Abstract
Background: Despite impressive advancements in early interventions in psychosis, there is an urgent need of robust neurobiological markers to improve the predictive value of psychosis transition. Available structural imaging literature in the field is undermined by several methodological caveats and a number of confounders such as exposure to antipsychotic treatment. Methods: Fourteen voxel-based morphometry studies of antipsychotic-naive subjects at enhanced clinical risk for psychosis (high risk [HR]) or experiencing a first-episode psychosis (FEP) were included. Formal meta-analysis of effect sizes and “signed differential mapping” voxel-based meta-analysis were combined to control the results for sample sizes, strength of individual findings, and confounding variables. Results: Formal effect size meta-analysis indicated consistent gray matter (GM) reductions both in subjects at enhanced clinical risk for psychosis and in first-episode subjects when compared with control groups. Voxel-based meta-analysis showed GM reductions in the temporal, limbic prefrontal cortex within the HR group and in the temporal insular cortex and cerebellum within the FEP group. Psychosis onset was characterized by GM decreases in temporal, anterior cingulate, cerebellar, and insular regions. GM alterations in the temporal regions directly related to severity of psychotic symptoms. There was no publication bias. Heterogeneity across studies was low. Sensitivity analyses confirmed robustness of the above results. Conclusions: Vulnerability to psychosis is associated with consistent GM decreases in prefrontal and temporolimbic areas. The onset of full disease is accompanied by temporoinsular, anterior cingulate, and cerebellar GM reductions. Neuroanatomical alterations in temporal regions may underlie the clinical onset of psychotic symptoms.
Keywords: neuroimaging, psychosis, MRI, VBM, high risk, first episode, prodromal, schizophrenia, dopamine
Introduction
Over the past decade, research on the psychosis risk syndrome (known variably as “clinical high risk (HR)” or “ultra HR” or “prodromal”) has exponentially progressed, allowing for preventive interventions to be feasible in clinical psychiatry.1 In the light of the severe functional, social, and economic long-term impact of psychoses, preventive interventions have been welcomed with a warm enthusiasm, and the number of new clinical services devoted to people at enhanced risk for psychosis has grown up worldwide. Ultimately, such clinical and research interest has lead to the proposal to include the HR syndrome as a new diagnosis in the coming Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V).2 However, despite these promises, validity of psychosis risk criteria is still highly discussed, and the problem of false positives undermines the benefits of preventive interventions.3 There is thus urgent need of reliable neurobiological markers underlying transition from a risk state to established psychosis. Neuroimaging techniques have promised to address this issue,4 and the HR for psychosis has been associated with alterations in the structure,5,6 function,7–9 connectivity,10 and neurochemistry11–13 of the brain4,14(for a review or structural findings, see ref.5 and for reviews of functional findings, see ref.7,8). However, despite the advancements of basic research in neuroscientific investigations, the diagnosis of HR state is nowadays still based on psychopathological criteria15 because of inconsistent and conflicting findings across individual imaging studies.14 A number of factors may contribute to heterogeneity across imaging findings; however, exposure to antipsychotic may play a prominent confounding role. Recent evidence has indicated even short-term treatment with antipsychotics can affect both the function16 and the structure17 of the brain during the early phases of the illness.
Our first aim was to address some of the methodological caveats present in the previous voxel-based meta-analyses controlling the results for the potential confounding effect of antipsychotic treatment. We have thus selected only antipsychotic-naive subjects at enhanced clinical risk for psychosis or experiencing a first episode of disease. Next, we have combined a voxel-based meta-analysis with a formal meta-analysis, weighting the results for sample sizes of individual studies and controlling for moderator variables. We tested the hypothesis that psychosis onset was associated with specific gray matter (GM) changes in prefrontal and temporal areas and that these would be correlated with psychotic symptoms.
Methods
Our first aim was to conduct a robust meta-analysis of GM changes underlying psychosis onset, so we have adopted a multi-steps methodological approach. At the level of selection procedures, we have controlled for the potential effect of antipsychotic treatment by choosing stringent inclusion criteria and focusing on antipsychotic-naive patients only. As available voxel-based meta-analytical packages methods do not allow controlling for the strength of results, we have additionally performed a formal meta-analysis of effect sizes to address robustness of individual findings, publication biases, and heterogeneity. Finally, we have employed signed differential mapping (SDM) to analyze the spatial coordinates obtained from the database and controlling the results for a number of potential moderators.
Selection Procedures
Search Strategies.
A systematic search strategy was used to identify relevant studies. Two independent researchers conducted a 2-step literature search. First, we carried out a Medline search to identify putative voxel-based morphometry (VBM) studies in subjects at enhanced clinical risk for psychosis or with a first episode of psychosis. The search was conducted in February 2011, and no time span was specified for date of publication. We used the following search terms: “VBM,” “psychosis risk,” “prodromal psychosis,” and “first-episode psychosis (FEP).” In a second step, the reference lists of the articles included in the review were manually checked for relevant studies not identified by computerized literature searching. There was no language restriction, though all included articles were in English.
Selection Criteria.
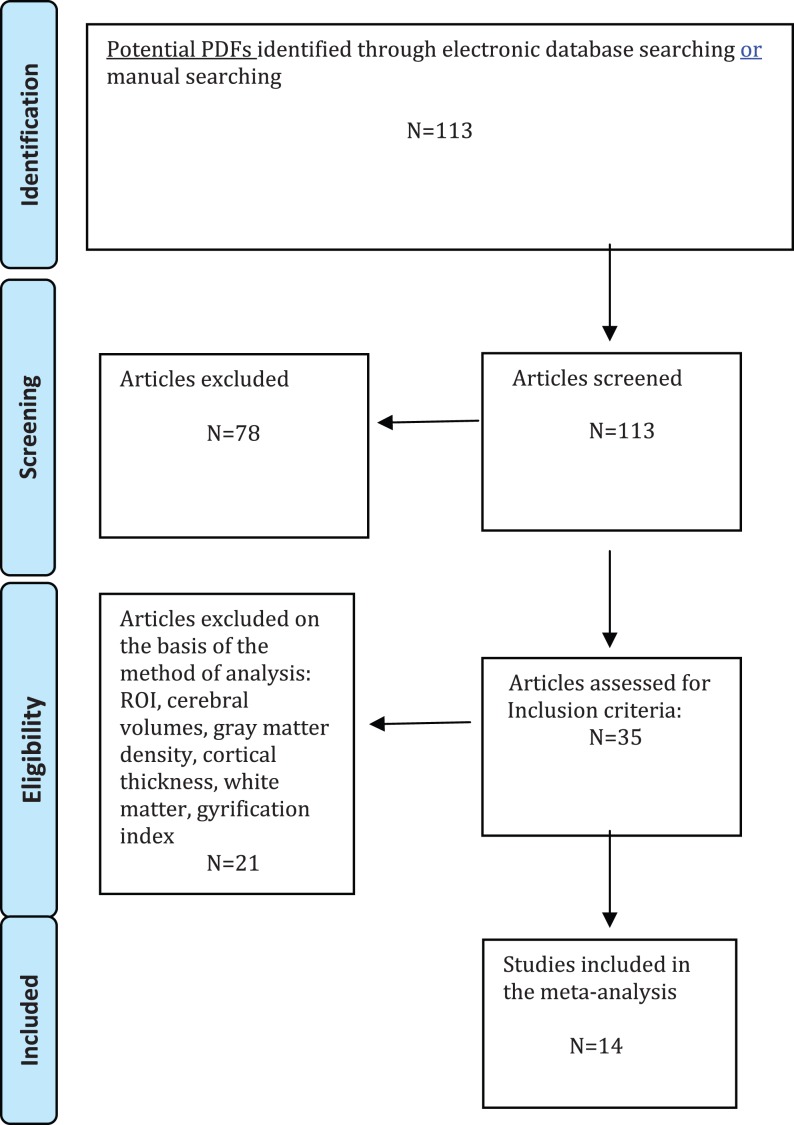
Studies were included according to the following criteria: (a) being an original article in a peer-reviewed journal, (b) have enrolled an antipsychotic-naive patient group (subjects at enhanced clinical risk for psychosis according to established criteria—HR, see below—or subjects with a FEP) and a matched control group, (c) have employed structural neuroimaging in conjunction with whole brain VBM. Studies reporting only region of interests (ROIs) findings were not included in the present meta-analysis. Similarly, we did not use coordinates relative to analyses employing small volume corrections (SVC) in preselected ROIs. Authors of studies where Talairach or Montreal Neurologic Institute coordinates (necessary for the voxel-level quantitative meta-analysis) were not explicitly reported were contacted to reduce the possibility of a biased sample set. In cases where the same or similar samples were used in separate articles, we only included data from the analysis of the largest sample. Studies were independently ascertained and checked by the 2 researchers, and inclusion and exclusion criteria were evaluated by consensus. To achieve a high standard of reporting, we have adopted “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” guidelines18 and the revised QUOROM Statements (Quality Of Reporting Of Meta-analyses)19 (see figure 1).
Fig. 1.
Search strategy used for the inclusion of the studies considered in the current meta-analysis.
Recorded Variables.
The recorded variables for each article included in the meta-analysis were: disease stage (first episode, HR), sample size, gender, mean age of participants, imaging package employed, IQ, duration of untreated psychosis/illness (DUP/DUI), handedness, and magnet intensity. Additionally, we recorded the statistical significance of the main findings and the method employed to correct the whole-brain results for multiple comparisons. Results were comprehensively reported in tables to assist the reader in forming an independent view on the following discussion.
Statistical Analysis
Meta-Analysis of Individual Effect Sizes.
The available voxel-based packages do not allow for weighting of the results based on the level of statistical significance reported in each study for a specific contrast. This means that it is not possible to exactly determine the relative strengths of GM differences between patients and controls. To address the strength of GM changes in the HR and FEP cohorts, we have computed a preliminary meta-analysis of individual effect sizes by using Comprehensive Meta-Analysis Software version 2 (Biostat, Inc., Englewood, NJ). This package employs the same computational algorithms used by the Cochrane Collaborators to weight studies. For each contrast included in the DSM meta-analysis, we have extracted the patient-control statistical difference (p or t value), and as a measure of effect size, we have adopted the Hedges’ g, in order to correct for bias from small sample sizes.20 We employed random effects models because they are more conservative than fixed-effect models and argued to better address heterogeneity between studies and study populations, allowing for greater flexibility in parsing effect size variability. Moreover, they are less influenced by extreme variations in sample size.21 The effect sizes were computed separately in the FEP and in the HR samples and then an overall estimate across groups was provided. The influence of potential moderators such as age, gender (percentage of females), and magnet intensity on the overall meta-analytical estimates was addressed in meta-regressions. Heterogeneity among study point estimates was assessed with the Q statistic with magnitude of heterogeneity being evaluated with the I 2 index.22 Publication bias was examined by visually inspecting funnel plots and applying the regression intercept of Egger et al.23 In this way, we assessed whether there was a tendency for selective publication of studies based on the nature and direction of their results. In addition, we used the fail-safe procedure24 to generate a number of unpublished studies that would be needed to move estimates to a nonsignificant threshold. To assess robustness of the results, we performed sensitivity analyses by sequentially removing each study and rerunning the analysis.
Voxel-Wise Meta-Analysis.
Prior of conducting the voxel-based meta-analysis, a strict selection of the reported peak coordinates of GM differences was applied by only including those that appear statistically significant at the whole-brain level (no SVCs). We have also carefully checked the same statistical threshold throughout the whole brain was used within each included study. This is intended to avoid biases toward liberally thresholded brain regions because it is not uncommon in neuroimaging studies that the statistical threshold for some ROI is rather more liberal than for the rest of the brain. SDM was recently employed to analyze GM changes in VBM studies (www.sdmproject.com/software/).25 SDM has the advantage over other meta-analytical tools of using all the foci information from contributing studies, of including both positive and negative findings in the same map, and of allowing meta-regressions to controls for moderators. The SDM methods have been described in detail elsewhere26 and are only briefly summarized here. First, a map of the differences in GM is separately recreated for each study. This includes limiting voxel values to a maximum to avoid biases toward studies reporting various coordinates in proximity and reconstructing both increases and decreases of GM in the same map. Second, meta-analytic maps were obtained by voxel-wise calculating the corresponding statistics from the study maps, weighted by the squared root of the sample size of each study so that studies with large sample sizes contribute more. The statistical significance of each voxel is determined using standard randomization tests.25,26 The influence of age, percentage of female patients, magnet intensity, and Positive and Negative Syndrome Scale (PANSS) total scores on GM differences was addressed in meta-regression analyses. Age of patients was included in its linear and quadratic forms (age and age squared, the latter obtained from age mean and variance) because the developmental trajectories of some brain regions during psychosis onset may be not linear.
Standard SDM within groups meta-analyses26 were conducted separately in FEP and HR subjects to describe the differences in GM between patients and healthy controls. Next, we contrasted HR and FEP by calculating the between-groups difference in each voxel and determining its statistical significance using a randomization test.26 These analyses were complemented with additional analyses to assess the robustness of the findings.26 These included descriptive analyses of quartiles to find the actual proportion of studies reporting results in a particular brain region (regardless of P values) and jackknife sensitivity analyses to assess the replicability of the results. Results were thresholded at P < .001 uncorrected, which has been found to be empirically equivalent to P < .05 corrected for multiple comparisons under different conditions.27 Additionally, we applied an extent threshold of Ke > 20 voxels.
Results
Inclusion Criteria for the HR Population
The HR studies included in the present study had recruited the participants on the basis of validated criteria developed to identify individuals with an enhanced risk for psychosis at a clinical phase when first symptoms and/or impairments emerge. These might present as “attenuated psychotic symptoms28” that are present below the threshold of full psychosis, “brief and self-limiting psychotic symptoms,”28 or a significant decrease in functioning in the context of a “genetic risk for schizophrenia” (Genetic Risk and Deterioriation syndrome)28 as well as early subjective disturbances of cognitive processes and the perception of the self and the world (BS, Basic Symptoms).29 Three interview measures have been developed to operationalize the UHR criteria: the Comprehensive Assessment of At Risk Mental State,30 the Structured Interview for Prodromal Syndromes,31 and the Basel Screening Instrument for Psychosis,32 while basic symptoms are usually assessed with the Bonn Scale for the Assessment of Basic Symptoms33 and the Schizophrenia Proneness Instrument, Adult Version.34
Number of Studies Found
Fourteen studies met inclusion criteria for the current meta-analysis (figure. 1). Specifically, we included 198 antipsychotic-naive subjects at HR for psychosis (mean age 22.5 years, SD 5.2) matched with 254 controls (mean age 23 years, SD 5.7; P > .05). The second cohort was relative to 206 antipsychotic-naive FEP subjects (mean age 26.4 years, SD 2.9) matched with 202 controls (mean age 26.7 years, SD 3.2; P > .05). Majority of studies was performed on a 1.5 Tesla Magnetic Resonance Imaging scanner and employed Statistical Parametric Mapping as imaging package. Most of them (79%) reported whole-brain findings corrected for multiple comparisons. Details of the included studies are presented in online supplementary table 1.
Formal Meta-Analysis of Effect Sizes
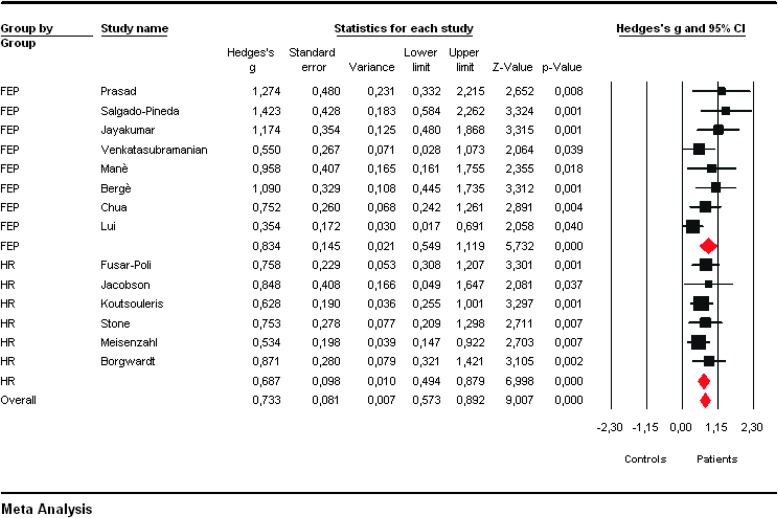
All VBM studies but one35 reported significant GM decreases in the patient group (FEP or HR) as compared with controls. Two VBM studies in FEP subjects reported both GM increases and decreases36,37 in patients as compared with controls. Meta-analysis of effect sizes showed no consistent GM increases in the patient groups as compared with control groups (P > .05). Conversely, overall Hedges’g scores indicated consistent GM reductions both in subjects at HR for psychosis (Hedges’s g =0.687, 95%CI 0.494–0.879, Z = 6.998, P < .001) and in FEP subjects (Hedges’g = 0.834, 95%CI 0.549–1.119, Z = 5.732, P < .001) when compared with controls (figure 2). No significant effect for magnet intensity, IQ, DUP/DUI, handedness, gender, and age was detected.
Fig. 2.
Formal meta-analysis of individual effect sizes (strenght of gray matter decreases in patients as compared with controls) across the voxel-based morphometry studies included in the database. Positive values indicate gray matter decreases in patients as compared to controls.
Visual inspection of funnel plots revealed no obvious evidence of publication bias. Quantitative evaluation of publication bias, as measured by the Egger intercept, was nonsignificant (P = 0.319). Finally, the fail-safe procedure determined that 386 unpublished studies would be needed to bring the overall meta-analytic estimate to a nonsignificant threshold. Robustness of meta-analytic findings was examined by sequentially removing each study and reanalyzing the remaining data set (producing a new analysis for each study removed). No study affected the overall Hedge’s g estimate more than 6%. The pattern of differences across the subanalyses remained essentially unchanged in direction and magnitude. According to the criteria set by Higgins and Thompson,38 heterogeneity in published studies was small in magnitude and statistically nonsignificant (Q = 14.258; P = 0.356; I 2 = 8.826).
Voxel-Wise Meta-Analysis
Within Groups Comparisons.
High Risk
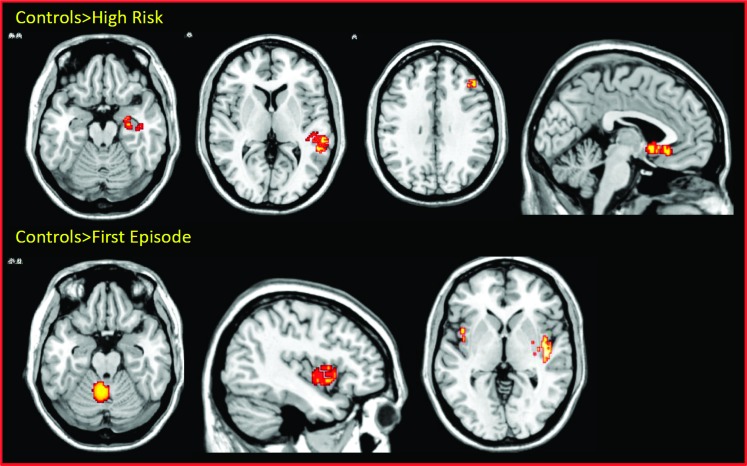
We detected significant GM reductions in with controls in a region spanning the right middle temporal and superior temporal gyri (BA41; P < 0.001), in the right parahippocampal gyrus and hippocampus (P < 0.001), in the left anterior cingulate (P < 0.001), and in the right middle frontal gyrus (table 1 and figure 3). No significant GM increases were found in the HR as compared with the control group.
Table 1.
Regional Differences in Gray Matter Volumes in Antipsychotic-naive VBM Studies Underlying Psychosis Onset
| Clusters | Side | BA | Maximum | |||||
| Coordinates | SDM | P | Ke | |||||
| Within groups | ||||||||
| High risk subjects | ||||||||
| HR < controls | ||||||||
| Middle/Superior temporal gyrus | R | 22 | 50 | −30 | 10 | 0.331 | <.00005 | 157 |
| Parahippocampal gyrus | R | 20 | 30 | −10 | −20 | 0.323 | <.0005 | 63 |
| Anterior cingulate | L | 25 | −2 | 18 | −4 | 0.316 | <.0005 | 75 |
| Middle frontal gyrus | R | 9 | 42 | 32 | 30 | 0.312 | <.0005 | 81 |
| First-episode subjects | ||||||||
| FEP < controls | ||||||||
| Superior temporal gyrus | R | 38 | 45 | 0 | −13 | 0.563 | <.00005 | 319 |
| Insula | L | 13 | −48 | 8 | 2 | 0.332 | <.0005 | 28 |
| Cerebellum | L | AL | −4 | −52 | −26 | 0.342 | <.0005 | 95 |
| Between groups | ||||||||
| FEP < HR | ||||||||
| Cingulate | R | 32 | 16 | 10 | 36 | 0.365 | <.0005 | 211 |
| Cerebellum | L | AL | −4 | −52 | −26 | 0.342 | <.0005 | 225 |
| Superior temporal gyrus | R | 22 | 48 | −16 | 6 | 0.322 | <.0005 | 197 |
| Insula | L | 13 | −42 | 10 | 2 | 0.341 | <.005 | 195 |
Note: HR, high risk; FEP, First-episode; SDM, signed differential mapping; AL, anterior lobe; Ke, cluster extent. No significant clusters of gray matter changes were observed for the following contrasts: HR > C, FEP > C, FEP > HR.
Fig. 3.
Within-groups gray matter (GM) changes. Displayed clusters show GM reductions in the high risk (above) and first episode subjects (below) as compared with healthy controls. The left of the picture is the left on the brain.
First Episode Psychosis
We detected significant GM reductions in FEP subjects as compared with controls in a wide cluster extending from the right superior temporal gyrus to the right insula (P < 0.00005), in the left insula (P < 0.0005), and in the left cerebellum (P < 0.0005; table 1 and figure 3). No significant GM increases were found in the FEP as compared with controls.
Between Groups Comparisons.
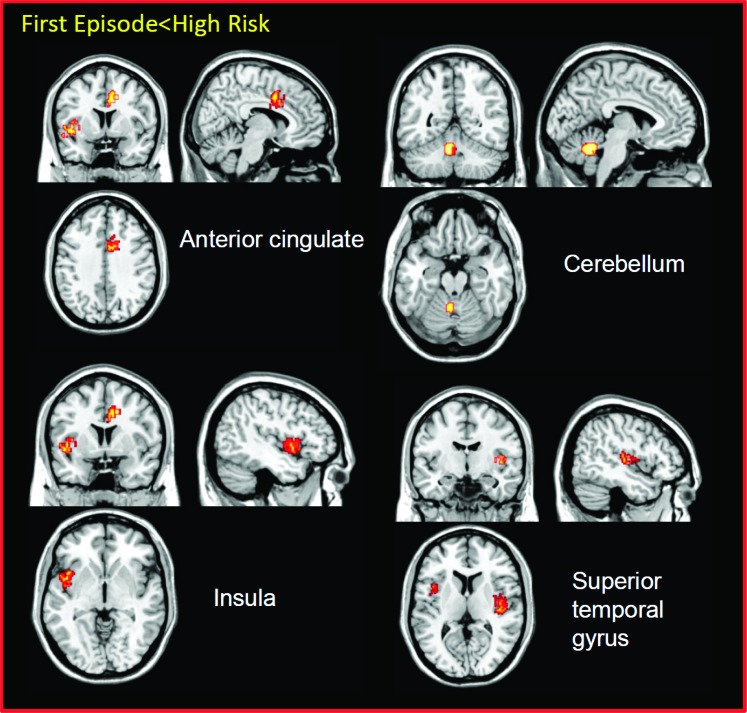
There were significant GM reductions in the FEP group as compared with the HR group in the right superior temporal gyrus (P < 0.0005), in the right anterior cingulate (P < 0.0005), in the left cerebellum (P < 0.0005), and in the left insula (P < 0.005; table 1 and figure 4). Conversely, no significant GM increases were observed in the FEP group as compared with the HR group.
Fig. 4.
Gray matter (GM) reductions underlying psychosis onset. Displayed clusters show GM decreases in the first episode as compared with the high-risk group. The left of the picture is the left on the brain.
Effect of Moderators.
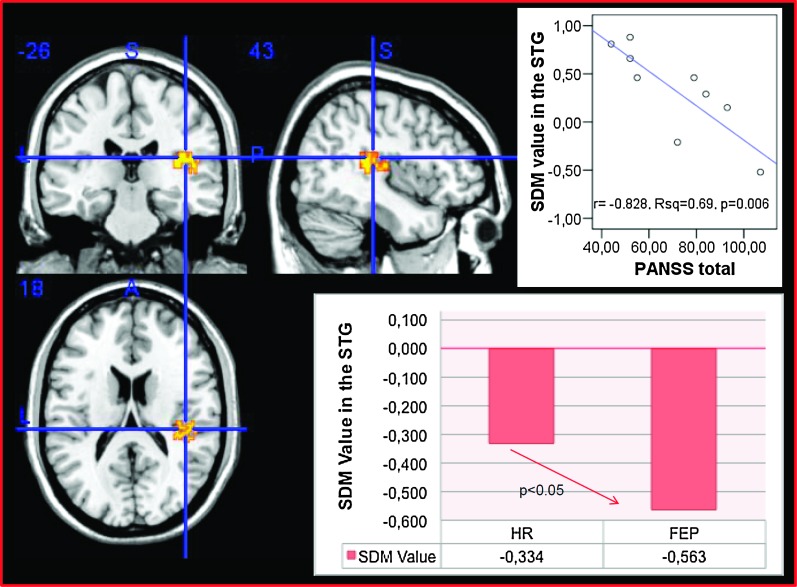
There were no significant effects for age, gender, magnet intensity, IQ, DUI/DUP, and handedness on the GM differences described above here. However, we detected a statistically significant correlation between GM changes and symptoms in the right superior temporal gyrus (x = 43, y = 18, z = −26, r = −0.828, R2 = 0.69, P = .006, see figure 5). Across the whole sample (HR + FEP), GM decreases were associated with elevation of the PANSS total score. This correlation survived correction for multiple comparisons and remained significant after checking for potential outliers with the Cook’s d test.
Fig. 5.
Voxel-wise meta-regression showing a negative correlation between gray matter volume (GM) in the right superior temporal gyrus (SDM value) and severity of psychotic symptoms (PANSS) underlying the disease onset. The left of the picture is the left on the brain. The bar graphs show the signed differential mapping (SDM) values of GM volume (as compared with healthy controls) in the right superior temoral gyrus in each group.
Discussion
To our best knowledge, this is the largest whole-brain structural meta-analysis exploring GM changes in antipsychotic naive subjects in relation to psychosis onset. Formal meta-analysis of individual effect sizes showed a consistent pattern of GM decreases in the patient groups as compared with controls. Voxel-based meta-analysis identified GM reductions in the right temporal, limbic prefrontal cortex within the HR group and in the temporoinsular cortex and cerebellum within the FEP subjects. Psychosis onset was characterized by GM volume reduction in right temporal and left anterior cingulate, cerebellar, and insular regions. GM reductions in the temporal regions were inversely correlated with severity of psychotic symptoms.
We adopted a multiple-step approach with the encompassing objective of providing reliable neuroanatomical maps of psychosis onset. First, at the stage of studies selection, we decided to include whole-brain (VBM) studies only avoiding ROI approaches (ie, ROIs or even SVC). Additionally, we carefully checked that the majority of studies included have employed some statistical method to correct the whole-brain results for multiple comparisons. The specific aim of the present meta-analysis however was to control for the potential confounding effect of medications. Thus, we have selectively included studies enrolling antipsychotic-naive subjects only. There is converging evidence indicating chronic antipsychotic treatment can influence GM volume in established psychosis.17,39 Recent structural imaging studies have further clarified that antipsychotic exposure can affect GM volume even at the onset of the disease, in the early phases of psychosis, influencing the structure of temporal and prefrontal cortex.17 In line with these findings, functional imaging studies have indicated that short-term or acute antipsychotic treatment can alter the neurophysiological cortical response during cognitive functioning.16 In a second step, we have computed a formal meta-analysis of individual effect sizes to test the magnitude of individual GM changes and overall replicability of imaging findings. To our best knowledge, this is the first time a formal effect-size meta-analysis is combined with a voxel-location statistical approach, to respectively ascertain both robustness and location of brain abnormalities underlying psychosis onset. The observed effectsize for gray matter decrease (0.7), according to the criteria established by Cohen is considered large, and this may reflect true neurobiological changes in the patient groups. In a final step, we have decided to employ SDM over other voxel-based meta-analytical packages because it allows weighting the results for sample sizes and addressing the confounding effect of moderators in meta-regression analyses.
To describe reliable neuroanatomical maps of psychosis onset, the key contrast was the comparison between the antipsychotic-naive HR group with the antipsychotic-naive FEP group. We found GM reductions underlying the onset of disease, with volume loss within the anterior cingulate, cerebellar, and temporoinsular regions, in line with previous analyses suggesting a trend toward GM loss.40 While we found more widespread GM volume reductions in the right hemisphere in HR and FEP patients compared with controls, when comparing FEP and HR directly, the left hemisphere was more affected. Consequently, GM reductions rather than increases seem to characterize the onset of psychosis. These alterations are independent of illness duration and antipsychotic treatment because they were observed in drug-naive subjects, and they were controlled for several confounders.
With respect to the anatomical localization of GM loss, reduction in anterior cingulate volume has been observed in psychotic disorders in association with impairments in emotional processing and higher executive performances (for a review, see ref. 41). The anterior cingulate is crucial for integrating cognitive and emotional processes in support of goal-directed behaviour. The functional diversity of the anterior cingulate, which encompasses executive, social cognitive, and affective functions, suggests that abnormalities in the region may partly explain the difficulties in cognitive and emotional integration that characterize the clinical manifestations of psychosis.42 Neuropathological research has supported a core role for anterior cingulate dysfunction in psychosis revealing alterations in the cellular and synaptic architecture of the region.43 A recent SDM voxel-based meta-analysis confirmed anterior cingulate (and insular) GM reductions in subjects presenting a first episode of psychosis, suggesting that the general salience network is abnormal from the onset of the illness in schizophrenia.44 Our group has previously showed anterior cingulate alterations are already evident prior the onset of disease during the prodromal phase and play a crucial role in psychosis transition.6 There is also specific functional imaging evidence indicating abnormal anterior cingulate engagement in the early phases of psychosis,45,46 in subjects at genetic risk for psychosis, 47,48 and in subjects at clinical risk for psychosis.6,9 Of interest, anterior cingulate function and structure has been reported to be especially sensitive to remedial antipsychotic treatment in psychosis.49,50 As there is evidence indicating that few weeks of antipsychotic treatment modulate the anterior cingulate response51,52 and as the latter has been associated with the longitudinal functional outcomes in at risk subjects,53 the question of the functional significance of dynamic prefrontal changes in the prodromal phases of psychosis may have some potential clinical implications for preventive interventions.
Work suggesting that cerebellar abnormalities occur in schizophrenia has been slowly accumulating for several decades.54 The cerebellum participates in neural circuits that perform higher cognitive functions of the sort mediated by heteromodal association cortices. It is connected to many regions of the cerebral cortex by a cortico-cerebellar-thalamic-cortical circuit playing a crucial role in this distributed circuit and coordinate or modulate aspects of cortical activity.54 In line with these premises, structural abnormalities in the cerebellum have been widely observed in HR subjects with subsequent development of psychosis.55 Similarly, involvement of the insular cortex is a common finding in neuroanatomical studies of schizophrenia. The insula is a cortical structure with extensive connections to many areas of the cortex and limbic system. It integrates external sensory input with the limbic system and is integral to the awareness of the body’s state.56 Many deficits observed in schizophrenia involve these functions and may relate to insula pathology, including the processing of both visual and auditory emotional information, pain, and neuronal representations of the self. Additional evidence confirms that insula alterations are crucial to the development of frank psychosis from an HR state.57,58
Finally, alterations in the superior temporal gyrus and its subregions have been shown in psychosis and appear to be specifically involved in the generation of hallucinations and thought disorders (for a review, see ref. 59). The most striking finding of our study was of a significant whole-brain meta-analytical correlation between brain structure and symptoms in the superior temporal gyrus, with GM reductions being associated with elevation of psychotic symptoms. As the HR group showed temporal GM decreases compared to controls, these alterations may reflect preexisting vulnerability. However, a decrease was also observed when the HR group was compared with the FEP group, suggesting that there may be active progressive changes of the temporal cortex during the transition period into psychosis.60 This is in line with available evidence of progressive structural and neurochemical abnormalities in temporal cortices during psychosis onset.7,61 Our correlation is of particular interest as the superior temporal gyrus is known to be implicated in the genesis of positive psychotic symptoms, as suggested by early structural imaging studies.62 The superior temporal gyrus contains several important structures of the brain, including primary auditory cortex in Heschl’s gyrus and auditory association cortical areas in the anterior portion of planum temporale.59 These regions have been thought of as candidates for the neural basis of language-related psychotic symptoms such as auditory hallucinations and thought disorders in patients with schizophrenia.63 In line with the above findings, a recent meta-analysis of functional imaging studies confirmed abnormal neural activity in the superior temporal gyrus of schizophrenic patients during auditory hallucinations.64
Limitations of the current study are well acknowledged. The small sample size, although similar to those of previous voxel-based meta-analyses,65 limited the power of our inferences, in particular subanalyses and meta-regressions. Furthermore, the present meta-analysis aimed to reveal differences in GM at specific brain coordinates rather than differences in volumes of prespecified ROIs. To achieve robust whole-brain results and to avoid selective reporting bias, we did not include ROIs data in this meta-analysis nor any contrasts that employed ROIs or even small-volume corrections. However, although the VBM provides an unbiased approach to establish the presence of regional differences in GM by surveying the whole brain, its limitations relate to the difficulty of spatially normalizing brains, the robustness of standard parametric tests and the interpretation of the results.66 In particular, VBM is sensitive to systematic shape differences attributable to misregistration from the spatial normalization procedure.66 Meta-analyses of brain volumes may also be vulnerable for biases in the literature, with selective outcome reporting and selective analyses reporting being possible explanations. An additional issue concerns the methodological differences of VBM studies. These include differences in smoothing kernel size, slice thickness, statistical threshold, and modulation used in preprocessing of VBM. The most important caveat of VBM imaging meta-analyses is the differential association with the various endophenotypes of the illness. The observed neuroanatomical differences may reflect the composite psychopathological status of the HR group, which includes true HR subjects (who will later develop psychosis) and subjects who are at HR but will not become psychotic.67 The cross-sectional design of the included studies prevented to clarify their long-term clinical outcome and the extent to which the observed findings relate to the subsequent onset of psychosis remains to be determined. To address biases and limit heterogeneity, we have controlled the effect of variables such as age, gender, and symptoms, but other factors like substance abuse and cognitive functioning could potentially play a confounding role. Additionally, it was not possible to use premorbid adjustment, race, and educational level as covariate as only a few studies have clearly reported them.
Conclusions
On the basis of available imaging literature, psychosis onset is characterized by consistent temporoinsular, anterior cingulate, and cerebellar GM reductions. Structural alterations in temporal regions are associated with severity of psychotic symptoms.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Ruhrmann S, Schultze-Lutter F, Bechdolf A, Klosterkotter J. Intervention in at-risk states for developing psychosis. Eur Arch Psychiatry Clin Neurosci. 2010;260(suppl 2):S90–S94. doi: 10.1007/s00406-010-0139-5. [DOI] [PubMed] [Google Scholar]
- 2.Fusar-Poli P, Yung AR. Should attenuated psychosis syndrome be included in the DSM5? The Lancet. doi: 10.1016/S0140-6736(11)61507-9. In press. [DOI] [PubMed] [Google Scholar]
- 3.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: a meta-analysis of evidence. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.1472. In press. [DOI] [PubMed] [Google Scholar]
- 4.McGuire P, Howes OD, Stone J, Fusar-Poli P. Functional neuroimaging in schizophrenia: diagnosis and drug discovery. Trends Pharmacol Sci. 2008;29:91–98. doi: 10.1016/j.tips.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2010;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Borgwardt SJ, McGuire P, Fusar-Poli P, Radue EW, Riecher-Rossler A. Anterior cingulate pathology in the prodromal stage of schizophrenia. Neuroimage. 2008;39:553–554. doi: 10.1016/j.neuroimage.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 7.Smieskova R, Fusar-Poli P, Allen P, et al. Neuroimaging predictors of transition to psychosis. a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Fusar-Poli P, Perez J, Broome M, et al. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Broome MR, Matthiasson P, Fusar-Poli P, et al. Neural correlates of executive function and working memory in the ‘at-risk mental state’. Br J Psychiatry. 2009;194:25–33. doi: 10.1192/bjp.bp.107.046789. [DOI] [PubMed] [Google Scholar]
- 10.Crossley NA, Mechelli A, Fusar-Poli P, et al. Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Hum Brain Mapp. 2009;30:4129–4137. doi: 10.1002/hbm.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fusar-Poli P, Howes OD, Allen P, et al. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry. 2010;67:683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- 12.Fusar-Poli P, Stone J, Broome M, et al. Thalamic glutamate levels predicts cortical response during executive functioning in subjects at high risk for psychosis. Arch Gen Psychiatry. 2011;68:881–890. doi: 10.1001/archgenpsychiatry.2011.46. [DOI] [PubMed] [Google Scholar]
- 13.Fusar-Poli P, Howes OD, Allen P, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- 14.Fusar-Poli P, Allen P, McGuire P. Neuroimaging studies of the early stages of psychosis: a critical review. Eur Psychiatry. 2008;23:237–244. doi: 10.1016/j.eurpsy.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Fusar-Poli P, Broome MR. Conceptual issues in psychiatric neuroimaging. Curr Opin Psychiatry. 2006;19:608–612. doi: 10.1097/01.yco.0000245750.98749.1b. [DOI] [PubMed] [Google Scholar]
- 16.Fusar-Poli P, Broome MR, Matthiasson P, Williams SC, Brammer M, McGuire PK. Effects of acute antipsychotic treatment on brain activation in first episode psychosis: an fMRI study. Eur Neuropsychopharmacol. 2007;17:492–500. doi: 10.1016/j.euroneuro.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Smieskova R, Fusar-Poli P, Allen P, et al. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia?—a systematic review. Curr Pharm Des. 2009;15:2535–2549. doi: 10.2174/138161209788957456. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. QUOROM Group. Br J Surg. 2000;87:1448–1454. doi: 10.1046/j.1365-2168.2000.01610.x. [DOI] [PubMed] [Google Scholar]
- 20.Hedges L, Holkin I. Statistical Methods for Meta-Analysis. New York, NY: Academic press; 1985. [Google Scholar]
- 21.Cooper H, Hedges L, Valentine J. Handbook of Research Synthesis and Meta-Analysis. New York, NY: Russell Sage Foundation; 2009. [Google Scholar]
- 22.Lipsey M, Wilson D. Practical Meta-analysis. Thousand Oaks, CA: Sage Publications; 2000. [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orwin R. A fail-safe N for effect size in meta-analysis. J Educ Stat. 1983;8:157–159. [Google Scholar]
- 25.Via E, Radua J, Cardoner N, Happe F, Mataix-Cols D. Meta-analysis of gray matter abnormalities in autism spectrum disorder: should Asperger disorder be subsumed under a broader umbrella of autistic spectrum disorder? Arch Gen Psychiatry. 2011;68:409–418. doi: 10.1001/archgenpsychiatry.2011.27. [DOI] [PubMed] [Google Scholar]
- 26.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 27.Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 2010;67:701–711. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- 28.Yung AR, Nelson B, Stanford C, et al. Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res. 2008;105:10–17. doi: 10.1016/j.schres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 30.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller TJ, McGlashan TH, Woods SW, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- 32.Riecher-Rossler A, Aston J, Ventura J, et al. [The Basel Screening Instrument for Psychosis (BSIP): development, structure, reliability and validity] Fortschr Neurol Psychiatr. 2008;76:207–216. doi: 10.1055/s-2008-1038155. [DOI] [PubMed] [Google Scholar]
- 33.Klosterkötter J, Gross G, Huber G, Wieneke A, Steinmeyer EM, Schultze-Lutter F. Evaluation of the Bonn scale for the assessment of basic symptoms. BSABS as an instrument for the assessment of schizophrenia proneness: a review of recent findings. Neurol Psychiatry Brain Res. 1997;5:137–150. [Google Scholar]
- 34.Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkötter J. Schizophrenia Proneness Instrument, Adult Version (SPI-A) Rome, Italy: Giovanni Fioriti Editore s.r.l.; 2007. [Google Scholar]
- 35.Jacobson S, Kelleher I, Harley M, et al. Structural and functional brain correlates of subclinical psychotic symptoms in 11-13 year old schoolchildren. Neuroimage. 2010;49:1875–1885. doi: 10.1016/j.neuroimage.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Salgado-Pineda P, Baeza I, Perez-Gomez M, et al. Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage. 2003;19(2 pt 1):365–375. doi: 10.1016/s1053-8119(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 37.Mane A, Falcon C, Mateos JJ, et al. Progressive gray matter changes in first episode schizophrenia: a 4-year longitudinal magnetic resonance study using VBM. Schizophr Res. 2009;114:136–143. doi: 10.1016/j.schres.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 39.Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan RCK, Di X, McAlonan GM, Gong Q. Brain anatomical abnormalities in high-risk individuals, first episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2009;37:177–188. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophr Res. 2007;93:1–12. doi: 10.1016/j.schres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Fornito A, Yucel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35:973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73:79–89. doi: 10.1016/j.schres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Tan HY, Choo WC, Fones CS, Chee MW. fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. Am J Psychiatry. 2005;162:1849–1858. doi: 10.1176/appi.ajp.162.10.1849. [DOI] [PubMed] [Google Scholar]
- 46.Boksman K, Theberge J, Williamson P, et al. A 4.0-T fMRI study of brain connectivity during word fluency in first-episode schizophrenia. Schizophr Res. 2005;75:247–263. doi: 10.1016/j.schres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 47.Whalley HC, Simonotto E, Moorhead W, et al. Functional Imaging as a Predictor of Schizophrenia. Biol Psychiatry. 2006;60:454–462. doi: 10.1016/j.biopsych.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Callicott JH, Egan MF, Mattay VS, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- 49.Stip E, Mancini-Marie A, Letourneau G, et al. Increased grey matter densities in schizophrenia patients with negative symptoms after treatment with quetiapine: a voxel-based morphometry study. Int Clin Psychopharmacol. 2009;24:34–41. doi: 10.1097/YIC.0b013e32831daf6c. [DOI] [PubMed] [Google Scholar]
- 50.Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology. 2009;34:2675–2690. doi: 10.1038/npp.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snitz BE, Macdonald A, III, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- 52.Lahti AC, Holcomb HH, Weiler MA, et al. Clozapine but not haloperidol Re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology. 2004;29:171–178. doi: 10.1038/sj.npp.1300312. [DOI] [PubMed] [Google Scholar]
- 53.Fusar-Poli P, Broome M, Woolley J, et al. Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: longitudinal VBM-fMRI study. J Psychiatr Res. 2011;45:190–198. doi: 10.1016/j.jpsychires.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 56.Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr Res. 2010;123:93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi T, Wood SJ, Yung AR, et al. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. 2009;111:94–102. doi: 10.1016/j.schres.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 58.Smieskova R, Allen P, Simon A, et al. Different duration of at-risk mental state associated with neurofunctional abnormalities. a multimodal imaging study [published online ahead of print Sep 06, 2011] Hum Brain Mapp. doi: 10.1002/hbm.21360. doi:10.1002/hbm.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun J, Maller JJ, Guo L, Fitzgerald PB. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res Rev. 2009;61:14–32. doi: 10.1016/j.brainresrev.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi T, Wood SJ, Yung AR, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 61.Howes O, Bose S, Turkheimer F, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry. 2011;16:885–886. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- 63.Allen P, Laroi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32:175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 64.Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- 65.Leung M, Cheung C, Yu K, et al. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophr Bull. 2009;37:199–211. doi: 10.1093/schbul/sbp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mechelli A, Price C, Friston J, Ashburner J. Voxel-based morphometry of the human brain: methods and Applications. Curr Med Imag Rev. 2005;1:0–9. [Google Scholar]
- 67.Yung AR, Yuen HP, Berger G, et al. Declining transition rate in ultra high risk (prodromal) services: dilution or reduction of risk? Schizophr Bull. 2007;33:673–681. doi: 10.1093/schbul/sbm015. [DOI] [PMC free article] [PubMed] [Google Scholar]