was 0.81, which was considered acceptable by the training team. The Social and Occupational Functioning Assessment Scale (SOFAS)17 was used to determine the level of social and occupational functioning. This scale, ranging from 0 to 100, is a modified version of the Global Assessment of Functioning (GAF) scale, separating the measures of social and occupational functioning from the measures of symptoms and psychological functioning. Three subgroups of ARMS patients are identified: genetic risk (schizotypy or a first-degree relative with a psychotic disorder, both with recently marked social decline), attenuated psychotic symptoms (subclinical psychotic symptoms, not fulfilling the criteria of psychosis), and patients who have experienced a brief limited intermittent psychosis (full-blown psychosis of ≤1 week with spontaneous remission). Details of the scoring system are described elsewhere.15
3. Schedules for Clinical Assessment in Neuropsychiatry (SCAN),18 to diagnose the patients who transitioned to psychosis.
4. Positive and Negative Syndrome Scale (PANSS),19 to assess the severity of psychotic symptoms after transition.
5. Depression was assessed with the Beck Depression Inventory (BDI-2)20 and with the Calgary Depression Scale (CDS).21
6. Social Interaction Anxiety Scale (SIAS).22
7. Manchester Short Assessment of Quality of Life (MANSA).23
8. Social and Occupational Functioning Assessment Scale (SOFAS),17 to assess overall functioning in a single score (0–100).
9. Personal Beliefs on Illness Questionnaire-Revised (PBIQ-R).24 This has 5 subscales: control over symptoms; entrapment by illness; loss of autonomy; social participation; and shame.
Setting
Participants were recruited at 4 sites in the Netherlands: (1) 93 patients at Mental Health Centre PsyQ Haaglanden, the Hague, (2) 51 patients at Academic Medical Centre and Mental Health Centre PsyQ, Amsterdam, (3) 27 patients at Mental Health Centre Rivierduinen, Leiden and surroundings, and (4) 30 patients at Mental Health Institute Friesland in the province of Friesland.
Procedure
The self-rating of subclinical psychotic symptoms on the PQ was followed by an interview-based rating with the CAARMS: a gold standard for diagnosing both psychosis and ARMS. If patients fulfilled every criterion, they were asked to participate in the study. All subjects were treated for the disorder for which they were seeking help. In addition, they were informed that they showed a risk profile for developing future mental problems and could be offered a preventive intervention. After adequately describing the study to the subjects, written informed consent was obtained. Persons aged ≤16 years also required consent from a parent or guardian.
Randomization
Randomization was stratified by site in order to rule out factors relating to the institutions, therapists, and location. The random allocation lists were generated by a web-based automated randomization system. A numeric balance across conditions was guaranteed by performing the randomization separately for each research site, in random-permuted blocks of 10. The allocation list was kept in a remote secure location, and an independent person randomly allocated the included patients after they signed informed consent. The randomization status was confirmed by fax to the randomization bureau by the different sites. Those who performed research assessments were kept blind to randomization. The assessors started each assessment by stating that the patient should not talk about therapy or therapist. In case the blinding was broken, the assessor was when possible replaced. This was reported and performed 5 times.
Interventions
The intervention used here is based on the protocol by French and Morrison25 that we have enriched with psycho-education on dopamine and cognitive biases. Both the experimental and the control group were treated with evidence-based active treatment for the axis 1 or 2 disorder from which they were suffering. The experimental group was given an add-on treatment that focused on subclinical psychosis. The protocol by French and Morrison (as a generic CBT protocol) has much in common with the active treatments, as CBT is the treatment recommended for many axis 1 disorders. For this reason we enriched the protocol with education on dopamine supersensitivity, explaining how this affects perception (hypersalience for trivial stimuli) and thinking (more intrusions, more causal reasoning over coincidences, stronger data-gathering bias, etc.). Furthermore, exercises were added to experience cognitive biases; becoming aware of cognitive biases may lead to corrected secondary appraisals.
The biases addressed are the following:
1. data-gathering bias, mainly characterized by jumping to conclusions
2. selective attention to threatening stimuli
3. confirmatory bias, moderating delusion formation
4. negative expectation bias, leading to increased distress levels, as well as underrating of one’s capacities
5. covariance bias, in which the chance of a causal relationship between independent events is overrated.
CBTuhr had a maximum provision of 26 weekly sessions. The mean number of sessions was 10: 16 patients had no sessions at all; 21 had 1–5 sessions; 16 had 6–11 sessions; and 45 had 12–25 sessions. Behavioral goals are to consolidate school and work attendance, foster interaction with friends and relatives, and, if applicable, to reduce cannabis use. A manual of the protocol can be obtained on request from the first author, and a book on the treatment of ARMS is in preparation. The therapists were all experienced in CBT for psychosis and were trained in the use of the protocol. There were 6 therapists in the Hague, 4 in Amsterdam, 2 in the province Friesland, and 4 in the Leiden region. One was a psychiatrist trained in CBT and all the others psychologists. The experience varied from 1 to 26 years.
Treatment Fidelity
The therapists were closely supervised: The group supervision sessions were scheduled every 2 weeks and a therapist attended at least once a month. The progress of the protocol was checked in the supervision sessions. A random selection of the taped therapy sessions was rated with the Revised Cognitive Therapy Scale.26 The scores of all therapists were (at least) at competent level (17.5% was competent; 55% proficient; and 27.5% expert level).
Participants
Patients were eligible for inclusion if the following criteria were met: (1) age 14–35 years; (2) a genetic risk or CAARMS scores in the range of the ARMS; and (3) an impairment in social functioning (a score on the SOFAS of 50 or less, and/or a reduction by 30% on the SOFAS for at least 1 month in the past year). Patients were excluded if they met any of the following criteria: (1) current or previous use of antipsychotic medication with ≥15mg cumulative haloperidol equivalent; (2) severe learning impairment; (3) problems due to an organic condition; (4) insufficient competence in the Dutch language; and (5) history of psychosis.
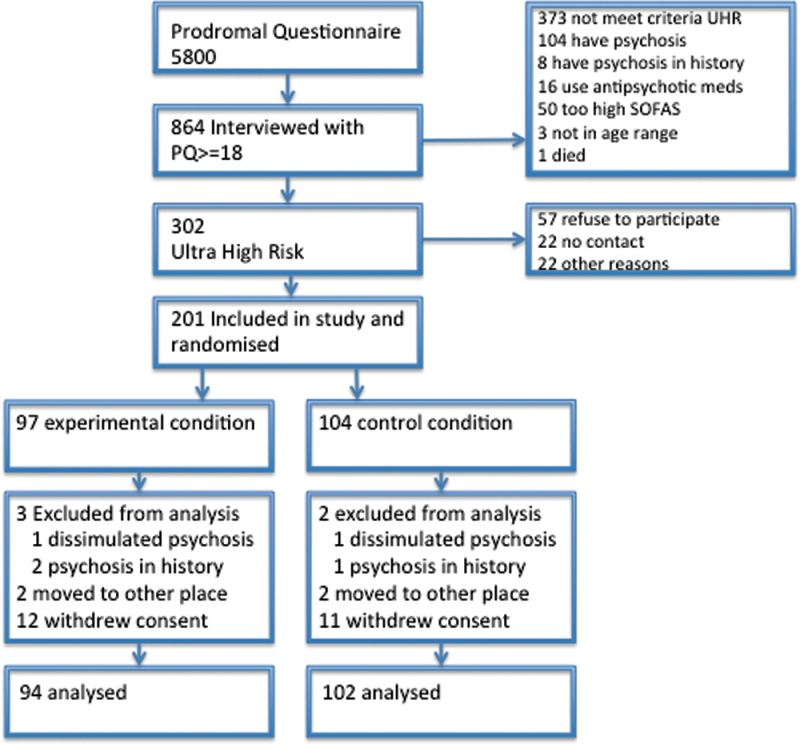
A total of 5,705 patients were screened with the PQ between February 2008 and February 2010 (figure 1). Of these, 864 patients with a score ≥18 on subclinical positive symptoms were interviewed with the CAARMS. The CAARMS interview revealed 104 patients to be psychotic even though their condition was not recognized during intake at the mental health institutions or by general practitioners at referral. A total of 302 patients fulfilled the criteria of being at risk. Of these latter patients, 201 were included in the study: 98 were randomized to the CBTuhr plus TAU condition and 103 were randomized to the TAU condition. Each patient was treated during 6 months and followed-up during 18 months. However, 15 patients withdrew their informed consent shortly after inclusion in the study: 7 patients from a rural site withdrew from CBTuhr because the intervention was provided in only 1 place, and they found the traveling too time consuming.
Fig. 1.
Flowchart of the study participants.
During the study, 5 patients were removed. Two of these patients made a transition in the first month and admitted during assessment of psychosis, that they were already psychotic at baseline. One was allocated to the CBTuhr condition and one to TAU. At baseline they had dissimulated their symptom levels with the purpose of being enrolled in the study. During transition assessments, another 3 patients revealed they had been treated with antipsychotic medications for a psychotic disorder in the past. Two of the patients were allocated to CBTuhr and one to TAU. These 5 patients thus fulfilled the exclusion criteria and were removed from the study at the moment this became clear; the decisions were made by the assessors who were blind to randomization. A total of 15 patients were lost to follow-up in the CBTuhr plus TAU condition and 13 patients were lost to follow-up in the TAU condition.
The patients were diagnosed by the routine psychiatric diagnostic procedures of the mental health services. The diagnoses were anxiety disorders (53), depression (52), mixed anxiety and depression (10), personality disorders (15), attention deficit hyperactivity disorder (13), addiction problems (12), eating disorders (11), posttraumatic stress disorder (10), oppositional defiant disorder (6), Asperger syndrome (5), relationship problems (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM V]-codes) (5), and other problems (9). Most treatments offered were CBT, pharmacotherapy, and group and couples therapy.
Primary Outcome
The primary outcome of this study was the transition to psychosis. The transition is defined by the CAARMS criteria and diagnosis was verified using the SCAN. Measurements were performed at baseline and at 2, 4, 6, 9, 12, 15, and 18 months, or at the moment a therapist or research assistant informed the researchers that a transition had (probably) taken place. The severity of psychosis, if a transition had taken place, was measured with the PANSS.
Secondary Outcomes
The secondary outcomes were depression, anxiety, quality of life, social functioning, and personal beliefs about illness. The secondary outcomes were measured at baseline, and at 6, 12, and 18 months in all patients who did not transition to psychosis.
Statistical Analyses
The analyses were conducted on an intention-to-treat basis using SPSS 18 software. The primary outcome is analyzed according to intention-to-treat and calculated using Kaplan-Meier survival statistics. People lost to follow-up were coded conservatively as nontransitions. Analyses on the secondary outcome measures cannot be done by linear mixed-modeling analysis because the missing values after a transition are not random: in the TAU group, more people made a transition than in the CBTuhr group. Analyses on the secondary outcome measures were based on the 164 patients who did not make a transition to psychosis during the study period. Changes over time were assessed by univariate tests of variance of the data at 6, 12, and 18 months, with baseline scores as covariate of the people who were nontransitions at that measurement moment. Chi square linear-by-linear test was performed to assess the discrete outcomes (in remission, at risk, psychosis) at 18-month follow-up. Numbers needed to treat were calculated for prevention of transition and attaining remission status.
Results
Baseline Data
The groups were compared on demographic characteristics and symptom measures at baseline. No significant differences were found (table 1).
Table 1.
Demographic Characteristics of the Experimental and Control Group
| Experimental (n = 98) | Control (n = 103) | Test Statistic | P value | |
|---|---|---|---|---|
| Age (y), M (SD) | 22.9 (5.6) | 22.6 (5.5) | t(199) = 0.365 | .715 |
| Education in years, M (SD) | 13.7 (2.5) | 14.0 (2.8) | t(193) = −0.926 | .355 |
| Sex ratio, M/F | 49/49 | 50/53 | χ2 (1) = 0.043 | .836 |
| Marital and living conditions | χ2 (2) = 0.705 | .703 | ||
| Single | 71 | 78 | ||
| With partner | 22 | 22 | ||
| Divorced | 5 | 3 | ||
| Employment/school | χ2 (4) = 4.86 | .303 | ||
| Paid job | 45 | 37 | ||
| Unpaid job | 5 | 9 | ||
| School | 28 | 29 | ||
| Unemployed | 12 | 19 | ||
| Otherwise | 8 | 4 | ||
| BDI depression: M (SD) | 21.0 (11.8) | 22.4 (12.8) | t(197) = −0.779 | .437 |
| CDS depression | 6.0 (4.9) | 6.3 (4.7) | t(193) = −0.430 | .667 |
| SIAS anxiety | 31.1 (16.5) | 32.3 (17.4) | t(197) = −0.490 | .625 |
| PBIQ-R dysfunctional beliefs | 73.2 (15.1) | 75.2 (17.5) | t(196) = −0.886 | .377 |
| CAARMS positive symptoms | 10.2 (3.0) | 10.3 (2.5) | t(199) = 0.112 | .911 |
| CAARMS negative symptoms | 7.0 (3.3) | 7.3 (3.6) | t(198) = −0.561 | .575 |
| CAARMS distress | 173.1 (74.6) | 171.0 (75.2) | t(199) = 0.354 | .724 |
| SOFAS social functioning | 46.4 (4.8) | 45.6 (5.1) | t(199) = 0.994 | .321 |
| MANSA quality of life | 51.9 (12.4) | 51.6 (12.7) | t(192) = 0.274 | .785 |
Notes: BDI, Beck Depression Inventory; CDS, Calgary Depression Scales; SIAS, Social Interaction Anxiety Scale; PBIQ, Personal Beliefs about Illness Questionnaire; CAARMS, Comprehensive Assessment of At-Risk Mental States; SOFAS, Social and Occupational Assessment Scale; MANSA, Manchester Short assessment of Quality of Life.
Primary Outcome
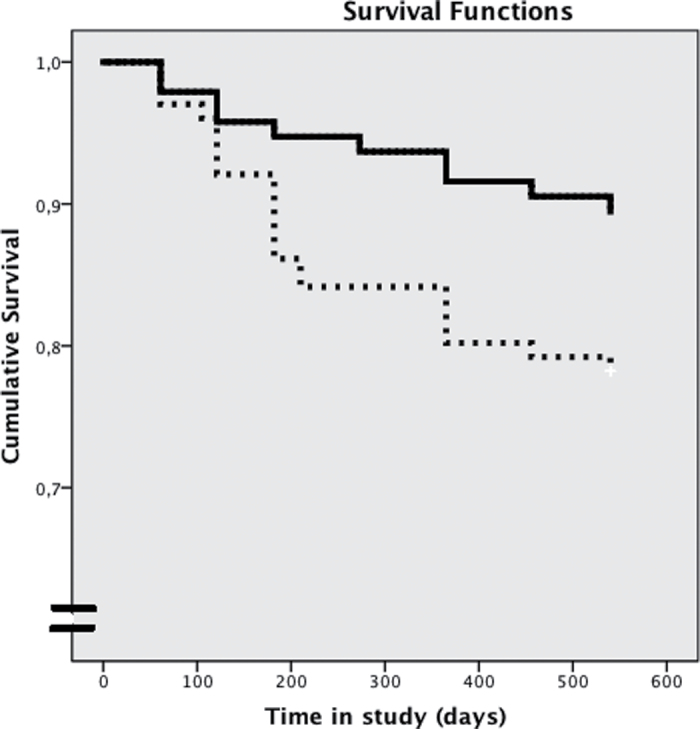
In the survival analyses, those who were lost to follow-up were conservatively considered as nontransitions. The Kaplan-Meier curves showed a significant difference between CBTuhr and control patients (Log rank test χ2 (1) = 5.575, P = .03). Mean survival time CBTuhr was 509.9 days (95% confidence interval [CI]: 488.3–531.6). Mean survival time TAU was 469.3 days (95% CI: 439.9–498.7). The odds ratio was 0.522 (95% CI: 0.188–0.948). In the CBTuhr condition, 5 patients at 6 months, 9 patients at 12 months, and 10 patients at 18 months cumulatively made the transition to psychosis. In the TAU condition, 14 patients at 6 months, 20 patients at 12 months, and 22 patients at 18 months made the transition to psychosis. Overall, 16.3% of the patients developed a psychotic episode. Survival curves are shown in figure 2. Sensitivity analyses were performed. If we include the 2 subjects (one in each condition) that dissimulated psychosis at baseline, the Kaplan-Meier remains significant (Log rank test χ2 (1) = 4.260, P = .04). Mean survival time CBTuhr is 504.9 days (95% CI: 481.3–528.6). Mean survival time TAU was 464.9 (95% CI: 434.5–495.3). The odds ratio was 0.445 (95% CI: 0.204–0.971).
Fig. 2.
Survival of patients in the experimental condition (CBT for ultrahigh risk patients [CBTuhr]) and control condition (treatment as usual [TAU]) during 18-month follow-up. Solid line, CBTuhr group; dotted line, TAU group. Log rank test χ2 (1) = 5.575, P = .03.
In the intention-to-treat analysis, including the 2 dissimulating patients and the 3 patients who actually relapsed to a second-episode of psychosis (2 in CBTuhr and 1 in TAU), the Kaplan-Meier showed a trend (Log rank test χ2 (1) =3.338, P = .06). Mean survival time CBTuhr was 500.8 days (95% CI: 476.0–525.5). Mean survival time TAU was 461.2 (95% CI: 430.3–492.2). The odds ratio was 0.503 (95% CI: 0.240–1.056).
After transition to psychosis, the DSM-IV diagnoses were schizophrenia, paranoid type (19); schizophrenia, disorganized type (2); psychotic disorder not otherwise classified (3); brief psychotic disorder (1); schizo-affective disorder (1); depression with psychotic features (4); and bipolar disorder (2).
All patients who transitioned fulfilled the PANSS criteria for psychosis (14 had 1 positive symptom intensity of 4; 12 had an intensity score of 5; 5 had an intensity score of 6; and 1 person had missing data).
Secondary Outcomes
Because the patients who transitioned to psychosis were not a random selection, a regular linear mixed-modeling analysis was ruled out. Univariate analyses showed no significant differences at 6, 12, and 18 months between the nontransitions in the experimental and the control groups on measures of general depression, anxiety, quality of life, and social functioning (table 2). The results on distress experienced by subclinical psychotic symptoms (CAARMS; F(1,159) = 6.46, P = .012) and feelings of being entrapped by the disease process (PBIQ-R; F(1,141) = 4.33, P = .039) were significant at the end of treatment at 6 months and favored the CBTuhr treatment.
Table 2.
Psychopathology Scores in the CBTuhr and TAU Groups
| Baseline, M (SD) | End of Treatment, M (SD) | 12-mo F-U, M (SD) | 18-mo F-U, M (SD) | |||||
|---|---|---|---|---|---|---|---|---|
| CBTuhr (n = 95) | TAU (n = 101) | CBTuhr (n = 80) | TAU (n = 90) | CBTuhr (n = 75) | TAU (n = 76) | CBTuhr (n = 71) | TAU (n = 69) | |
| CAARMS Intensity, 0–24 | 10.2 (3.0) | 10.3 (2.5) | 7.9 (4.3) | 8.5 (3.9) | 6.1 (4.7) | 5.9 (4.2) | 4.1 (4.2) | 4.9 (3.5) |
| CAARMS Frequency, 0–24 | 12.3 (3.6) | 12.7 (4.0) | 9.2 (4.6) | 10.5 (4.6) | 7.7 (5.5) | 7.7 (5.1) | 5.2 (5.5) | 6.9 (5.0) |
| CAARMS Distress, 0–400 | 173.1 (74.6) | 171.0 (75.2) | 105.7 (86.4) | 135.8 (85.2) | 87.2 (78.9) | 91.0 (87.1) | 71.9 (88.9) | 73.9 (78.2) |
| BDI, 0–63 | 20.8 (11.8) | 22.4 (12.9) | 15.2 (10.5) | 17.4 (14.4) | 11.3 (9.5) | 12.6 (11.4) | 9.6 (9.4) | 11.3 (11.1) |
| Clin. Depr., BDI ≥19 | 57.4% | 58.0% | 34.3% | 39.5% | 21.3% | 30.5% | 13.4% | 20.6% |
| SIAS, 0–76 | 31.0 (16.5) | 32.2 (17.3) | 25.5 (13.9) | 26.7 (16.6) | 20.7 (12.5) | 20.7 (15.6) | 22.2 (13.8) | 20.3 (15.2) |
| Clin. Soc. Ph., SIAS ≥36 | 41.5% | 42.0% | 27.5% | 23.7% | 15.5% | 17.9% | 20.0% | 16.9% |
| MANSA, 12–84 | 51.9 (12.4) | 51.6 (12.7) | 50.0 (12.7) | 50.0 (13.4) | 61.1 (12.3) | 60.6 (12.8) | 57.0 (12.2) | 55.5 (14.4) |
| SOFAS, 0–100 | 46.4 (4.8) | 45.6 (5.1) | 53.8 (9.7) | 51.5 (10.6) | 56.8 (11.8) | 57.0 (13.3) | 61.6 (12.8) | 59.6 (13.7) |
| PBIQ-R | ||||||||
| Control, 5–30 | 13.7 (3.1) | 13.6 (3.3) | 11.6 (3.2) | 12.6 (3.4) | 10.8 (3.4) | 11.4 (3.4) | 10.1 (3.2) | 10.8 (3.4) |
| Shame, 6–36 | 14.5 (3.8) | 14.8 (3.9) | 13.6 (3.7) | 14.5 (4.1) | 12.7 (3.9) | 12.3 (4.1) | 12.3 (3.6) | 12.7 (4.1) |
| Entrapment, 6–36 | 15.8 (3.6) | 16.2 (4.1) | 13.9 (3.9) | 15.3 (4.0) | 12.9 (3.8) | 13.3 (4.2) | 12.4 (4.2) | 12.9 (4.3) |
| Loss, 7–43 | 18.3 (4.2) | 19.2 (4.9) | 16.3 (4.4) | 17.8 (4.7) | 15.4 (4.3) | 15.5 (4.9) | 14.9 (4.0) | 15.8 (4.8) |
| Participation, 5–30 | 10.7 (3.1) | 11.3 (3.6) | 9.7 (2.7) | 10.5 (3.4) | 9.1 (3.1) | 8.9 (3.2) | 8.9 (3.0) | 9.3 (3.5) |
Notes: F-U, follow-up; CAARMS Intensity, Intensity of subclinical psychotic symptoms on the CAARMS (unusual thought content, nonbizarre ideas, perceptual abnormalities, disorganised speech); CAARMS Frequency, Frequency of subclinical psychotic symptoms on the CAARMS; CAARMS Distress, distress due to subclinical psychotic symptoms on the CAARMS; Clin. Depr., Moderate to Severe Depression on the BDI; SIAS, Social Interaction Anxiety Scale; Clin. Soc. Ph., Clinical Social Phobia; MANSA, Manchester short assessment of Quality of Life; SOFAS, Social functioning assessment scale; PBIQ-R, Personal Beliefs on Illness Questionnaire-Revised; TAU, treatment as usual; CBTuhr, CBT for ultrahigh risk patients. Bold numbers indicate significant differences (P < .05) on univariate analyses with baseline score as a covariate.
At-risk Status at 18-month Follow-up
Patients in both conditions tended to show marked improvement. At the end of treatment at 6 months, 35% were in remission of ARMS; at the 12-month follow-up, 48% had remitted; and at 18-month follow-up, 63% had remitted. Clinical depression as assessed with the BDI-2 decreased from almost 60% at baseline to less than 20% at 18-month follow-up, and clinical social phobia as assessed with the SIAS decreased from over 40% at baseline to less than 20% at 18-month follow-up.
The CBTuhr group had a higher remission rate of ARMS (70.4% remission of ARMS; 17.3% ARMS; 12.3% psychosis) than the TAU group (57.0% remission of ARMS; 19.4% ARMS; 23.7% psychosis). The chi square linear-by-linear showed that the CBTuhr group was overrepresented at the good end (remission) and underrepresented at the worse end (psychosis; χ2 (1) = 4.27, P = .039).
The number needed to treat (NNT) for preventing transition to psychosis was 9 (95% CI: 4.7–89.9) and to accomplish remission of ARMS was 7 (95% CI: 3.7–71.2). With inclusion of the 5 violations to exclusion criteria, the number NNT for preventing transition to psychosis is 10 and no longer significant. NNT Benefit = 4.9–∞ and NNT Harm = 186.7–∞. The NNT to accomplish remission of ARMS becomes 8 (95% CI: 3.8–396.4).
Discussion
The present study shows that the number of transitions to psychosis could be reduced by about 50% with a CBT intervention targeting cognitive biases. This is in accordance with other studies, which also achieved positive results by employing different interventions including pharmacotherapy,27 CBT,28–30 and omega-3 fatty acids.31 Some interventions combined more interventions at the same time.32–34 However, most of these positive results await replication in larger sample sizes or could not be confirmed in a large trial that suffered from a low transition rate.35
In the present study no differences were found between the CBTuhr and TAU group in outcome measures assessing the severity of depression and anxiety. In both groups, depression and anxiety severity scores were reduced at the follow-up assessments. In both conditions, the TAU was active treatment, and this might explain the positive effect on anxiety and depression. Also, after analyzing some of the secondary outcome measures on an intention-to-treat basis with pattern-mixture analysis, similar results emerged. This finding is comparable to other studies.28,29,32,35,36
This study has a transition rate of 16.3%, which is higher than most recent trials with reported rates of 6%–9%.28,35,36 There are several explanations for this. The first is our method of screening all consecutive help-seeking people at secondary mental health services with the PQ in 3 of the 4 participating research sites. The subgroup recruited by screening was much more transition prone than the subgroup recruited by referral only.37 The subsample recruited by screening also reported more depression and anxiety symptoms, and a greater decline in social functioning. Although the SOFAS and GAF are not 100% comparable, social functioning seems to be poorer in the present study (SOFAS = 46) than in the studies by Addington et al (GAF = 59),28 Yung et al (GAF = 56)36 and Morrison et al (GAF = 51).35 Another possible explanation is that screening not only recruited new cases of patients with UHR status (incidence) but also recruited patients with a long-term persistent subclinical psychotic symptoms (prevalence), who were detected for the first time. For instance, several patients reported that they had been help-seeking for a longer period of time and that earlier treatment of depression or anxiety disorder was not experienced as being effective for their symptoms. Because the group with persistent subclinical symptoms is more prone to transition,38 this could also have contributed to the higher transition rate in the screened than in the referred subsample.
Both the experimental and the control group were treated for their nonpsychotic disorder. If reducing the emotional arousal as a consequence of therapy would also prevent the transition to psychosis, then we would expect to find no difference between the 2 conditions. However, because an effect was found, this means that treating the current disorder might be beneficial, and that our CBTuhr has a specific effect on treating subclinical psychotic symptoms and preventing transition to psychosis. At the end of treatment (6-month follow-up assessment), more patients in the CBTuhr group reported the subclinical symptoms to be less distressful and reported feeling less entrapped by the subclinical symptoms than did the control group. Moreover, remission from ARMS is also demonstrated; this enhances the opportunity for social inclusion, as described in the goals of early intervention. Perhaps remission from ARMS could be a goal in itself because reduced social functioning also plays role in the definition of ARMS.
Severe mental illness has high societal costs as a result of an early start in combination with low mortality rates. While most somatic chronic diseases (eg, malignancies, cardiovascular diseases) are illnesses of old age, psychiatric diseases mainly emerge at adolescence (ie, 75% of adult psychiatric disorders start before age 25 years, with 50% of onsets occurring before age 15 years). Furthermore, 60% of health-related disability in 15- to 34-year-olds is due to mental illness or substance abuse.39 Although the effects in this study are only modest in absolute numbers, a focus on prevention, reducing the impact of severe mental disorders, and social inclusion may prove to be cost-effective.
The strength of our study is that it was performed largely in secondary mental health services. In addition, a relatively large number of UHR subjects was included and followed. In our opinion, the results can be generalized to other mental health services.
A limitation of the study is that patients who transitioned to psychosis were not followed in the same way as those who did not. The reason for this is that psychopathology data collected after transition may be influenced by the use of antipsychotic medication and can no longer be evaluated as a function of the original condition (CBTuhr and TAU). As a result, the secondary analyses were performed on nontransitioners and can be biased and/or underpowered. The 2 significant findings at the end of treatment can be due to type 1 error because we did not correct for multiple testing.
Also the removal of 5 violations against the exclusion criteria is a limitation. We presented full intention-to-treat analyses that showed that the significant difference between the groups is reduced to a tendency.
The fact that the experimental group had more therapy sessions than the control group during the experimental period of 6 months is also a limitation of this study.
Another limitation is the relatively short follow-up of 18 months; however, a 4-year follow-up of the complete study cohort is taking place to further assess symptom status and social function.
In this group of UHR patients, a CBT intervention specifically targeting the appraisal of subclinical symptoms and teaching awareness of cognitive biases reduced the number of transitions to psychosis and increased the number of patients who no longer experience subclinical psychotic symptoms.
Funding
Netherlands Health Research Council, ZonMW (120510001).
Acknowledgments
The authors thank the research assistants and therapists from the participating institutions: Sven van Amstel, David van den Berg, Petra Bervoets, Nynke Boonstra, Marion Bruns, Sarah Eussen, Gitty de Haan, Mischa van der Helm, Ceres Horsmeijer, Martijn Huijgen, Lianne Kampman, Aaltjse Malda, Carin Meijer, Julia Meijer, Roeline Nieboer, Bianca Raaijmakers, Marleen Rietveld, Nadia van der Spek, Annelies van Strater, Tinie van der Tang, Zhenya Tatkova, Jenny van der Werf, Swanny Wierenga, Annemieke Zwart. Gratitude also goes to the people who consulted on this study: Paul French, Rachel Loewy, Kees Korrelboom, Tony Morrison, Niels Smits, Lucia Valmaggia, and Alison Yung. We also thank PsyQ Haaglanden, Parnassia, Rivierduinen, PsyQ Amsterdam, Academic Medical Centre Amsterdam, and Mental Health Institute Friesland for participating in the study and for support in housing. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl 1998. 172 14–20 [PubMed] [Google Scholar]
- 2. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry 2005. 39 964–971 [DOI] [PubMed] [Google Scholar]
- 3. Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res 2003. 60 21–32 [DOI] [PubMed] [Google Scholar]
- 4. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry 2008. 65 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGorry PD, Nelson B, Amminger GP, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry 2009. 70 1206–1212 [DOI] [PubMed] [Google Scholar]
- 6. de Koning MB, Bloemen OJ, van Amelsvoort TA, et al. Early intervention in patients at ultra high risk of psychosis: benefits and risks. Acta Psychiatr Scand 2009. 119 426–442 [DOI] [PubMed] [Google Scholar]
- 7. Yung AR, Nelson B. Young people at ultra high risk for psychosis: a research update. Early Interv Psychiatry 2011. 5(52–57 [DOI] [PubMed] [Google Scholar]
- 8. Seeman P. All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci Ther 2011. 17 118–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris R, Griffiths O, Le Pelley ME, Weickert TW. Attention to irrelevant cues is related to positive symptoms in schizophrenia [published online ahead of print January 20, 2012]. Schizophr Bull. doi:10.1093/schbul/sbr192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doll BB, Hutchison KE, Frank MJ. Dopaminergic genes predict individual differences in susceptibility to confirmation bias. J Neurosci 2011. 31 6188–6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore SC, Sellen JL. Jumping to conclusions: a network model predicts schizophrenic patients’ performance on a probabilistic reasoning task. Cogn Affect Behav Neurosci 2006. 6 261–269 [DOI] [PubMed] [Google Scholar]
- 12. Cools R. Dopaminergic control of the striatum for high-level cognition. Curr Opin Neurobiol 2011. 21 402–407 [DOI] [PubMed] [Google Scholar]
- 13. Moritz S, Woodward TS. Metacognitive training in schizophrenia: from basic research to knowledge translation and intervention. Curr Opin Psychiatry 2007. 20 619–625 [DOI] [PubMed] [Google Scholar]
- 14. Freeman D, Garety P, Kuipers E, et al. Delusions and decision-making style: use of the Need for Closure Scale. Behav Res Ther 2006. 44 1147–1158 [DOI] [PubMed] [Google Scholar]
- 15.Rietdijk J, Dragt S, Klaassen R, et al. A single blind randomized controlled trial of cognitive behavioural therapy in a help-seeking population with an At Risk Mental State for psychosis: the Dutch Early Detection and Intervention Evaluation (EDIE-NL) trial. Trials. 2010;11:30. doi: 10.1186/1745-6215-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loewy RL, Bearden CE, Johnson JK, Raine A, Cannon TD. The prodromal questionnaire (PQ): preliminary validation of a self-report screening measure for prodromal and psychotic syndromes. Schizophr Res 2005. 77 141–149 [DOI] [PubMed] [Google Scholar]
- 17. Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry 1992. 149 1148–1156 [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization Schedules for Clinical Assessment in Neuropsychiatry. Version 2.1. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987. 13 261–276 [DOI] [PubMed] [Google Scholar]
- 20. Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 21. Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res 1992. 6 201–208 [DOI] [PubMed] [Google Scholar]
- 22. Mattick RP, Clarke JC. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behav Res Ther 1998. 36 455–470 [DOI] [PubMed] [Google Scholar]
- 23. Priebe S, Huxley P, Knight S, Evans S. Application and results of the Manchester Short Assessment of Quality of Life (MANSA). Int J Soc Psychiatry 1999. 45 7–12 [DOI] [PubMed] [Google Scholar]
- 24. Birchwood M, Mason R, MacMillan F, Healy J. Depression, demoralization and control over psychotic illness: a comparison of depressed and non-depressed patients with a chronic psychosis. Psychol Med 1993. 23 387–395 [DOI] [PubMed] [Google Scholar]
- 25. French P, Morrison AP. Early Detection and Cognitive Therapy for People at High Risk of Developing Psychosis: A Ttreatment Approach Chichester, West Sussex, England: John Wiley & Sons; 2004. [Google Scholar]
- 26. James IA, Blackburn IM, Reichelt FK. Manual of the Revised Cognitive Therapy Scale (CTS-R) Northumberland, UK: Tyne and Wears NHS Trust; 2006. [Google Scholar]
- 27. McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry 2006. 163 790–799 [DOI] [PubMed] [Google Scholar]
- 28. Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophr Res 2011. 125 54–61 [DOI] [PubMed] [Google Scholar]
- 29. Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry 2004. 185 291–297 [DOI] [PubMed] [Google Scholar]
- 30. Morrison AP, French P, Parker S, et al. Three-year follow-up of a randomized controlled trial of cognitive therapy for the prevention of psychosis in people at ultrahigh risk. Schizophr Bull 2007. 33 682–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amminger GP, Schäfer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry 2010. 67 146–154 [DOI] [PubMed] [Google Scholar]
- 32. McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry 2002. 59 921–928 [DOI] [PubMed] [Google Scholar]
- 33. Nordentoft M, Thorup A, Petersen L, et al. Transition rates from schizotypal disorder to psychotic disorder for first-contact patients included in the OPUS trial. A randomized clinical trial of integrated treatment and standard treatment. Schizophr Res 2006. 83 29–40 [DOI] [PubMed] [Google Scholar]
- 34. Bechdolf A, Wagner M, Ruhrmann S, et al. Preventing progression to first-episode psychosis in early initial prodromal states. Br J Psychiatry 2012. 200 22–29 [DOI] [PubMed] [Google Scholar]
- 35.Morrison AP, French P, Stewart SL, et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ. 2012;344:e2233. doi: 10.1136/bmj.e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yung AR, Phillips LJ, Nelson B, et al. Randomized controlled trial of interventions for young people at ultra high risk for psychosis: 6-month analysis. J Clin Psychiatry 2011. 72 430–440 [DOI] [PubMed] [Google Scholar]
- 37. Rietdijk J, Klaassen R, Ising H, et al. Detection of people at risk of developing a first psychosis: comparison of two recruitment strategies. Acta Psychiatr Scand. 2012;126:21–30 [DOI] [PubMed] [Google Scholar]
- 38. Dominguez MD, Wichers M, Lieb R, Wittchen HU, van Os J. Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: an 8-year cohort study. Schizophr Bull 2011. 37 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hickie IB. Youth mental health: we know where we are and we can now say where we need to go next. Early Interv Psychiatry 2011. 5(63–69 [DOI] [PubMed] [Google Scholar]