Abstract
Chronic stress has been suggested to influence the pathogenesis of Alzheimer’s disease (AD); however, the mechanism underlying this influence remains unknown. In this study, we created a triple transgenic mouse model that overexpresses corticotrophin-releasing factor (CRF) and human amyloid-β protein precursor (AβPP), to investigate whether increases in the expression of CRF can mimic the effects of stress on amyloid metabolism and the neurodegeneration. Tg2576 mice that overexpresses human AβPP gene were crossbreed with Tetop-CRF (CRF) mice and CaMKII-tTA (tTA) mice to create a novel triple transgenic mouse model that conditioned overexpresses CRF in forebrain and overexpresses human AβPP (called AβPP+/CRF+/tTA+, or TT mice). Then we evaluated serial neuro-anatomical and behavioral phenotypes on TT mice using histological, biochemical, and behavioral assays. TT mice showed a Cushingoid-like phenotype starting at 3 months of age. At 6 months of age, these mice demonstrated increases in tissue-soluble amyloid-β (Aβ) and Aβ plaques in the cortex and hippocampus, as compared to control mice. Moreover, TT mice characterized substantial decreases in dendritic branching and dendritic spine density in pyramidal neurons in layer 4 of the frontal cortex and CA1 of the hippocampus. Finally, TT mice showed significantly impaired working memory and contextual memory, with a modest increase in anxiety-like behavior. Our results suggested genetic increases in the brain of CRF expression mimicked chronic stress on the effects of amyloid deposition, neurodegeneration, and behavioral deficits. The novel transgenic mouse model will provide a unique tool to further investigate the mechanisms between stress and AD.
Keywords: Alzheimer’s disease, amyloid-β, cognitive function, corticotrophin-releasing factor, neurodegeneration, stress
INTRODUCTION
There is a growing appreciation of the impact of environmental factors on the pathogenesis of Alzheimer’s disease (AD), particularly the late-onset sporadic form of this disease [1-3]. In patients with AD, psychosocial stress, mediated by changes in the hypothalamic-pituitary-adrenal (HPA) axis, has been suggested as one of these environmental factors [3-8]. Changes in the HPA axis in AD patients includes increases in plasma cortisol levels [9-11], correlations between increases in plasma cortisol levels and cognitive decline [3]. Furthermore, the expression of corticotrophin releasing factor (CRF), including reductions in CRF-immunoreactive neurons in the frontal and temporal cortices [12, 13] coupled with increases in the density of postsynaptic CRF receptors in postmortem tissue samples [14, 15]. While these changes are sometimes interpreted as evidence of HPA axis disinhibition triggered by AD-related hippocampal degeneration, the mechanism underling the relationship between stress and the pathogenesis of AD remains unclear.
Genetically manipulated mice that imitate some of the neuropathological changes of AD provide a unique opportunity to investigate the influence of environmental factors, such as stress, on the pathogenesis of AD at the cellular and molecular levels. The Tg2576 mouse is arguably the most well-known mouse model of AD. This mouse overexpresses human amyloid-β protein precursor (AβPP) 695 and demonstrates amyloid-β (Aβ) plaque deposition in the cortex and hippocampus and memory-related behavioral deficits at 9-10 months of age [16, 17]. Recent studies indicated that behavioral stressors increase the production of Aβ and Aβ plaques throughout the hippocampus and cortex of stressed Tg2576 and AβPP V717I–CT100 mice [18, 19]. Furthermore, treatment of triple transgenic AβPP/PS1/MAPT mice with dexamethasone, a glucocorticoid receptor agonist, increases brain AβPP and Aβ levels as well as BACE (β secretase) and the β-CTF of AβPP [20]. Our group reported that behavioral stressors increase interstitial levels of Aβ via the activation of CRF receptors (CRFR1) and concomitant increases in neuronal activity [21, 22].
CRF and its receptors are critical regulators of HPA axis activity in response to stress, and have been shown to modulate neuronal activity throughout the cortex and hippocampus [23, 24]. In order to test the hypothesis that increases in CRF expression may be one of the mechanisms by which environmental stressors influence the behavioral and histological pathogenesis of AD, we created then evaluated a novel triple transgenic mouse model that conditionally overexpresses the CRF and human AβPP. These novel mice significantly increased in tissuesoluble Aβ and Aβ plaques, decreased in dendritic branching and dendritic spine density, and impaired working memory. Our findings suggest that conditionally overexpressed CRF mediated amyloid deposition, neurodegeneration, and behavioral deficits, which mimicked chronic stress induced neuropathological changes in the mouse model of AD [22, 25].
METHODS AND MATERIALS
Breeding and genotyping of transgenic mouse lines
All animal procedures were done in accordance with the NIH and Animal Studies Committee at Northwestern University guidelines. We currently maintain three single transgenic mouse colonies, Tg2576, Tetop-CRF and CaMKII-tTA. The details of the procedure to create Tetop-CRF and CaMKII-tTA have been described previously [26]. We crossbred Tg2576 mice (AβPP+, male) with either a Tetop-CRF+ or CaMKII-tTA+ mouse to obtain either an AβPP+/CRF+ or AβPP+/tTA+ mouse line. We crossed these mice with either a CamKII-tTA+ or Tetop-CRF+ mouse to obtain an AβPP+/CRF+/tTA+ mouse line (referred to as TT mouse). For control of the inducible tetracycline-off/on system, mice “on doxycycline” were fed doxycycline chow (200 mg doxycycline/1 kg; Research Diets) from weaning for 6 months to suppress CRF transgene expression.
Corticosterone assay
Blood was collected by rapid retro-orbital phlebotomy at 5 : 45 AM before light on and 5 : 45 PM before light off. Plasma concentration of corticosterone was measured using ELISA kit according to published methods [26]. The animal number of each group = 5 for this measurement.
CRF measurement
Free CRF levels from tissue samples were analyzed using an ELISA kit (COSMO BIO Co., Tokyo, Japan). The brain tissue was prepared by homogenization and centrifuged (15,000 rpm/min, 20 min) at 4°C. Supernatant was transferred into a glass tube in an ice bath. Methanol was applied into an extraction column for conditioning and then drained by aspiration. The column was equilibrated by two washes with distilled water, and the brain supernatant was added to the column with a pipette. The volume of the supernatant applied was recorded. The column was aspirated slowly, washed with distilled water and finally eluted twice with methanol. The eluate was collected in a glass tube, dried in a centrifugal vaporizer, and the resulting residue (sample for assay) was stored at −20°C until the assay is performed. The assay procedure was detailed in the ELISA kit. The animal number of each group = 3–5 for this measurement.
AβPP and Aβ in brain tissue
Mice were rapidly (1 min) perfused with 0.9% saline at 6 months of age. The brain was then dissected, and one hemisphere was frozen at −80°C for biochemical analyses. The remaining hemisphere was fixed in 4% paraformaldehyde for morphological analysis. ELISA kits (Invitrogen) were used to measure soluble and insoluble Aβ42in the cortex and hippocampus according to the manufacturer’s instructions. Western blots was performed to measure AβPP levels. The primary antibodies were used for immunoblotting at the following dilutions: AβPP 22C11 (1 : 8000), AβPP C-terminal (2.F2.19B4) (1 : 10000), Beta-tubulin III (1 : 1000). The animal number of each group = 5 for these measurement.
Aβ plaque identification
The detailed methods have been described in our previous studies [22, 27]. The animals were perfused transcardially with 1% heparinized 0.01 M phosphate buffer (PBS) for 2 min and then 4% paraformaldehyde for 25–30 min. Brains were removed and post-fixed at 4°C using the same fixative with 30% sucrose for 48 h. The brains were dissected and embedded in Tissue-Tek embedding medium (Electron Microscopy Sciences, Hatfield, PA), and cut into 35 μm thick sections in the coronal plane using a cryostat (Leica CM 1850 UV, Nussloch, Germany). Selected sections were rinsed with 0.1 M PBS (pH 7.4) and incubated in a blocking solution of 5% normal goat serum for 1 h. Sections were then incubated overnight in the primary antibody for Aβ at 4°C (rabbit polyclonal antibody, 1 : 1000, Biosource, Camarillo, CA). After PBS washing, the sections were incubated in biotinylated anti-rabbit secondary antibody for 2 h at room temperature (RT), then in an avidin-biotin complex for 1 h at RT (Vector Laboratories, Burlingame, CA). Aβ-like immunoreactivity was visualized using a DAB kit (Vector Laboratories, Burlingame, CA). To confirm the presence of compact (fibrillar) Aβ plaques in selected sections, we stained floating sections using a 1% thioflavine S aqueous solution for 5 min and then differentiated in 70% alcohol for 3–5 min. Aβ plaques were determined separately in two brain areas, the cerebral cortex and hippocampal formation. Cortex was defined as all areas dorsal to the rhinal fissure and dorsal or lateral to the corpus callosum and external capsule. The hippocampal formation was defined as the hippocampus proper plus the dentate gyrus and subiculum. We used two different staining methods to quantify the density of Aβ plaques, and measurement of total Aβ plaque area (i.e., amyloid burden) in each brain was evaluated using the CAST stereological program. The animal number of each group = 5 for Aβ plaque measurement.
Measurements of dendritic spine size and density
At 6 months of age, mice were sacrificed and their brains were collected and subjected to Golgi staining (FD Rapid GolgiStain Kit; FD Neurotechnologies) according to the manufacturer’s instructions. Briefly, the brains were immediately removed and rinsed in 0.1 M phosphate buffer. Brains were immersed in a Golgi-Cox solution. The mixture of solutions was replaced once after 12 h of initial immersion, with storage at room temperature in darkness for 2–3 weeks. After the immersion period in the Golgi-Cox solution, brains were transferred to a cryoprotectant solution and stored at 4°C for at least 48 h in the dark before cutting. Brains were rapidly frozen with dry ice and cut in the coronal plane at approximately 150 μm thickness on a cryostat. The sections were transferred onto gelatin-coated slides. Cut sections were air dried at room temperature in the dark. After drying, sections were rinsed with distilled water and were subsequently stained in a developing solution and dehydrated, cleared and coverslipped. The pyramidal neurons in the layer 4 of the frontal cortex (up to dorsal hippocampal area), the pyramidal neurons of CA1 in the dorsal hippocampus and neurons in the medial-dorsal thalamic nucleus were selected for dendritic analysis. Twenty-five neurons (10 from the cortex; 10 dorsal hippocampus, and 5 from thalamus) of each animal were selected by stereological program (CAST) for dendritc branching and spine density measurement. Dendritic branching was evaluated using Sholl analysis, measuring total dendritic length and number of intersections at concentric circles at increasing distance from the soma (1/2 diameter = 150 μm) [28, 29]. Spines were identified and characterized [30]. Spine density was expressed as the number of spines per 10 μm of dendrite length. Five mice in each group were used for dendrite branching and spine density measurements.
Behavioral testing
All behavior testing was done by experimenters blinded to genotype and treatment in doxycycline experiments. Eight-ten mice in each group were used for the behavioral tests.
Spontaneous alternation
The apparatus consists of a three-arm (5 × 21 × 15.5 cm) Y maze. The mouse was placed on the center region facing one of the closed arms where it was allowed to freely explore the arms for 5 min. Each mouse completes one trial and is then placed back in its home cage. An entry was defined as the presence of all four paws of the animal in the given arm. The sequence of arm entries was recorded by two trained observers; a successful alternation was defined as entry into all three arms in consecutive choices. The apparatus was cleaned between trials to remove any olfactory cues.
Fear conditioning task
Detailed methods have been described in our previous studies [31]. Animals were trained and tested in two Plexiglas conditioning chambers (26 × 18 × 18 cm) (Med Associates Inc., Georgia, VT), with a metal grid floor, within a larger sound-attenuating chamber. On day 1, training took place in the first chamber which contained a cup containing mint extract placed beneath the grid floor. Freezing behavior, defined as no movement (ambulation, sniffing or stereotypy) other than respiration, was recorded every 10 s for 5 min. After the first 2 min, a 20 s, 80 dB, a 2800 Hz tone was presented, and during the last 2 s of the tone, the animals received a 0.5 mA continuous footshock. This procedure was repeated two more times at 1 min intervals. On day 2, animals were returned to the chamber, and the amount of freezing behavior in response to context (i.e., memory for context) was recorded every 10 s for 8 min. On day 3, the animals were placed in a second chamber (scented with coconut with the grid floor covered with polyurethane). Freezing behavior was again recorded for 2 min. Then, the 80 dB, 2800 Hz tone was represented continuously for 8 min, and freezing behavior in response to the cue was recorded.
Sensitivity to the footshock was tested on the day following completion of the cued conditioning evaluation. The animals were returned to the first conditioning chamber for 2 min and exposed to a series of 2 s shocks, beginning with an intensity of 0.05 mA. The shock intensity was increased by 0.05. The level of shock required to evoke flinching, running, vocalization, and jumping will be determined, once the threshold for each of these responses has been determined the exposures will stop, or if the threshold reaches 1.0 mA, for example, if all behavioral responses are observed at a level of 0.25 mA, that is determined to be the threshold for that animal and no further shocks are administered. This measure is used to determine if perception of the shock stimulus is consistent across experimental groups.
Light-dark preference
The apparatus consists of a shuttle box separated into two chambers by a guillotine door. One compartment is dark and the other is illuminated with a bright stimulus light (500 lux); both chambers contain a metal grid floor. Photobeams installed in each chamber detect the location and locomotor activity of the mice and report that activity to the Graphic State computer program, which controls the experimental processes. All of the testing equipment is housed within a sound-attenuating chamber, which contains a fan that provides 68 dB of background noise. On testing day, the animal was placed in the dark chamber with the sliding door closed and allowed to explore for 1 min. At this point, the sliding door opened and the animal was allowed to explore both the dark and light chambers for 10 min.
Data analysis
We used two-way ANOVA or an unpaired two-way Student-Test to assess measures of Aβ, plaque deposition, spine density, and biochemical and immunohistochemical indices (p < 0.05). When genotype (i.e., TT, CT, AβPP versus WT), drug condition (i.e., doxycycline versus vehicle), genotype * drug interactions were found, post-hoc analyses were performed using Fisher’s Protected Least Squares Design (PLSD) tests.
RESULTS
Establishing a AD mouse model with conditional CRF overexpression
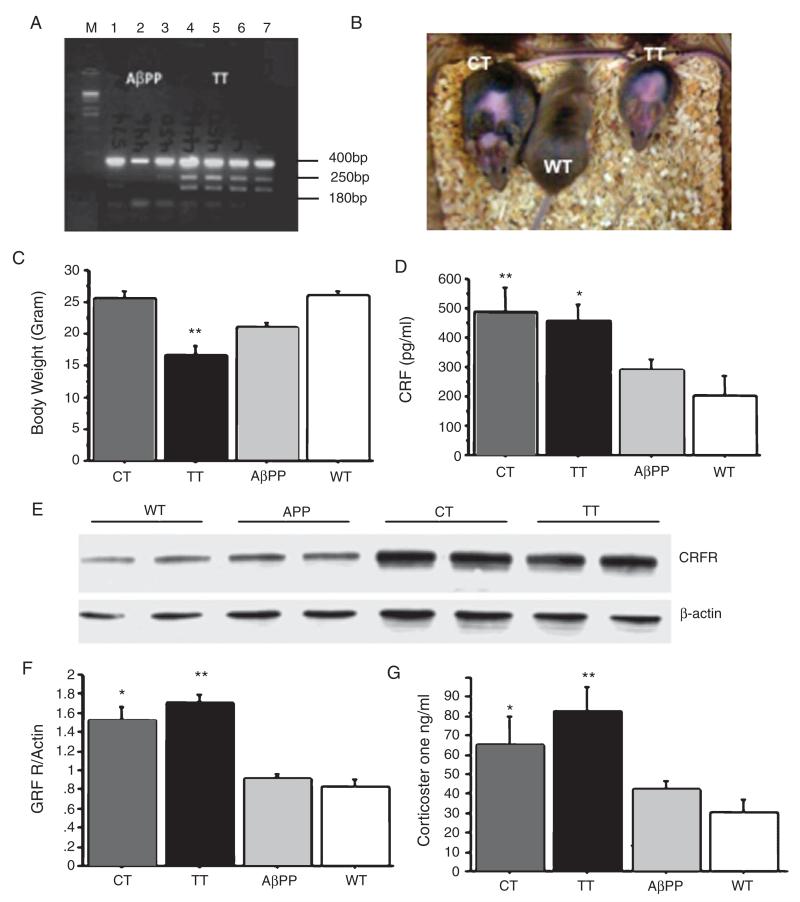
A triple transgenic mouse that overexpresses human AβPP as well as CRF in the forebrain in the absence of doxycycline administration was produced by first crossbreeding Tg2576 males (referred to as AβPP+) with Tetop-CRF+ or CaMKII-tTA+ females to obtain either AβPP+/CRF+ or AβPP+/tTA+ mice. These two lines of mice were then crossed with either a CaMKII-tTA+ or Tetop-CRF+ mouse to produce the triple transgenic mouse line (AβPP+/CRF+/tTA+ mouse line, referred to as TT). The electrophoresis gel pictured (Fig. 1A) verifies that these triple transgenic mice contained the human AβPP, tetop-CRF and CaMKII-tTA genes. CRF+/tTA+ (referred to as CT) mice were produced as previously described [26].
Fig. 1.
Characterization of the novel triple transgenic mouse (TT). A) Electrophoresis gel analysis indicating the presence of AβPP, Tetop-CRF, and CaMKII-tTA DNA in TT mice. B) At 6 months of age, a Cushingoid-like phenotype with hair loss and an olive-shaped body was observed in both CT and TT mice. C) TT mice had significantly reduced body weights, starting from weaning (21 days) to 6 months of age (p < 0.001, n = 8–10 for each group). D) A significant effect of genotype on CRF protein levels in the cortex and hippocampus was observed (F(3,18) = 4.48, p = 0.03; n = 3–5 for each group); with post-hoc testing (corrected for multiple tests) suggesting that both CT and TT mice had significant increases in CRF levels compared to AβPP+ and wild type mice (post-hoc p < 0.05 in both cases). E, F) A significant effect of genotype on CRF receptor 1 (CRFR1) protein levels in the cortex and hippocampus was observed (F(3,18) = 4.48, p = 0.04, n = 3–5 for each group), with both CT and TT mice again showing significant increases as compared to AβPP and wild type mice (post-hoc p < 0.05 in both cases). G) A significant effect of genotype on basal plasma corticosterone at 6 months of age (*p < 0.05, and **p < 0.01). AβPP (or APP): amyloid-β protein precursor; CRF: corticotrophin releasing factor; Tetop-CRF: corticotrophin releasing factor gene under the control of tetracycline/doxycycline operator, CaMKII-tTA: tetracycline transactivator gene under the control of Ca2+/calmodulin-dependent protein kinases II promoter; CT: Tetop-CRF+/CaMKII-tTA+ mice or CRF+/tTA+ mice; TT: AβPP+/Tetop-CRF+/CaMKII+ mice or AβPP+/CRF+/tTA+ mice; WT: wild type control mice.
By 3–4 months of age, a Cushingoid phenotype was observed in both CT and TT mice, characterized by hair loss and an olive-shaped body (Fig. 1B and C). In addition, TT mice had significantly reduced body weights (Fig. 1C). Levels of both CRF and CRFR expression, as measured by enzyme-linked immunosorbent assay (ELISA) and Western blot, were increased in the cortex and hippocampus of both TT and CT mice (Fig. 1D-F). Increases in basal plasma corticosterone levels were also detected in CT and TT mice (Fig. 1G).
Increases in tissue Aβ levels and Aβ plaque deposition
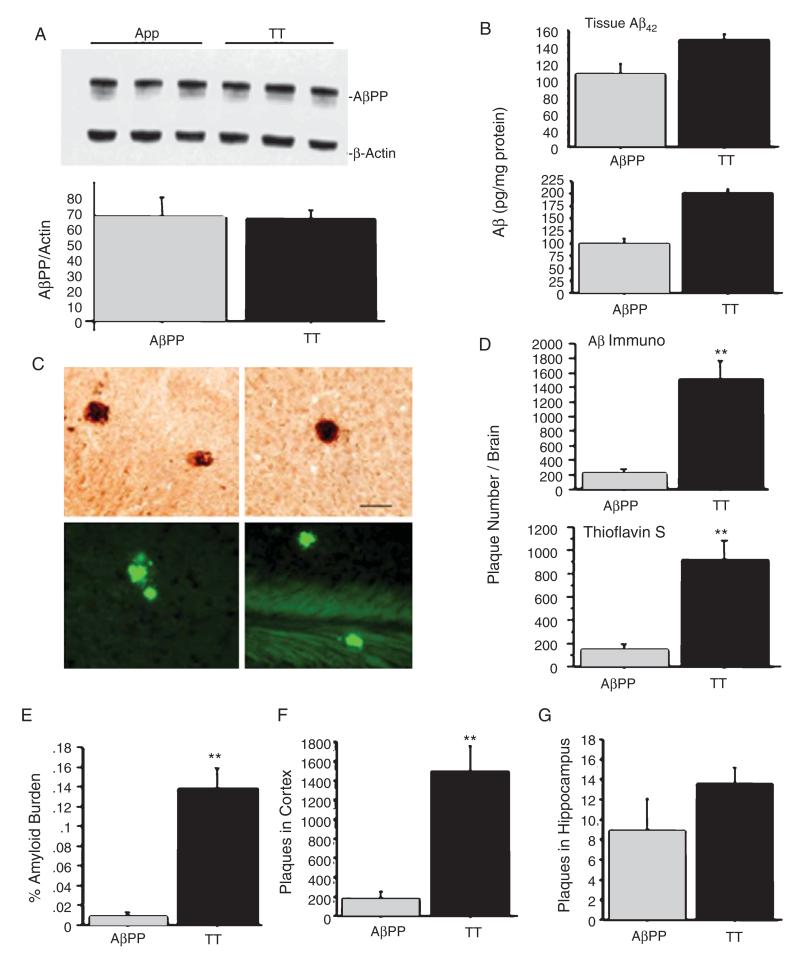
Before investigating whether CRF forebrain overexpression exacerbates pathological and behavioral outcomes, we first evaluated AβPP and Aβ expression in TT mice. Western blot analysis showed that AβPP levels were not increased in TT mice as compared to levels in AβPP+ mice (Fig. 2A). ELISA analysis indicated that levels of tissue soluble (Fig. 2B, upper panel) and insoluble Aβ42(Fig. 2B, lower panel) were significantly increased in the cortex and hippocampus of TT mice as compared to the levels in AβPP+ mice at 6 months of age. Using immunohistochemical staining, we found that these Aβ plaques were distributed predominately in the cortex of TT mice (Fig. 2C, two upper panels). Thioflavin S staining confirmed that the observed plaques were composed of amyloid (Fig. 2C, two lower panels). Aβ plaques were less commonly observed in the hippocampus of TT mice, but not in any other brain areas. Quantitative analysis of Aβ plaque deposition indicated that both Aβ plaque number and burden were increased in the cortex and hippocampus of TT mice as compared to AβPP mice (Fig. 2D-G). These results suggest that CRF overex-pression increases Aβ production from AβPP and/or slows Aβ degradation without affecting AβPP expression in TT mice. Since CT and wild type mice were absent of human AβPP gene, there were no Aβ plaques in these mice therefore we excluded these two groups in the results.
Fig. 2.
Analysis of AβPP and Aβ deposition in TT and AβPP+ mice. A) AβPP levels were similar in TT and AβPP+ mice (an unpaired two way Student-Test, p = 0.93) at 6 months of age. B) ELISA analysis shows that TT mice had higher levels of both soluble (p = 0.033) (upper panel) and insoluble (p = 0.0024) (lower panel) Aβ42 levels at 6 months of age compared to levels in AβPP+ mice. C) Aβ immunohistochemical staining revealed substantial Aβ plaque deposition in the cortex of TT mice at 6 months of age (upper panels, Bar = 50 μm), but not at 3 months of age. Thioflavine staining revealed a similar distribution of plaque deposition (lower panels). D, E) Quantitative analysis of Aβ plaque deposition indicates a significant effect of genotype on Aβ plaque number ((p < 0.0001) by immunostaining and thioflavine S staining, D) and amyloid burden (p < 0.0001, E). F, G) TT mice showed dramatic increases in plaque numbers and burden in the cortex, but not in the hippocampus, as compared to AβPP+ mice (**p < 0.01).
Decreases in dendritic numbers, branching, and spine density
As CRF has been reported to affect neuronal development and survival [28], we investigated the effect of CRF overexpression on neuronal architecture in the presence or absence of AβPP production. We compared dendritic branch number, length, and spine density across the groups of animals using Golgi staining in forebrain regions where CRF expression was increased (e.g., cortex and hippocampus) as well as non-forebrain regions (e.g., thalamus). We found a significant decrease in the number of cortical dendritic branch terminals (but not in primary or secondary branches) (Fig. 3A-C) in TT, CT and AβPP+ mice as compared to wild type mice. In addition, dendritic spine density was significantly decreased in the cortex and hippocampus but not in the thalamus of TT, CT, and AβPP+ mice as compared to those in wild type mice. In both of these comparisons, TT mice showed the significant lowest values compared to all other genotypes (Fig. 3D-G).
Fig. 3.
Evaluation of the dendrites spines in TT, CT, AβPP+, and WT mice. A) Golgi staining shows similar gross visual morphology of neuron and dendritic branching for TT, CT, AβPP+ and WT mice at 6 months of age. Scale bar = 50 μm. B) Sholl analysis (i.e., method of concentric circles) for pyramidal neurons in the cortex reveals a significant effect of genotype (F(3,61) = 15.84, p < 0.000), distance from the soma (F(4,244) = 535.23, p < 0.0001), and a genotype x distance interaction (F(12,2444) = 1.83, p = 0.044) on dendritic arborization. The number of dendrite branches in the intersections was significantly decreased in TT mice starting at 60 μm from the soma. C) There is no difference in the number of the primary branches. D) Representative images of pyramidal neuron spines in the cortex of CT, TT, AβPP+ and WT mice from individual spines identified and counted under 100X magnification using stereological methods. Scale bar = 10 μm. E, F) A significant effect of genotype was found on spine density in the frontal cortex (E) (F(3,278) = 22.07, p < 0.0001), and hippocampus (F) (F(3,136) = 71.28, p < 0.0001). Spine density is expressed as number of spines per 10 μm dendrite length. Post-hoc testing showed significant decreases spine density in CT, TT, and AβPP+ mice as compared to WT mice (p < 0.01) in the frontal cortex and hippocampus, with TT mice having lower values as compared to CT and AβPP+ mice (p < 0.05) (*p < 0.05 compared to WT; #p < 0.05 compared to CT and AβPP+ mice). G) No effect of genotype was observed on spine density in the thalamus.
Exacerbation of cognitive function deficits and increases in anxiety-like behavior
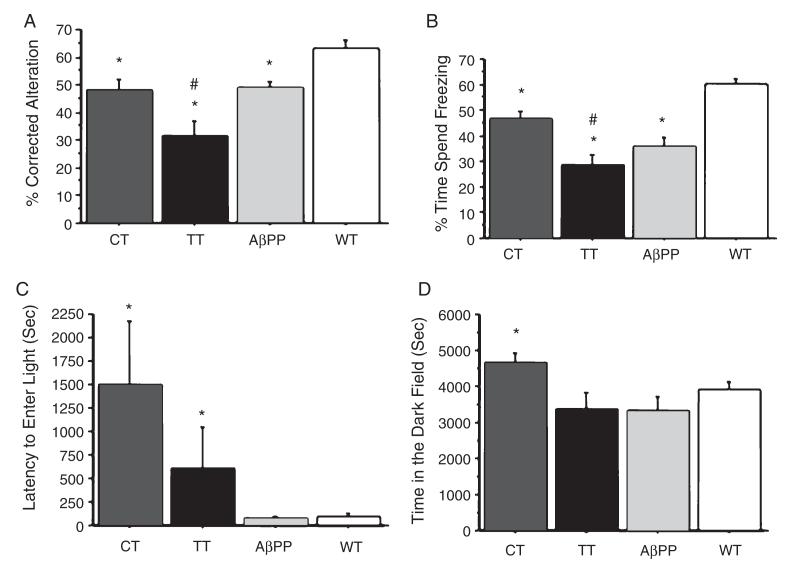
To assess whether changes in Aβ production, Aβ plaque deposition, and neuronal architecture induced by forebrain CRF overexpression were accompanied by changes in memory-related behavior, we compared performance on tests of spontaneous alternation and contextual fear conditioning across the groups of mice at 6 months of age. The spontaneous alternation, measured by a Y-arm maze testing an animal’s tendency to enter novel arms, is commonly used to test spatial working memory. In our study, TT, CT, and AβPP+ mice demonstrated significant decreases in the percentage (%) of correct alternations as compared to alternations in WT mice at 6 months of age (Fig. 4A). The largest decreases in spontaneous alternation performance were observed in TT mice and were significantly different as compared to other genotypes at 6 months of age (Fig. 4A). Similarly, we observed significant deficits in contextual memory but not cued memory in the fear-conditioning test (i.e., freezing behavior in response to context and cue) in TT and AβPP+ mice as compared to CT and WT mice at 6 months of age (Fig. 4B, data not shown the cueinduced memory results), with TT mice again showing the largest decrease in freezing behavior.
Fig. 4.
Memory and anxiety behavioral analysis of TT mice. In the spontaneous Y-maze alternation task, there was a significant effect of genotype on the percentage (%) of correct alternations at 6 months of age (F(3,32) = 6.92; p = 0.001). A) Post-hoc testing showed that CT, TT, and AβPP+ mice demonstrated decreases in correct alternations as compared to WT mice (*p < 0.05), while TT mice demonstrated significant decreases in correct alternations as compared to CT, AβPP+, and WT mice (#p < 0.05). Similarly, in conditioned fear testing, there was a significant effect of genotype on freezing behavior in response to context (F(3,62) = 5.352, p = 0.0024) at 6 months of age. B) Post-hoc tests again showed that CT, TT, and AβPP+ mice demonstrated less freezing behavior than WT mice (*p < 0.05), while TT mice demonstrated less freezing behavior than CT and WT mice (#p < 0.05), but not AβPP+ mice. The light-dark (L:D) preference test showed a significant effect of genotype on the latency to enter the light chamber (F(3,32) = 3.08, p = 0.04) and overall time in the dark chamber (F(3,32) = 3.84, p = 0.02). C) Post-hoc testing indicated that CT and TT mice had longer latencies to enter the light chamber as compared to AβPP+ and WT. D) CT mice spent more time in the dark chamber as compared to TT, AβPP+, and WT mice (*p < 0.05 as compared to WT mice; #p values <0.05 as compared to CT and AβPP+ mice.
To assess the effect of CRF overexpression on anxiety-like behavior, we measured behavioral responses in the light:dark (L:D) preference test. Consistent with an anxiogenic phenotype, at 6 months of age we observed increases in latency to enter the illuminated compartment in both CT and TT mice, as compared to AβPP+ and WT mice (Fig. 4C). In addition, CT, but not TT mice, spent longer time in the dark chamber compared to AβPP and WT mice (Fig. 4D).
Doxycycline prevents CRF overexpression-induced Aβ levels and cognitive deficits
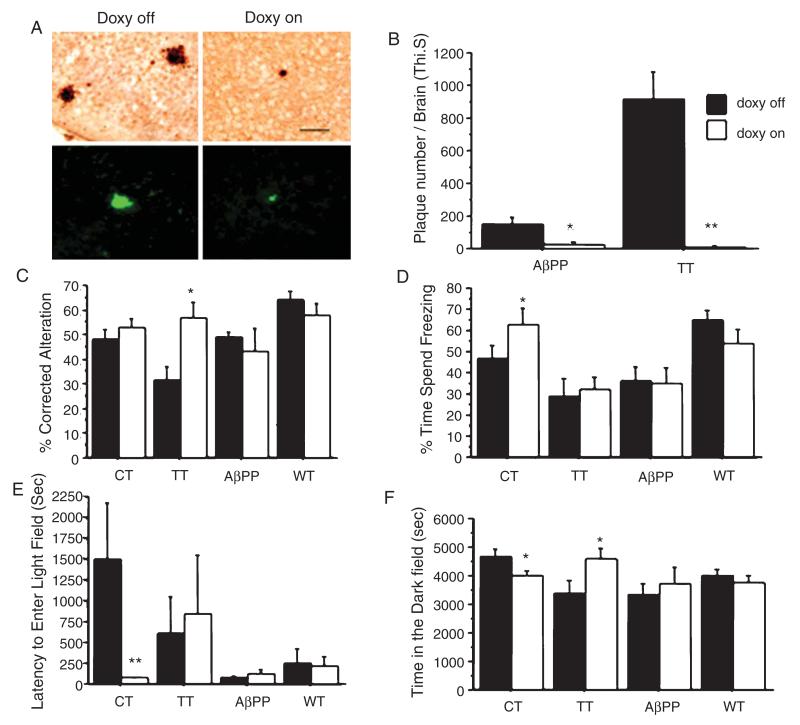
To confirm that increases in Aβ plaque deposition and behavioral deficits could be reversed in part or in whole by decreases in forebrain CRF expression, we administered doxycycline in the chow of TT mice from weaning until 6 months of age. In the CT model, we previously demonstrated that doxycycline effectively inhibits CRF overexpression [26]. Here, we found TT and CT mice treated with doxycycline fail to express the Cushingoid-like phenotype (i.e., mice no longer have the hair loss and change in body shape) and have normal body weights compared to wild type mice. Moreover, Aβ deposition was reversed in TT mice treated with doxycycline (Fig. 5A and B). Finally, doxycycline treatment improved performance with variable results on tests by significantly increasing the % of corrected the alternation in TT mice in spontaneous alteration test (Fig. 5C), and significantly increasing the freezing time in CT mice in contextual memory test (Fig. 5D). Moreover, the anxiety like behavior was reversed in CT mice by significantly decreasing the latency to enter the light field (Fig. 5E) and decreasing the time spent in the dark field (Fig. 5F).
Fig. 5.
Doxycycline reverses the stress induced pathological and behavioral phenotypes in TT mice. A) Fewer Aβ plaques in the cortex and hippocampus were detected by Aβ immunohistochemistry and thioflavine S staining in TT mice fed doxycycline in their chow from weaning until 6 months of age, (scale bar = 50 μm). B) A quantitative analysis of thioflavine S stained sections shows significant effects of genotype (F(1,21) = 17.93, p = 0.0004), drug (F(21,1) = 33.8, p < 0.0001), and a genotype X drug interaction (F(1,21) = 19.09, p = 0.0003). Post-hoc testing indicated that doxycycline almost-completely blocked Aβ plaque deposition in TT mice. Doxycycline also influenced Aβ plaque deposition in AβPP+ mice. C) We also observed a significant effect of genotype (F(3,66) = 4.6, p = 0.005) and a genotype * drug interaction (F(3,66) = 4.46, p = 0.0065) on spontaneous alternation behavior, with post-hoc testing indicated that TT mice improved their performance during doxycycline administration. D) A genotype effect (F(3,72) = 6.659, p = 0.0005), but neither a drug effect nor genotype * drug interaction was observed for contextual memory behavior. Post-hoc analysis of freezing behavior indicates that CT but not the TT mice improved their contextual memory test after 6 months of doxycycline administration. E, F) Doxycycline also reversed the anxiety phenotype as measured by decreased the latency to enter light field and decreased time in the dark field in CT mice. (*p < 0.05; **p < 0.01 compared to the groups with doxycycline off. Dox: doxycycline.
DISCUSSION
Previous studies using different transgenic mouse models of AD indicate that behavioral stressors can increase the production of Aβ and accelerate its incorporation into Aβ plaques [18-20]. Moreover, CRF has been implicated in mediating the effects of behavioral stressors [18, 20-22, 32, 33]. However, research in this area has been studied with exogenously administered CRF because of the limitation that chronic CRF administration cannot pass through the blood brain barrier. To our knowledge, our present studies are the first to use a genetic approach to investigate the effects of consistent increases in the release of CRF on Aβ metabolism, Aβ plaque deposition, neurodegenerative process and their behavioral consequences. Creating a novel mouse model with a Tg2576 genetic background that conditionally overexpresses CRF in the forebrain (referred to AβPP+/CRF+/tTA+ mice or TT mice) allows us to directly control intrinsic increases in CRF and linked such increases with changes in Aβ metabolism and deposition.
Using this mouse model, we found that overexpression of CRF increased tissue levels of Aβ, increased Aβ plaque deposition, and accelerated dendritic degeneration. These pathological changes were accompanied by the appearance of behavioral deficits related to working memory induced by Y-maze and contextual memory induced by fear conditioning tests. The TT mice showed Cushingoid-like phenotypes with increased plasma corticosterone, suggesting that genetically overexpressed CRF made an impact on the function of the HPA axis. However, the reduced body weight in the TT mice is unusual. Perhaps overexpression of CRF in hypothalamic regions that regulate appetite/food intake are responsible for this effect. The TT mouse exhibited characteristics highly analogous to the effects of behavioral stressors, in particular chronic isolation stress, on the AβPP+ mouse model (Tg2576 mouse line). Table 1 summarizes the shared characteristics of TT mice and stressed AβPP+ mice [18, 22, 25]. Increased tissue Aβ levels and accelerated Aβ-plaque deposition appeared in both TT and stressed AβPP+ mice at 6 months of age, which is 2-3 months earlier than regular AβPP+ mice. The distribution of amyloid plaques was somewhat different in that cortical plaques were most prominent in TT mice while plaques were more evenly distributed throughout the cortex and hippocampus as well as the striatum and corpus callosum in stressed AβPP+ mice [18]. This difference in plaque distribution may be due to the forebrain-specific expression of the CaMKIIα promoter in the TT mouse, which was used to drive the overexpression of CRF. Next, we will investigate patterns of the effect of CRF on tissue Aβ and Aβ-plaque deposition with increasing age up to 18 months. The results further support the hypothesis that stress and stress factors, particularly CRF, will influence neuropathogenesis and cognitive function in AD [18, 20-22, 31, 32].
Table 1.
Comparison of TT mice and chronic isolation stressed Tg2576 mice
| Animal (6 months of age) | Cognitive deficits | Anxiety | Tissue Ap | Ap plaques | Synaptic loss |
|---|---|---|---|---|---|
| TT (ApPP+/CRF+/tTA) | ++++ | + | ++++ | +++++ | ++++ |
| CT (CRF+/tTA+) | +++ | ++++ | - | - | ++ |
| Stressed ApPP (iso-Tg2576) | ++++ | + | ++++ | ++++ | +++ |
| Regular ApPP (reg-Tg2576) | ++ | ± | ++ | ± | + |
±: Few animals showing the phenotype. +: Some animals showing the phenotype.
++, +++: Most animals; moderate behavioral deficits, tissue Ap levels, Ap plaque and synaptic loss.
++++, +++++: All animals; severe behavioral deficits, Ap levels, Ap plaque and synaptic loss.
Our findings provide a plausible basis for the regulation of Aβ metabolism by CRF. Since our data suggests that AβPP is not increased in TT mice, it is likely that CRF increases Aβ by increasing the conversion rate of AβPP to Aβ and/or decreasing Aβ degradation. Further investigating C-terminals fragments could help determine whether β-secretase or γ-secretase cleavage of AβPP is affected. CRF-induced increases in Aβ and CRF expression itself may cause synapse loss, which may be one of the biological bases of the behavioral impairment. CRF and its receptors are critical regulators of HPA axis activity in response to stress and widespread neuromodulators, particularly in the cortex and hippocampus [23, 34]. Therefore, overexpression of CRF in the brain may influence Aβ metabolism either directly through neuromodulator activity or indirectly through downstream changes in HPA axis activity. In future experiments, we will perform adrenalectomies to block downstream changes and to distinguish between these two pathways.
It has been accepted that Aβ metabolism is regulated by neuronal activity, which may be increased by CRF [21]. CRF exerts its cellular effects by activating one of its two known G-protein-coupled receptors (GPCRs), CRFR1 and CRFR2 [35]. In fact, CRF binds to CRFR2 with ~40-fold lower affinity; therefore, most of its activity can be attributed to activation of CRFR1 [36]. CRFR stimulation primarily activates the cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) signaling cascade in neuronal systems [37, 38], though CRF receptors also can couple to the phospholipase C (PLC)-protein kinase C (PKC) pathway [38, 39]. Stressors induce the release of CRF from the hypothalamus, which in turn initiates the stress response pathway [40]. Also, stressors can induce a large increase in catecholamine release in the prefrontal cortex, which in turn increases cAMP and protein kinase C (PKC) intracellular signaling. This can lead to reduced prefrontal neuronal firing and impairments in working memory [41, 42]. In addition, elevated CRF receptor binding during periods of increased activity can raise PKC levels above the threshold for synaptic modification.
Recent studies indicate that PKC plays an important role in the pathophysiology of AD [43-50]. For example, increases in PKC activity stimulate Aβ peptide production and tau protein hyperphosphorylation [50]. Dysregulation of PKC-MAPK signaling increases Aβ level in the brain [48]. However, PKA/PKC and other GPCRs signaling pathways might regulate α-secretase-mediated cleavage of AβPP [49], and a novel type of PKC, PKCe, activates the Aβ degrading activity of endothelin converting enzyme type 1 (ECE-1), which might be mediated via the mitogen-activated protein kinase (MAPK) pathway as well. The highest level of adenylate cyclase activation is in the cerebral cortex, an area that is profoundly involved in AD [51]. This neuroanatomical specificity supports the hypothesis that CRFR1 receptors play a role in the pathogenesis of AD [49]. Future studies using our model to investigate cAMP-PKA and/or PLC/PKC signaling pathways involved in CRF-mediated effects on AD-like pathology are necessary.
A recent study also indicated that stress in Tg2576 mice markedly increased metabolic oxidative stress and down-regulated the expression of matrix metalloproteinase-2 (MMP-2), a potent Aβ degrading enzyme, in the brain. These stress effects were reversed by CRFR1 antagonist NBI 27914 [33]. Therefore, we need elucidation of whether high reactive oxygen species levels are regulated in the brain of TT mice and to test whether CRFR1 antagonists could revise stress or CRF induced neuropathological changes. CRFR1 antagonists are currently being evaluated in clinical trials for their efficacy in the treatment of major depression, anxiety, post-traumatic stress disorder, and substance abuse disorders [52]. However, their potential therapeutic value for neurodegenerative disorders has not yet been explored. The effects of CRFR1 antagonists on the metabolism of Aβ and Aβ plaque deposition will be facilitated by the availability of our novel TT animal model.
Dendritic spines are critical structural and functional units of neurons. In our prior studies of stressed AβPP+ mice, we found that neuronal degeneration was related to plaque deposition [27]. There is also increasing evidence that both behavioral stressors and stress-related hormones can induce the disintegration of dendritic arborization [53-55] and dendritic spine density [28, 29, 56]. In addition, a direct effect of CRF on spine density was found to be selective to the area of CA3 apical dendritic spines in the hippocampus, associated with a loss of excitatory synapses, the site of long-term potentiation and learning and memory function deficits [56]. In the present study, we found either AβPP or CRF overexpression influenced dendritic architecture by decreasing the terminal branches and spine densities but not the primary and secondary branches in the layer 4 of the frontal cortex and the pyramidal cells of CA1 of dorsal hippocampus. In TT mice with combined expression of AβPP and CRF, the degree of spine loss in the cortex and hippocampus was much more prominent. Spine loss and regression have been reported in human studies in the context of normal aging [57] and neurodegenerative disorders, including AD [58, 59]. The integrity of dendrites and their arborization are regulated by a variety of extracellular factors including neurotransmitters, growth factors, and hormones [29, 60]. Reduced spine number would result in the reduction of the total postsynaptic area of excitatory synapses, which in turn would influence receptor density, synaptic signaling [61], and the number of functional synapses [60]. Eventually, these changes may influence overall synaptic plasticity and function [62, 63]. Finally, synaptic plasticity is believed to underlie learning and memory functions [63, 64]. Thus, decreases in spine number induced by changes in CRF and AβPP/Aβ release may serve as conceivable substrates for the deficits in working and contextual memory function detected in the TT, CT and AβPP+ mice.
In summary, the results of the present study suggest that intrinsic increases in CRF may be the key regulator by which behavioral stressors influence Aβ metabolism and plaque deposition in mouse models of AD and perhaps in patients with sporadic AD as well. The TT mice we created provide a unique opportunity to further investigate the link between CRF stimulation and Aβ production through specific signal transduction pathways, which would offer insights into the CNS mechanisms that govern Aβ regulation and amyloid plaque production. In future studies, we will use the TT animal model to address such questions and to test the efficacy of CRFR antagonists as potential therapeutics for AD.
ACKNOWLEDGMENTS
This work was supported by MH060883 (JGC) and AG025824 (JGC and HD).
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1021).
REFERENCES
- [1].Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- [2].Tsolaki M, Kounti F, Karamavrou S. Severe psychological stress in elderly individuals: A proposed model of neurodegeneration and its implications. Am J Alzheimers Dis Other Demen. 2009;24:85–94. doi: 10.1177/1533317508329813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weiner MF, Vobach S, Olsson K, Svetlik D, Risser RC. Cortisol secretion and Alzheimer’s disease progression. Biol Psychiatry. 1997;42:1030–1038. doi: 10.1016/s0006-3223(97)00165-0. [DOI] [PubMed] [Google Scholar]
- [5].de Souza-Talarico JN, Chaves EC, Nitrini R, Caramelli P. Stress and coping in older people with Alzheimer’s disease. J Clin Nurs. 2009;18:457–465. doi: 10.1111/j.1365-2702.2008.02508.x. [DOI] [PubMed] [Google Scholar]
- [6].Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- [7].Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- [8].Hasegawa T. Prolonged stress will induce Alzheimer’s disease in elderly people by increased release of homocysteic acid. Med Hypotheses. 2007;69:1135–1139. doi: 10.1016/j.mehy.2007.02.034. [DOI] [PubMed] [Google Scholar]
- [9].Swanwick GR, Kirby M, Bruce I, Buggy F, Coen RF, Coakley D, Lawlor BA. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer’s disease: Lack of association between longitudinal and cross-sectional findings. Am J Psychiatry. 1998;155:286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- [10].Umegaki H, Ikari H, Nakahata H, Endo H, Suzuki Y, Ogawa O, Nakamura A, Yamamoto T, Iguchi A. Plasma cortisol levels in elderly female subjects with Alzheimer’s disease: A cross-sectional and longitudinal study. Brain Res. 2000;881:241–243. doi: 10.1016/s0006-8993(00)02847-x. [DOI] [PubMed] [Google Scholar]
- [11].Rehman HU. Role of CRH in the pathogenesis of dementia of Alzheimer’s type and other dementias. Curr Opin Investig Drugs. 2002;3:1637–1642. [PubMed] [Google Scholar]
- [12].Whitehouse PJ, Vale WW, Zweig RM, Singer HS, Mayeux R, Kuhar MJ, Price DL, De Souza EB. Reductions in corticotropin releasing factor-like immunoreactivity in cerebral cortex in Alzheimer’s disease, Parkinson’s disease, and progressive supranuclear palsy. Neurology. 1987;37:905–909. doi: 10.1212/wnl.37.6.905. [DOI] [PubMed] [Google Scholar]
- [13].Pomara N, Singh RR, Deptula D, LeWitt PA, Bissette G, Stanley M, Nemeroff CB. CSF corticotropin-releasing factor (CRF) in Alzheimer’s disease: Its relationship to severity of dementia and monoamine metabolites. Biol Psychiatry. 1989;26:500–504. doi: 10.1016/0006-3223(89)90071-1. [DOI] [PubMed] [Google Scholar]
- [14].De Souza EB. Corticotropin-releasing factor receptors: Physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- [15].Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature. 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- [16].Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- [17].Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- [18].Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- [19].Jeong YH, Park CH, Yoo J, Shin KY, Ahn SM, Kim HS, Lee SH, Emson PC, Suh YH. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer’s disease model. FASEB J. 2006;20:729–731. doi: 10.1096/fj.05-4265fje. [DOI] [PubMed] [Google Scholar]
- [20].Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci U S A. 2007;104:10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dong H, Yuede CM, Yoo HS, Martin MV, Deal C, Mace AG, Csernansky JG. Corticosterone and related receptor expression are associated with increased betaamyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155:154–163. doi: 10.1016/j.neuroscience.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- [24].Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dong H, Csernansky JG. Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. J Alzheimers Dis. 2009;18:459–469. doi: 10.3233/JAD-2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kolber BJ, Boyle MP, Wieczorek L, Kelley CL, Onwuzurike CC, Nettles SA, Vogt SK, Muglia LJ. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J Neurosci. 2010;30:2571–2581. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dong H, Martin MV, Chambers S, Csernansky JG. Spatial relationship between synapse loss and beta-amyloid deposition in Tg2576 mice. J Comp Neurol. 2007;500:311–321. doi: 10.1002/cne.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, Baram TZ. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hering H, Sheng M. Dendritic spines: Structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- [31].Dong H, Yuede CM, Coughlan C, Lewis B, Csernansky JG. Effects of memantine on neuronal structure and conditioned fear in the Tg2576 mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2008;33:3226–3236. doi: 10.1038/npp.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rissman RA, Lee KF, Vale W, Sawchenko PE. Corticotropin-releasing factor receptors differentially regulate stress-induced tau phosphorylation. J Neurosci. 2007;27:6552–6562. doi: 10.1523/JNEUROSCI.5173-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee KW, Kim JB, Seo JS, Kim TK, Im JY, Baek IS, Kim KS, Lee JK, Han PL. Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice via generation of metabolic oxidative stress. J Neurochem. 2009;108:165–175. doi: 10.1111/j.1471-4159.2008.05769.x. [DOI] [PubMed] [Google Scholar]
- [34].Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: Implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Haug T, Storm JF. Protein kinase A mediates the modulation of the slow Ca(2+)-dependent K(+) current, I(sAHP), by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J Neurophysiol. 2000;83:2071–2079. doi: 10.1152/jn.2000.83.4.2071. [DOI] [PubMed] [Google Scholar]
- [36].Wood SK, Woods JH. Corticotropin-releasing factor receptor-1: A therapeutic target for cardiac autonomic disturbances. Expert Opin Ther Targets. 2007;11:1401–1413. doi: 10.1517/14728222.11.11.1401. [DOI] [PubMed] [Google Scholar]
- [37].Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: New molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- [40].Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- [41].Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, Gobeske KT, Sweatt JD, Manji HK, Arnsten AF. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- [42].Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impairment: Implications for mental illness. Learn Mem. 2008;15:551–564. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- [43].Choi DS, Wang D, Yu GQ, Zhu G, Kharazia VN, Paredes JP, Chang WS, Deitchman JK, Mucke L, Messing RO. PKCepsilon increases endothelin converting enzyme activity and reduces amyloid plaque pathology in transgenic mice. Proc Natl Acad Sci U S A. 2006;103:8215–8220. doi: 10.1073/pnas.0509725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].de Barry J, Liegeois CM, Janoshazi A. Protein kinase C as a peripheral biomarker for Alzheimer’s disease. Exp Gerontol. 2010;45:64–69. doi: 10.1016/j.exger.2009.10.015. [DOI] [PubMed] [Google Scholar]
- [45].Lee W, Boo JH, Jung MW, Park SD, Kim YH, Kim SU, Mook-Jung I. Amyloid beta peptide directly inhibits PKC activation. Mol Cell Neurosci. 2004;26:222–231. doi: 10.1016/j.mcn.2003.10.020. [DOI] [PubMed] [Google Scholar]
- [46].Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis. 2006;9:309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- [47].Nelson TJ, Cui C, Luo Y, Alkon DL. Reduction of beta-amyloid levels by novel protein kinase C(epsilon) activators. J Biol Chem. 2009;284:34514–34521. doi: 10.1074/jbc.M109.016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kim T, Hinton DJ, Choi DS. Protein kinase C-regulated abeta production and clearance. Int J Alzheimers Di. 2011;2011:857368. doi: 10.4061/2011/857368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Thathiah A, De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nat Rev Neurosci. 2011;12:73–87. doi: 10.1038/nrn2977. [DOI] [PubMed] [Google Scholar]
- [50].da Cruz e Silva OA, Rebelo S, Vieira SI, Gandy S, da Cruz e Silva EF, Greengard P. Enhanced generation of Alzheimer’s amyloid-beta following chronic exposure to phorbol ester correlates with differential effects on alpha and epsilon isozymes of protein kinase C. J Neurochem. 2009;108:319–330. doi: 10.1111/j.1471-4159.2008.05770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Martin B, Lopez de Maturana R, Brenneman R, Walent T, Mattson MP, Maudsley S. Class II G protein-coupled receptors and their ligands in neuronal function and protection. Neuromolecular Med. 2005;7:3–36. doi: 10.1385/nmm:7:1-2:003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- [54].Alfarez DN, Karst H, Velzing EH, Joels M, Krugers HJ. Opposite effects of glucocorticoid receptor activation on hippocampal CA1 dendritic complexity in chronically stressed and handled animals. Hippocampus. 2008;18:20–28. doi: 10.1002/hipo.20360. [DOI] [PubMed] [Google Scholar]
- [55].Donohue HS, Gabbott PL, Davies HA, Rodriguez JJ, Cordero MI, Sandi C, Medvedev NI, Popov VI, Colyer FM, Peddie CJ, Stewart MG. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: A stereological and three-dimensional ultrastructural study. Neuroscience. 2006;140:597–606. doi: 10.1016/j.neuroscience.2006.02.072. [DOI] [PubMed] [Google Scholar]
- [56].Chen Y, Rex CS, Rice CJ, Dube CM, Gall CM, Lynch G, Baram TZ. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci U S A. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci U S A. 2009;106:16877–16882. doi: 10.1073/pnas.0908706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Calabrese B, Wilson MS, Halpain S. Development and regulation of dendritic spine synapses. Physiology (Bethesda) 2006;21:38–47. doi: 10.1152/physiol.00042.2005. [DOI] [PubMed] [Google Scholar]
- [61].Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat Rev Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- [62].Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lin B, Kramar EA, Bi X, Brucher FA, Gall CM, Lynch G. Theta stimulation polymerizes actin in dendritic spines of hippocampus. J Neurosci. 2005;25:2062–2069. doi: 10.1523/JNEUROSCI.4283-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]