Abstract
Background
Psychotic disorders are associated with widespread reductions in white matter (WM) integrity. However, the stage at which these abnormalities first appear and whether they are correlates of psychotic illness, as opposed to an increased vulnerability to psychosis, is unclear. We addressed these issues by using diffusion tensor imaging (DTI) to study subjects at ultra high risk (UHR) of psychosis before and after the onset of illness.
Methods
Thirty-two individuals at UHR for psychosis, 32 controls, and 15 patients with first-episode schizophrenia were studied using DTI. The UHR subjects and controls were re-scanned after 28 months. During this period, 8 UHR subjects had developed schizophrenia. Between-group differences in fractional anisotropy (FA) and diffusivity were evaluated cross sectionally and longitudinally using a nonparametric voxel-based analysis.
Results
At baseline, WM DTI properties were significantly different between the 3 groups (P < .001). Relative to controls, first-episode patients showed widespread reductions in FA and increases in diffusivity. DTI indices in the UHR group were intermediate relative to those in the other 2 groups. Longitudinal analysis revealed a significant group by time interaction in the left frontal WM (P < .001). In this region, there was a progressive reduction in FA in UHR subjects who developed psychosis that was not evident in UHR subjects who did not make a transition.
Conclusions
People at UHR for psychosis show alterations in WM qualitatively similar to, but less severe than, those in patients with schizophrenia. The onset of schizophrenia may be associated with a progressive reduction in the integrity of the frontal WM.
Keywords: vulnerability, high risk, first episode, schizophrenia, diffusion, DTI
Introduction
Functional neuroimaging studies suggest that there are alterations in the functional connectivity between different brain regions in schizophrenia1 and that these abnormalities are evident at the first episode of illness and in people at ultra high risk (UHR) of developing psychosis.2,3 Similarly, structural neuroimaging studies have shown that there are global and regional changes in white matter (WM) volume in schizophrenia4 and that these are also evident at the onset of psychosis5 and in UHR subjects.6 Approximately 20%–40% of people at UHR of psychosis develop psychosis within 24 months,7,8 and this subgroup shows more marked reductions in gray matter volume in the inferior frontal9,10 and medial temporal cortex11 at presentation than UHR subjects who do not subsequently develop psychosis. Furthermore, repetition of scanning in these subjects indicates that some of these baseline abnormalities progress with the later transition to psychosis10,12 and that these longitudinal magnetic resonance imaging (MRI) changes are not evident in UHR subjects who do not become psychotic. However, the extent to which these gray matter changes are related to alterations in WM integrity is unclear.
Diffusion tensor imaging (DTI) can measure the direction and degree of water diffusion as well as its anisotropy (ie, its relative value along ordered structures such as axon bundles vs across them). Anisotropy (commonly reported as fractional anisotropy [FA]) can be altered by pathologic factors, such as demyelination and axonal membrane deterioration, and so is often used as an index of WM integrity.13 The diffusivity along axons provides complementary information regarding WM structure. The axial (parallel) diffusivity corresponds to the amount of diffusion measured along the direction of principal diffusion, while the radial (perpendicular) diffusivity corresponds to the average diffusion in the perpendicular plane. Axial and radial diffusivity have recently been reported to change following axonal degeneration and demyelination in animal models14; however, there is no evidence as to whether this holds true in humans.
Several DTI studies have reported reduced WM integrity in chronic schizophrenia,15 particularly in tracts connecting the frontal and temporal lobes,15,16 as well in first-episode psychosis (FEP).17–19 There have only been a few DTI studies in subjects at UHR of psychosis. These have been limited to cross sectional comparisons and have given inconsistent results.20 One study reported a reduction of FA in the WM of frontal lobe,21 while another found a reduction in the superior longitudinal fasciculus (SLF).22 Peters et al23 used tractography to assess the uncinate and arcuate fasciculi, cingulum bundle, and corpus callosum but did not find any differences between UHR and controls. Only one previous DTI study has subdivided UHR subjects in terms of their clinical outcome. UHR subjects who later became psychotic had lower FA at presentation than healthy controls in the WM of the left frontal lobe, in a region that includes the anterior thalamic radiation and the inferior frontooccipital fasciculus.24 Compared with UHR subjects who did not become psychotic, they had lower FA in the WM lateral to the right putamen and in the left superior temporal gyrus but higher FA in left posterior temporal WM.24 To date, there have been no longitudinal DTI studies in UHR subjects. Hence, the extent to which abnormalities in WM integrity at first presentation may progress with the subsequent onset of psychosis is unknown.
The aim of the present study was to assess WM integrity in individuals at UHR for psychosis, using both cross sectional and longitudinal analyses. We tested 2 hypotheses. The first was that relative to controls, UHR individuals would show qualitatively similar WM abnormalities to patients in the FEP but that the magnitude of these abnormalities would be less severe. Our second prediction was that within the UHR group, individuals who later developed psychosis would show more marked abnormalities at baseline than those that did not, and these abnormalities would progress longitudinally because they made the transition to psychosis.
To date, DTI studies in subjects at increased risk of psychosis have focused on measuring FA.20 A subsidiary aim of the present study was to also assess WM integrity in UHR subjects using measures of radial and axial diffusivity. Our corresponding hypothesis was that reductions in FA in UHR subjects would be associated with altered diffusivity.
Methods
Participants
UHR Group.
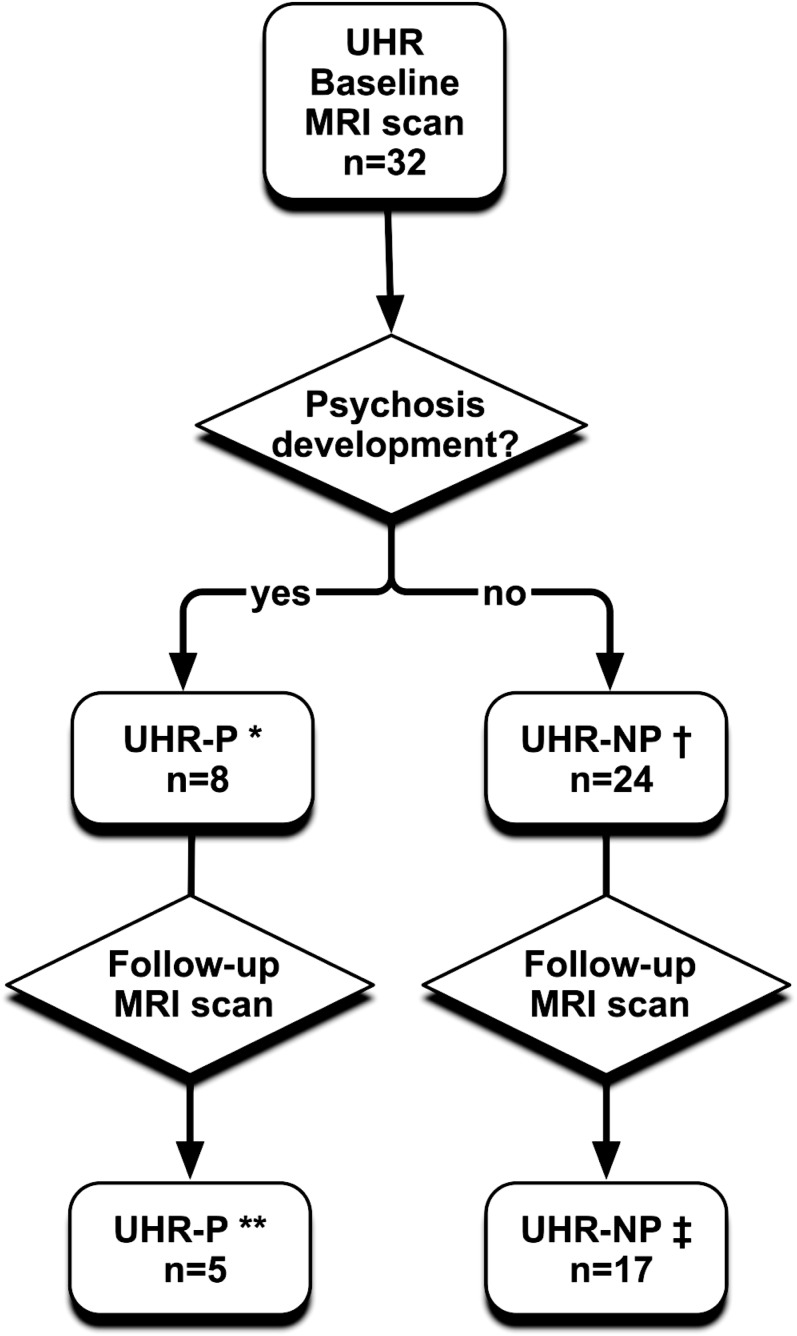
Individuals meeting the Personal Assessment and Crisis Evaluation criteria for the at-risk mental state (n = 32) were recruited from Outreach and Support in South London (OASIS).7 The diagnosis was based on assessment by 2 experienced clinicians using the Comprehensive Assessment for the At-Risk Mental State (CAARMS).25 All UHR subjects were naive to antipsychotic medication at the time of the baseline scan. They were followed clinically at monthly intervals during the first year, at 3 monthly intervals during the second and third years, and annually thereafter. Twenty-two UHR subjects completed both clinical follow-up and MRI scan (see figure 1). During this period, 5 developed psychosis and 17 did not. Transition to psychosis was defined according to the criteria in the CAARMS, and a diagnosis of schizophrenia was confirmed at reassessment 12 months after transition using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (SCID).26 Within the transition subgroup (n = 5), the mean interval between the baseline and follow-up MRI scans was 2 years, while in the nontransition subgroup (n = 17), it was 2.4 years. At the time of the second MRI scan, 3 of the transition subgroup and 2 of the nontransition subgroup were receiving antipsychotic medication (Quetiapine).
Fig. 1.
Detailed characteristics of the UHR group. *Age (mean ± SD): 22.75 ± 2.92; gender (male/females): 7/1. **Age (mean ± SD): 25.18 ± 3.70; gender (male/females): 4/1; 3 subjects were receiving antipsychotic medication (Quetiapine). †Age (mean ± SD): 23.67 ± 4.13; gender (male/females): 12/12. ‡Age (mean ± SD): 27.03 ± 4.33; gender (male/females): 7/10; 2 subjects were receiving antipsychotic medication (Risperidone, Quetiapine).
First-Episode Group.
Patients from the same geographical area as the UHR sample who had recently presented with an FEP (n = 15) were recruited from the Lambeth Early Onset (LEO) Service. All met DSM-IV criteria for a schizophreniform psychosis27 at the time of scanning and met SCID (DSM-IV) criteria for schizophrenia26 when re-assessed 12 months later. Six of the patients were medication naive, and 9 had received less than 3 weeks of treatment with low doses of atypical antipsychotic medication (mean dose in chlorpromazine equivalents = 189 mg/d; mean duration of treatment = 11.6 d).
Controls.
Healthy volunteers (n = 32) were recruited from the same geographical area, as the clinical subjects via local advertisements, or from the friends of the clinical subjects.
Handedness was assessed using the Edinburgh Handedness Inventory.28 Intellectual function (IQ) was estimated using the Wechsler Adult Intelligence Scale, 3rd Edition (WAIS-III).29 The severity of symptoms in both clinical groups was assessed with the Positive and Negative Symptoms Scale30 on the day of scanning by a psychiatrist (J.B.W) trained in its use. Level of function was assessed using the Global Assessment of Functioning scale.27 Exclusion criteria included history of neurological disorder, history of alcohol, or other substance misuse disorder according to DSM-IV criteria. Participants gave written informed consent to participate in the study, which was reviewed and approved by the Joint South London and Maudsley and Institute of Psychiatry Research Research Ethics Committee.
MRI Acquisition
Magnetic resonance imaging was performed on all participants on a 1.5 Tesla GE SIGNA NVi scanner (General Electric, Milwaukee, WI). Following localizer, calibration, and structural scans, DTI data were collected using a cardiac-gated single-shot echo-planar sequence with 60 contiguous 2.5 mm thick near axial slices, matrix size 96 × 96 over a 24 cm field of view, zero-filled during reconstruction to a 128 × 128 matrix, final reconstructed voxel size = 1.875 × 1.87.5 × 2.5 mm3; time echo = 107 ms; time repetition = 15 R-R intervals. At each scan location, 7 images without diffusion gradients were acquired together with 64 diffusion-weighted images (b = 1300 s.mm−2) with diffusion sensitization directions distributed over a (hemi)sphere.
Image Analysis
Images were corrected for head movement, eddy currents, and skull-stripped using FSL (http://www.fmrib.ox.ac.uk/fsl/). The 6 elements of the diffusion tensor were calculated for each voxel using linear regression.13 FA was calculated and data from all subjects realigned to the bicommissural line of a target image (chosen from the study participants) using an affine followed by nonlinear registration. The nonlinear transformation was then applied to each tensor component, and the warped components were recombined into a single tensor file. In order to allow for the effects of this reorientation, the local tensor orientation was adjusted using the “preservation of principal direction” algorithm implemented within Camino (http://cmic.cs.ucl.ac.uk/camino). Finally, realigned diffusion tensor images of all subjects were used to create a population-specific template to which each subject's images were then (re-)normalized; this final stage of processing was performed with “dti-tk” toolkit (http://www.nitrc.org/projects/dtitk) and is described in more detail below.
Population-Specific Template Creation
A template was created from our cohort of study (patients and controls) in order to avoid the bias associated with using a standard template from a normal population, which is not representative of our patient group. The population-specific template was generated from tensor images, rather than scalar images, such as FA, using an unbiased diffeomorphic method.31 Whole tensor-based registration was chosen over FA-based registration because this approach has been reported to align WM regions better than scalar-based registration methods.31,32 Additionally, templates for FA, axial, and radial diffusivity were calculated from the final tensor template.13
Normalization to the Population-Specific Template
Images were normalized to the population-specific tensor template using a high-dimensional approach.31 FA, axial, and radial diffusivity maps were calculated.13 Finally, the International Consortium for Brain Mapping white matter labels atlas (ICBM-DTI-81)33 was registered to the 3 population-specific templates (FA, axial, and radial diffusivity) using an affine followed by a nonlinear registration and used as brain mask to restrict subsequent analyses to WM only.
Group Mapping Analysis
The main analysis of the data was performed using XBAM v4, a software package developed at King's College London, Institute of Psychiatry (http://brainmap.co.uk) which implements a nonparametric approach based on permutations to minimize assumptions of data normality and is based on median statistics to control for outlier effects. Relative to parametric analyses, the nonparametric approach has the additional advantage of allowing test statistics incorporating spatial information such as 3D cluster mass (the sum of suprathreshold voxel statistics), which are generally more powerful than other possible test statistics but for which no parametric approximation is known.
For the cross sectional analysis, between-group differences in the DTI indices of interest (FA, parallel, and perpendicular diffusivity) were estimated by fitting an ANOVA model at each voxel, whereas for the longitudinal analysis, data were analyzed using a nonparametric repeated measures ANOVA.
To test for the interaction between group and time between scans, a series of nonparametric factorial analyses of variance were used. For each analysis, a voxel-level significance threshold within XBAM was initially set to .05 to give maximum sensitivity and to avoid type II errors, and voxels where nonparametric testing of the model gave evidence for rejection of the null hypothesis were highlighted. Three-dimensional spatial clusters generated from the voxels thus highlighted were then subject to more rigorous statistical testing of their cluster mass. At this stage, a cluster mass threshold was computed from the distribution of cluster masses in the permutated data, such that the expected number of type I error clusters under the null hypothesis was less than one over the whole brain. Finally, as multiple group-wise analyses tests were performed on the 3 diffusion indices, results of the cluster analyses were corrected for multiple comparisons in order to further control for false positives.
Localization of Findings
Because the population-specific tensor template was not in the Montreal Neurological Institute (MNI) space, significant clusters were warped into the standard MNI space. Scalar image templates were coregistered to the IIT2 DTI brain template34 using an initial affine registration followed by a nonlinear registration. The transformations so calculated were then applied to the group mapping results. The most likely anatomic localization of each cluster highlighted was finally determined by reference to the IIT2 DTI brain template in the spatial coordinates of the ICBM-152 brain template.34
Statistical Analysis of Clinical and Demographic Data
Group-wise measures of clinical and sociodemographic variables were analyzed using one-way ANOVA, t test, and chi-square test where appropriate (PASW 18; SPSS Inc, Chicago, IL). When significant differences were found, the Tukey's honestly significant difference test for pairwise comparisons was applied. Before statistical tests, the data were checked for assumptions of normality and equality of variances. If these assumptions were violated, the Mann–Whitney U test was used.
Demographic and Clinical Variables
A summary of demographic and clinical variables is reported in table 1. Eight UHR subjects (25%) developed psychosis (UHR-P) subsequent to baseline (see figure 1). Five of this UHR-P subgroup were re-scanned after the onset of psychosis, along with 17 (of the 24) UHR subjects who had not developed psychosis. Eight (25%) of the 32 healthy controls were also re-scanned. The investigators tried to contact all of the subjects who had been scanned at baseline. However, some were not contactable, and others declined to be re-assessed. The mean interval between the baseline and follow-up scans in the total UHR sample was 15 months (range 32), while in the UHR-P and UHR-NP subgroups, it was 23.7 (range 15.8) and 29.3 (range 32) months, respectively. The mean interscan interval in controls was 45.7 months (range 28 months). The interscan interval did not differ between the UHR-P and UHR-NP groups but was significantly longer in controls than UHR subjects (P < .001).
Table 1.
Demographic Characteristics of the Experimental Groups
| Variable | Baseline | Follow-up | |||||
| FEP | UHR | HC | Group Comparison | UHR | HC | Group Comparison | |
| Group size | 15 | 32 | 32 | — | 22 | 8 | — |
| Age at DTI scan: years (SD) | 24.1 (5.2) | 23.4 (3.8) | 25.9 (5) | F 2,79 = 1.888, P = .158 | 26.6 (4.2) | 29.6 (4.3) | F 1,29 = 2.87, P = .10 |
| Gender (male/female) | 14/1 | 19/13 | 28/5 | χ2 (2) = 8.54, P = .01 | 11/11 | 7/1 | χ2 (1) = 3.48, P = .06 |
| Handedness (right/left/ambivalent)a | 13/0/2 | 31/1/0 | 32/0/0 | χ2 (2) = 4.87, P = .08 | 21/1/0 | 8/0/0 | χ2 (1) = 15.39, P = .22 |
| IQ (SD)b | 97.8 (6.7) | 97.2 (8.9) | 103.1 (6.2) | F 2,75 = 5.301, P = .007 | 97.1 (9.3) | 103.2 (7.2) | F 1,29 = 2.81, P = .11 |
Note: FEP, first episode of psychosis; UHR, ultra high risk; HC, healthy controls; WAIS-III, Wechsler Adult Intelligence Scale III.
Measured with the Edinburgh Handedness Inventory.
IQ was missing for 4 subjects.
At the time of the follow-up scan, 77% of the UHR sample were still antipsychotic naive (and were not taking any other psychotropic medication), while 23% of them were receiving antipsychotic treatment. Within the UHR-P and UHR-NP subgroups, the corresponding figures were 40% and 60%, and 88.2% and 11.8%, respectively. In the longitudinal analysis, because the interscan interval was longer in controls than UHR subjects, the time between scans was used as a covariate but in order to allow for the potential confounding effects of correlations between the covariate and independent variables this introduces, we also repeated the longitudinal analysis without covariate adjustment.
Results
Cross Sectional Comparisons at Baseline
FEP vs UHR vs Healthy Controls.
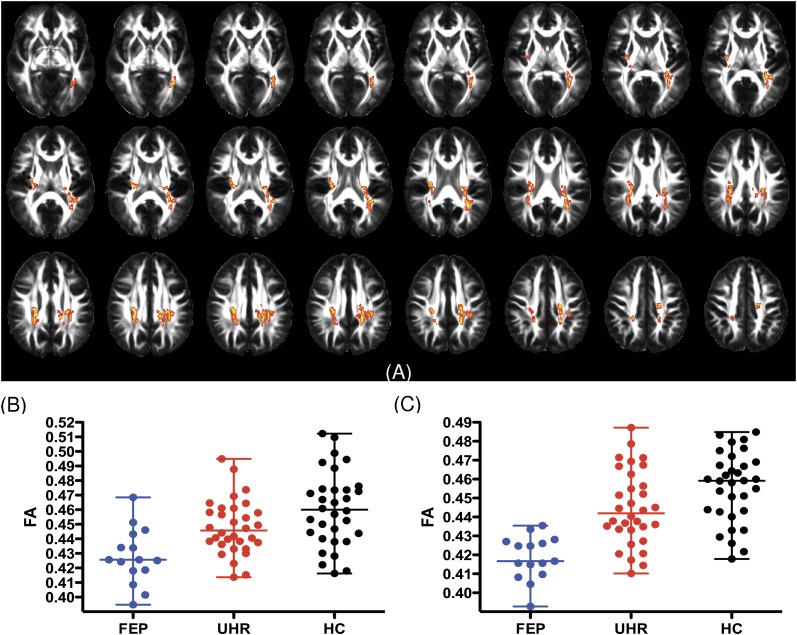
At baseline, there were significant linear relationships for differences across the 3 groups in FA (for a detailed description of the results, see figure 2A and online supplementary table S1) in 2 clusters. In both clusters, FA was lowest in the FEP group, highest in controls, and intermediate in the UHR group (see figure 2B). The first cluster comprised voxels in areas corresponding to the splenium and body of the corpus callosum, the left inferior and SLF, and the left inferior frontooccipital fasciculus (for details, see online supplementary table S1). The second cluster included the right external capsule, the retrolenticular part of the right internal capsule, and the right posterior corona radiata (for details, see online supplementary table S1).
Fig. 2.
Linear changes in FA across three groups. Figure (A) displays areas of reduced fractional anisotropy (FEP < UHR < HC); images are radiologically oriented (participant's left is to the right). The figure (B and C) shows differences in FA for significant clusters (median ± range).
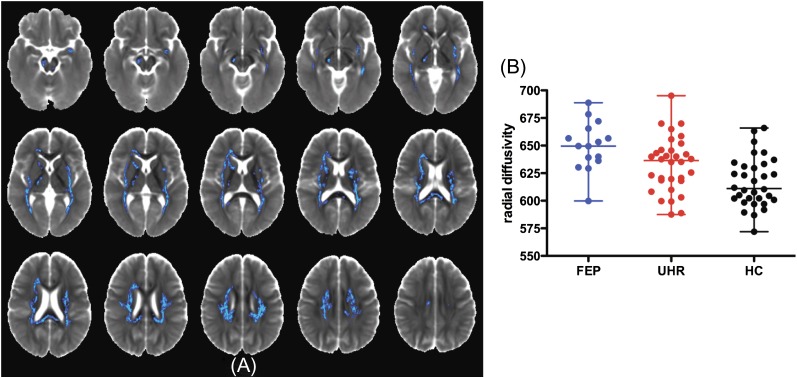
There was a larger set of regions where there was a significant linear relationship for radial diffusivity across the groups, with it being greatest in the FEP group, lowest in controls, and intermediate in the UHR group (see figure 3).
Fig. 3.
Linear changes in RD across 3 groups. Figure (A) displays areas of increased radial diffusivity (FEP > UHR > HC); images are radiologically oriented (participant's left is to the right). The figure (B) shows differences in RD for significant clusters (median ± range). Radial diffusivity is measured in 10−6 mm2/s.
Linear relationship for axial diffusivity differences was evident in many clusters (see online supplementary figure S4). In some clusters, axial diffusivity was highest in the FEP group and lowest in the controls, but in others, the opposite relationship applied.
Post hoc paired comparisons revealed that differences in FA, radial, and axial diffusivities were driven by differences between the FEP and control groups, while the differences between the UHR group and controls were not significant. There were no FA or RD clusters where the linear relationship across groups was in the opposite direction (eg, FA highest in FEP and radial diffusivity lowest in FEP).
Findings at Baseline in UHR Subjects Who Later Developed Psychosis
Within the UHR group, there were no differences in FA, axial, or radial diffusivity between subjects who developed psychosis (UHR-P) and those who did not (UHR-NP). Similarly, there were no significant differences between the UHR-P group and healthy controls.
Longitudinal Analysis
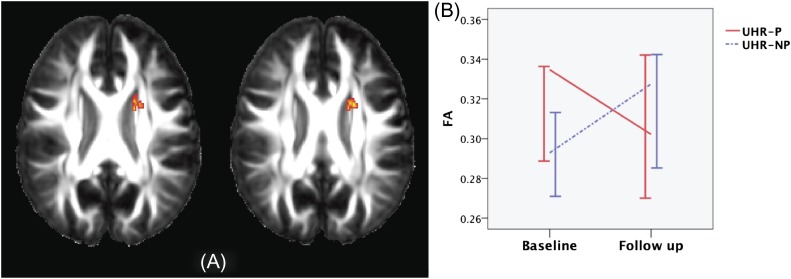
There was a significant group (UHR-P vs UHR-NP) by time (baseline vs follow-up scan) interaction on FA in a cluster spanning the anterior limb of the left internal capsule (ALIC), body of the corpus callosum, left superior corona radiata, and left superior frontooccipital fasciculus (P = .0009). In this cluster, there was a longitudinal reduction in FA in the UHR-P group but a slight increase in the UHR-NP group (see figure 4), although these within-group changes were not themselves significant. This result did not change when the “nuisance covariate” (time between scans) was excluded from the analysis. There were no significant groups by time interactions for axial or radial diffusivity.
Fig. 4.
Group × time interaction analysis results. Fractional anisotropy map (A) indicating the areas where there is an interaction between group membership and time between scans. Image is radiologically oriented (participant's left is to the right). Plot of FA values (median ± 95% CI) extracted from the significant cluster (B).
Cross Sectional Comparisons at Follow-up
At follow-up, there were no significant differences in FA between the UHR subjects who had developed psychosis and the UHR subjects who had not. However, there was greater radial diffusivity in one cluster in middle cerebellar peduncle (P < .001), while axial diffusivity was reduced in the middle cerebellar peduncle and left cerebral peduncle (P = .001) but greater in the right superior and posterior corona radiata, splenium, and body of the corpus callosum (P < .0004; for details, see online supplementary table S2).
Discussion
Our first hypothesis was that the UHR state would be associated with alterations in WM integrity qualitatively similar to, but less severe than, those seen in schizophrenia. Consistent with this hypothesis, we found that, at baseline, FA, radial diffusivity, and axial diffusivity values in UHR subjects were intermediate relative to those in the first-episode patients and controls. These differences were evident in regions of WM corresponding to the major associative fibers that connect fronto-parieto-temporal (SLF) and fronto-parieto-occipital regions (inferior frontooccipital fasciculus), commissural fibers (corpus callosum), and cortico-subcortical pathways (corona radiata, corticospinal tract, and corticopontine tract). The findings in the SLF corroborate previous findings in FEP18,35 and UHR populations.22
Our results suggest that structural abnormalities in fronto-temporo-parietal connections are present before the onset of psychosis, in line with functional connectivity findings in UHR subjects.3 These abnormalities are comparable to those reported in DTI studies of patients with FEP.18,19,35 We also found reduced FA in the splenium of the corpus callosum in the UHR group. A previous study in UHR subjects did not find differences in this region,23 whereas reduced FA in the splenium of the callosum has been reported in some studies of FEP.17,19
Our second hypothesis was that DTI abnormalities would be more marked in UHR subjects who later developed psychosis than in those who did not. Consistent with this prediction, we found a significant interaction between “group” (UHR-P and UHR-NP) and time, which post hoc analyses indicated was driven by a longitudinal reduction in FA the UHR-P group (figure 4). This difference was evident in a region that comprised the left anterior limb of the internal capsule (ALIC), left corona radiata, left superior frontooccipital fasciculus, and anterior body of the corpus callosum. To our knowledge, this is the first evidence that transition to psychosis in UHR subjects is associated with longitudinal changes in WM integrity. The ALIC is traversed by axonal fibers that connect the thalamus to the prefrontal cortex and form part of a circuit linking the frontal lobe and basal ganglia. WM abnormalities have previously been reported in the ALIC,18 body of the corpus callosum,18 and along the superior occipitofrontal fasciculus19 in FEP, and oligodendrocyte abnormalities have been described in the prefrontal WM in schizophrenia.36 The localization of our findings is also consistent with those in previous DTI studies of individuals at high genetic risk of schizophrenia.37,38
We did not find significant differences at baseline between UHR subjects who subsequently developed psychosis and UHR subjects who did not. However, a recent DTI study24 reported that, at presentation, the former subgroup had lower FA than the latter in the right putamen and left superior temporal lobe but higher FA in a posterior part of the left temporal lobe. This difference in findings may reflect differences in the nature of the respective UHR samples. Although the total number of UHR subjects was similar, a greater proportion developed psychosis in the previous study, which may have provided more power when comparing the UHT-P and UHT-NP subgroups. On the other hand, in the Bloemen study,24 the proportion of UHR subjects who had already been treated with antipsychotics was significantly greater in the UHR-P than in the UHR-NP group, whereas at baseline, all our UHR subjects were medication naive. The potentially confounding effects of medication are discussed further below. The UHR subjects in the previous study were also around 4 years younger than in our study, and the pattern of DTI findings in patients who develop psychosis appears to vary according to the age of the subject at illness onset.39 A previous volumetric study of WM in UHR subgroups found that, at baseline, individuals who developed psychosis (UHR-P) significantly differ from subjects who did not (UHR-NP).6 There are several possible explanations for this difference in findings. Methodological differences between our study and the one by Walterfang et al6 may account for these inconsistencies. Another possible explanation for this might be that the pathophysiological processes underlying WM volume changes in psychosis may not be reflected by changes in FA.40 Our results suggest that further research is needed to understand how volumetric and diffusion changes relate to each other in UHR individuals.
We also predicted that alterations in FA in UHR subjects would be associated with changes in axial and radial diffusivity. This hypothesis was confirmed, although the relationship between alterations in FA and in diffusivity varied according to the site of the findings (see online supplementary table S3). Alterations in radial diffusivity have been associated with demyelination in animal models.14 However, we were not able to directly assess myelination. While increased radial diffusivity may suggest axonal damage,14 in some regions, this was associated with increased axial diffusivity, which is evident when fibers are tightly aligned within the WM. In other areas, radial diffusivity was increased despite no alteration in FA, while in the ALIC, there was reduced FA but no diffusivity changes. Further work is required to clarify the basis of these different relationships between FA and diffusivity.
We acknowledge some limitations to our study. Although larger than those in most previous DTI studies of UHR subjects, our sample was still modest, and the findings require confirmation in a larger group. Given the small number of females involved in the study, we could not assess the effects of gender on DTI results. Because 3 of the UHR subjects who developed psychosis had been started on antipsychotic medication by the time of the follow-up scan, we cannot exclude the possibility that the longitudinal findings in this subgroup were influenced by treatment after the onset of psychosis. However, previous DTI studies in schizophrenia have not identified a clear effect of antipsychotic medication on FA.15 Furthermore, a recent meta-analysis of antipsychotic-naive voxel-based morphometry (VBM) studies showed that cortical volume loss observed at the onset of psychosis is independent of antipsychotic treatment.41 Group-mapping analyses of FA maps have been criticized for inaccuracies in the alignment of individual images to the template.42 We used a diffeomorphic deformable tensor-based image registration31 because it registers DTI images better than other techniques,32 reduces the susceptibility to false positives due to tensor shape confounds, and improves the sensitivity to detect anisotropy changes.31 However, we acknowledge that while misregistration can be minimized by the use of voxel-based31 or tract-based42 analysis techniques, it can never be completely eliminated, and results should therefore always be interpreted in light of this potential confound. Data were analyzed using a whole brain rather than a region of interest (ROI) approach because the existing DTI literature indicates that multiple tracts are affected in schizophrenia15 and in UHR populations.20
In conclusion, these data suggest that the UHR state is associated with reduced WM integrity in similar areas to those affected in FEP, but to a lesser degree. Furthermore, we have provided the first evidence that the onset of psychosis in UHR subjects may be associated with a longitudinal progression of abnormalities in the left frontal WM.
Funding
This work was supported by a Wellcome Trust Research Training Fellowship awarded to Dr F.C. (086636/Z/08/Z); a Medical Research Council New Investigator Award conferred to Dr E.B. (G0901310). Dr S.B. was supported by a Joint MRC/Priory clinical research training fellowship (G0501775). This research was in part supported by GlaxoSmithKline, but with no restriction to data access, analysis, or presentation. Prof G.J.B. received honoraria for teaching from General Electric during the course of this study.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
Thanks go to all the clients, staff, and referrers of both OASIS and LEO. Professor P.K.M. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The NIHR Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, Kings College London, supported this project. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.McGuire PK, Frith CD. Disordered functional connectivity in schizophrenia. Psychol Med. 1996;26:663–667. doi: 10.1017/s0033291700037673. [DOI] [PubMed] [Google Scholar]
- 2.Benetti S, Mechelli A, Picchioni M, Broome M, Williams S, McGuire P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132(pt 9):2426–2436. doi: 10.1093/brain/awp098. [DOI] [PubMed] [Google Scholar]
- 3.Crossley NA, Mechelli A, Fusar-Poli P, et al. Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Hum Brain Mapp. 2009;30:4129–4137. doi: 10.1002/hbm.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Chua SE, Cheung C, Cheung V, et al. Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res. 2007;89:12–21. doi: 10.1016/j.schres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Walterfang M, McGuire PK, Yung AR, et al. White matter volume changes in people who develop psychosis. Br J Psychiatry. 2008;193:210–215. doi: 10.1192/bjp.bp.107.043463. [DOI] [PubMed] [Google Scholar]
- 7.Broome MR, Woolley JB, Johns LC, et al. Outreach and support in south London (OASIS): implementation of a clinical service for prodromal psychosis and the at risk mental state. Eur Psychiatry. 2005;20:372–378. doi: 10.1016/j.eurpsy.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: a meta-analysis of evidence. Arch Gen Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 9.Borgwardt SJ, Riecher-Rossler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 11.Mechelli A, Riecher-Rossler A, Meisenzahl EM, et al. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch Gen Psychiatry. 2011;68:489–495. doi: 10.1001/archgenpsychiatry.2011.42. [DOI] [PubMed] [Google Scholar]
- 12.Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 14.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2005;58:921–929. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Gasparotti R, Valsecchi P, Carletti F, et al. Reduced fractional anisotropy of corpus callosum in first-contact, antipsychotic drug-naive patients with schizophrenia. Schizophr Res. 2009;108:41–48. doi: 10.1016/j.schres.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Iglesias R, Tordesillas-Gutierrez D, Barker GJ, et al. White matter defects in first episode psychosis patients: a voxelwise analysis of diffusion tensor imaging. Neuroimage. 2010;49:199–204. doi: 10.1016/j.neuroimage.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Cheung V, Cheung C, McAlonan GM, et al. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2008;38:877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- 20.Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? J Psychiatr Res. 2010;44:993–1004. doi: 10.1016/j.jpsychires.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Peters BD, Schmitz N, Dingemans PM, et al. Preliminary evidence for reduced frontal white matter integrity in subjects at ultra-high-risk for psychosis. Schizophr Res. 2009;111:192–193. doi: 10.1016/j.schres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66:562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters BD, de Haan L, Dekker N, et al. White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology. 2008;58:19–28. doi: 10.1159/000154476. [DOI] [PubMed] [Google Scholar]
- 24.Bloemen OJ, de Koning MB, Schmitz N, et al. White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol Med. 2010;40:1297–1304. doi: 10.1017/S0033291709991711. [DOI] [PubMed] [Google Scholar]
- 25.Yung A, Phillips L, McGorry P, et al. Comprehensive Assessment of At-Risk Mental States (CAARMS) Melbourne, Australia: University of Melbourne, Department of Psychiatry, Personal Assessment and Crisis Evaluation Clinic; 2002. [Google Scholar]
- 26.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-IV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 28.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D. Wechsler Adult Intelligence Scale—Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 30.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Avants BB, Yushkevich PA, et al. High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: an example study using amyotrophic lateral sclerosis. IEEE Trans Med Imaging. 2007;26:1585–1597. doi: 10.1109/TMI.2007.906784. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Gupta A, Liu Z, et al. DTI registration in atlas based fiber analysis of infantile Krabbe disease. Neuroimage. 2011;55:1577–1586. doi: 10.1016/j.neuroimage.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S, Peng H, Dawe RJ, Arfanakis K. Enhanced ICBM diffusion tensor template of the human brain. Neuroimage. 2011;54:974–984. doi: 10.1016/j.neuroimage.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szeszko PR, Ardekani BA, Ashtari M, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- 36.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 37.Munoz Maniega S, Lymer GK, Bastin ME, et al. A diffusion tensor MRI study of white matter integrity in subjects at high genetic risk of schizophrenia. Schizophr Res. 2008;106:132–139. doi: 10.1016/j.schres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Hoptman MJ, Nierenberg J, Bertisch HC, et al. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophr Res. 2008;106:115–124. doi: 10.1016/j.schres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Kyriakopoulos M, Perez-Iglesias R, Woolley JB, et al. Effect of age at onset of schizophrenia on white matter abnormalities. Br J Psychiatry. 2009;195:346–353. doi: 10.1192/bjp.bp.108.055376. [DOI] [PubMed] [Google Scholar]
- 40.Chan WY, Yang GL, Chia MY, et al. White matter abnormalities in first-episode schizophrenia: a combined structural MRI and DTI study. Schizophr Res. 2010;119:52–60. doi: 10.1016/j.schres.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr134. doi:10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.