Abstract
Type II endometrial carcinomas are a highly aggressive group of tumour subtypes that are frequently associated with inactivation of the TP53 tumour suppressor gene. We show that mice with endometrium-specific deletion of Trp53 initially exhibited histological changes that are identical to known precursor lesions of type II endometrial carcinomas in humans and later developed carcinomas representing all type II subtypes. The mTORC1 signalling pathway was frequently activated in these precursor lesions and tumours, suggesting a genetic cooperation between this pathway and Trp53 deficiency in tumour initiation. Consistent with this idea, analyses of 521 human endometrial carcinomas identified frequent mTORC1 pathway activation in type I as well as type II endometrial carcinoma subtypes. mTORC1 pathway activation and p53 expression or mutation status each independently predicted poor patient survival. We suggest that molecular alterations in p53 and the mTORC1 pathway play different roles in the initiation of the different endometrial cancer subtypes, but that combined p53 inactivation and mTORC1 pathway activation are unifying pathogenic features among histologically diverse subtypes of late stage aggressive endometrial tumours.
Keywords: clear cell, endometrial carcinoma, mouse model, p53, serous
INTRODUCTION
Carcinomas of the endometrium, the mucosa that lines the uterus, are the most frequently diagnosed malignancies of the female genital tract (Jemal et al, 2011). Endometrial carcinomas are classified in a dualistic model based on histological appearance, clinical behaviour and molecular differences (Di Cristofano & Ellenson, 2007). Type I tumours are endometrioid endometrial adenocarcinomas (AdCas), which account for 80% of endometrial malignancies and are characterized by a relatively high-survival rate (85% at 5 years), due partly to their frequent early detection. These tumours frequently exhibit mutations or alterations in oncogenes and tumour suppressor genes, including PTEN, PIK3CA, PIK3R1, PIK3R2, LKB1, TSC2 and KRAS, that result in activation of the PI3K-AKT-mTORC1 signalling pathway (Catasus et al, 2009; Cheung et al, 2011; Konopka et al, 2011; Lu et al, 2008; Oda et al, 2005; Salvesen et al, 2009; Tashiro et al, 1997; Urick et al, 2011; Velasco et al, 2006). Type I tumours are associated with excessive oestrogen stimulation, frequently harbour mutations in CTNNB1 and exhibit microsatellite instability (Di Cristofano & Ellenson, 2007). While roughly half of poorly differentiated, high-grade type I tumours exhibit TP53 mutations, low-grade tumours rarely display TP53 mutations, suggesting that loss of function of the p53 tumour suppressor protein is a late event in the progression of type I disease (Lax et al, 2000).
Type II endometrial tumours develop mainly in elderly women and comprise three different subtypes; serous AdCas, clear cell AdCas and undifferentiated carcinomas, each representing approximately 5–10% of all endometrial carcinoma cases (Di Cristofano & Ellenson, 2007; Prat et al, 2007). Despite their relative rarity, these tumours contribute to nearly half of the total number of endometrial cancer-related deaths due to their highly invasive and metastatic nature. Carcinosarcomas (also known as malignant mixed Müllerian tumours) represent approximately 1–3% of all endometrial malignancies and comprise both malignant epithelial and mesenchymal components. These tumours mostly arise as a result of de-differentiation of high-grade type I or type II AdCas and follow a highly aggressive clinical course that has a very poor outcome (Abeln et al, 1997; Wada et al, 1997).
Type II endometrial carcinomas and carcinosarcomas are associated with different sets of gene mutations to type I carcinomas. Mutations in PTEN or KRAS and microsatellite instability are rare in type II tumours (Catasus et al, 2009; Lax et al, 2000; Tashiro et al, 1997), but there is strong correlative evidence that mutations in TP53 are involved at the earliest stage of tumour development. TP53 mutations are found in up to 95% of cases of endometrial serous AdCa (Fadare & Zheng, 2009; Zheng et al, 2011). Endometrial serous AdCas are believed to arise from precursor lesions termed endometrial glandular dysplasia (EmGD) that progress to a non-invasive cancer termed endometrial intraepithelial carcinoma (EIC; Fadare & Zheng, 2009; Zheng et al, 2007, 2004, 2011). Approximately 50–75% of these lesions display TP53 mutations (Jarboe et al, 2009; Jia et al, 2008; Liang et al, 2004; Zhang et al, 2009). Clusters of cells displaying strong p53 immunoreactivity can also be infrequently observed in some morphologically normal endometrial glands, suggesting that TP53 mutation represents the earliest step of serous AdCa formation (Jarboe et al, 2009; Zhang et al, 2009). TP53 mutations also occur at high frequency in endometrial clear cell AdCas (An et al, 2004; Fadare et al, 2006; Lax et al, 1998) and in carcinosarcomas (Abeln et al, 1997; Kounelis et al, 1998; Soong et al, 1999; Szukala et al, 1999; Taylor et al, 2006; Wada et al, 1997). Thus, in contrast to type I tumours, type II tumours are frequently characterized by early mutations in TP53.
We present functional genetic evidence showing that p53 suppresses the formation of type II endometrial tumours in mice and have employed a large cohort of human endometrial carcinomas to demonstrate molecular similarities between aggressive type I and type II tumours.
RESULTS
Analysis of mice with genitourinary tract-specific Trp53 deletion
The Ksp1.3-Cre transgene drives Cre expression widely in the kidney epithelium and in the embryonic precursor structures that give rise to most of the epithelia of the adult genitourinary system (Frew et al, 2008; Shao et al, 2002). In the uterus, Ksp1.3-Cre activity results in a mosaic pattern of gene deletion in the endometrial epithelial cells of the lumen and glands (Frew et al, 2008). We attempted to generate a mouse model of endometrial type II carcinomas by crossing Trp53fl/fl mice (Jonkers et al, 2001), in which loxP sites are present in intron 1 and intron 10 of the Trp53 gene, the mouse homologue of human TP53, to mice expressing the Ksp1.3-Cre transgene. PCR-based assays of genomic DNA from the kidney, uterus and epididymis of these Ksp1.3-Cre/+;Trp53fl/fl mice confirmed the genotype and the presence of Cre-mediated recombination of the loxP-flanked Trp53 allele (Fig 1A). Real-time PCR analysis demonstrated reduction in Trp53 mRNA abundance in these tissues in Cre-expressing mice (Fig 1B). Cohorts of littermate +/+; Trp53fl/fl (hereafter referred to as WT) and Ksp1.3-Cre/+;Trp53fl/fl (hereafter referred to as Trp53Δ/Δ) mice were analysed at 9–10 weeks (WT, n = 5; Trp53Δ/Δ, n = 7), 24–35 weeks (WT, n = 8; Trp53Δ/Δ, n = 21), 47–58 weeks (WT, n = 5; Trp53Δ/Δ, n = 10) and 65–79 weeks (WT, n = 22; Trp53Δ/Δ, n = 33). Trp53Δ/Δ mice developed normally and at 10 weeks of age displayed no gross morphological alterations in the kidney (Fig 1D), uterus (Fig 1H and Supporting Information Fig 1D, G, M and P), ureter, epididymis, vas deferens or vesicular glands (unpublished observations). Moreover, both male and female Trp53Δ/Δ mice were normally fecund and fertile.
Figure 1. Type II endometrial tumours formed in older Ksp1.3-Cre; Trp53fl/fl mice.
- A. Upper panels: genotyping PCR of tail DNA to detect the Ksp1.3-Cre transgene (+ denotes present and − denotes absent) or the Trp53 floxed (fl) or wild-type (+) allele. Lower panels: PCR to detect Cre-mediated recombination at the Trp53 locus using DNA isolated from kidney, epididymis and uterus of the same mice used in the upper panels. The position of the recombined band is shown by Δ.
- B. Real-time quantitative PCR analysis of Trp53 mRNA abundance (normalized to 18S RNA abundance) in indicated tissues from wild-type (white bars) or Trp53Δ/Δ (black bars) mice.
- C-H. Haematoxylin and eosin stain of sections of kidneys (C and D), epididymis (E and F) and uterus (G and H) from wild-type or Trp53Δ/Δ mice aged 10 weeks (C, D, G and H) or 6 months (E and F). (I–N) Examples of tumours that arose in 14–16-month-old knockout mice. Arrowheads in (F) highlight atypical nuclei. All scale bars represent 50 µm.
- I-N. Serous AdCa with papillary growth pattern (I), Clear cell AdCa with papillary growth pattern (J), Serous AdCa with acinar growth pattern (K), Clear cell AdCa with acinar growth pattern (L). Carcinosarcoma displaying mixed clear cell and (heterologous) chrondrosarcoma components (M), Undifferentiated carcinoma (N).
Trp53Δ/Δ mice displayed no pathological alterations in the kidney, ureter, bladder, vas deferens or vesicular glands up until at least 79 weeks of age. Regions of the epithelium of the corpus epididymis of male Trp53Δ/Δ mice frequently developed a vacuolated appearance and displayed substantial nuclear atypia at around 6 months of age (Fig 1F). To our knowledge, these lesions are unlike any reported lesions in humans. Consistent with the very low frequency of tumour formation in the human epididymis, these lesions did not progress to tumours in any of the 14 male mice in the oldest cohort and were not further studied.
While no tumours were observed in female mice in the cohorts up until approximately 1 year of age, 16 of 19 female mice in the 65–79 week cohort developed a total of 26 independent endometrial tumours of various histological appearances (Table 1 and Fig 1I–N)). Individual mice frequently exhibited multiple independent endometrial tumours of different histological appearances (Supporting Information Fig 2). Human serous AdCas of the uterus typically have complex, branching papillae with cellular budding and usually broad, thick fibrovascular cores, but occasionally thin to delicate cores. The nuclei are usually highly atypical (Silverberg et al, 2003). Many of the mouse lesions displayed almost identical morphologies with high-grade nuclei, papillary or micropapillary growth patterns and were regarded as serous AdCas. AdCas composed mainly of clear or hobnail cells arranged in solid, tubulo-cystic or papillary patterns were classified as clear cell AdCas. Twelve tumours arose in the lumen of the uterus and exhibited papillary morphology with characteristics of either serous AdCa (Fig 1I) or clear cell AdCa (Fig 1J), or in some instances a mixture of both. In contrast, 11 tumours that arose from the glandular component of the endometrium displayed an acinar pattern of growth and displayed either serous or clear cell AdCa morphologies (Fig 1K and L). Three carcinosarcomas with mixed clear cell AdCa components and either chondrosarcoma (Fig 1M) or rhabdomyosarcoma components also arose. Additionally, two undifferentiated carcinomas, containing a mixture of regions of solid, tubulo-cystic and squamous growth patterns (Fig 1N) arose. Of these latter five lesions, four had invaded tissues beyond the uterus and one of these had metastasized throughout the peritoneal cavity, displaying nodules of tumour growth on the liver, intestines and peritoneal wall. Carcinosarcomas and mixed carcinomas are believed to result from the de-differentiation of high-grade type I or type II carcinomas (Abeln et al, 1997; Wada et al, 1997). Supporting this idea, two of the three tumours of this type that arose in Trp53Δ/Δ mice were very large tumours (Table 1 and Supporting Information Fig 3) that contained regions of either clear cell or undifferentiated carcinoma. One carcinosarcoma contained a very large region of ‘pure’ tubulo-cystic clear cell AdCa that likely represents the initial large lesion from which the carcinosarcoma component is derived (Supporting Information Fig 3). PCR analysis of genomic DNA isolated from 4 of 4 macro-dissected tumours displayed Cre-mediated recombination at the Trp53 locus (unpublished observations), indicating that these tumours arose from Trp53 knockout cells. Thus, three different histological subtypes of type II endometrial carcinomas, as well as carcinosarcomas, arose in Trp53Δ/Δ mice at high frequency.
Table 1.
Summary of endometrial tumour incidence, histological subtype, growth pattern, nuclear grade, tumour size and staining intensity for antibodies against phospho-Tyr1248-ErbB2 (P-ErbB2), phospho-Thr202/Tyr204-ERK1/2 (P-ERK), phospho-Ser473-AKT (P-AKT), phospho-Thr421/Ser424-p70 S6 Kinase (P-S6K), phospho-Ser240/244-ribosomal S6 protein (P-S6) and phospho-Thr37/46-4E-BP1 (P-4E-BP1)
| Mouse ID | Age (weeks) | Tumour ID | Tumour entity | Histological subtype (s) | Growth patterns | Nuclear grade | Diameter (mm) | P-ErbB2 | P-ERK | P-AKT | P-S6K | P-S6 | P-4E-BP1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 310 | 65 | 21 | EIC | Serous | Micropapillary, acinar, cribriform | High | 0.5 | + | + | +++ | + | + | + |
| 279 | 72 | 2 | AdCa | Serous | Micropapillary, acinar, cribriform | High | 4.0 | + | + | + | + | +++ | + |
| 279 | 72 | 5 | AdCa | Serous, partly clear cell | Micropapillary, acinar, cribriform, hobnailing | High | 5.5 | + | + | + | + | +++ | + |
| 306 | 69 | 17 | AdCa | Serous, partly clear cell | Micropapillary, acinar, cribriform, diffuse | High | 13.0 | + | + | + | + | +++ | + |
| 285 | 67 | 6 | AdCa | Serous | Acinar, cribriform | High | 3.7 | + | + | + | + | +++ | + |
| 281 | 72 | 11 | AdCa | Serous | Micropapillary, acinar, cribriform | High | 1.3 | + | + | + | + | + | + |
| 310 | 65 | 20 | AdCa | Serous | Micropapillary, acinar, cribriform | High | 1.7 | + | + | + | + | + | + |
| 281 | 72 | 9 | AdCa | Serous | Acinar, cribriform | High | 3.3 | + | + | + | + | + | + |
| 279 | 72 | 4b | EIC | Serous, partly clear cell | Papillary, hobnailing | High | 0.3 | + | + | + | + | +++ | + |
| 287 | 67 | 15 | AdCa | Clear cell | Papillary | Low | 1.0 | + | + | + | +++ | +++ | +++ |
| 287 | 67 | 16 | AdCa | Serous | Micropapillary, acinar, cribriform | High | 0.9 | + | + | + | +++ | +++ | + |
| 288 | 67 | 23a | EIC | Serous | Acinar, cribriform | High | 0.4 | + | + | +++ | +++ | +++ | + |
| 281 | 72 | 8 | AdCa | Serous, partly clear cell | Papillary, micropapillary | High | 3.1 | + | + | +++ | +++ | +++ | +++ |
| 323 | 68 | 14 | AdCa | Serous, partly clear cell | Micropapillary, papillary, hobnailing | Low | 2.3 | + | +++ | +++ | +++ | +++ | +++ |
| 388 | 71 | 25 | AdCa | Serous, partly clear cell | Micropapillary, papillary, hobnailing | High | 1.4 | + | +++ | +++ | +++ | +++ | +++ |
| 280 | 72 | 27 | AdCa | Serous, partly clear cell | Micropapillary, papillary, hobnailing | High | 1.1 | + | +++ | +++ | +++ | +++ | +++ |
| 282 | 72 | 19 | AdCa | Serous, partly clear cell | Papillary, micropapillary | Low | 4.5 | + | + | +++ | +++ | +++ | +++ |
| 279 | 72 | 4a | EIC | Serous | Micropapillary, acinar | High | 0.5 | +++ | + | + | + | +++ | + |
| 320 | 68 | 12 | EIC | Serous | Micropapillary, papillary, acinar | High | 0.5 | + | + | + | +++ | + | + |
| 389 | 71 | 7 | AdCa | Serous | Micropapillary, acinar, cribriform | High | 4.0 | + | + | + | + | + | + |
| 281 | 72 | 10 | AdCa | Serous | Micropapillary, acinar, cribriform, diffuse | High | 8.2 | + | + | + | + | + | +++ |
| 323 | 68 | 13 | Carcinosarcoma | With heterologous component | Acinar, cribriform, signet ring like, solid, diffuse | High | 13.0 | + | +++/+ | +/− | +/− | +/− | +++/− |
| 278 | 58 | 22 | Carcinosarcoma | With heterologous component | Tubulo-cystic, solid, diffuse | High | 16.0 | +/− | +++/− | +/− | +/− | + | +++/− |
| 288 | 67 | 23b | Carcinosarcoma | With heterologous component | Solid, diffuse | High | 2.5 | – | +/− | +/− | +/− | +/− | +++/− |
| 275 | 75 | 18 | Carcinoma | Undifferentiated | Solid, tubulo-cystic, squamous | High | 11.0 | + | – | +++/− | – | + | +/− |
| 280 | 72 | 26 | Carcinoma | Undifferentiated | Solid | High | 15.0 | – | +/− | +++/− | – | + | +++ |
AdCa, adenocarcinoma; EIC, endometrial intraepithelial carcinoma; +, same staining intensity as non-tumourous endometrium; +++, upregulated staining compared to non-tumourous endometrium; −, no staining; /, mixed staining within tumour.
Identification of tumour precursor lesions in Trp53Δ/Δ mice
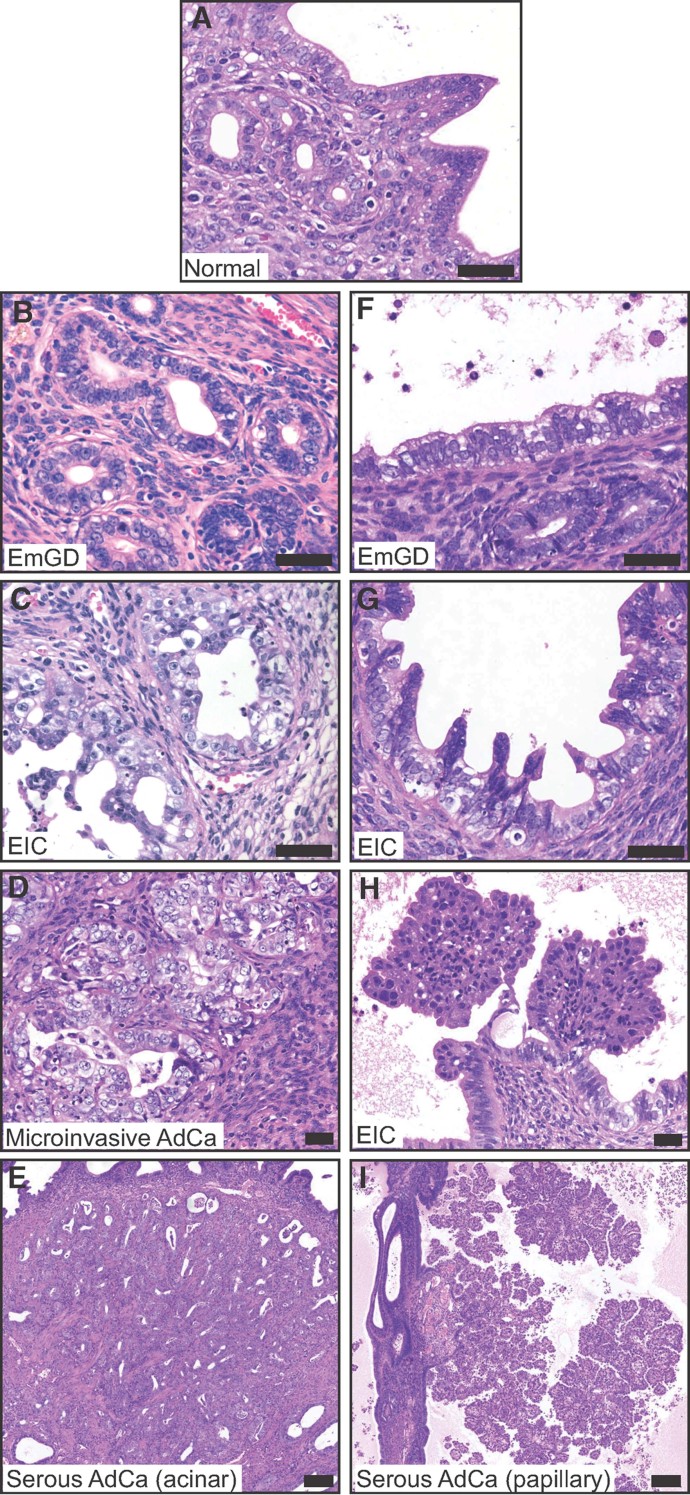
Analysis of the non-tumourous endometrium of tumour-bearing mice identified a series of putative tumour precursor lesions in the surface epithelium of the lumen and in the epithelium of endometrial glands. In longitudinal histological sections of uteri from 16 mice that contained 26 macroscopically visible tumours we identified 169 and 191 independent sites of EmGD in the epithelium lining the glands (Fig 2B) and the lumen (Fig 2F), respectively. EmGD represents the earliest morphologically identifiable serous carcinoma precancerous lesion in humans (Yi & Zheng, 2008; Zheng et al, 2004). The EmGD lesions in the lumen and glands of Trp53Δ/Δ mice are histologically identical to human EmGD and are characterized by enlarged and hyperchromatic nuclei, prominent nucleoli, nuclear crowding and loss of basal nuclear localization. In humans, EIC is believed to arise from EmGD and appears to be the immediate precursor of serous AdCa (Ambros et al, 1995; Sherman et al, 1995). Serous EIC is specifically associated with concurrent serous AdCa of the endometrium but may also be observed in association with clear cell AdCa (Ambros et al, 1995; Lax et al, 1998). EIC exhibits single or multiple layers of malignant cells that have replaced the endometrial surface epithelium or gland. The human EIC cells have pleomorphic nuclei and frequent mitotic figures, resembling the cells of high-grade tumours (Sherman et al, 1995). Endometrial glands and lumen in Trp53Δ/Δ mice also displayed numerous sites of EIC (46 and 54, respectively), characterized by extensive nuclear atypia, multi-layered cellular growths and the presence of micropapillary projections (Fig 2C and G). A novel apparent tumour precursor lesion that we term ‘EIC with papillary structures’ was also evident in the lumen surface epithelium. This lesion, which was identified at 20 discrete sites in these sections, is characterized by a papillary growth pattern with a fibrovascular core (Fig 2H). The epithelial components of some of these papillary EIC lesions exhibited typical clear cell morphology with tubulocystic growth pattern, cuboidal cells with clear cytoplasm and ‘hobnail’ cells with apical nuclei that bulge into the lumen. Other lesions displayed serous morphology with micropapillary features, large vesicular nuclei with coarse chromatin and prominent nucleoli. Thus, these EIC lesions appear to be precursor lesions of clear cell and serous AdCas with papillary growth patterns. An equivalent intermediate lesion, that we call ‘microinvasive adenocarcinoma’, was observed 10 times in the glandular compartment of the endometrium (Fig 2D). Based on their morphologies and relative frequencies of occurrence, it appears that these lesions represent a spectrum of histological changes that occur in the progression from a normal epithelium to a carcinoma, consistent with the model proposed for human endometrial serous AdCa (Zheng et al, 2004). AdCas with a papillary growth pattern in our model appear to form exclusively from the surface epithelium of the lumen (Fig 2I), whereas AdCas with an acinar growth pattern arise from endometrial glands (Fig 2E). Supporting the progression model, and consistent with observations in human disease (Zheng et al, 2004), the endometrial epithelium directly surrounding several papillary AdCas (Fig 3G shows an example) displayed a continuous transition from normal epithelium (Fig 3J) to EmGD (Fig 3M) to EIC (Fig 3P) to a papillary AdCa growth pattern (Fig 3P). Furthermore, all mice in the 24–29-week-old cohort displayed some regions of EmGD (Supporting Information Fig 1E, H, N and Q) and all mice in the 47–58-week-old cohort frequently displayed widespread regions of EmGD and/or EIC (Supporting Information Fig 1F, I, O and R) but did not display any tumours. Thus, as they age, Trp53-deficient mice progressively display a spectrum of endometrial histological alterations ranging from low-nuclear grade dysplasia through to invasive high-grade carcinomas. These mice therefore represent the first model that accurately reproduces the progression and hallmark morphological features of human type II endometrial carcinomas.
Figure 2. Precursor lesions and progression model of tumour formation.
- A. Normal endometrial glands and lumenal surface epithelium.
- B-E. Examples of lesions illustrating the proposed progression model from EmGD arising in glands (B), to EIC in the glands (C) to microinvasive AdCa (D) to serous AdCa with acinar growth pattern (E).
- F-I. Examples of lesions illustrating the proposed progression model from EmGD arising in lumenal surface epithelium (F), to EIC in the lumen (G) to EIC with papillary growth (H) to serous AdCa with papillary growth pattern (I). Scale bars in A–H depict 50 µm and in E and F depicts 200 µm.
Figure 3. Upregulation of P-S6 and P-4E-BP1 at transition zones of serous AdCa.
(A, D, G, J, M, and P) Haemotoxylin and eosin stain (H&E), (B, E, H, K, N and Q) P-S6 IHC stain and (C, F, I, L, O and R) P-4E-BP1 IHC stain
- A-F. Two examples of EIC with papillary growth.
- G-I. Low-power image of a serous AdCa and dysplastic adjacent epithelia.
- J-L. Non-dysplastic endometrial surface epithelium from a region that is continuous with the upper epithelium but outside of the fields shown in (G–I).
- M-O. Zoom of boxed region 1 from (G) to (I) showing EmGD.
- P-R. Zoom of boxed region 2 from (G) to (I) showing the transition from EmGD (multi-layered epithelium below/left of the arrowhead) to EIC/serous AdCa (epithelium above/right of the arrowhead). Scale bars in (G–I) represent 200 µm, in all other panels 50 µm.
Molecular analyses of Trp53 mutant endometrial tumours
Since the Ksp1.3-Cre transgene drives gene deletion during embryogenesis (Frew et al, 2008; Shao et al, 2002) but tumours do not arise until approximately 14–16 months of age, Trp53 appears to be largely dispensable for the normal formation and proliferative homeostasis of the endometrium. It is likely that additional mutations must occur that cooperate with Trp53 mutation to initiate and drive tumour progression. Since 17–29% of type II endometrial tumours in humans are characterized by amplifications of the ERBB2 gene (Konecny et al, 2009; Morrison et al, 2006; Nofech-Mozes et al, 2008) we analysed ErbB2 protein expression and activation status using immunohistochemical (IHC) staining of sections of mouse uteri containing a total of 26 type II tumours. One small glandular clear cell EIC displayed intense staining for ErbB2 and for phospho-Tyr1248-ErbB2, demonstrating that the expression of this oncogenic tyrosine kinase receptor is highly upregulated and activated (Table 1 and Supporting Information Fig 4). However, no other tumours showed upregulation of ErbB2 expression or activity, implicating pathways other than ErbB2 as playing a predominant pathogenic role in these tumours.
To investigate the activation status of other signalling pathways that may be dysregulated, IHC staining was conducted using antibodies that recognize phosphorylated forms of various signalling molecules. A positive score was given to a tumour either if the intensity of staining or the frequency of staining in the epithelial cells was higher than non-tumourous endometrium in the same section (see Supporting Information Fig 5 for an example) Markers of activation of the AKT-mTORC1 signalling axis, including phospho-Ser473-AKT (P-AKT), phospho-Thr421/Ser424-p70 S6 Kinase (P-S6K), phospho-Ser240/244-ribosomal S6 protein (P-S6) and phospho-Thr37/46-4E-BP1 (P-4E-BP1) were upregulated in a large percentage of the tumours (Table 1). 21 of 26 tumours displayed positive staining for at least one marker of the AKT-mTORC1 signalling pathway and 9 of these were positive for two or more markers. In contrast, only 5 of 24 tumours were positive for phospho-Thr202/Tyr204-ERK1/2 (Table 1), one was positive for phospho-Ser217/221-MEK1/2 and none for phospho-Thr183/Tyr185-SAPK/JNK (unpublished observations). Thus, type II endometrial tumours in Trp53Δ/Δ mice frequently display over-activation of the AKT-mTOR C1 signalling cascade but more rarely of the MAPK signalling cascades. A particularly striking correlation between activation of the AKT-mTORC1 signalling pathway and tumour subtype was observed in the group of papillary lesions, including serous and clear cell AdCas as well as larger EIC lesions (Table 1).
We took advantage of this correlation as well as the strong and specific IHC staining pattern of P-S6 and P-4E-BP1 as sensitive downstream markers of activation of mTORC1 to further investigate the sequence of events that leads to the formation of papillary AdCas. Small clusters of elevated staining for P-S6 were frequently observed in regions of EmGD lesions in glands (Supporting Information Fig 6A and B) and in the lumen (Supporting Information Fig 6C and D) of endometria from Trp53Δ/Δ mice. However, regions of EmGD were also observed that did not display elevated staining for P-S6 (Supporting Information Fig 6E and F). Sites of elevated staining corresponded to 18 and 12%, in the glands and lumen, respectively, of the total number of identified EmGD lesions in the same samples, suggesting that mTORC1 pathway activation is not required for the transition from a normal epithelium to EmGD. Strikingly however, all papillary EIC lesions, even those papillary outgrowths that comprised only a few cells (Supporting Information Fig 6G and H), were strongly positive for P-S6 (Fig 3B and E) and P-4E-BP1 (Fig 3C and F). Moreover, in three large papillary tumours where it was possible to identify a continuous transition in the epithelium from normal to EmGD to EIC to serous or clear cell AdCa, elevated staining for P-S6 (Fig 3H and Q) and for P-4E-BP1 (Fig 3I and R) was observed precisely at the transition between EIC and papillary AdCa. These data demonstrate that mTORC1 pathway activation is not necessary for the formation of EmGD or EIC, but suggest that they are associated with, or drive, papillary outgrowth, a frequent feature of aggressive clear cell and serous endometrial AdCas.
Activation of the PI3K-AKT-mTORC1 pathway occurs frequently in all subtypes of human endometrial tumours
We constructed two human endometrial carcinoma tissue microarrays (TMA; see Supporting Information Table 1), in total comprising tumour punches from 463 endometrioid AdCas (type I), 34 serous AdCas (type II), 19 clear cell AdCas (type II), 16 undifferentiated carcinomas (type II) and 16 carcinosarcomas. A poor patient outcome was independently associated with type II carcinomas or carcinosarcomas (Supporting Information Fig 7A), older age at diagnosis (Supporting Information Fig 7B), high-grade (see later in Fig 6G) or high-FIGO stage (see later in Fig 6J), validating the TMAs against known clinical parameters of these tumour types. We immunohistochemically stained the TMAs using antibodies against proteins or epitopes that reveal the activation status of the PI3K and/or mTORC1 signalling pathways (p110α, PTEN, P-AKT, phospho-Ser9-GSK3β (P-GSK3β), phospho-Ser2448-mTOR (P-mTOR), TSC1, TSC2, P-S6K and P-4E-BP1), or have been previously proposed to be differentially expressed between different subtypes of endometrial carcinoma (PAX8, IMP3 and p16; Mhawech-Fauceglia et al, 2010; Tong et al, 2010; Yemelyanova et al, 2009) or have been shown to predict a poor prognosis (p53, ErbB2; Catasus et al, 2009; Konecny et al, 2009; Morrison et al, 2006). Examples of tumour spots displaying different IHC scores for p53 or displaying negative (0) and strong (3) IHC scores for the other antibodies are shown in Supporting Information Fig 8.
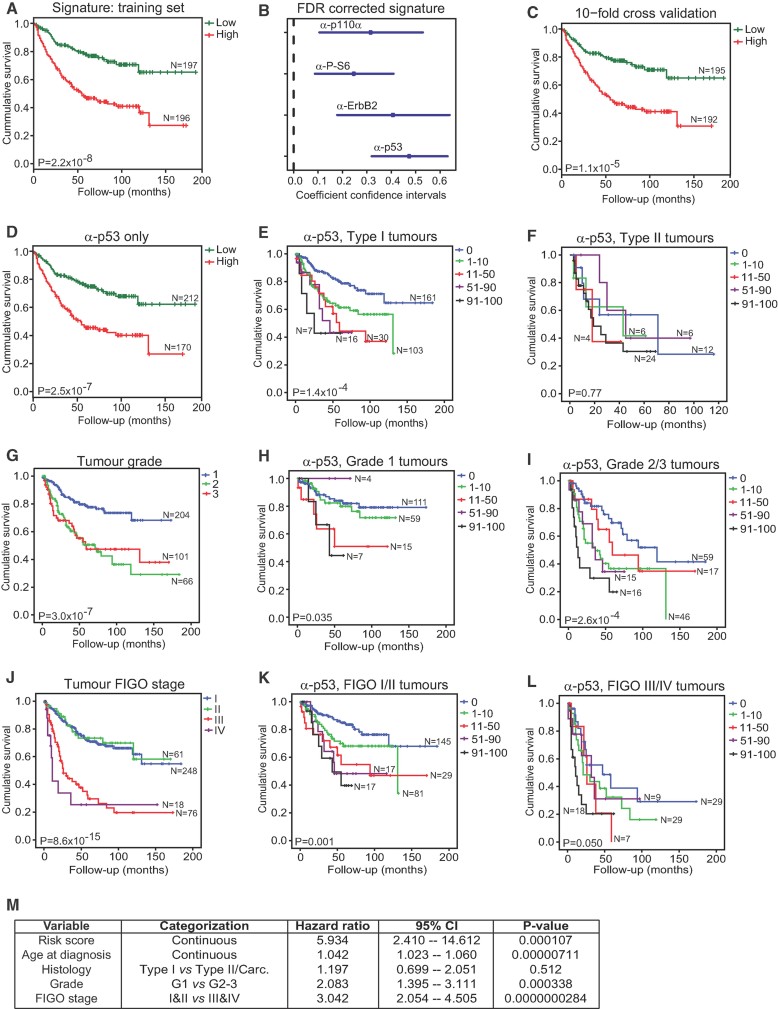
Figure 6. p53 is the best predictor of patient survival in endometrial cancers.
- A. Kaplan–Meier plots of low-risk and high-risk patients using a four-marker risk-model signature for endometrial carcinoma based on staining patterns for p110α, P-S6, ErbB2 and p53.
- B. Contribution of each marker to the signature represented by coefficients and confidence intervals.
- C. Patient stratification based on 10-fold cross-validation of the linear risk score model.
- D-L. Patient stratification based on the median split of p53 expression frequency only (D). Kaplan–Meier survival plots of subgroups of patients based on p53 staining in type I tumours (E), p53 staining in type II tumours (F), tumour grade (G), p53 staining in grade 1 tumours (H), p53 staining in grade 2 and 3 tumours (I), tumour FIGO stage (J), p53 staining in FIGO stage I and II tumours (K) and p53 staining in FIGO stage III and IV tumours (L). Log Rank (Mantel–Cox) tests were conducted to test for equality in the survival expectation of each group. N-values represent the number of patients in each group.
- M. Multivariate Cox regression analysis of factors possibly influencing overall survival of patients with endometrial carcinoma (n = 360, number of events = 112, 50 observations deleted due to missing values). Carc., carcinosarcoma.
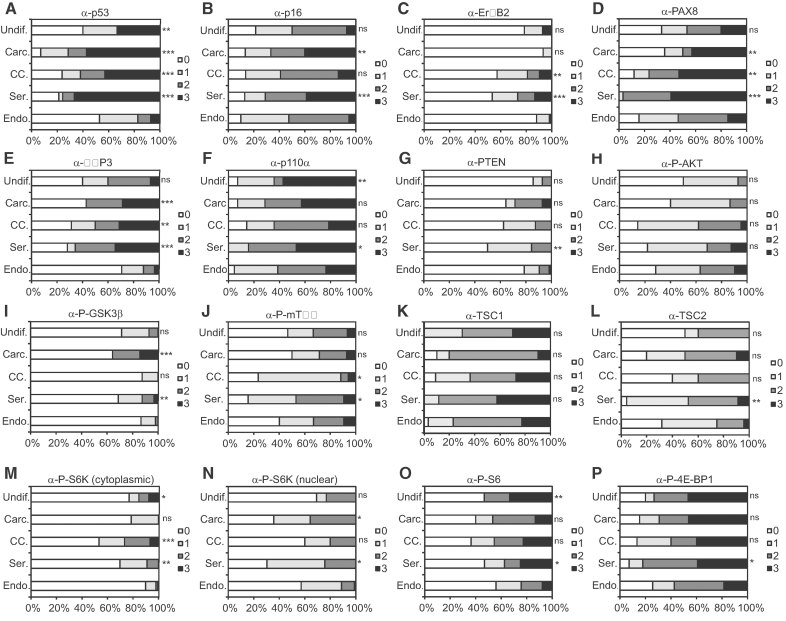
Consistent with our mouse genetic observations that Trp53 suppresses type II endometrial tumours, tumours displaying frequent and strong nuclear p53 expression, an indicator of TP53 mutation or aberrant stabilization, occurred more frequently in serous AdCas, clear cell AdCas, carcinosarcomas and undifferentiated carcinomas than in endometrioid AdCas (Fig 4A). High levels of p16 expression were more frequent in serous AdCas and carcinosarcomas than in endometrioid AdCas (Fig 4B). High levels of ErbB2 expression were more frequent in serous AdCas and clear cell AdCas than in endometrioid AdCas (Fig 4C). PAX8 and IMP3 expression are more frequently high in serous AdCas, clear cell AdCas and carcinosarcomas than in endometrioid tumours (Fig 4D and E). Thus, subsets of type I and type II tumours displayed different, as well as overlapping, molecular profiles.
Figure 4. Identification of molecular discriminants of tumour subtype.
TMAs were stained with antibodies against (A) p53, (B) p16, (C) ErbB2, (D) PAX8, (E) IMP3, (F) p110α, (G) PTEN, (H) P-AKT, (I) P-GSK3β, (J) P-mTOR, (K) TSC1, (L) TSC2 (M) P-S6K (cytoplasmic staining), (N) P-S6K (nuclear staining), (O) P-S6 and (P) P-4E-BP1. Graphs depict cumulative frequency of staining score for each tumour subtype. Fisher's exact test was used to compare frequency of expression of each marker in each subtype of endometrial cancer pairwise with endometrioid carcinoma. *p < 0.05, **p < 0.01 or ***p < 0.001, ns, no significant difference. Endo, endometrioid adenocarcinoma; Ser, serous adenocarcinoma; CC, clear cell adenocarcinoma; Carc, carcinosarcoma; Undif, undifferentiated carcinoma.
Consistent with the known frequent mutational activation of the PI3K-AKT-mTORC1 signalling pathway in type I endometrioid AdCas this group of tumours frequently displayed moderate or strong expression of p110α (Fig 4F), P-AKT (Fig 4H), P-GSK3β (Fig 4I), P-mTOR (Fig 4J), P-S6K (Fig 4M and N), P-S6 (Fig 4O) and P-4E-BP1 (Fig 4P), as well as frequent lack or low level of PTEN staining (Fig 4G). Consistent with the findings in our mouse model, serous AdCas, clear cell AdCas, carcinosarcomas and undifferentiated carcinomas also frequently displayed a similar PI3K pathway activation signature. Indeed, the frequency of type II tumours that displayed high levels of expression of PI3K pathway activation markers (or low levels of expression of PTEN) were always either statistically indistinguishable from the frequency displayed by type I tumours or in many cases were more frequent. Thus, dysregulation of the PI3K-AKT-mTORC1 signalling pathway is a unifying molecular feature of many type I and type II endometrial tumours.
p53 status strongly predicts patient survival in endometrial carcinomas
Given the overlapping molecular features between subsets of type I and type II tumours, we sought to identify pathogenic factors and diagnostic markers for aggressive cases of endometrial carcinomas in general, irrespective of histological subtype. Kaplan–Meier survival curves for each marker are presented in Fig 5. High levels or frequency of expression of p53 (Fig 5A), p16 (Fig 5B), IMP3 (Fig 5E), p110α (Fig 5F), P-GSK3β (Fig 5I), P-S6 (Fig 5O) or P-4E-BP1 (Fig 5P) all independently predicted poor survival, emphasizing the importance of p53 and the PI3K-mTORC1 pathway in controlling the aggressiveness of endometrial tumours.
Figure 5. Identification of individual molecular predictors of patient outcome in endometrial carcinoma.
Kaplan–Meier plots of patient survival over time after diagnosis when patients were grouped according to IHC staining frequency (% strongly positive cells) for (A) p53 or IHC score for (B) p16, (C) ErbB2, (D) PAX8, (E) IMP3, (F) p110α, (G) PTEN, (H) P-AKT, (I) P-GSK3β, (J) P-mTOR, (K) TSC1, (L) TSC2, (M) P-S6K (cytoplasmic staining), (N) P-S6K (nuclear staining), (O) P-S6 or (P) P-4E-BP1. Log Rank (Mantel–Cox) tests were conducted to test for equality in the survival expectation of each group. N-values represent the number of patients in each group.
We systematically assessed if multiple combinations of any of these IHC markers may represent a ‘signature’ that is a superior predictor of survival. To overcome the frequent problem of missing values in TMA datasets we developed a novel statistical approach based on a learning model that is invariant to missing values and results in an easily interpretable and practically applicable linear model. This learning model generated a hazard score for each endometrial carcinoma patient based on combinations of marker stainings. The final model was able to split the patients into a high-risk group and a low-risk group based on staining patterns for p110α, P-S6, ErbB2 and p53 (Fig 6A). The difference between these groups was highly statistically significant and the individual contribution of each marker to this signature is shown by the coefficient confidence intervals depicted in Fig 6B. The model was validated by a 10-fold cross-validation strategy (Fig 6C). A multivariate Cox regression model was then learned to assess the influence of the risk score, age at diagnosis, histological tumour subtype, tumour grade and FIGO stage on the overall survival rate (Fig 6M). In this model, the risk score was the best predictor of poor outcome (p = 0.000107) with a hazard ratio of 5.93 (95% confidence interval 2.41–14.61). Hence in cases with increased risk score, the probability of death was approximately six times higher than in patients with low-risk scores. Age at diagnosis, tumour grade and FIGO stage also had a significant effect on overall survival, albeit with lower hazard ratios than the risk score (Fig 6M). Detailed analysis of the signature model revealed that p53 is the dominating predictor and that the risk for an individual patient can be equally well assessed by using p53 staining status alone as by using the entire signature (Fig 6D). Thus, these analyses demonstrated that p53 status is by far the most important factor in predicting survival of patients with endometrial carcinomas.
Learning models were also separately applied to type I and type II tumours in order to predict a risk score for each endometrial carcinoma patient (Supporting Information Fig 9). The model for type I endometrial tumours was able to split the patients into a high-risk group and a low-risk group based on staining patterns for p110α, P-AKT, ErbB2 and p53, with p53 again being the dominating predictor. In contrast, the model learned for type II tumours revealed that P-S6K was the only IHC-based marker necessary to predict the outcome of patients. p53 did not show a significant effect on overall survival in type II tumours.
We next subdivided the endometrial carcinoma patients into the categories that are currently used for endometrial carcinoma diagnosis, namely histological subtype, tumour grade and FIGO stage and investigated the prognostic power of our risk signature (p110α, P-S6, ErbB2 and p53) and of p53 IHC staining alone in each of these groups. In each cohort of patients, either p53 staining alone (Fig 6D–L) or the four-marker signature (Supporting Information Fig 10) was able to predict poor patient outcome. Almost half of all type I endometrioid tumours, which are typically considered to be less aggressive tumours, displayed some degree of strong nuclear p53 staining and this strongly predicted a poor outcome for this subset of patients (Fig 6E). In contrast, p53 staining was not predictive of survival of patients with type II endometrial carcinomas (Fig 6F), likely because the vast majority of these tumours contain TP53 mutations and perhaps because some of the non-p53 staining tumours may have lost p53 function by gene deletion or truncating mutation, which cannot be detected in our assay. Importantly, while conventional histopathological analysis predicts a better clinical outcome for grade 1 tumours in comparison to grade 2 or 3 tumours (Fig 6G), or for FIGO stage I and II tumours in comparison to FIGO stage III and IV tumours (Fig 6J), grouping according to p53 IHC score within these subgroups was able to further stratify these patients according to risk of death and predicted a poor survival outcome in the early grade (Fig 6H) and stage (Fig 6K) tumours, as well as in the more advanced grade (Fig 6I) and stage (Fig 6L) tumours. In this study, p53 immunoreactivity therefore represents a highly sensitive and predictive marker that assists with risk stratification of endometrial carcinomas.
To validate that our p53 IHC score indeed reflects the presence of TP53 mutations we performed deep sequencing analyses (Supporting Information Table 2) of TP53 exons 5–8, in which mutations were detected previously with high frequency (http://www.sanger.ac.uk/genetics/CGP/cosmic). Tumour DNA was isolated from endometrioid (n = 56) or serous (n = 7) endometrial carcinomas that displayed different p53 IHC scores. An average of 2059 sequencing reads per amplicon provided insight into the heterogeneity of TP53 mutations present in each individual tumour. The number of independent TP53 gene mutations that cause coding alterations (Fig 7A) and the cumulative frequencies at which these mutations were represented in the DNA population (Fig 7B) were similar in all groups of tumours, regardless of subtype or p53 IHC score. However, the frequency of the most abundant (dominant) TP53 mutation in each tumour correlated excellently with the p53 IHC score (Spearman's rho = 0.6113, p < 0.0001). Groups of tumours that displayed an IHC score of over 50% contained dominant TP53 mutations that were present at high frequencies in the tumour DNA population (Fig 7C). Bioinformatic assessment of the predicted effect of these mutations on the function of the p53 protein revealed that tumours with a high p53 IHC score more frequently contain dominant mutations that are predicted to have a highly detrimental effect on protein function (Fig 7D). Moreover, there is a statistically significant association between the predicted severity of the TP53 mutation and the abundance of the mutation within the DNA population of an individual tumour (Fig 7E). Finally, the presence of a dominant TP53 mutation at a frequency of greater than 33% predicted a poor patient outcome (Fig 7F). These findings demonstrate a strong correlation between the p53 IHC score and presence of TP53 gene mutation and suggest that in both type I and serous endometrial carcinomas that TP53 gene mutations that inhibit p53 protein function provide a selective advantage to tumour cells, resulting in their enrichment in the tumour population and generation of a highly aggressive tumour.
Figure 7. Deep sequencing of TP53 exons 5–8 in endometrial carcinomas.
- Total number of different mutations affecting the coding sequence in endometrioid or serous carcinomas grouped according to p53 IHC score. There are no significant differences between groups.
- Cumulative frequency of sequencing reads exhibiting one or more mutations. Scores of over 100% indicate that multiple independent mutations occur in the tumour. There are no significant differences between groups.
- Frequency at which the dominant mutation is represented in the tumour DNA.
- Percentage of tumours for which the dominant mutations depicted in C are predicted to have no impact or a low, medium or high-negative functional impact on the p53 protein.
- Frequency at which the dominant mutation is represented in an individual tumour, grouped according to predicted functional impact of the mutation. In A, B, C and E, statistically significant differences between groups were calculated using a one-way ANOVA and Bonferroni's multiple comparison test and depicted as *p < 0.05, **p < 0.01 or ***p < 0.001.
- Kaplan–Meier survival plots of patients based on the frequency of occurrence of the dominant TP53 mutation.
DISCUSSION
We present an accurate mouse model of type II subtypes of endometrial carcinomas that will facilitate future molecular and clinical studies of these highly aggressive cancer subtypes. Molecular genetic studies have demonstrated that different subtypes of human type II endometrial carcinomas exhibit frequent and early inactivation or mutation of the TP53 tumour suppressor gene. We show that deletion of the Trp53 gene in the mouse endometrium results in the formation of tumours with very high penetrance at around 14–16 months of age, providing strong functional genetic evidence that Trp53 functions as a tumour suppressor gene in type II endometrial carcinomas. These tumours are not only histologically identical to their human counterparts, but arise from a series of similar morphologically identifiable precursor lesions. Lesions that are identical to the EmGD and EIC lesions that are known to be precursors of human endometrial serous AdCa are observed in our mouse model. Thus, this model allows the study of the sequence of molecular and cell biological alterations that occur during the development and progression of type II endometrial tumours.
Trp53 gene deletion under the control of the Ksp1.3-Cre transgene occurs during embryogenesis and induces gene deletion widely in the kidney and genitourinary tract, yet tumours arise only in the endometrium in old mice, suggesting that additional genetic alterations must accumulate and cooperate with Trp53 deficiency to initiate cancer. The absence of tumours in tissues other than the endometrium likely reflects the very low rate of cellular turnover in these tissues in comparison to the cyclic nature of cellular proliferation in the endometrium, which presumably allows mutations to frequently accumulate in the absence of p53 function. Indeed, we identified activation of components of the PI3K and/or mTORC1 signalling cascade in the majority of the type II endometrial tumours that arose in Trp53 mutant mice. Taking advantage of the fact that our mouse model allows analysis of numerous apparent tumour precursor lesions we identified in some tumours that activation of the mTORC1 pathway is precisely associated with the transition from an intraepithelial proliferation pattern to a papillary growth pattern, suggesting that secondary genetic alterations that lead to activation of the mTORC1 pathway may act to drive progression of tumours from EmGD or EIC precursor lesions.
Prompted by these observations, TMA analysis of a large cohort of endometrial cancers demonstrated that activation of the PI3K-AKT-mTORC1 signalling pathway occurs not only in type I carcinomas, which are known to be characterized by frequent activation of this pathway (Catasus et al, 2009; Cheung et al, 2011; Konopka et al, 2011; Lu et al, 2008; Oda et al, 2005; Salvesen et al, 2009; Tashiro et al, 1997; Urick et al, 2011; Velasco et al, 2006), but also at an equivalent or often higher frequency in serous AdCas, clear cell AdCas, undifferentiated carcinomas and carcinosarcomas. Moreover, IHC markers of activation of this pathway independently predict poor patient survival, highlighting the importance of this signalling pathway in endometrial tumour behaviour. Thus, PI3K and/or mTORC1 pathway activation appears to be a frequent pathogenic feature of all endometrial carcinoma subtypes, not only of type I tumours. An important question for future research will be to characterize what causes the hyperactivation of PI3K-mTORC1 pathway signalling in type II tumours as PTEN mutations are known to be very rare in this group of tumours (An et al, 2004; Catasus et al, 2009; Salvesen et al, 2009; Tashiro et al, 1997). Interestingly, we have previously observed a high rate of loss of PTEN immunoexpression in type II endometrial carcinomas (Dellas et al, 2009), suggesting that mechanisms other than deletion or mutation, for example promoter hypermethylation or microRNA-mediated silencing, may potentially contribute to PTEN inactivation in type II cancers. Several studies have also identified amplifications or activating mutations in the genes encoding the catalytic and regulatory subunits of PI3K, namely PIK3CA, PIK3R1 and PIK3R2 in both type I and type II tumours (Catasus et al, 2009; Cheung et al, 2011; Salvesen et al, 2009; Urick et al, 2011).
While numerous previous smaller studies investigating the significance of TP53 mutation status or p53 immunoreactivity have demonstrated that p53-deficient endometrial carcinomas tend to have a poor survival outcome, these studies in most cases failed to demonstrate a prognostic importance of p53 in multivariate survival analyses (reviewed in Lee et al, 2009). By analysing the largest patient cohort to date we show that the immunoexpression level of p53, which has been best described as being involved in the pathogenesis of aggressive type II tumours, is also a powerful predictor of poor patient survival in type I tumours. Deep sequencing of exons 5–8 of the TP53 gene showed a strong correlation between the p53 IHC staining score and TP53 gene mutation in many tumours. The absence of highly abundant TP53 mutations in a sub-fraction of tumours that exhibit a strong p53 IHC score is likely attributable to TP53 mutations in other exons that were not sequenced here or due to aberrant stabilization of p53 due to alterations in one of the many regulatory pathways that control p53 protein stability and function. We demonstrate that TP53 mutations that are predicted to have a strong detrimental functional impact on the p53 protein are frequently enriched in the population of tumour cells in type I or type II endometrial tumours, consistent with the idea that these mutations provide a proliferative advantage to the individual tumour cells that harbour these mutations. In summary, irrespective of endometrial carcinoma subtype, grade or FIGO stage, we find that strong p53 nuclear immunoreactivity represents an excellent marker to stratify patients into poor outcome groups, even in low-grade and low-FIGO stage tumours. We suggest that these patients, who would otherwise be stratified into a relatively low-risk group, should actually be considered as high-risk patients who would benefit from additional monitoring and therapy, possibly including the use of drugs to inhibit the PI3K pathway and/or strategies to kill p53 deficient tumour cells (Lane et al, 2010). We suggest that further clinical studies should investigate the possible value of performing p53 immunohistochemistry as a routine procedure to assist with risk stratification of endometrial carcinomas.
Given that aggressive type I and type II endometrial carcinomas exhibit similar patterns of p53 inactivation and PI3K pathway activation, we argue that the differences between these two subtypes may in fact be minimal and encourage that these aggressive tumour types should be viewed as being ‘molecularly similar’ rather than ‘histologically different’. One of the major differences between the pathogenesis of type I and type II endometrial cancers may lie in their origin and the order of occurrence of mutations in TP53 and in the PI3K pathway. Precursor lesions of type I endometrioid carcinomas, termed complex atypical hyperplasia, frequently exhibit loss of expression or mutational inactivation of PTEN, demonstrating that PI3K pathway activation is a very early event in type I tumour formation (Hayes et al, 2006; Tantbirojn et al, 2008). The presence of functional wild-type p53 in these early lesions likely suppresses aggressive tumour formation. Our clinical data strongly support the notion that the mutation of TP53 in type I tumours converts them to an aggressive tumour type, reflected in the poor survival of patients whose type I tumours exhibited frequent strong p53 nuclear immunoreactivity. In contrast, type II tumours arise from precursor lesions that already lack p53 function, making them highly prone to very rapidly form aggressive tumours in response to ‘second hit’ mutations, such as those in the PI3K pathway or ERBB2, leading to a very poor clinical outcome for these patients. Consistent with these ideas, uterine-specific double knockout of Pten and Trp53 caused tumours to form more rapidly than in mice harbouring deletion of Pten alone (Daikoku et al, 2008). Unfortunately, the histological appearance of these double knockout tumours was not reported. Moreover, sequencing of PTEN, PIK3CA and TP53 in human endometrial carcinomas revealed that mutations in either PTEN or PIK3CA together with concomitant mutation in TP53 predicted a worse patient outcome than for tumours with TP53 mutation alone (Catasus et al, 2009).
The paper explained
PROBLEM:
Endometrial carcinomas of the uterus are the most common tumour type that develops in the female genital tract. The most frequent form of these tumours are so-called type I endometrioid carcinomas which typically have a relatively good prognosis. On the other hand, the rarer but more aggressive type II endometrial carcinomas, comprising serous carcinomas, clear cell carcinomas and undifferentiated carcinomas, account for a disproportionate number of endometrial cancer-associated deaths. The molecular causes of these different forms of cancer remain incompletely understood.
RESULTS:
We find that deletion of the Trp53 tumour suppressor gene in the endometrium of mice causes the formation of all subtypes of type II endometrial carcinomas. The accuracy of this mouse model is reflected by the fact that tumours arise from dysplastic precursor lesions that are identical to those that are believed to represent the first steps in the development of human endometrial cancers. We identify that dysregulation of the PI3K-mTOR signaling pathway plays an important role in the development of type II tumours in mice and in humans and that the status of the p53 tumour suppressor protein is a very powerful predictor of the aggressiveness of both type I and type II subtypes of human endometrial tumours.
IMPACT:
This highly accurate mouse model of type II endometrial carcinomas will facilitate future studies of the causes of these cancers.
In summary, our data derived from Trp53 deficient mice and from human tumour samples form the basis of a new understanding of tumour formation in the endometrium. We suggest that PI3K-mTOR pathway activation and TP53 inactivation play different roles in the initiation of type I and type II endometrial cancer initiation but that combined signalling pathway alterations in both PI3K and/or mTORC1 and p53 are a frequent unifying pathogenic feature of late stage tumours of both subtypes.
MATERIALS AND METHODS
Mouse genetics and analyses
Trp53fl/fl mice (FVB.129-Trp53tm1Brn; Jonkers et al, 2001) from the NCI Frederick Mouse Repository were crossed to C57BL/6J Ksp1.3-Cre mice (Shao et al, 2002) obtained from Dr. Peter Igarashi (UT Southwestern, USA) to generate +/+;Trp53fl/fl (WT) and Ksp1.3-Cre/+;Trp53fl/fl (Trp53Δ/Δ) mice. The Ksp1.3-Cre transgene was genotyped as described (Shao et al, 2002). Genotyping of the Trp53fl allele and detection of Cre-mediated recombination at the Trp53 locus were performed as described (Jonkers et al, 2001). Real-time PCR was performed using the following primer pairs for Trp53 (5′-GCGTAAACGCTTCGAGATGTT-3′ and 5′-TTTTTATGGCGGGAAGTAGACTG-3′) and for 18S rRNA (5′-GTTCCGACCATAAACGATGCC-3′ and 5′-TGGTGGTGCCCTTCCGTCAAT-3′). Littermate controls were used in all experiments. Mouse experiments were conducted in accordance with the ethical guidelines of the Canton of Zurich.
Antibodies
The following antibodies were used: PI3K p110α (Cell Signaling Technology, #4255), PTEN (Dako, M3627), phospho-Ser473-AKT (Cell Signaling Technology, #4051), phospho-Ser9-GSK3β, phospho-Ser2448-mTOR (Cell Signaling Technology, #2976), phospho-Thr421/Ser424-p70 S6 Kinase (Cell Signaling Technology, #9204), phospho-Ser240/244-ribosomal S6 protein (Cell Signaling Technology, #2215), phospho-Thr37/46-4E-BP1 (Cell Signaling Technology, #2855), p53 (Dako, M7001), p16 (Santa Cruz Biotechnology, sc-56330), human ERBB2 (Novacastra Laboratories, NCL-L-CBE-356), mouse ERBB2 (Abcam, ab2428), phospho-Tyr1248-ERBB2 (Sigma Aldrich, SAB4300061), phospho-Thr202/Tyr204-ERK1/2 (Cell Signaling Technology, #4376), phospho-Ser217/221-MEK1/2 (Cell Signaling Technology, #9121), phospho-Thr183/Tyr185-SAPK/JNK (Cell Signaling Technology, #9251), PAX8 (Protein Tech Group, 10336-1-AP), IMP3 (Dako, M3626), TSC1 (Abcam, ab59273) and TSC2 (Abcam, ab32936).
Tissue microarrays
TMAs containing 339 (Basel TMA) and 182 (Zurich TMA) formalin-fixed, paraffin-embedded endometrial cancer tissues were constructed as previously described (Dellas et al, 2009; Kononen et al, 1998; Lebeau et al, 2008). Median follow-up of the cohort from Basel was 31.5 months (1–184), and 44.5 months (1–124) for the cohort from Zurich. The study for both cohorts was approved by the scientific ethics committee from Basel and Zurich (approval no.: KEK-ZH-NR: 2010-0358). The endometrial carcinoma patients were treated in academic centers and outpatient clinics. Patients with endometrial cancer who had localized disease were treated by hysterectomy and bilateral salpingo-oophorectomy (with or without pelvic and paraaortic lymphadenectomy). Adjuvant intravaginal radiation therapy was postoperatively given when invasion of the myometrium or histologic grade 3 tumour was found. All carcinomas were staged according to the 7th edition of the International Union Against Cancer (UICC) and American Joint Committee on Cancer (AJCC) TNM system (Edge et al, 2010). Histopathologic subtype was determined by two authors (H. M. and R. C.) with the aid of IHC staining in questionable cases. Histologic grading was according to the FIGO grading system based on the ratio of glandular or papillary structures versus solid tumour growth (grade 1, <5% solid tumour; grade 2, 6–50% solid; and grade 3, >50% solid; Creasman, 2009). Follow-up information about metastasis, and causes of death were obtained from the participating departments of gynecology at the University Hospital Basel, University Hospital Zurich, Cantonal Hospital in Liestal/Basel-County and Women's Hospitals in Loerrach and Rheinfelden, and from the Cancer Registries of Basel and Zurich. Survival time was calculated from the time of biopsy diagnosis to the death of patients.
Immunohistochemistry
Three micrometres of TMA or mouse tissue sections were subjected to antigen retrieval (microwave oven for 10 min at 250 W) and immunohistochemistry was carried out in a NEXES immunostainer (Ventana, Tucson, AZ, USA). Two surgical pathologists (P. J. W. and K. I.) performed a blinded evaluation of the immunostained slides without knowledge of clinical data. For the analysis of p53 staining, tumours were scored in five groups based on the extent of p53 positivity by determining the frequency of tumour cells displaying strong nuclear staining (0, 1–10, 11–50, 51–90 and 91–100%). Focal weak nuclear p53 staining was regarded as background and scored as 0%. ERBB2 expression was scored according to the DAKO HercepTest criteria. Cytoplasmic immunoreactivity of p16, IMP3, P-GSK3β, P-S6, P-S6K, P-4E-BP1, PTEN, P-AKT, P-mTOR and nuclear immunoreactivity of p110α, PAX8 and P-S6K was estimated using a semi-quantitative four-step scoring system (0–3): 0, negative; 1, weak positive; 2, strong positive; 3, very strong positive.
Deep sequencing analysis
DNA was isolated using the DNeasy Blood & Tissue Kit (Qiagen) from punches taken from the same region on the paraffin blocks as used for TMA construction. Fifty nanograms of DNA was used as input for TP53 PCR amplification (AmpliTaq Gold, Roche) with fusion primers including multiplex identifiers (MID; see Supporting Information). Amplicons were checked on a 1% agarose gel for proper amplification. Amplicon processing was conducted as described by the Amplicon Library Preparation and emPCR (Lib-A) Method GS Junior Titanium Series manual from Roche. Shortly, amplicons were purified using Agencourt AMPure XP beads (Beckman Coulter) and quantified with the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen). Forty-five amplicons (9 patients with five amplicons each) were mixed in equimolar ratio per sequencing run. Emulsion PCR (emPCR) was performed with 106 molecules and 5,00,000 enriched beads were loaded on a 454 Junior Sequencer from Roche. Demultiplexing and variant calling was done with the Amplicon Variant Analyser (AVA) software from Roche. Variants with zero reads in either forward or reverse direction were discarded. Additionally, only variants with at least 50 reads were included in the analysis. Variants were further analysed using the Mutation Assessor software (http://mutationassessor.org/) and scored as 3, 2 and 1 with high, medium and low-functional impact, respectively. Deletions, insertions and missense mutations generating a STOP codon were scored as high-impact mutations (score 3). Statistical analysis involved a one-way ANOVA with subsequent Bonferroni's multiple comparison test.
Statistical analyses
The univariate proportional hazard models used to generate risk scores are described in detail in Supporting Information. A multivariable Cox regression model was learned for testing the independent prognostic relevance of the risk score and other variables. The proportionality assumption for all variables was assessed with log-negative-log survival distribution functions. Non-parametric Kaplan–Meier estimators were used to analyse overall survival. Differences between survival estimates were assessed with the log-rank test (LRT). A statistical association between clinic-pathological and molecular parameters was tested using a two-sided Fisher's exact test. In case of multiple statistical tests the Bonferroni–Holm procedure was applied.
Acknowledgments
We thank Silvia Behnke, Martina Storz, Susanne Dettwiler, Annette Bohnert, Sonja Brun-Schmid, Adriana von Teichman and Marcel Glönkler for excellent technical assistance and Simone Holdermann for critical reading of the manuscript. This work was supported by an SNF Förderungsprofessur grant to IJF and a Baugarten Foundation grant to PJW.
Supporting Information is available at EMBO Molecular Medicine Online.
The authors declare that they have no conflict of interest.
Author contributions
IJF, PJW and WK designed the experiments; IJF, PJW, KI, TJF, SG, MR and NF conducted and analysed the experiments; PJW, AN, MR, RC and HM conducted the histopathological analyses; AD, DF and HM generated the TMAs; IJF and PJW wrote the manuscript with the assistance of all authors.
For more information
General information about endometrial cancers http://www.cancer.gov/cancertopics/types/endometrial
Mutation database for human type II endometrial carcinomas: http://www.sanger.ac.uk/perl/genetics/CGP/cosmic?action=byhist&s=4&hn=carcinoma&sn=endometrium&sh=mixed_endometrioid_and_clear_cell_carcinoma&sh=mixed_serous_and_clear_cell_carcinoma&sh=carcinosarcoma-malignant_mesodermal_mixed_tumour&sh=mixed_serous_and_endometrioid_and_clear_cell_carcinoma&sh=clear_cell_carcinoma&sh=mixed_serous_and_endometrioid_carcinoma&sh=serous_carcinoma&sh=undifferentiated_carcinoma
Corresponding author laboratory webpage http://www.physiol.uzh.ch/research/GrFrew.html
Supplementaary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Abeln EC, Smit VT, Wessels JW, de Leeuw WJ, Cornelisse CJ, Fleuren GJ. Molecular genetic evidence for the conversion hypothesis of the origin of malignant mixed mullerian tumours. J Pathol. 1997;183:424–431. doi: 10.1002/(SICI)1096-9896(199712)183:4<424::AID-PATH949>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Ambros RA, Sherman ME, Zahn CM, Bitterman P, Kurman RJ. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol. 1995;26:1260–1267. doi: 10.1016/0046-8177(95)90203-1. [DOI] [PubMed] [Google Scholar]
- An HJ, Logani S, Isacson C, Ellenson LH. Molecular characterization of uterine clear cell carcinoma. Mod Pathol. 2004;17:530–537. doi: 10.1038/modpathol.3800057. [DOI] [PubMed] [Google Scholar]
- Catasus L, Gallardo A, Cuatrecasas M, Prat J. Concomitant PI3K-AKT and p53 alterations in endometrial carcinomas are associated with poor prognosis. Mod Pathol. 2009;22:522–529. doi: 10.1038/modpathol.2009.5. [DOI] [PubMed] [Google Scholar]
- Cheung LWT, Hennessy BT, Li J, Yu S, Myers AP, Djordjevic B, Lu Y, Stemke-Hale K, Dyer MD, Zhang F, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011;1:170–185. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, Ellenson LH, Dey SK. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68:5619–5627. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellas A, Jundt G, Sartorius G, Schneider M, Moch H. Combined PTEN and p27kip1 protein expression patterns are associated with obesity and prognosis in endometrial carcinomas. Clin Cancer Res. 2009;15:2456–2462. doi: 10.1158/1078-0432.CCR-08-1732. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Ellenson LH. Endometrial carcinoma. Annu Rev Pathol. 2007;2:57–85. doi: 10.1146/annurev.pathol.2.010506.091905. [DOI] [PubMed] [Google Scholar]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. New York, USA: Springer; 2010. Gynecologic sites; pp. 403–418. [Google Scholar]
- Fadare O, Zheng W. Insights into endometrial serous carcinogenesis and progression. Int J Clin Exp Pathol. 2009;2:411–432. [PMC free article] [PubMed] [Google Scholar]
- Fadare O, Liang SX, Ulukus EC, Chambers SK, Zheng W. Precursors of endometrial clear cell carcinoma. Am J Surg Pathol. 2006;30:1519–1530. doi: 10.1097/01.pas.0000213296.88778.db. [DOI] [PubMed] [Google Scholar]
- Frew IJ, Minola A, Georgiev S, Hitz M, Moch H, Richard S, Vortmeyer AO, Krek W. Combined VHLH and PTEN mutation causes genital tract cystadenoma and squamous metaplasia. Mol Cell Biol. 2008;28:4536–4548. doi: 10.1128/MCB.02132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MP, Wang H, Espinal-Witter R, Douglas W, Solomon GJ, Baker SJ, Ellenson LH. PIK3CA and PTEN mutations in uterine endometrioid carcinoma and complex atypical hyperplasia. Clin Cancer Res. 2006;12:5932–5935. doi: 10.1158/1078-0432.CCR-06-1375. [DOI] [PubMed] [Google Scholar]
- Jarboe EA, Pizer ES, Miron A, Monte N, Mutter GL, Crum CP. Evidence for a latent precursor (p53 signature) that may precede serous endometrial intraepithelial carcinoma. Mod Pathol. 2009;22:345–350. doi: 10.1038/modpathol.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jia L, Liu Y, Yi X, Miron A, Crum CP, Kong B, Zheng W. Endometrial glandular dysplasia with frequent p53 gene mutation: a genetic evidence supporting its precancer nature for endometrial serous carcinoma. Clin Cancer Res. 2008;14:2263–2269. doi: 10.1158/1078-0432.CCR-07-4837. [DOI] [PubMed] [Google Scholar]
- Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- Konecny GE, Santos L, Winterhoff B, Hatmal M, Keeney GL, Mariani A, Jones M, Neuper C, Thomas B, Muderspach L, et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br J Cancer. 2009;100:89–95. doi: 10.1038/sj.bjc.6604814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- Konopka B, Janiec-Jankowska A, Kwiatkowska E, Najmola U, Bidzinski M, Olszewski W, Goluda C. PIK3CA mutations and amplification in endometrioid endometrial carcinomas: relation to other genetic defects and clinicopathologic status of the tumors. Hum Pathol. 2011;42:1710–1719. doi: 10.1016/j.humpath.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Kounelis S, Jones MW, Papadaki H, Bakker A, Swalsky P, Finkelstein SD. Carcinosarcomas (malignant mixed mullerian tumors) of the female genital tract: comparative molecular analysis of epithelial and mesenchymal components. Hum Pathol. 1998;29:82–87. doi: 10.1016/s0046-8177(98)90394-x. [DOI] [PubMed] [Google Scholar]
- Lane DP, Cheok CF, Lain S. p53-based cancer therapy. Cold Spring Harb Perspect Biol. 2010;2:a001222. doi: 10.1101/cshperspect.a001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax SF, Pizer ES, Ronnett BM, Kurman RJ. Clear cell carcinoma of the endometrium is characterized by a distinctive profile of p53, Ki-67, estrogen, and progesterone receptor expression. Hum Pathol. 1998;29:551–558. doi: 10.1016/s0046-8177(98)80002-6. [DOI] [PubMed] [Google Scholar]
- Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000;88:814–824. [PubMed] [Google Scholar]
- Lebeau A, Grob T, Holst F, Seyedi-Fazlollahi N, Moch H, Terracciano L, Turzynski A, Choschzick M, Sauter G, Simon R. Oestrogen receptor gene (ESR1) amplification is frequent in endometrial carcinoma and its precursor lesions. J Pathol. 2008;216:151–157. doi: 10.1002/path.2405. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Kim TJ, Kim DS, Choi CH, Lee JW, Lee JH, Bae DS, Kim BG. p53 alteration independently predicts poor outcomes in patients with endometrial cancer: a clinicopathologic study of 131 cases and literature review. Gynecol Oncol. 2009;116:533–538. doi: 10.1016/j.ygyno.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Liang SX, Chambers SK, Cheng L, Zhang S, Zhou Y, Zheng W. Endometrial glandular dysplasia: a putative precursor lesion of uterine papillary serous carcinoma. Part II: molecular features. Int J Surg Pathol. 2004;12:319–331. doi: 10.1177/106689690401200405. [DOI] [PubMed] [Google Scholar]
- Lu KH, Wu W, Dave B, Slomovitz BM, Burke TW, Munsell MF, Broaddus RR, Walker CL. Loss of tuberous sclerosis complex-2 function and activation of mammalian target of rapamycin signaling in endometrial carcinoma. Clin Cancer Res. 2008;14:2543–2550. doi: 10.1158/1078-0432.CCR-07-0321. [DOI] [PubMed] [Google Scholar]
- Mhawech-Fauceglia P, Herrmann FR, Rai H, Tchabo N, Lele S, Izevbaye I, Odunsi K, Cheney RT. IMP3 distinguishes uterine serous carcinoma from endometrial endometrioid adenocarcinoma. Am J Clin Pathol. 2010;133:899–908. doi: 10.1309/AJCPQDQXJ4FNRFQB. [DOI] [PubMed] [Google Scholar]
- Morrison C, Zanagnolo V, Ramirez N, Cohn DE, Kelbick N, Copeland L, Maxwell GL, Fowler JM. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J Clin Oncol. 2006;24:2376–2385. doi: 10.1200/JCO.2005.03.4827. [DOI] [PubMed] [Google Scholar]
- Nofech-Mozes S, Khalifa MA, Ismiil N, Saad RS, Hanna WM, Covens A, Ghorab Z. Immunophenotyping of serous carcinoma of the female genital tract. Mod Pathol. 2008;21:1147–1155. doi: 10.1038/modpathol.2008.108. [DOI] [PubMed] [Google Scholar]
- Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65:10669–10673. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]
- Prat J, Gallardo A, Cuatrecasas M, Catasus L. Endometrial carcinoma: pathology and genetics. Pathology. 2007;39:72–87. doi: 10.1080/00313020601136153. [DOI] [PubMed] [Google Scholar]
- Salvesen HB, Carter SL, Mannelqvist M, Dutt A, Getz G, Stefansson IM, Raeder MB, Sos ML, Engelsen IB, Trovik J, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci USA. 2009;106:4834–4839. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol. 2002;13:1837–1846. doi: 10.1097/01.asn.0000016444.90348.50. [DOI] [PubMed] [Google Scholar]
- Sherman ME, Bur ME, Kurman RJ. p53 in endometrial cancer and its putative precursors: evidence for diverse pathways of tumorigenesis. Hum Pathol. 1995;26:1268–1274. doi: 10.1016/0046-8177(95)90204-x. [DOI] [PubMed] [Google Scholar]
- Silverberg SG, Mutter GL, Kurman RJ, Kubik-Huch RA, Nogales F, Tavassoli FA. Tumours of the uterine corpus: epithelial tumours and related lesions. In: Tavassoli FA, Devilee P, editors. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press; 2003. pp. 217–232. [Google Scholar]
- Soong R, Knowles S, Hammond IG, Michael C, Iacopetta BJ. p53 protein overexpression and gene mutation in mixed Mullerian tumors of the uterus. Cancer Detect Prev. 1999;23:8–12. doi: 10.1046/j.1525-1500.1999.00062.x. [DOI] [PubMed] [Google Scholar]
- Szukala SA, Marks JR, Burchette JL, Elbendary AA, Krigman HR. Co-expression of p53 by epithelial and stromal elements in carcinosarcoma of the female genital tract: an immunohistochemical study of 19 cases. Int J Gynecol Cancer. 1999;9:131–136. doi: 10.1046/j.1525-1438.1999.09905.x. [DOI] [PubMed] [Google Scholar]
- Tantbirojn P, Triratanachat S, Trivijitsilp P, Niruthisard S. Detection of PTEN immunoreactivity in endometrial hyperplasia and adenocarcinoma. J Med Assoc Thai. 2008;91:1161–1165. [PubMed] [Google Scholar]
- Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, Li J, Parsons R, Ellenson LH. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- Taylor NP, Zighelboim I, Huettner PC, Powell MA, Gibb RK, Rader JS, Mutch DG, Edmonston TB, Goodfellow PJ. DNA mismatch repair and TP53 defects are early events in uterine carcinosarcoma tumorigenesis. Mod Pathol. 2006;19:1333–1338. doi: 10.1038/modpathol.3800654. [DOI] [PubMed] [Google Scholar]
- Tong GX, Devaraj K, Hamele-Bena D, Yu WM, Turk A, Chen X, Wright JD, Greenebaum E. PAX8: A marker for carcinoma of Mullerian origin in serous effusions. Diagn Cytopathol. 2010;39:567–574. doi: 10.1002/dc.21426. [DOI] [PubMed] [Google Scholar]
- Urick ME, Rudd ML, Godwin AK, Sgroi D, Merino M, Bell DW. PIK3R1 (p85alpha) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 2011;71:4061–4067. doi: 10.1158/0008-5472.CAN-11-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco A, Bussaglia E, Pallares J, Dolcet X, Llobet D, Encinas M, Llecha N, Palacios J, Prat J, Matias-Guiu X. PIK3CA gene mutations in endometrial carcinoma: correlation with PTEN and K-RAS alterations. Hum Pathol. 2006;37:1465–1472. doi: 10.1016/j.humpath.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Wada H, Enomoto T, Fujita M, Yoshino K, Nakashima R, Kurachi H, Haba T, Wakasa K, Shroyer KR, Tsujimoto M, et al. Molecular evidence that most but not all carcinosarcomas of the uterus are combination tumors. Cancer Res. 1997;57:5379–5385. [PubMed] [Google Scholar]
- Yemelyanova A, Ji H, Shih Ie M, Wang TL, Wu LS, Ronnett BM. Utility of p16 expression for distinction of uterine serous carcinomas from endometrial endometrioid and endocervical adenocarcinomas: immunohistochemical analysis of 201 cases. Am J Surg Pathol. 2009;33:1504–1514. doi: 10.1097/PAS.0b013e3181ac35f5. [DOI] [PubMed] [Google Scholar]
- Yi X, Zheng W. Endometrial glandular dysplasia and endometrial intraepithelial neoplasia. Curr Opin Obstet Gynecol. 2008;20:20–25. doi: 10.1097/GCO.0b013e3282f2fd50. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liang SX, Jia L, Chen N, Fadare O, Schwartz PE, Kong B, Zheng W. Molecular identification of “latent precancers” for endometrial serous carcinoma in benign-appearing endometrium. Am J Pathol. 2009;174:2000–2006. doi: 10.2353/ajpath.2009.081085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Liang SX, Yu H, Rutherford T, Chambers SK, Schwartz PE. Endometrial glandular dysplasia: a newly defined precursor lesion of uterine papillary serous carcinoma. Part I: morphologic features. Int J Surg Pathol. 2004;12:207–223. doi: 10.1177/106689690401200302. [DOI] [PubMed] [Google Scholar]
- Zheng W, Liang SX, Yi X, Ulukus EC, Davis JR, Chambers SK. Occurrence of endometrial glandular dysplasia precedes uterine papillary serous carcinoma. Int J Gynecol Pathol. 2007;26:38–52. doi: 10.1097/01.pgp.0000228138.56222.4e. [DOI] [PubMed] [Google Scholar]
- Zheng W, Xiang L, Fadare O, Kong B. A proposed model for endometrial serous carcinogenesis. Am J Surg Pathol. 2011;35:e1–e14. doi: 10.1097/PAS.0b013e318202772e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.