Abstract
Drug resistance continues to be a major barrier to the delivery of curative therapies in cancer. Historically, drug resistance has been associated with over-expression of drug transporters, changes in drug kinetics or amplification of drug targets. However, the emergence of resistance in patients treated with new-targeted therapies has provided new insight into the complexities underlying cancer drug resistance. Recent data now implicate intratumoural heterogeneity as a major driver of drug resistance. Single cell sequencing studies that identified multiple genetically distinct variants within human tumours clearly demonstrate the heterogeneous nature of human tumours. The major contributors to intratumoural heterogeneity are (i) genetic variation, (ii) stochastic processes, (iii) the microenvironment and (iv) cell and tissue plasticity. Each of these factors impacts on drug sensitivity. To deliver curative therapies to patients, modification of current therapeutic strategies to include methods that estimate intratumoural heterogeneity and plasticity will be essential.
Keywords: chemotherapy, clonal variants, drug resistance, intratumoural heterogeneity, tumour cell plasticity
Cancer drug resistance contributes to treatment failure
Cure or control of disseminated disease remains the greatest challenge facing cancer clinicians/scientists, and the greatest cause of patient mortality. Advances in surgery and radiation oncology provide cures for many forms of malignancy. However, these advances are unlikely to produce substantial improvements in cures for patients with metastatic disease. The control of malignancies beyond the primary site of the tumour, requires systemic therapeutic strategies. Systemic chemotherapy-based treatments for cancer emerged in the 1940s to 1960s amid considerable resistance from the clinical community (DeVita & Chu, 2008). Single drug treatments for cancer were widely practiced until the 1960's when DeVita and coworkers championed the concept of combination chemotherapy. The rationale for their approach was to combine agents with different modes of action, thereby increasing the likelihood of synergistic anti-cancer effects (Devita & Schein, 1973). By the mid-1970's, combination chemotherapy had increased the complete remission rate for Hodgkin's lymphoma from 20 to 80% and for lymphosarcoma from 15% to over 50% (Devita & Schein, 1973). With few exceptions, combination chemotherapy is now standard practice when treating both primary and secondary tumours. Despite these advances, a significant fraction of advanced human malignancies remain refractory to curative attempts with conventional chemotherapeutics mainly due to inherent or acquired drug resistance.
In the last decade, there has been a large effort to identify specific mutations within tumours that could be exploited as therapeutic targets. We now have clinical experience with several new classes of ‘targeted’ and ‘non-targeted’ therapies such as anti-angiogenic drugs, anti-stromal drugs, immune modulators, epigenetic modifiers and inhibitors of various growth factors and their signalling pathways. Patient response to these drugs has varied from profound curative responses (Kwak et al, 2010; Rosti et al, 2012) through to transient (Flaherty et al, 2010; Sampson et al, 2010) or poor responses (Garraway & Janne, 2012). Despite the success of targeted and non-targeted approaches to treating cancers, the emergence of resistant disease continues to be a significant cause of patient mortality.
Tumour Drug resistance can be inherent or acquired and is mediated by multiple biochemical processes operating individually or in combination (Fodale et al, 2011). Known factors that lead to drug resistance include (i) induction of drug transporters, (ii) activation of DNA repair, (iii) changes in drug metabolism, (iv) gene amplification or mutation of target proteins and (v) changes in survival/apoptotic pathways. The ATP-binding cassette (ABC) family of drug transporters comprises 48 genes that code for transmembrane pumps that are selectively involved in the efflux of small molecule drugs and toxins (Fukuda & Schuetz, 2012). Some of these transporters have been shown to play a specific role in pumping cytotoxic drugs out of the cell preventing the accumulation of cytotoxic concentrations within the cell and hence invoking drug-resistance (Fukuda & Schuetz, 2012). DNA repair pathways comprise a complex network of proteins able to sense DNA damage (e.g.: ATM, ATR, Chk1/2, BRCA1 or p53) through to the machinery required to repair the damage. Drug metabolizing enzymes such as the cytochrome P450 family of enzymes or the glucuronyl transferases are responsible for the biotransformation of many anti-cancer drugs and their activity contributes to the modulation of intracellular drug levels. This contributes to the sensitivity of cancer cells to cytotoxic drugs. In addition to these regulators of drug sensitivity, gene amplification of receptors, such as EGF receptor, targeted by tyrosine kinase inhibitors can often compromise the efficacy of therapies. Finally, the relative activities of pro-apoptotic and anti-apoptotic (survival) pathways contribute to the sensitivity of a cancer cell to a cytotoxic stimulus (see Chonghaile & Letai, 2008; Engelman, 2009). Combined, these broad, overlapping, mechanisms are the main biochemical determinants of cancer cell sensitivity to cytotoxic drugs (excluding anatomical or diffusional considerations). Therapeutic strategies to modify drug transporters (see Haar et al, 2012), drug metabolism and survival pathways (see Chonghaile & Letai, 2008; Engelman, 2009) have all been developed and trialed in patients. However, further resistance frequently occurs followed by disease relapse and progression. Thus, knowing the main biochemical contributors to resistance has not led to the development of tests that are predictive of tumour behaviour, nor has it led to substantive improvements in patient outcomes.
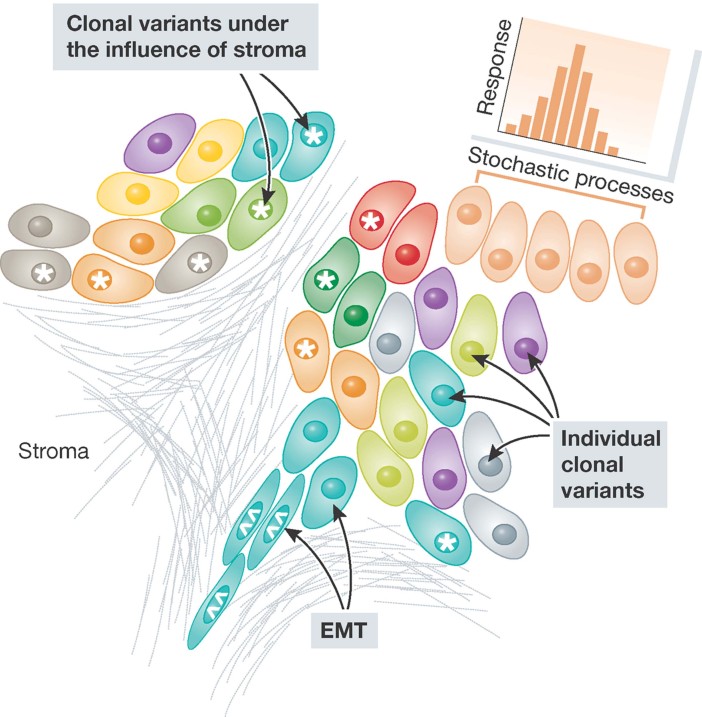
The emergence of resistance to the diverse range of drugs available for cancer treatment is indicative of the dynamic nature of tumour tissue. Recent evidence emerging from studies in which the tumour environment was interrogated suggests that a more fundamental driver of resistance is intratumoural heterogeneity (Ding et al, 2012; Navin et al, 2011; Ruiz et al, 2011; Xu et al, 2012). These studies highlight the likelihood that tumours comprise cancer cells that vary in their sensitivity to chemotherapeutics due to genotypic or phenotypic variation. This is an important finding because the basis of resistance has significant implications for the management of cancer patients. Heterogeneity, in this context, refers to variation in tissue response, tissue composition, tissue physiology, tissue phenotype and tissue genotype. For example, genomic heterogeneity may arise through heritable genetic and epigenetic mechanisms and is exemplified by the presence of discrete clonal variants within a tumour (Navin et al, 2011), the potential presence of tumour initiating subpopulations of cells (Bonnet & Dick, 1997; Lapidot et al, 1994) or cells with a characteristic ‘mutator’ phenotype (see Kolodner et al, 2011). Phenotypic heterogeneity may result from genomic heterogeneity but also can result from stroma/tumour cell interactions, tumour cell/tumour cell interactions or simply as a result of the stochastic nature of biological processes (Fig 1). All of these contributors to intratumoural heterogeneity are likely to be operative simultaneously and highlight why resistance is likely to be dynamic.
Figure 1. Scheme depicting the basis for heterogeneity in drug responses within a tumour.
The different clonal variants present within tumours are represented by the different coloured cells. Heterogeneity, within a clonal variant, due to stromal interaction is marked by * whilst heterogeneity attributable to plasticity/EMT is marked by ⁁⁁. Finally, heterogeneity in an identical clone due to stochastic variation is marked.
Glossary
Clonal variant
Genetically/epigenetically distinct variants of tumour cells within an individual tumour that had a common cell of origin.
Combination chemotherapy
The use of a defined cocktail of chemotherapeutics that individually have different modes of action but when delivered in combination have greater anti-cancer effects.
Conventional chemotherapeutic
Therapeutic agents that are designed to induce a cytotoxic, cytostatic or immune response regardless of the underlying defects that contribute to cancer development.
Intratumoural heterogeneity
Refers to the variation in genome, epigenome, proteome and cell and tissue behaviour that is found within an individual tumour and its stromal constituents.
Plasticity
The ability of a cell to reversibly and flexibly change lineage or to modify cell behaviour through alteration in differentiation programming.
Stochastic process
A process that is determined by random events.
Systems biology
The field of biology that attempts to explain cell, tissue and organism behaviour based on a knowledge of all the interactions that occur between the biological networks within the system.
Targeted therapies
Therapeutic agents that modulate the activity of specific molecules which a cancer is known to be dependent upon.
Biological determinants of intratumoural heterogeneity
Understanding the biological and genetic basis for how cells acquire heterogeneity has important implications for how we manage patients. The acquisition of intratumoural heterogeneity is frequently modelled on evolutionary principles (Gerlinger et al, 2012; Polyak, 2007). In these models, a tumour is assumed to derive from a single founder cell that has acquired a mutation in a critical gene. This mutation is passed on to progeny that are subject to further lesional events, resulting in the production of progeny that continue to acquire genetic/epigenetic mutations leading to a fully transformed malignancy comprising many clonal variants (Ding et al, 2012; Gerlinger et al, 2012; Polyak, 2007). Two models of tumour evolution have been proposed; the cancer stem cell model and the clonal evolution model (Polyak, 2007; Shackleton et al, 2009). Both accommodate the generation and expansion of genotypic and phenotypic variants within tumours. The major point of difference between the models is whether tumour initiating and self-renewal activity is restricted to a fixed subpopulation of cells or is shared by all the variant clones within the tumour.
The cancer stem cell model proposes that there is a fixed, rare, subpopulation of cancer cells that possess stem-like activity with respect to their self-renewal capacity and ability to initiate a tumour in xenotransplant models (Bonnet & Dick, 1997; Lapidot et al, 1994; Shackleton et al, 2009). This model is strongly supported by data from human leukaemias such as acute myeloid leukaemia (AML) (Bonnet & Dick, 1997; Lapidot et al, 1994). Cells enriched from patients with AML could be divided into populations that differed in the expression of specific surface molecules. The bulk of AML cells was CD32−CD38− and, when injected into immunocompromised mice, could not initiate a tumour. By contrast, the rare CD32+CD38− fraction of cells could initiate tumours with high efficiency (Bonnet & Dick, 1997; Lapidot et al, 1994). There have now been a number of studies in other tumour types, including pancreatic cancer, breast cancer, head and neck cancer and medulloblastoma, which have also reported the presence of rare stem-like cells within tumours (Al-Hajj et al, 2003; Bao et al, 2006; Li et al, 2007; Prince et al, 2007). In contrast, the clonal evolution model allows most, if not all, cells to retain a capacity for self-renewal and tumour initiating activity. Thus, from a therapeutic point of view, the cancer stem cell model would require ablation of the cancer stem cell population to invoke a cure whereas the clonal evolution model would require the ablation of all clonal variants to invoke a cure. However, both models allow for the generation of genotypically discrete clonal variants that could differ with respect to chemotherapeutic sensitivity. Recent reports have indicated that tumour cell subpopulations can trans-differentiate into one another indicating that the rigid requirement of the cancer stem cell model, that tumour initiating activity is restricted to a fixed population of tumour cells, may be questioned (Chaffer et al, 2011; Gupta et al, 2011; Roesch et al, 2010). Whilst, these studies have only been reported for breast cancer and melanoma, they do provide an important conceptual framework to unify the two models.
Evidence implicating intratumoural heterogeneity as a driver of chemotherapy resistance in cancer patients
Intratumoural heterogeneity is evident in human cancers and most likely contributes to differing chemotherapeutic responses. Hence, to improve cure rates, an understanding of the contribution by intratumoural heterogeneity to drug resistance is essential. The contributors to intratumoural heterogeneity are genetic variation, stochastic processes, microenvironment and cell and tissue plasticity (Fig 1). The evidence for the role of each of these is discussed below.
Genetic variation and heterogeneous intratumoural drug responses
The introduction of many new targeted therapies to clinical practice provides support for the role of intratumoural heterogeneity in the loss of drug sensitivity. For example, a number of studies have recently reported the use of comparative genomic hybridization analysis and next generation sequencing to analyse individual tumour cells isolated from primary breast cancers (Navin et al, 2011), pancreatic adenocarcinomas (Ruiz et al, 2011), acute myeloid leukaemia (Ding et al, 2012) and renal cell carcinoma (Gerlinger et al, 2012; Xu et al, 2012). There is now definitive evidence showing that primary human tumours contain genetically distinct subpopulations of tumour cells. In primary breast cancers, glioblastomas, melanomas and renal cell carcinomas, clonal variants not only exist within tumours but are also confined to different sub-anatomic sites within the tumours (Gerlinger et al, 2012; Navin et al, 2011; Snuderl et al, 2011; Takata et al, 2000). Moreover, exome sequencing of single cells isolated from a renal cell carcinoma showed that only 30% of the genetic lesions within a tumour are common to all the cancer cells (Gerlinger et al, 2012; Xu et al, 2012). Finally, several independent studies have demonstrated that multiple clonal variants exist within established head and neck cancer cell lines (Cameron et al, 2010; Erlich et al, 2012; Poth et al, 2010). Significantly, these variants differed with respect to their transcriptomic profile, their sensitivity to chemotherapy, their ability to initiate tumours, and their ability to interact with one another to initiate tumours (Cameron et al, 2010; Erlich et al, 2012; Poth et al, 2010). Combined, these studies unequivocally show that genetically distinct variants of tumour cells exist within individual tumours in multiple tumour types.
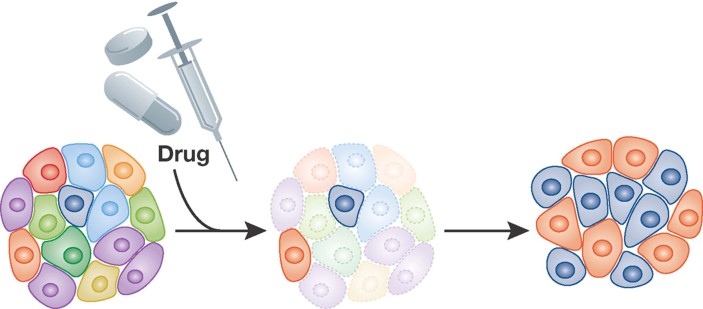
There is increasing evidence demonstrating a role for intratumoural heterogeneity in drug resistance. Many patients have an immediate response to conventional cytotoxic therapies, which can be followed by recurrence and resistance to rechallenge with the same chemotherapeutic agents (DeVita & Chu, 2008; Garraway & Janne, 2012). In some instances, a relapsed tumour may be sensitive to a different chemotherapy protocol and thus patients may undergo multiple cycles of differing chemotherapeutic cocktails in pursuit of a sustained response (DeVita & Chu, 2008; Garraway & Janne, 2012). Similar clinical scenarios have been observed with the newer targeted therapies. For example, the first generation BCR/ABL kinase inhibitor, imatinib, or the V600E mutant-specific BRAF inhibitor, Vemurafenib produce profound initial responses in patients followed in many instances by the development of resistance (Flaherty et al, 2010; Rosti et al, 2012; Villanueva et al, 2010). In chronic myleogenous leukaemia, imatinib resistance is frequently associated with tumour cells that no longer harbour imatinib-sensitive mutations in the BCR-ABL kinase (Garraway & Janne, 2012; Michor et al, 2005). Switching patients to second-generation drugs with broader specificity, such as dasatinib can overcome this resistance (Rosti et al, 2012). In the instance of BRAF V600E mutant-specific therapies, resistance arises in a sub-population of cells in which IGF1 receptor signalling has been deregulated (Villanueva et al, 2010). Similarly, the recent trial of a vaccine against an EGF receptor mutation in glioblastoma demonstrated a similar transient response (Sampson et al, 2010). These trials clearly show the clinical effectiveness of targeted therapies. However, they also show that a paradoxical weakness of targeted therapies may be the highly selective nature of their action. Thus, tumours act as a repository of genetically variant transformed cells that differ in their sensitivity to targeted therapies (Fig 2).
Figure 2. Model depicting the selective resistance of specific clonal variants in response to a chemotherapeutic.
Clonal variants, of varying chemotherapeutic sensitivity are represented by different colours.
Emergence of drug resistance in patients receiving targeted or non-targeted therapy is consistent with the presence of pre-existing variants of tumour cells with varying drug sensitivities. This is supported by molecular studies showing the presence of sequence-verified tumour cell variants within individual human tumours (Navin et al, 2011; Ruiz et al, 2011). Whilst intratumoural genetic heterogeneity clearly has a capacity to drive resistance it is ironic to note that drug treatment may contribute to intratumoural genetic heterogeneity. A recent study sequenced tumour cells from acute myeloid leukaemia (AML) patients prior to and following treatment and relapse (Ding et al, 2012). Relapse was accompanied by the emergence of drug resistant clones (Ding et al, 2012). Moreover, in one patient alone they found 330 tumour-specific mutations, 78 relapse-specific mutations and only 5 mutations that were shared between the primary and relapsed tumours (Ding et al, 2012). In total, eight patients were sequenced and in all instances they found that chemotherapy altered the mutational and variant composition of the tumour resulting in genetically distinct tumour cell variants in treated patients (Ding et al, 2012). These data indicate that the mutagenic properties of some of the therapies currently in use could contribute to heterogeneity and hence could contribute to resistance.
Stochastic processes contribute to heterogeneous intratumoural drug responses
The natural variation that occurs within any cell population is often overlooked as a source of variation in chemotherapy. A series of studies by Sorger and colleagues (Albeck et al, 2008; Spencer et al, 2009) showed the extent of variation that can occur within a genetically identical population of tumour cells in response to a cytotoxic stimulus. They demonstrated that the cytotoxic ligand TRAIL displayed considerable variability with respect to the time and extent of cell death. Using cells stably expressing proteins in the extrinsic apoptosis pathways, they showed that the time to apoptosis, within any culture of genetically identical cancer cells, varied and could be described by a normal distribution. Those cells at extreme ends of the distribution spectrum responded very differently to the same dose of TRAIL (Spencer et al, 2009). This is not an isolated observation. Gascoigne & Taylor (2008) reported a similar finding by measuring the response to antimitotic chemotherapeutics in a number of cancer cell-lines (Gascoigne & Taylor, 2008). Combined, these studies indicate that genetically identical cells under identical physical conditions differ in their response to a given chemotherapeutic to an extent that may impact on clinical response.
Microenvironmental factors contribute to heterogeneous intratumoural drug responses
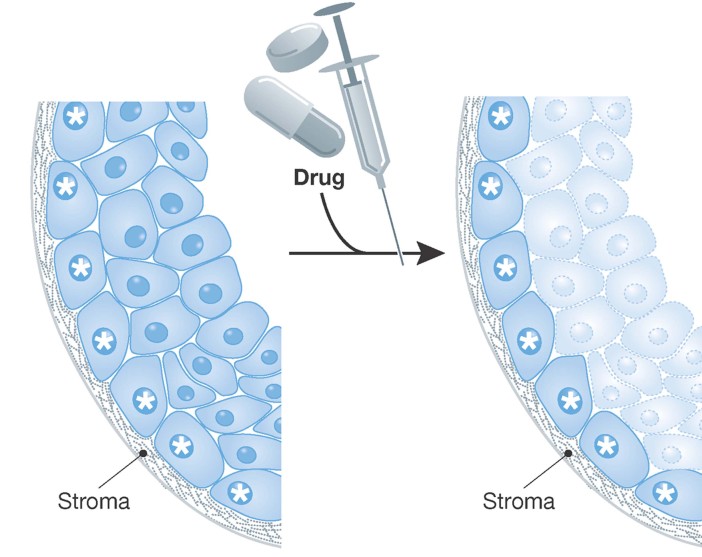
There is evidence that the tumour stroma actively contributes to heterogeneous tumour behaviour and, in particular, chemosensitivity. Stromal components can constitute greater than 50% of tumour mass. Tumour stroma comprises cellular and non-cellular components such as fibroblasts, immunocytes and structural proteins/fibres through to cells and tissue associated with more complex structures such as blood vessels, muscles, bone marrow or nerves. Stromal elements directly control tumour cell behaviour and chemotherapeutic responses. For example, Muranen et al (2012) showed that treatment of breast and ovarian cancer cell lines with PI3K/mTOR inhibitors led to a rapid cytotoxic response. However, they also observed that a small population of cells consistently survived in 3D tissue culture systems. The surviving cancer cells were characterized by their close proximity and interaction with the matrigel in which the cultures were grown (Muranen et al, 2012). Stromal elements and stromal substitutes such as matrigel are known to engage cellular receptors such as integrins. In this instance, Muranen et al (2012) showed that PI3K/mTOR inhibitors induced IGF1 receptor and EGF receptor, on those cells which contacted the stroma. This led to the activation of antiapoptotic pathways (e.g.: BCl2) resulting in drug resistance (Muranen et al, 2012). Significantly, treatment of mice with EGF receptor or IGF1 receptor inhibitors resulted in improved drug responses to the PI3K/mTOR inhibitors in animal models of breast and ovarian cancer (Muranen et al, 2012). Two important concepts arise from this. Firstly, drug resistance may be attributable to a subpopulation of tumour cells that, through their contact with the basement membrane, have acquired drug resistance (Fig 3). Secondly, these data show that chemoresistance can be manipulated pharmacologically. Similarly, non-small cell lung carcinoma (NSCLC) and breast cancers are associated with significant stromal infiltration. In particular, expression of proteins such as the integrins and their basement membrane ligands, laminins, are overexpressed and disrupted in their expression pattern (Desgrosellier & Cheresh, 2010). Laminin/integrin ligation is known to activate intracellular pathways such as NFkB, MAPK/ERK, PTEN/PI3K/Akt, resulting in suppression of the cytotoxic response of breast, oral or NSCLC cells to anoikis (Weaver et al, 2002), etoposide (Sethi et al, 1999; Weaver et al, 2002), doxorubicin (Sethi et al, 1999) or cisplatin (Sansing et al, 2011). Thus, the interaction of tumour cell surface receptors with adjacent stromal elements can induce drug resistant behaviour in adjacent tumour cells (Fig 3). The importance of stroma-mediated chemosensitivity has been recognized and is the basis for the development, and clinical trial, of agents such as the RGD-based inhibitors of integrins (e.g.: cilengitide) in cancer patients (Vermorken et al, 2011).
Figure 3. Model depicting the impact of stromal interactions on the sensitivity of identical clonal variants to chemotherapeutics.
Identical clonal variants are shown. Those cells that interact with the stroma are marked by a star.
Given the contribution of stromal elements to tumour cell behaviour it was quickly realized that fibroblasts associated with cancer tissue were different, phenotypically (Dicker et al, 2002; Elkabets et al, 2011; Place et al, 2011) and genomically (Eng et al, 2009; Hu et al, 2005; Qiu et al, 2008) from fibroblasts associated with normal tissues. The origin of this heterogeneity is unclear. A recent study shows that cancers can be infiltrated by stromal cells derived from the bone marrow (Elkabets et al, 2011). Thus, heterogeneity could arise in the resident tissue fibroblasts or result from infiltration with fibroblasts of a different origin. Despite the complexity of the origins of cancer associated stromal cells it is clear that tumour behaviour is dependent upon their presence and thus they offer an opportunity for therapeutic intervention. For example, it has been shown that cancer stroma could have a profound indirect effect on the chemosensitivity of pancreatic cancer cells (Olive et al, 2009; Provenzano et al, 2012). The insensitivity of pancreatic cancers to gemcitabine was due, in part or wholly, to the presence of a non-permeable stromal barrier that restricted the ability of gemcitabine to reach the tumour cells. It was shown that the use of Hedgehog antagonist, IPI-926, or the hyaluronic acid disrupter, PEGPH20, collapsed the stroma allowing gemcitabine to reach the tumour cells and induce tumour cell death (Olive et al, 2009; Provenzano et al, 2012). In this instance, the resistance to gemcitabine was directly attributable to the anatomic heterogeneity within the tumour.
Tumour stroma contributes indirectly to chemotherapeutic sensitivity by regulating tumour development/progression and by exerting selection pressure on the evolving tumour. In this way the stromal elements dictate the genetic/epigenetic/phenotypic composition of the tumour and thereby modulate chemotherapeutic sensitivity. Arguably the best example of the pro-tumourigenic activity of stromal elements is seen by the establishment and growth of tumour cells at metastatic sites. For example, it has been shown that primary tumour cells are able to contribute to the establishment of a premetastatic niche at distant sites, which in turn, serves to attract and nurture the growth of tumour cells that have left the site of the primary malignancy (Kaplan et al, 2006). The best example of this would be skeletal osteoclasts which are essential to the establishment and growth of breast cancer cells at distant sites within the skeleton (Guise et al, 2006; Mundy, 2002). The relationship between the breast cancer cells and the osteoclasts is often referred to as a ‘vicious cycle’ because primary breast cancer cells release growth factors such as RANKL which stimulate the growth and maturation of distant skeletal osteoclasts which in turn resorb bone releasing matrix-associated growth factors such as TGFβ1 that attract and promote the growth of breast cancer cells in the bone (Guise et al, 2006; Mundy, 2002). The establishment of skeletal metastases significantly reduces patient lifespan and ablation of osteoclasts, using bisphosphonates, significantly reduces patient morbidity and increases lifespan such that it is now standard of care for advanced metastatic breast cancer (Coleman, 2011). These data provide a strong line of biological and clinical evidence showing the importance of the tumour stroma to tumour cell growth and the enormous clinical value of targeting this process. It is noteworthy that recent studies have shown that metastatic foci of medulloblastoma are genetically divergent from tumour cells of the primary lesion (Wu et al, 2012) suggesting that the establishment of metastatic foci may be selective for specific variants of the primary tumour that have an inherent or acquired capacity to migrate to, or take up residence, in the premetastatic niche. Thus, the metastatic stroma and presence of genetically distinct metastatic variants will contribute to the differing chemosensitivities of metastatic lesions.
The innate immune system is an active participant in the development of tumours. M1 macrophages, for example, are tumour-suppressive and associated with good tumour responses to therapy whilst M2 macrophages are pro-tumourigenic and associated with tumour progression (Mosser & Edwards, 2008). The relationship between macrophages and chemotherapeutic response has now been demonstrated in breast cancer. Recent data have shown that a high tumour associated macrophage to T lymphocyte ratio in primary breast cancers was associated with a poor prognosis (DeNardo et al, 2011). DeNardo et al (2011) showed that high levels of colony stimulating factor-1 in breast cancer led to the recruitment of tumour associated macrophages which, in turn, suppressed the tumour-suppressive activity of T lymphocytes and inhibited taxane-mediated cytotoxicity. Pharmacological inhibition of tumour associated macrophage infiltration led to the sensitization of breast cancer cells to cytotoxic drugs confirming their causal association with drug resistance (DeNardo et al, 2011). Thus, there is clinical and experimental evidence to show that the local tumour immune system contributes to chemotherapeutic responses.
Contribution of tumour cell plasticity to heterogeneous intratumoural drug responses
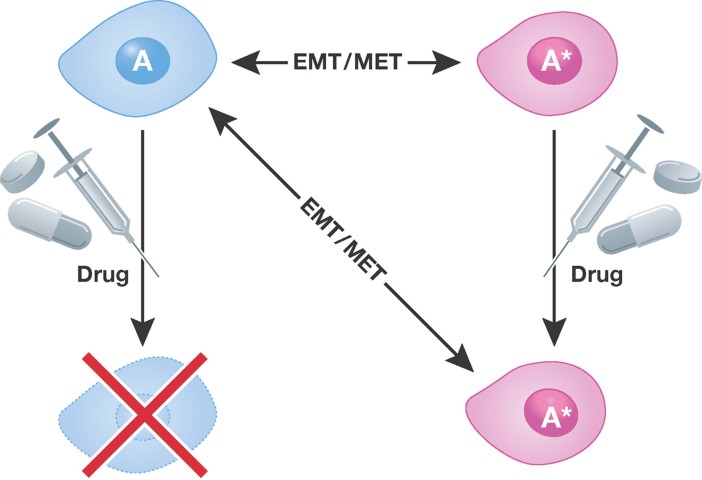
Tumour cells display considerable plasticity and this plasticity extends to sensitivity to chemotherapeutics (Fig 4). In cancer, plasticity refers to the ability of a cell to reversibly change lineage or to modify cell behaviour beyond the normal differentiation programme of that cell. Plasticity relating to lineage transition is generally silenced in adult tissues with the exception of some stem cell compartments (Tang, 2012). Thus, the reinstatement of plasticity in cancer cells reflects a pathological consequence of changes in the tumour cells or in the adjacent tumour environment. From a therapeutic point of view, plasticity is a confounding factor since cancer cells that respond to a particular cytotoxic therapy may be insensitive to chemotherapy if they have changed their phenotype. The best-described example of cancer cell plasticity is the continuum observed in the epithelial to mesenchymal transition (EMT) and the reverse of this process, the mesenchymal to epithelial transition (MET) (reviewed in Nieto, 2011). Studies of the EMT have revealed a causal link between the EMT and the acquisition of stem-like activities and chemoresistance. For example, the mesenchymal phenotype in lung, pancreatic, and head and neck cancer cells is associated with insensitivity to the clinically approved EGFR-targeted agent erlotinib/Tarceva (Thomson et al, 2005; Yauch et al, 2005). In particular, lung carcinoma cell lines, which have undergone an EMT, exhibit reduced sensitivity to erlotinib due to reduced dependence on the EGFR pathway (Thomson et al, 2008; Yao et al, 2010). Moreover, studies of drug sensitivity in various cancer cells, before or after the EMT, show that following a mesenchymal transition cancer cells are resistant to TRAIL (McConkey et al, 2009), radiation (Bao et al, 2006; Nieto, 2011), paclitaxel (Cheng et al, 2007), and cisplatin (Hsu et al, 2010; Latifi et al, 2011). Passage through the EMT is regulated at a transcriptional level by a suite of transcription factors such as Zeb1, Twist, Snail and Slug (Arumugam et al, 2009; Nieto, 2011) that are responsible for the phenotypic changes that accompany the EMT. In particular, the loss of E-Cadherin expression is the classic marker of the EMT and is controlled by Snail/Twist and Zeb1. Significantly, these same factors induce drug resistance (Arumugam et al, 2009; Nieto, 2011).
Figure 4. Model depicting impact of tumour cell plasticity on chemotherapeutic sensitivity.
In this model a cell may give rise to individual tumour cells of different lineage that differ in their sensitivity to a chemotherapeutic agent.
Plasticity is not restricted to EMT (Thompson & Haviv, 2011). Recent work has shown that different pathological subtypes of breast cancer cells are able to give rise to one another (Chaffer et al, 2011; Gupta et al, 2011). Specifically, basal, ductal and stem-like cancer cell populations were isolated from two different breast cancer cell-lines indicating that established cell lines can stably retain intratumoural heterogeneity. Moreover, the authors showed that each of the different cell subpopulations could give rise to all three lineages in approximately the same proportions observed in the unsorted population. It can be concluded that the different subpopulations are not fixed in their phenotype. Of relevance to the present review, it was reported that the stem-like cells were chemo-resistant to paclitaxel and 5-fluorouracil and that expansion of the other subtypes of cells following chemotherapeutic exposure was due to the resistance of the stem cell fraction (Gupta et al, 2011). Interestingly, they found that all three populations of cells could initiate tumour formation in vivo. Moreover, Roesch et al (2010) showed that melanoma cells can be divided into slow and fast replicating populations. The slow-cycling population represented a small fraction of the melanoma cells and was characterized by high levels of expression of the demethylase enzyme Jarid1B (Roesch et al, 2010). Both Jarid1B+ve and Jarid1B−ve melanoma cells could initiate tumours in vivo and could give rise to mixed populations of Jarid1B+ve and Jarid1B−ve melanoma cells (Roesch et al, 2010). However, knockdown of Jarid1B reduced self-renewal suggesting that Jarid1B+ve cells had stem-like qualities. Unfortunately, the chemo-sensitivity of the Jarid1B+ve and Jarid1B−ve populations was not examined (Roesch et al, 2010). Although these studies are very recent and have not yet been validated in other cancer types, they provide important insight into how intratumoural heterogeneity evolves and how this may relates to drug responses (Fig 4).
Challenges posed by tumour heterogeneity
Whilst there has been some success with therapies targeting pathways identified from profiling whole tumours (Flaherty et al, 2010; Sampson et al, 2010), such strategies are yet to deliver widespread improvements in cure or long-term survival. A major limitation of global profiling of tumours is the inability to identify clonal variant-specific lesions, potential tissue interactions or plasticity within a tumour. Since these factors are significant drivers of cancer drug resistance it is essential to develop methods to estimate their potential contribution in any tumour (see Pending issues).
Estimating intratumoural heterogeneity will be challenging. Heterogeneity varies between and within tumour types as well as within an individual tumour. Indeed, it has been reported that within a tumour type there is <5% commonality of genetic lesions (e.g.: Kan et al, 2010; Wood et al, 2007). This suggests that profiling individual tumour types, using single cell sequencing techniques, to estimate intratumoural heterogeneity and molecular targets, may be needed for target-directed personalized therapies in the future. Unfortunately, it remains unclear how many genetically distinct variants may exist within tumours at any time. Identifying driver mutation targets may prove to be the easier part of this process since the ability to sequence the genomes of individual tumour cells, is now possible (Navin et al, 2011; Ruiz et al, 2011; Xu et al, 2012). Whether it will have the sensitivity to quantitate the number of different variants present within an individual tumour remains unknown. However, the efficacy of existing targeted therapies against their cognate target cells would suggest that approaches that target multiple targets from multiple variants will invoke good clinical responses.
An unresolved and more challenging complication relates to the plasticity of tumour cells coupled with the instability that drives genetic heterogeneity. Plasticity and genome instability give rise to variant tumour behaviour and will remain major barriers to the delivery of curative therapies. Furthermore, it will be important to develop strategies that can modify tumour cell plasticity. In this regard, such strategies would need to either inhibit the transition to chemoresistant states or, encourage cells to retain or acquire a chemo-sensitive phenotype. Because of the complexity of tumours it will be important to develop experimental systems that allow us to model processes that promote intratumoural heterogeneity/plasticity and trial management strategies in these models as a prelude to defining clinical management protocols in patients. The complexity of tumours would suggest that this will require integrated systems-biology-based platforms in which we can input data relating to the complexity and plasticity of an individual patients tumour and output a clinical management strategy based on the identification of multiple potential targets. In this instance, strategies that combine target-directed therapies with non-targeted/ablative therapies may help to simultaneously reduce tumour burden, plasticity and complexity. Similarly, agents that modify immune cell function or stroma may also provide powerful adjuncts to targeted and conventional chemotherapeutics.
An additional issue relating to therapeutic approaches to cancer treatment relates to whether all tumour cells need to be ablated or whether a subclinical burden of disease is an acceptable endpoint. Tumour dormancy studies show that patients can harbour cancer cells without manifesting clinical disease (Paez et al, 2012). However, it is likely that the threshold for tolerance of cancer cells will be dependent upon the nature of the transformations in the tumour cells (Leung & Brugge, 2012), the plasticity of the tumour cells or the interactions of the tumour cells with the adjacent environment. These are important issues since a curative therapy would need to reduce the tumour burden and heterogeneity to a level that is associated with a low risk of recurrence.
Pending issues
Develop methods to estimate existing tumour heterogeneity
Develop techniques to quantitate the relative abundance of tumour variants
Develop models that recapitulate the effects of genetic variants, stroma, tissue plasticity and stochastic processes on chemotherapeutic sensitivity
Develop therapeutic strategies to collapse tumour heterogeneity
Develop platforms to track changing heterogeneity during treatment
Develop systems biology-based platforms that predict tumour complexity and drug sensitivity
Develop cost-effective platforms to individualise clinical management of patients.
Acknowledgments
NS is supported by a Fellowship from the Cancer Council Queensland. MH and FS are supported by Career Development Awards from the National Health and Medical Research Council of Australia (no. 569512 and 546155 respectively).
The authors declare that they have no conflict of interest.
References
- Albeck JG, Burke JM, Aldridge BB, Zhang M, Lauffenburger DA, Sorger PK. Quantitative analysis of pathways controlling extrinsic apoptosis in single cells. Mol Cell. 2008;30:11–25. doi: 10.1016/j.molcel.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prsospective identification of tumourigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam T, Ramachandran V, Fourneir KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Cameron S, Dahler A, Jabbar I, Endo-Munoz L, Thomas G, Poth K, Rickwood D, Guminski A, Saunders N. Tumour initiating activity and tumour morphology of HNSCC is modulated by interactions between clonal variants within the tumour. Lab Invest. 2010;90:1594–1603. doi: 10.1038/labinvest.2010.131. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27((Suppl 1)):S149–S157. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. The use of bisphosphonates in cancer treatment. Ann New York Acad Sci. 2011;1218:3–14. doi: 10.1111/j.1749-6632.2010.05766.x. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:52–65. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVita VT, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643–8653. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- Devita VT, Schein PS. The use of drugs in combination for the treatment of cancer: rationale and results. New Engl J Med. 1973;288:998–1006. doi: 10.1056/NEJM197305102881905. [DOI] [PubMed] [Google Scholar]
- Dicker AJ, Serewko MM, Russell T, Rothnagel JA, Strutton GM, Saunders NA. Isolation, from a basal cell carcinoma, of a functionally distinct fibroblast-like cell type, that overexpresses ptch. J Invest Dermatol. 2002;118:859–865. doi: 10.1046/j.1523-1747.2002.01739.x. [DOI] [PubMed] [Google Scholar]
- Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, Ritchey JK, Young MA, Lamprecht T, McLellan MD, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabets M, Gifford AM, Scheel C, Nilsson B, Reinhardt F, Bray MA, Carpenter AE, Jirstrom K, Magnusson K, Ebert BL, et al. Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J Clin Invest. 2011;121:784–799. doi: 10.1172/JCI43757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C, Leone G, Orloff MS, Ostrowski MC. Genomic alterations in tumor stroma. Cancer Res. 2009;69:6759–6764. doi: 10.1158/0008-5472.CAN-09-0985. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targetting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Erlich RB, Kherrouche Z, Rickwood D, Endo-Munoz L, Cameron S, Dahler A, Hazar-Rethinam M, De long LM, Wooley K, Guminski A, et al. Preclinical evaluation of dual PI3K-mTOR inhibitors and histone deacetylase inhibitors in head and neck squamous cell carcinoma. Br J Cancer. 2012;106:107–115. doi: 10.1038/bjc.2011.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman AA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. New Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodale V, Pierobon M, Liotta L, Petricon E. Mechanism of cell adaptation: when and how do cancer cells develop chemoresistance. Cancer J. 2011;17:9–95. doi: 10.1097/PPO.0b013e318212dd3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Schuetz JD. ABC transporters and their role in nucleoside and nucleotide drug resistance. Biochem Pharmacol. 2012;83:1073–1083. doi: 10.1016/j.bcp.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Janne PA. Circumventing cancer drug resistance in the era of personalised medicine. Cancer Discov. 2012;2:214–226. doi: 10.1158/2159-8290.CD-12-0012. [DOI] [PubMed] [Google Scholar]
- Gascoigne KE, Taylor SS. Cancer cells display profound intra and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–222. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. New Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Haar CP, Hebbar P, Wallace GC, Das A, Vandergrift WA, Smith JA, Giglio P, Patel SJ, Ray SK, Banik NL. Drug resistance in glioblastoma: a mini review. Neurochem Res. 2012;37:1192–1200. doi: 10.1007/s11064-011-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DSS, Lan HY, Huang CH, Tai SK, Chang SY, Tsai TL, Chiang CC, Tzeng CH, Wu KJ, Kao JY, et al. Regulation of excision repair cross-complementation group 1 by snail contributes to cisplatin resistance in head and neck cancer. Clin Cancer Res. 2010;16:4561–4571. doi: 10.1158/1078-0432.CCR-10-0593. [DOI] [PubMed] [Google Scholar]
- Hu M, Yao J, Cai L, Bachman KE, Brule F, Velculescu V, Polyak K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the “pre-metastatic niche”: within bone and beyond. Cancer Metastasis Rev. 2006;25:521–529. doi: 10.1007/s10555-006-9036-9. [DOI] [PubMed] [Google Scholar]
- Kolodner RD, Cleveland DW, Putnam CD. Aneuploidy drives a mutator phenotype in cancer. Science. 2011;333:942–943. doi: 10.1126/science.1211154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak EL, Bang YJ, Carnidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Janne PA, Costa DB, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifi A, Abubaker K, Castrechini N, Ward AC, Lionque C, Dobill F, Kumar J, Thompson EW, Quinn MA, Findlay JK, et al. Cisplatin treatment of primary and metastatic epithelial ovarian carcinomas generates residual cells with mesenchymal stem cell-like profile. J Cell Biochem. 2011;112:2850–2864. doi: 10.1002/jcb.23199. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri M, Dick J. A cell initiating human acute myeloid leukemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Leung CT, Brugge JS. Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature. 2012;482:410–414. doi: 10.1038/nature10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- McConkey DJ, Choi W, Marquis L, Martin F, Williams SB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, Nowak MA. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–1270. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- Muranen T, Selfors LM, Worster DT, Iwanicki MC, Song L, Morales FC, Gao S, Mills GB, Grugge JS. inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell. 2012;21:227–239. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, Cook K, Stepansky A, Levy D, Esposito D, et al. Tumour evolution inferred by single cell sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:16.1–16.30. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez D, Labonte MJ, Bohanes P, Zhang W, Benhanim L, Ning Y, Waktsuki T, Loupakis F, Lenz HJ. Cancer dormancy: a model of early dissemination and late cancer recurrence. Clin Cancer Res. 2012;18:645–653. doi: 10.1158/1078-0432.CCR-11-2186. [DOI] [PubMed] [Google Scholar]
- Place AE, Huh SJ, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13:227–237. doi: 10.1186/bcr2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K. Breast cancer: origins and evolution. J Clin Invest. 2007;117:3155–3163. doi: 10.1172/JCI33295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poth K, Guminski A, Thomas G, Jabbar I, Saunders NA. Treatment with cisplatin induces a transient increase in tumourigenic potential in resistant HNSCC cells. Mol Cancer Ther. 2010;9:2430–2439. doi: 10.1158/1535-7163.MCT-10-0258. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targetting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Hu M, Sridhar A, Opeskin K, Fox S, Shipitsin M, Trivett M, Thompson ER, Ramakrishna M, Gorringe KL, et al. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet. 2008;40:650–655. doi: 10.1038/ng.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, Herlyn M. A Temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosti G, Castagnetti F, Gugliotta G, Palandri F, Baccarani M. Second-generation BCR-ABL inhibitors for frontline treatment of chronic myeloid leukemia in chronic phase. Crit Rev Oncol Hematol. 2012;82:159–170. doi: 10.1016/j.critrevonc.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Ruiz C, Lenkiewicz E, evers L, Holley T, Robeson A, Kiefer J, Demeure MJ, Hollingsworth MA, Shen M, Prunkard D, et al. Advancing a clinically relevant perspective of the clonal nature of cancer. Proc Natl Acad Sci USA. 2011;108:12054–12059. doi: 10.1073/pnas.1104009108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansing HA, Sarkeshik A, Yates JR, Patel V, Gutkind JS, Yamada KM, Berrier AL. Integrin αβ1, ανβ, α6β effectors p130Cas, Src and talin regulate carcinoma invasion and resistance. Biochem Biophys Res Commun. 2011;406:171–176. doi: 10.1016/j.bbrc.2011.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance. Nat Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Snuderl M, Fazlollahi L, Le LP, Nitta M, Zhelyazkova BH, Davidson CJ, Akhavanfard S, Cahill DP, Aldape KD, Betensky RA, et al. Mosaic amplification of multiple recepttor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20:810–817. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Spencer SL, Gaudet S, Albeck JG, Burke JM, Sorger PK. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature. 2009;459:428–433. doi: 10.1038/nature08012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M, Morita R, Takehara K. Clonal heterogeneity in sporadic melanomas as revealed by loss of heterozygosity analysis. Int J Cancer. 2000;85:492–497. [PubMed] [Google Scholar]
- Tang DG. understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22:457–472. doi: 10.1038/cr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson S, Buck E, Petti F, Griffin G, Brown E, Ramnarine N, Iwata KK, Gibson N, Haley JD. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–9462. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- Thomson S, Petti F, Sujka-Kwok I, Epstein D, Haley JD. Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis. 2008;25:843–854. doi: 10.1007/s10585-008-9200-4. [DOI] [PubMed] [Google Scholar]
- Thompson EW, Haviv I. The social aspects of EMT-MET plasticity. Nat Med. 2011;17:1048–1049. doi: 10.1038/nm.2437. [DOI] [PubMed] [Google Scholar]
- Vermorken JB, Guigay J, Mesia R, Trigo JM, Keilholz U, Kerber A, Bethe U, Picard M, Brummendorf TH. Phase I/II trial of cilengitide with cetuximab, cisplatin and 5-fluorouracil in recurrent and/or metastatic squamous cell cancer of the head and neck: findings of the phase I part. Br J Cancer. 2011;104:1691–1696. doi: 10.1038/bjc.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva J, Vultur A, Lee JT, Somasundaram R, Kalabis MF, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargetting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, lelievre S, Lakins JN, Chrenek MA, Jones JCR, Giancotti F, Werb Z, Bissell MJ. β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- Wu X, Northcott PA, Dubuc A, Dupuy AJ, Shih DJH, Witt H, Croul S, Bouffet E, Fults DW, Eberhart CG, et al. Clonal selection drives genetic divergence of metatstatic medulloblastoma. Nature. 2012;482:529–533. doi: 10.1038/nature10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Hou Y, Yin X, Bao L, Tang A, Song L, Li F, Tsang S, Wu K, Wu H, et al. Single-cell exome sequencing reveals singl-nucleotide mutation characteristics of a kidney tumor. Cell. 2012;148:886–895. doi: 10.1016/j.cell.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B, Lindsted T, Schlederer M, Johns C, Altorki N, Mittal V, et al. TGF-{beta} IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci USA. 2010;107:15535–15540. doi: 10.1073/pnas.1009472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Januario T, Eberhard DA, Cavet G, Zhu W, Fu L, Pham TQ, Soriano R, Stinson J, Seshagiri S, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005;11:8686–8698. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]