Abstract
The possible involvement of potato (Solanum tuberosum L.) starch-branching enzyme I (PSBE-I) in the in vivo synthesis of phosphorylated amylopectin was investigated in in vitro experiments with isolated PSBE-I using 33P-labeled phosphorylated and 3H end-labeled nonphosphorylated α(1→4)glucans as the substrates. From these radiolabeled substrates PSBE-I was shown to catalyze the formation of dual-labeled (3H/33P) phosphorylated branched polysaccharides with an average degree of polymerization of 80 to 85. The relatively high molecular mass indicated that the product was the result of multiple chain-transfer reactions. The presence of α(1→6) branch points was documented by isoamylase treatment and anion-exchange chromatography. Although the initial steps of the in vivo mechanism responsible for phosphorylation of potato starch remains elusive, the present study demonstrates that the enzyme machinery available in potato has the ability to incorporate phosphorylated α(1→4)glucans into neutral polysaccharides in an interchain catalytic reaction. Potato mini tubers synthesized phosphorylated starch from exogenously supplied 33PO43− and [U-14C]Glc at rates 4 times higher than those previously obtained using tubers from fully grown potato plants. This system was more reproducible compared with soil-grown tubers and was therefore used for preparation of 33P-labeled phosphorylated α(1→4)glucan chains.
Starch is composed of the two polymers, amylose and amylopectin. The amylose molecules are essentially linear α(1→4)glucan chains, whereas the amylopectin molecules are highly branched and often contain small amounts of covalently bound phosphate (Hizukuri et al., 1970). Potato tuber starch is characterized by a high content of phosphate relative to cereal starches (Rooke et al., 1949; Hizukuri et al., 1970). The phosphate groups are located as monoesters at the C-6 (approximately 70%) and at the C-3 (approximately 30%) positions of the Glc residues (Hizukuri et al., 1970; Takeda and Hizukuri, 1982). Phosphorylation levels differ by 3-fold among potato varieties (Bay-Smidt et al., 1994) and strongly depend upon growth conditions (Nikuni et al., 1969). Small starch granules contain approximately 25% more bound phosphate per Glc residue than large granules, whereas the overall level of phosphorylation does not depend on tuber size (Nielsen et al., 1994).
In a previous study it was found that phosphorylation occurs concurrently with de novo synthesis of starch in potato (Solanum tuberosum L.) tuber discs (Nielsen et al., 1994). However, the mechanism underlying phosphorylation of starch remains elusive. Neither the identity of a phosphorylated intermediate, which could be incorporated in the α(1→4)glucan chains, nor the enzyme system responsible for its incorporation are known.
Since amylopectin is phosphorylated and amylose is not, it is of interest to determine whether the potato SBE (EC 2.4.1.18) can utilize phosphorylated glucans as a substrate. In the present study we have tested the possible involvement of SBE in the formation of phosphorylated starch. The normal mode of action of SBE is to catalyze the cleavage of an α(1→4)glucosidic linkage followed by a condensation of the released α(1→4)glucan to an acceptor chain thereby introducing an α(1→6)glucosidic linkage. The catalytic mechanism may involve sequential binding of the acceptor chain and then the donor chain (Borovsky et al., 1976) or, alternatively, binding of two α(1→4)glucan chains that have formed a double helix.
Glucans with a DP of less than 40 do not serve as a substrate for PSBE-I at 30°C (Borovsky et al., 1976). However, at lower temperatures, where double-helix formation is facilitated, shorter chains do serve as substrates, and the presence of branch points stimulates the rate of catalysis (Borovsky et al., 1975b). This would suggest that PSBE-I acts on an α(1→4)glucan double helix rather than on two unassociated α(1→4)glucan chains. Further support for this hypothesis has been provided by monitoring the association of PSBE-I to linear malto-oligosaccharides (Blennow et al., 1998b). Maximal association took place with chains with a DP of 10 to 15, which coincides with the minimal chain length of 10 Glc residues that are required for initial double-helix formation by linear maltooligosaccharides (Gidley and Bulpin, 1987). Accordingly, an involvement of SBE in the synthesis of phosphorylated amylopectin would require that the enzyme be able to use phosphorylated α(1→4)glucans having a DP of 10 to 15 or preferably larger as a substrate. Such phosphorylated glucans can be derived from potato tuber starch by debranching with isoamylase (Blennow et al., 1998a). A radiolabeled version of the glucans can be obtained by in vivo labeling beforehand of the starch-bound phosphate, as described by Nielsen et al. (1994). Using such 33P-labeled α(1→4)glucans and nonphosphorylated α(1→4)glucans labeled with 3H at the reducing end as the substrates, we demonstrate that PSBE-I catalyzes chain-transfer reactions using the phosphorylated linear glucans as donors to form branched phosphorylated polysaccharides.
MATERIALS AND METHODS
Chemicals and Reagents
Chemicals and phosphorylase a from rabbit muscle were supplied by Sigma, isoamylase was from Megazyme (Sydney, Australia), α-amylase (Termamyl Type L) was from Novo Nordisk A/S (Bagsværd, Denmark), and radiochemicals were from Amersham.
Plant Material and in Vitro Culture
Potato (Solanum tuberosum L. cv Dianella) plants were grown in the greenhouse as described previously (Nielsen et al., 1994). Tubers with a diameter of approximately 5 cm were harvested from 4-month-old plants, rinsed in tap water, and used immediately for incubation experiments.
Mini tubers were grown as described by Visser et al. (1994) with the following modification: Sterile, in vitro-grown plants, used as donor plants, were initially obtained by placing surface-sterilized stem sections from greenhouse-grown plants on shoot-inducing medium. The in vitro potato plants were grown at 22°C using a 14-h light period (160 μmol m−2 s−1). For tuber induction, a stem section (1 cm) with one resting auxiliary bud and one fully developed leaf was excised from a donor plant. The leaf was removed and the stem was transferred to tuber-inducing medium and placed in darkness at 14°C. After 4 weeks, the formed tubers (3 mm in diameter) were harvested and used immediately for experiments.
Radiolabeling Experiments
Incubation of Mini Tubers
Six mini tubers (each approximately 100 mg fresh weight) were cut into halves and incubated for 4 h (total volume: 100 μL) in 300 mm Glc and 3.7 MBq 33PO43− or in 300 mm sorbitol and 74 kBq [U-14C]Glc at room temperature. Three potato tuber discs (each approximately 100 mg fresh weight) were excised from soil-grown tubers (5 cm in diameter) as described previously (Nielsen et al., 1994) and incubated in the same way as the mini tubers.
Isolation of Starch Granules
Starch granules were isolated and washed as described previously (Nielsen et al., 1994), incorporating two additional washes of the starch in 10 mL of 100 mm phosphoric acid for 5 min at room temperature.
Isolation of 33P-Labeled Phosphorylated α(1→4)Glucans
Isolated 33P-labeled starch (approximately 10 mg) from the incorporation experiment was gelatinized (2 mL of water, 5 min, 100°C). After addition of sodium acetate (pH 4.0) to a final concentration of 50 mm, the starch was debranched by isoamylase (2 units, 2 h, 37°C). After the incubation period, the enzyme was inactivated by boiling (5 min) and the phosphorylated glucans produced were isolated using anion-exchange chromatography (DEAE-Sepharose, Pharmacia) as described in Blennow et al. (1998a). The 33P content of each fraction was quantified by liquid-scintillation counting. Total sugar content was determined using the phenol sulfuric acid method (Dubois et al., 1956). After separation of the neutral and phosphorylated glucans, the material was lyophilized and used immediately for experiments.
For enzymatic degradation, the phosphorylated glucans (0.1 mg) were dissolved in 0.5 mL of 5 mm Mes, 4 mm CaCl2, pH 6.5, and incubated with α-amylase (1 unit, 2 h, 25°C). After incubation, a 100-μL sample was immediately applied to a CarboPac PA-100 anion-exchange column (see below) and analyzed using the method described by Blennow et al. (1998a).
Synthesis of α(1→4)Glucans and Reduction with NaB(3H)4
α(1→4)Glucans were synthesized by incubating (37°C, 20 h) phosphorylase a from rabbit muscle (100 units) in 20 mL of 0.35 mm maltoheptaose, 60 mm Glc-1-P, and 1 mm AMP (pH 7.0). After inactivation of the enzyme (100°C, 5 min), the resulting glucan fraction was precipitated with 80% (v/v) ethanol, lyophilized, and stored at −20°C.
A modification of the method of Borovsky et al. (1976) was used to chemically synthesize a 3H-labeled nonphosphorylated α(1→4)glucan substrate. Neutral glucans (1 mg) in 100 μL of 0.1 n NaOH were reacted with 0.5 MBq NaB(3H)4 (25°C, overnight). To ensure quantitative reduction of all reducing ends, a surplus of unlabeled NaBH4 (2 mg in 200 μL of 0.1 n NaOH) was added and the reaction allowed to continue for an additional 2 h. Surplus reagent was destroyed by addition of 1 n HCl until no more hydrogen evolved. Precipitated borate was removed by application of the sample to an NAP-10 column (Pharmacia) and elution of the α(1→4)glucans was performed with 1.5 mL of 50 mm phosphate buffer (pH 7.5). The 3H-end-labeled α(1→4)glucan fraction was used immediately.
Chain-Transfer Experiment
PSBE-I was isolated to homogeneity from potato tubers by affinity chromatography. The isolated PSBE-I was free of amylases and other hydrolytic activities, as analyzed by activity measurements and zymogram analysis using the method described in Viksø-Nielsen et al. (1998). For chain-transfer experiments, PSBE-I (10 ng) was incubated (25°C, 2 h) with 33P-labeled phosphorylated α(1→4)glucans (1 mg, solubilized in 0.25 mL of 0.1 n NaOH and neutralized with 0.1 n HCl) and with 3H-labeled α(1→4)glucans (1 mg) in 0.5 mL of 50 mm sodium phosphate buffer, pH 7.5, 0.05% n-octylglucoside, and 0.1 mg/mL BSA.
The enzyme was inactivated by boiling (5 min), and the resulting product was applied to an anion-exchange column (1 × 5 cm, DEAE-Sepharose) equilibrated in 5 mm Mes, pH 8.0. Neutral glucans were washed off the column with 15 mL of water. The phosphorylated glucans were subsequently eluted using 15 mL of 100 mm NaCl and 10 mm HCl (1.5-mL fractions).
Gel-Permeation Chromatography
The molecular mass distribution of the substrates and the phosphorylated product obtained from the chain-transfer reaction was analyzed using a column (830 × 26 mm) of Sephacryl S-200 (Pharmacia) as described elsewhere (Blennow et al., 1998a) and calibrated using a mixture of linear α(1→4)glucans as molecular mass markers.
HPAEC
A DX 500 system (Dionex Corp., Sunnyvale, CA) equipped with an S-3500 autosampler and fitted with a CarboPac PA-100 column was used to analyze the isolated, neutral, and phosphorylated α(1→4)glucan chains (Blennow et al., 1998a).
Liquid-Scintillation Counting
The incorporation of 33PO43− and [U-14C]Glc into starch was measured using a WinSpectral 1414 liquid-scintillation counter (Wallac, Helsinki, Finland) with WinSpectral version 1.0 software and Ecoscint A scintillation liquid (National Diagnostics, Manville, NJ). Samples containing 3H and 33P were counted using a separate isotope library for each isotope and automatic correction of curve overlaps.
RESULTS
Incorporation of Phosphate into Starch
Incorporation of 33PO43− and [U-14C]Glc into starch using mini tubers was found to be 4-fold more effective than incorporation into potato tuber discs (Table I). The incorporation was linear with time for up to 4 h and continued for at least 14 h (data not shown). These results are similar to those previously reported with potato tuber discs (Nielsen et al., 1994). On this basis, the mini tuber system was chosen as the optimal experimental system for production of radiolabeled phosphorylated α-glucan chains.
Table I.
Incorporation of [U-14C]Glc and 33PO43− into starch using potato tuber discs and potato mini tubers after 4 h of incubation
| Tissue | Radioactivity
|
|
|---|---|---|
| [U-14C]Glc | 33PO43− | |
| dpm mg−1 α(1→4) glucan | ||
| Tuber discs | 250 ± 40 | 490 ± 70 |
| Mini tubers | 950 ± 60 | 2400 ± 120 |
Values are ± se (n = 3).
Isolation and Characterization of 33P-Labeled α(1→4)Glucan from Debranched Starch
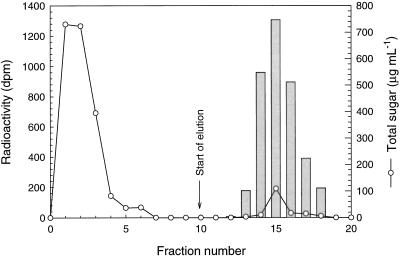
Phosphorylated α(1→4)glucan chains were isolated from isoamylase-debranched starch by anion-exchange chromatography. The neutral glucan chains were eluted from the column with 5 mm Tris-HCl, pH 7.5 (Fig. 1, fractions 1–6), and the phosphorylated glucan chains were subsequently eluted with 100 mm NaCl and 10 mm HCl, pH 2.0 (fractions 13–18).
Figure 1.
Isolation of 33P-labeled phosphorylated α(1→4)glucans by anion-exchange chromatography of isoamylase-debranched starch isolated from potato mini tubers. The phosphorylated glucans were eluted with 100 mm NaCl and 10 mm HCl, pH 2.0. Bars represent radioactivity originating from 33P-labeled phosphorylated α(1→4)glucans.
The isolated phosphorylated glucans were lyophilized, redissolved in 0.1 n NaOH, and fractionated by HPAEC. The possible occurrence of phosphorylated glucan chains carrying more than one phosphate group and a varying internal location of the phosphate group in each glucan chain combined with the variability in chain length resulted in a broad elution profile. The radioactivity eluted in the same fractions as the phosphorylated glucan (Fig. 2, A and B). To ensure that all the 33P label originated from phosphate groups bound to α(1→4)glucan, a fraction of the isolated 33P-labeled molecules was degraded with α-amylase and analyzed similarly. The elution profile obtained (Fig. 2C) was that expected from the conversion of phosphorylated glucan into a shorter oligosaccharide. The superimposable labeling pattern (Fig. 2D) documents that the 33P label detected in the nondegraded sample (Fig. 2B) is bound to the α(1→4)glucan chains.
Figure 2.
A, Elution profile of 33P-labeled phosphorylated α(1→4)glucan chains as determined by HPAEC/PAD. B, Distribution of 33P radioactivity in 1-mL fractions of the anion-exchange eluate of A. C, Elution of α-amylase limit phosphorylated α(1→4)glucan. D, Elution of 33P radioactivity in 1-mL fractions of the anion-exchange eluate in C. nC, Nanocoulombs.
Synthesis of α(1→4)Glucan using Phosphorylase a
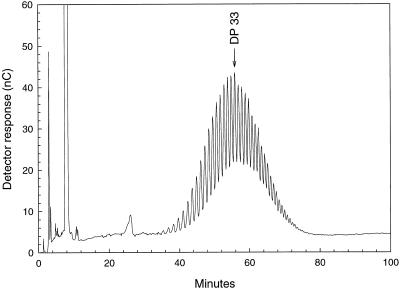
To create a second, well-defined substrate for PSBE-I, nonphosphorylated α(1→4)glucan was synthesized from Glc-1-P using phosphorylase a and maltoheptaose as a primer. The chain-length distribution of the precipitated glucan fraction was determined by HPAEC and revealed an approximately binomial distribution peaking at DP 33 (Fig. 3). To produce a 3H-labeled substrate distinguishable from the 33P-labeled phosphorylated glucan chains, the free anomeric center was reduced with NaB(3H)4.
Figure 3.
Distribution of neutral α(1→4)glucan chains synthesized with phosphorylase a from maltoheptaose and Glc-1-P as determined by HPAEC/PAD using a CarboPac PA-100 column. nC, Nanocoulombs.
Chain Transfer Catalyzed by PSBE-I
The 33P- and 3H-labeled α(1→4)glucans were tested as the substrates for PSBE-I, which was isolated to homogeneity (Fig. 4) by affinity chromatography (Viksø-Nielsen et al., 1998). After incubation of the 33P- and 3H-labeled glucans with PSBE-I, one-half of the reaction mixture was applied to an anion-exchange column to separate the neutral and phosphorylated products obtained from the chain-transfer process. As expected, the neutral products were exclusively 3H labeled. In contrast, the eluted phosphorylated products were co-labeled with 3H and 33P (Fig. 5A). This indicates that PSBE-I has catalyzed a chain-transfer reaction, resulting in the formation of a covalent linkage between the 33P- and 3H-labeled α(1→4)glucans.
Figure 4.
SDS-PAGE and zymogram of PSBE-I isolated to homogeneity by γ-cyclodextrin-affinity chromatography. Lane A, SDS-PAGE of isolated PSBE-I. Lane B, Zymogram of isolated PSBE-I. The location of the SDS-PAGE molecular weight markers (Mr 116,000, 66,000, 45,000, and 27,000) are indicated on the left.
Figure 5.
Anion-exchange chromatography (DEAE-Sepharose) of products obtained from a PSBE-I-catalyzed chain-transfer reaction. A, Products from PSBE-I-catalyzed chain-transfer reactions. B, Sample as in A debranched with isoamylase. Black bars represent radioactivity originating from 33P-labeled phosphorylated α(1→4)glucans. White bars represent radioactivity originating from 3H end-labeled groups.
To demonstrate the presence of α(1→6)linkages in the 3H/33P-labeled products, the second half of the original reaction mixture was debranched with isoamylase. Separation of the debranched glucans by anion-exchange chromatography revealed a clear separation of the 33P- and 3H-labeling into distinct peaks (Fig. 5B). This proves that PSBE-I has formed α(1→6)linkages between the 3H- and 33P-labeled glucans.
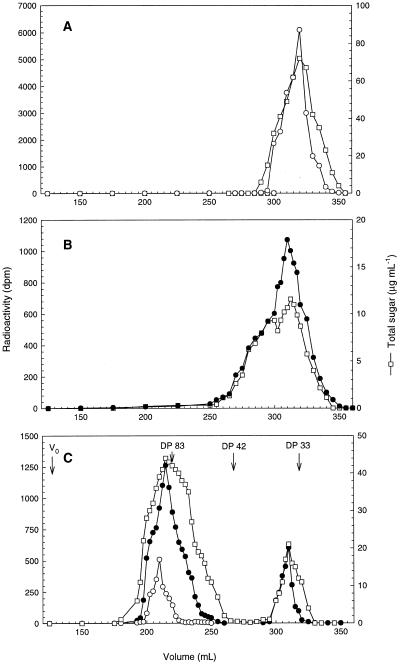
The molecular mass distribution of the 3H- and 33P-labeled substrates and the products generated in the chain-transfer process (Fig. 5A, fractions 11–16) were determined using gel-permeation chromatography (Fig. 6, A, B, and C, respectively). Two major groups of products were generated (Fig. 6C). The first group of products eluted around 210 mL and was labeled with 3H and 33P and had a main peak around DP 80 to 85. The broad peak obtained indicates that the products synthesized in the chain-transfer process are complex oligosaccharides most likely containing more than one branch point. The second minor group of products eluted at 320 mL and was exclusively labeled with 33P.
Figure 6.
Gel-permeation chromatography of substrates and products in the PSBE-I catalyzed chain-transfer reactions. A, Distribution of 3H end-labeled α(1→4)glucans (Fig. 3) used as a substrate for PSBE-I. B, Distribution of 33P-labeled phosphorylated α(1→4)glucans isolated from potato mini tubers (Fig. 1, fractions 13–18) used as a substrate for PSBE-I. C, Distribution of the products obtained from PSBE-I-catalyzed chain-transfer reactions (Fig. 5A, fractions 11–16) after removal of neutral chains by anion-exchange chromatography. The elution pattern of phosphorylase a-synthesized standards with a mean DP of 33, 42, and 83 is indicated. V0, Void volume. •, Radioactivity originating from 33P-labeled phosphorylated α(1→4)glucans; ○, radioactivity originating from the 3H-labeled end groups; and □, total sugar in μg mL−1.
DISCUSSION
In the present study we have demonstrated that potato mini tubers efficiently incorporate administered [U-14C]Glc and 33PO43− into a phosphorylated starch. Earlier, Nielsen et al. (1994) used potato tuber discs as a model system for analysis of starch biosynthesis. The mini tuber system is superior to the tuber discs system because phosphorylated starch is synthesized at a rate that is 4 times higher (Table I). Furthermore, the physiological status of the mini tubers is well defined because they are synchronized with respect to age and size (Visser et al., 1994), thus providing a highly reproducible experimental system. In contrast, the physiological status of tubers harvested from normal potato plants varies, since some tubers may be actively growing while others of the same size are resting, as evidenced by a much lower activity of the starch biosynthetic machinery. Mini tubers thus constitute a suitable experimental system for the production of 33P-labeled phosphorylated amylopectin-derived glucan chains. The elution profile of the phosphorylated glucans obtained by HPAEC/PAD (Fig. 2A) was similar to the elution profiles of phosphorylated glucans isolated from fully grown potato plants (Blennow et al., 1998a).
The marked preferential occurrence of phosphate in amylopectin compared with amylose suggests a functional link between the branching reactions and the mechanism of starch phosphorylation. The first studies on the substrate specificity of potato SBE using defined substrates were conducted by Borovsky and co-workers (Borovsky et al., 1975a, 1975b, 1976). However, there are no reports in the literature on investigations using phosphorylated glucans as a substrate for any of the isoforms of SBE.
To specifically monitor the occurrence of chain-transfer reactions, the 33P-labeled phosphorylated α(1→4)glucans were used in combination with nonphosphorylated 3H-end-labeled linear α(1→4)glucan chains (Fig. 6, A and B, respectively). The phosphorylated products were isolated by ion-exchange chromatography. Size fractionation by gel-filtration chromatography revealed the formation of dual-labeled (3H/33P) polysaccharides with masses in the range of 8,000 to 15,000 D, indicating that they are the products of chain-transfer reactions (Fig. 6C). The minor fraction in the mass range of 4,500 to 6,000 D (eluting around 320 mL) could be phosphorylated α(1→4)glucan chains that were not used by PSBE-I in the chain-transfer reaction or residual fragments cleaved by PSBE-I during the reaction.
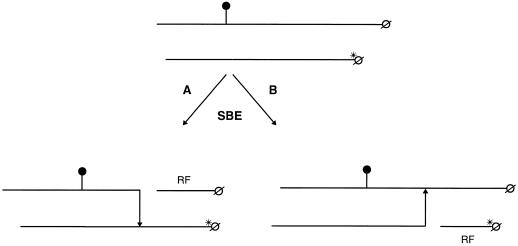
Two hypothetical PSBE-I catalyzed chain-transfer mechanisms are outlined in Figure 7. In reaction A, a product containing radioactivity originating from both the 3H-labeled end group and the 33P-labeled phosphate group is formed. Such products were indeed isolated (Fig. 5A), documenting that reaction A has taken place. Thus, we conclude that PSBE-I is capable of using a phosphorylated glucan as a donor chain. We can neither verify nor rule out that the enzyme also can use a phosphorylated glucan as an acceptor chain (reaction B). If reaction B proceeds, two mono-labeled products containing either 33P or 3H would be formed. In this case the 33P-labeled product would contain a negatively charged phosphate group, and this glucan would co-elute with the product formed in reaction A during anion-exchange chromatography. The third possibility of a chain-transfer reaction catalyzed by PSBE-I, namely an intra-chain transfer involving phosphorylated glucans, cannot be verified nor ruled out using the methods used in this study. A product originating from an intra-chain transfer process cannot be distinguished from the phosphorylated products isolated in Figure 5A, since it will co-elute with the other phosphorylated products due to the negatively charged phosphate group.
Figure 7.
Model of a simple inter-chain-transfer process mediated by potato SBE-I involving one phosphorylated α(1→4)glucan chain and one neutral chain. In reaction A, the phosphorylated α(1→4)glucan is used by PSBE-I as the donor chain. The phosphorylated α(1→4)glucan is cleaved and an α(1→6)linkage is formed to the 3H-labeled α(1→4)glucan. This reaction leaves an unlabeled residual fragment (RF). In reaction B, the phosphorylated chain is used as the acceptor chain by PSBE-I. In this reaction, the 3H-labeled end is cleaved off of the donor chain and subsequently an α(1→6)linkage is formed to the phosphorylated acceptor chain. This reaction leaves a residual fragment containing the 3H-labeled end group. Phosphate groups are indicated with •; ø represents the reducing end, whereas *ø represents a 3H-labeled end group. Arrows indicate the direction of formation of the α(1→6)glycosidic bond.
SBE is localized on the starch granule surface (Kram et al., 1993) or strongly bound to starch granules (Larsson et al., 1996) and supposedly would integrate soluble-phosphorylated α(1→4)glucans into amylopectin by reaction A (Fig. 7). This would require long and unbranched glucan chains protruding from the granule surface, as proposed by Lineback (1986), and the existence of phosphorylated, soluble glucans. The possible involvement of PSBE-I in introducing soluble α(1→4)glucans into amylopectin is supported by an observed increase in the pool of soluble glucans in potato tubers in which the activity of PSBE-I has been lowered by antisense techniques (Kossmann et al., 1997). α(1→4)Glucans have been proposed to affect starch synthesis in potato tubers (Denyer et al., 1996). Potato tubers do contain small amounts of soluble, branched α(1→4)glucans, which can be detected by HPAEC analysis (data not shown). These glucans are probably synthesized in the amyloplast stroma by soluble, starch synthases or by a plastidic phosphorylase isoform with an affinity for low-molecular-mass linear α(1→4)glucans (Steup, 1988). The amount of soluble glucans that can be obtained is too low to permit an analysis of their phosphate content. Soluble α(1→4)glucans may also originate from the trimming or editing of the amylopectin molecule by debranching enzymes or amylases (Ball et al., 1996; Mouille et al., 1996). If phosphorylated α(1→4)glucans are derived from a glucan-trimming process, the chain transfer of phosphorylated α(1→4)glucans mediated by PSBE-I may not be the primary way of phosphorylating starch. In this case, it would constitute a way to reintroduce the liberated phosphorylated α(1→4)glucans into amylopectin.
The current study documents that phosphorylated α(1→4)glucans participate in PSBE-I-catalyzed α-glucan-transfer reactions. It remains to be established whether this is a characteristic of potato SBEs and is thus a discriminating factor with respect to the formation of phosphorylated amylopectin, or whether SBEs from other plants likewise are able to utilize phosphorylated α(1→4)glucans for the production of phosphorylated amylopectin. The latter case would imply that the ability to form phosphorylated glucans is restricted to plants producing phosphorylated starch.
ACKNOWLEDGMENTS
We would like to thank Bente Wischmann and Anne Mette Bay-Smidt for their establishment and help with the mini tuber system.
Abbreviations:
- DP
degree of polymerization
- HPAEC
high-performance anion-exchange chromatography
- PAD
pulsed amperiometric detection
- PSBE-I
potato starch-branching enzyme I
- SBE
starch-branching enzyme
Footnotes
This work was financially supported by the European Union Fishery and Agiculture Industrial Research program and by the Danish Food Technology Program (Føtek II).
LITERATURE CITED
- Ball S, Guan H-P, James M, Myers A, Keeling P, Mouille G, Buléon A, Colonna P, Preiss J. From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell. 1996;86:349–352. doi: 10.1016/s0092-8674(00)80107-5. [DOI] [PubMed] [Google Scholar]
- Bay-Smidt A, Wischmann B, Olsen CE, Nielsen TH. Starch bound phosphate in potato as studied by a simple method for determination of organic phosphate and 31P-NMR. Stärke. 1994;46:167–172. [Google Scholar]
- Blennow A, Bay-Smidt AM, Olsen CE, Wischmann B, Møller BL. The degree of starch phosphorylation is related to the chain length distribution of the neutral and the phosphorylated chains of amylopectin. Carbohydr Res. 1998a;307:45–54. [Google Scholar]
- Blennow A, Viksø-Nielsen A, Morell MK. α-Glucan binding of potato tuber starch branching enzyme I as determined by tryptophan fluorescence quencing, affinity electrophoresis and steady state kinetics. Eur J Biochem. 1998b;252:331–338. doi: 10.1046/j.1432-1327.1998.2520331.x. [DOI] [PubMed] [Google Scholar]
- Borovsky D, Smith EE, Whelan WJ. Purification and properties of potato 1,4-α-d-glucan 6-α-(1, 4-α-glucano)-transferase. Eur J Biochem. 1975a;59:615–625. doi: 10.1111/j.1432-1033.1975.tb02490.x. [DOI] [PubMed] [Google Scholar]
- Borovsky D, Smith EE, Whelan WJ. Temperature-dependence of the action of Q-enzyme and the nature of the substrate for Q-enzyme. FEBS Lett. 1975b;54:201–205. doi: 10.1016/0014-5793(75)80074-3. [DOI] [PubMed] [Google Scholar]
- Borovsky D, Smith EE, Whelan WJ. On the mechanism of amylose branching by potato Q-enzyme. Eur J Biochem. 1976;62:307–312. doi: 10.1111/j.1432-1033.1976.tb10162.x. [DOI] [PubMed] [Google Scholar]
- Denyer K, Clarke B, Hylton C, Tatge H, Smith AM. The elongation of amylose and amylopectin chains in isolated starch granules. Plant J. 1996;10:1135–1143. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Gidley MJ, Bulpin PV. Crystallization of malto-oligosaccharides as models of the crystalline forms of starch. Carbohydr Res. 1987;161:291–300. [Google Scholar]
- Hizukuri S, Tabata S, Nikuni Z. Studies on starch phosphate. Part 1: estimation of glucose-6-phosphate residues in starch and the presence of other bound phosphate(s) Stärke. 1970;22:338–343. [Google Scholar]
- Kossmann J, Büttcher V, Abel GJW, Duwenig E, Emmermann M, Frohberg C, Lloyd JR, Lorberth R, Springer F, Welsh T and others. Starch biosynthesis and modifications of starch structure in transgenic plants. Macromol Symp. 1997;120:29–38. [Google Scholar]
- Kram AM, Oostergetel GT, van Bruggen EFJ. Localization of starch branching enzyme in potato tuber cells with the use of immunoelectron microscopy. Plant Physiol. 1993;101:237–243. doi: 10.1104/pp.101.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson CT, Hofvander P, Khoshnoodi J, Ek B, Rask L, Larsson H. Three isoforms of starch synthase and two isoforms of starch branching enzyme are present in potato tuber starch. Plant Sci. 1996;117:9–16. [Google Scholar]
- Lineback DR. Current concepts of starch structure and its impact on properties. J Jpn Soc Starch Sci. 1986;33:80–88. [Google Scholar]
- Mouille G, Maddelein M-L, Libessart N, Talaga P, Decq A, Delrue B, Ball S. Preamylopectin processing: a mandatory step for starch biosynthesis in plants. Plant Cell. 1996;8:1353–1366. doi: 10.1105/tpc.8.8.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TH, Wischmann B, Enevoldsen K, Møller BL. Starch phosphorylation in potato tubers proceeds concurrently with de novo biosynthesis of starch. Plant Physiol. 1994;105:111–117. doi: 10.1104/pp.105.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikuni Z, Hizukuri S, Kamagi K, Hasegava H, Moriwaki T, Fukui T, Doi K, Nara S, Maeda I. The effect of temperature during maturation period on the physico-chemical properties of potato and rice starches. Mem Sci Indstr Res Osaka Univ. 1969;26:1–27. [Google Scholar]
- Rooke HS, Lampitt LH, Jackson EM. The phosphorous compounds of wheat starch. Biochem J. 1949;45:231–236. doi: 10.1042/bj0450231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steup M. Starch degradation. In: Preiss J, editor. The Biochemistry of Plants. London, UK: Academic Press; 1988. pp. 255–296. [Google Scholar]
- Takeda Y, Hizukuri S. Location of phosphate groups in potato amylopectin. Carbohydr Res. 1982;102:321–327. [Google Scholar]
- Viksø-Nielsen A, Blennow A. Purification of starch branching enzyme from potato using γ-cyclodextrin affinity chromatography. J Chromatogr A. 1998;800:382–385. [Google Scholar]
- Visser RGF, Vreugdenhil D, Hendriks T, Jacobsen E. Gene expression and carbohydrate content during stolon to tuber transition in potatoes (Solanum tuberosum) Physiol Plant. 1994;90:285–292. [Google Scholar]