Abstract
Hydrogen sulfide (H2S), produced by cystanthionine-γ-lysase (CSE) in the cardiovascular system, has been suggested to be the third gasotransmitter in addition to nitric oxide (NO) and carbon monoxide (CO). The present study aimed to investigate the role of H2S in ischemic postconditioning (IPO) during the early period of reperfusion. IPO with 6 episodes of 10 sec reperfusion followed by 6 episodes of 10 sec ischemia (IPO 2’) was administered when reperfusion was initiated. Cardiodynamics and the concentration of H2S were measured at 1, 2, 3, 4, 5, 10, 20, 30, 60, 90 and 120 min of reperfusion. Lactate dehydrogenase (LDH) levels and infarct size were determined at the end of the reperfusion. The concentration of H2S was stable during the whole experiment in the control group, whereas it reached a peak at the first minute of reperfusion in the ischemia-reperfusion (IR) group. The concentration of H2S at the first minute of reperfusion in the IPO 2’ group was higher compared to that of the IR group, which correlated with cardioprotection including improved heart contractile function and reduced infarct size and LDH levels. However, the above effects of IPO 2’ were attenuated by pre-treatment with blockade of endogenous H2S production with DL-propargylglycine for 20 min prior to global ischemia. Furthermore, we found that other forms of IPO, IPO commencing at 1 min after reperfusion (delayed IPO) or lasting only for 1 min (IPO 1’), failed to increase the concentration of H2S and protect the myocardium. We conclude that the peak of endogenous H2S in the early reperfusion phase is the key to cardioprotection induced by IPO.
Keywords: hydrogen sulfide, postconditioning, ischemia-reperfusion injury, cardioprotection
Introduction
Ischemic postconditioning (IPO) is defined as a short series of repetitive cycles of brief reperfusion and re-occlusion of the coronary artery applied immediately at the onset of reperfusion. Its cardioprotective effects include the reduction of infarct size and the improvement of coronary artery endothelial dysfunction and neutrophil accumulation in the area at risk (1,2). However, the effects of IPO are determined by the time frame of the early reperfusion. A previous study (3) has reported that the infarct-sparing advantage of IPO and the reduction in malondialdehyde (MDA) and dihydroethidium (DHE) fluorescence intensity were lost when IPO was delayed for the 1-min period of reperfusion. These data suggest that the early moments of reperfusion during which immediate IPO is applied are crucial for its protective effects to take place. Cohen et al (4) proposed that the time frame for IPO was the first 2 min at the onset of reperfusion, as 1 min or delayed IPO was ineffective. Hausenloy et al (5) suggested that IPO may be mediated through the modulation of the mitochondrial permeability transition pore (mPTP), whose opening in the first few minutes of myocardial reperfusion mediates cell death. The significant burst of reactive oxygen species (ROS) and Ca2+ overload during the first minute of reperfusion are important manifestations of ischemic reperfusion injury (6), whereas IPO treatment reduces reperfusion injury by decreasing ROS generation and attenuating mitochondrial Ca2+ concentrations. However, the exact mechanisms for IPO warrant further investigation, as the ideal time frame for IPO has not yet been fully elucidated.
Hydrogen sulfide (H2S), as an endogenous material, has been characterized as the third gasotransmitter besides nitric oxide (NO) and carbon monoxide (CO) (7). In the cardiovascular system, H2S is predominantly generated by cystathionine-γ-lyase (CSE) (7,8). CSE mRNA expression has been reported to be higher in the rat myocardium than in the thoracic aorta, with enzyme activity in the naïve myocardium being 19 nmol/min/g protein (9). The level of H2S detected in rat serum was 46 μM (10). Thus, the heart is constantly bathed in a considerable amount of H2S generated by the cardiac myocytes. Previously, it has been reported that the concentration of endogenous H2S in plasma and myocardial tissue was significantly decreased in isoproterenol-induced myocardial injury (11,12). Additionally, H2S has been shown to protect the heart from myocardial ischemia-reperfusion (IR) injury in various studies (13–16). Furthermore, endogenous H2S has been shown to mediate the cardioprotection induced by IPO (17). Despite abundant support for H2S in the cardioprotective effects of IPO, the involvement of H2S in the early reperfusion phase of IPO has not been studied. In the present study, we hypothesized that increased endogenous H2S content in the coronary effluent during early reperfusion is indispensible for the beneficial effects of IPO.
Materials and methods
Materials
Our study conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publications No. 85-23, revised 1996).
Male Sprague-Dawley rats (230–270 g; n=40) were maintained in an air-filtered, temperature- (20–22°C) and light-(12-h light/dark cycle) controlled room, with a relative humidity of 50–52%. Rats were fed with standard commercial pellets and water ad libitum. DL-propargylglycine (PAG) was obtained from Sigma-Aldrich Co. Ltd. (St. Louis, MO, USA).
Langendorff isolated heart model
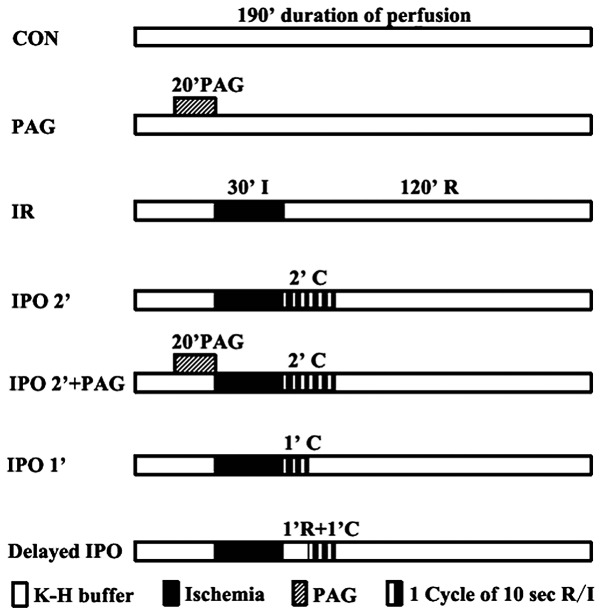
Isolated heart experiments were performed as previously described (18). Sprague-Dawley rats were anesthetized with sodium pentobarbital (50 mg/kg). After a midline sternotomy, the hearts were rapidly excised into ice-cold heparinized (5 U/ml) perfusate buffer. After removal of the lung and surrounding tissue, the aorta was rapidly cannulated with a 20-gauge, blunt-ended needle, and retrograde coronary perfusion was initiated at a constant pressure of 80 mmHg with modified Krebs-Henseleit buffer containing: 120 mM NaCl; 25 mM NaHCO3; 11 mM D-Glucose; 4.7 mM KCl; 1.2 mM MgSO4; 1.2 mM KH2PO4; and 2.5 mM CaCl2, pH 7.4). The perfusate buffer was saturated with a 95% O2 and 5% CO2 gas mixture at 37°C before use. A latex balloon was inserted into the left ventricle via the left atrium, inflated with distilled water and connected to Maclab System (Maclab, AD Instruments, Ltd., Colarado Springs, CO, USA). The left ventricular end diastolic pressure was set between 5 and 10 mm Hg. The balloon volume was unchanged throughout the experiment. Cardiodynamic function for left ventricular developed pressure (LVDP), rate pressure product (RPP), maximum gradient during systoles (+dP/dtmax) and minimum gradient during diastoles (−dP/dtmax) of the heart were continuously monitored with a computer-based data acquisition system. Prior to each experimental protocol, the isolated hearts were allowed to stabilize for 20 min at 37°C. Hearts were excluded from further study if after stabilization they failed to develop steady sinus rhythm or their left ventricular developed pressures were <60 mm Hg. Isolated rat hearts were perfused and stabilized for at least 20 min before recording data. Global ischemia was mimicked by stopping the perfusion of Krebs-Henseleit buffer, i.e., perfusion rate = 0 ml/min. The hearts were randomly divided into 7 groups according to the perfusion protocol as shown in Fig. 1. The control (CON) and PAG groups served as the negative controls in this study. Rat hearts were randomly divided into 7 groups (n=5, each group): i) CON, 190 min duration of perfusion; ii) PAG, treated with 2 mM PAG (the inhibition of endogenous H2S synthesis) for 20 min after 20 min stabilization, followed by perfusion for 150 min; iii) IR, after 40 min stabilization, hearts were administered 30 min global ischemia, followed by reperfusion for 120 min; iv) IPO 2’, 6 cycles of 10 sec reperfusion followed by 6 cycles of 10 sec ischemia immediately upon reperfusion (2 min total intervention); v) IPO 2’ + PAG: hearts were pre-treated with 2 mM PAG for 20 min prior to global ischemia and IPO treatment for 2 min; vi) IPO 1’, the algorithm of IPO was repeated for 3 cycles (1 min total intervention); vii) delayed IPO, hearts were reperfused for 1 min, after which the 6 cycles of IPO 2’ algorithm were applied.
Figure 1.
Schematic presentation of the experimental treatment protocol. White field, Krebs solution; black field, no-flow ischemia. DL-propargylglycine (PAG), an inhibitor H2S of synthesis, was administered 20 min prior to ischemic treatment. CON, control; IPO, ischemic postconditioning; I, ischemia; R, reperfusion; C, cycles.
H2S concentration measurement
The coronary venous effluent at 1, 2, 3, 4, 5, 10, 20, 30, 60, 90 and 120 min of reperfusion was collected from each group. Samples were diluted with deionised water (final volume, 500 μl) and added to a 1.5-ml tube containing zinc acetate (1% w/v, 250 μl) to trap H2S (13,19). Subsequently, N,N-dimethyl-p-phenylenediamine sulphate (20 μM; 133 μl) in 7.2 mol/l hydrogen chloride (HCl) was added, followed by the addition of FeCl3 (30 μM; 133 μl) in 1.2 mol/l HCl. Thereafter, trichloroacetic acid (10% w/v, 250 μl) was used to precipitate any proteins present. Samples were cleared by centrifugation (10,000 × g) and the A670 measured on aliquots from the resulting supernatant (300 μl) using an ultraviolet spectrometer 2450 (Shimadzu Corp., Kyoto, Japan). Various concentrations of sodium hydrogen sulfide (NaHS) were used to plot standard curve and calculate the concentration of H2S in samples (μmol·l−1).
Infarct size
Infarct size was assessed by triphenyltetrazolium chloride (TTC) staining (20). At the end of the IR protocol, the hearts were cut into 2-mm transverse slices. After incubation in 1% TTC in PBS (pH 7.4) solution for 30 min (37°C), the sections were immersed in formalin (4% w/v) for another 30 min. Images were scanned into a computer and total ventricular area as well as infarct area were determined by computerized planimetry (Adobe Photoshop, version CS3). The infarct size was expressed as a percentage of the total ventricular area (%).
Lactate dehydrogenase (LDH) content in coronary efflux
The LDH content in the coronary effluent was used as a biochemical marker of cardiomyocyte injury. For each heart, a baseline sample of coronary effluent was collected from the superfusate bath overflow during the final minute of equilibration (prior to the onset of global ischemia). Coronary effluent was then collected at the end of 120 min of reperfusion and a 1.5-ml aliquot was stored on ice for assay within 24 h. LDH content was determined with an automated spectrophotometric clinical assay using an ACE Chemistry Analyzer (Alfa Wassermann Inc., West Caldwell, NJ, USA).
Statistical analysis
All results are expressed as the means ± SD. Statistical analysis was performed using SPSS 13.0 software for Windows. Differences between groups were analyzed by one-way ANOVA followed by the Student Newman-Keuls test. P<0.05 was considered to indicate a statistically significant difference.
Results
Changes in H2S concentration in the coronary effluent
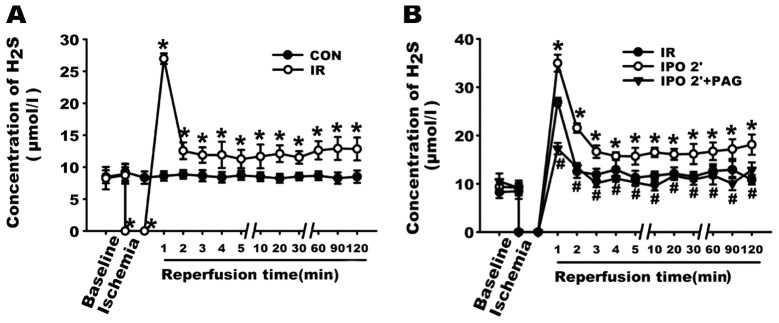
H2S is synthesized mainly by CSE in the cardiovascular system. However, it is unclear whether H2S can still be generated in ischemic heart. As shown in Fig. 2A, the concentration of H2S in the CON group was stable, ranging from 8.28±0.89 to 9.21±1.32 μmol/l. By contrast, after 30-min global ischemia, the concentration of H2S in the coronary effluent in the first minute of reperfusion reached a peak (26.96±0.83 μmol/l) of almost 4-fold the level of the baseline, which strongly suggests that during the 30 min of global ischemia, H2S was still produced and accumulated in the myocardium. On the basis of this result, we further examined the changes in H2S concentration in the coronary effluents. As shown in Fig. 2B, in the first minute after the resumption of flow, the concentration of H2S in the IR, IPO 2’, IPO 2’ + PAG group increased to 26.96±0.83, 34.97±1.74 and 17.26±1.20 μmol/l, respectively. Compared with the IR group, the concentration of H2S in the coronary effluent was significantly increased in the IPO 2’ group (P<0.05), indicating that the IPO 2’ treatment further increased the level of H2S in the coronary effluent during the first minute of reperfusion. However, in the IPO 2’ + PAG group, pre-treatment with PAG for 20 min prior to global ischemia abolished the effect of the IPO 2’ treatment on the content of H2S during the first minute of reperfusion. It should be noted that during the entire reperfusion process, the concentration of H2S in the coronary effluent in the IPO 2’ group was significantly higher than that in the IR and IPO 2’ + PAG group (all p<0.05).
Figure 2.
Change in H2S concentration (mean ± SD). (A) Effect of ischemia-reperfusion (IR) on H2S concentration. (B) Effect of ischemic postconditioning (IPO) 2’ on concentration of hydrogen sulfide (H2S) in the presence and absence of DL-propargylglycine (PAG). *P<0.05, compared with control (CON) (A) or IR (B) group; #P<0.05, compared with IPO 2’ group. n=5.
Changes in cardiodynamic function
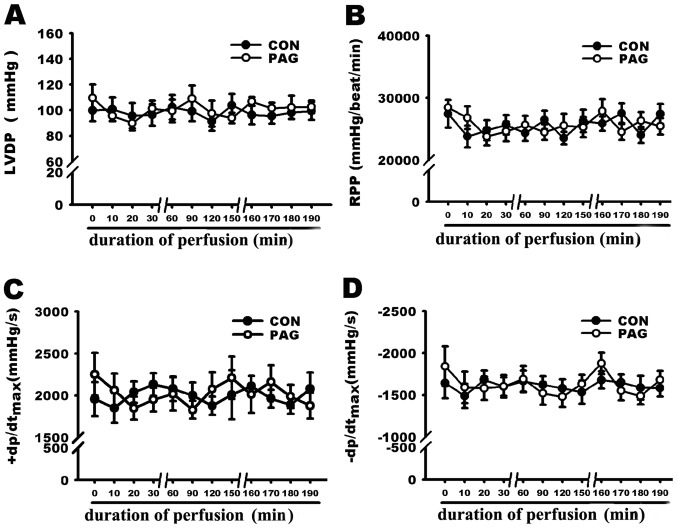
The effect of PAG on cardiodynamic function in the isolated rat hearts was examined. As shown in Fig. 3, the administration of PAG (2 mM) alone did not significantly influence cardiodynamic function including LVDP, RPP, +dP/dtmax and −dP/dtmax. PAG was therefore used in the following experiments to determine the involvement of endogenous H2S in the cardioprotection induced by IPO 2’ treatment.
Figure 3.
Effect of DL-propargylglycine (PAG) on cardiodynamics (mean ± SD). Mean data of cardiodynamics showing that 2 mM PAG alone did not alter the cardiodynamics significantly. (A) Left ventricular developed pressure (LVDP); (B) rate pressure product (RPP); (C) maximum gradient during systoles (+dP/dtmax); (D) minimum gradient during diastoles (−dP/dtmax). n=3 or 4.
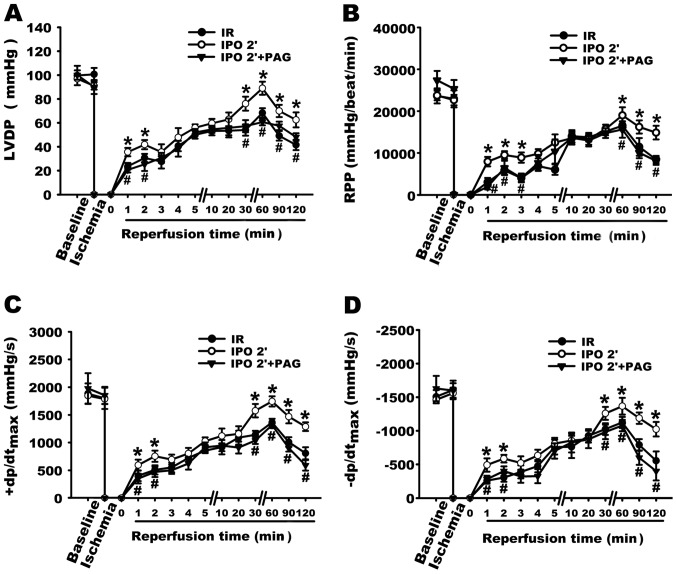
Cardiac mechanical function including LVDP, RPP, ±dp/dtmax was measured to determine the involvement of endogenous H2S in the cardioprotection induced by the IPO 2’ treatment. As shown in Fig. 4, the IPO 2’ treatment exerted a significant cardioprotective effect with the recovery of cardiac function following ischemia compared with that of IR. The recoveries at 120 min of LVDP, RPP, +dP/dtmax and −dP/dtmax in IPO 2’ group were 1.50, 1.76, 1.60 and 1.84-fold of the IR group, respectively (all P<0.05 compared with IR, n=5). However, pre-treatment with 2 mM PAG 20 min prior to global ischemia, which inhibited the production of endogenous H2S prior to and during ischemia, significantly diminished the cardioprotective effect of the IPO 2’ treatment by reducing LVDP, RPP, +dP/dtmax and −dP/dtmax to 0.77, 0.55, 0.46 and 0.39-fold of the IPO 2’ group, respectively (all P<0.05 compared with IPO 2’, n=5).
Figure 4.
Effect of ischemic postconditioning (IPO) 2’ on cardiodynamics in the presence or absence of DL-propargylglycine (PAG) (mean±SD). (A) Left ventricular developed pressure (LVDP); (B) rate pressure product (RPP); (C) maximum gradient during systoles (+dP/dtmax); (D) minimum gradient during diastoles (−dP/dtmax). *P<0.05, compared with ischemia-reperfusion (IR) group; #P<0.05, compared with IPO 2’ group. n=5.
Changes in infarct size
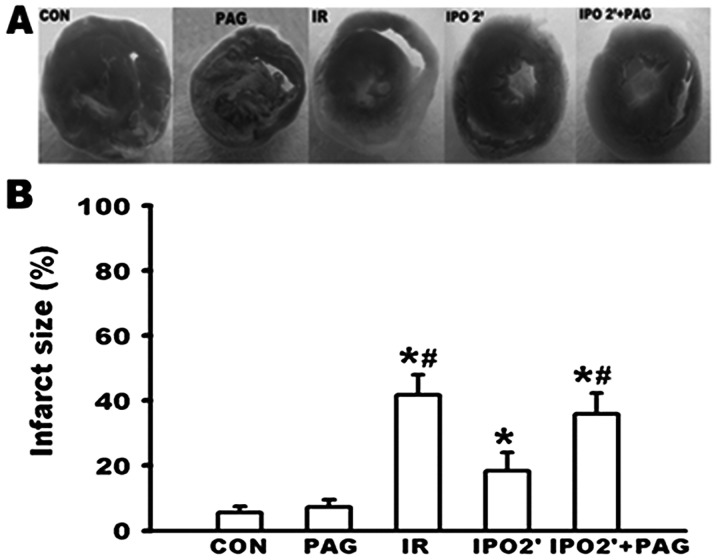
As shown in Fig. 5, no significant differences in infarct size were observed between the CON and PAG group. While an infarct size of 41.8±6.2% was caused in the IR group, the infarct size was significantly decreased to 18.3±5.7% in the IPO 2’ group (P<0.05). However, PAG pre-treatment in the IPO 2’ group resulted in an infarct size of 35.9±6.4%. This was significantly increased when compared to the IPO 2’ group (P<0.05), suggesting that PAG inhibited the cardioprotection observed by the IPO 2’ treatment.
Figure 5.
Effect of ischemic postconditioning (IPO) 2’ on myocardial infarction in the presence or absence of DL-propargylglycine (PAG) (mean ± SD). (A) Representative photographs of infarcted heart slices stained with triphenyltetrazolium chloride. Infarcted tissue appears pale, while viable tissue stains red. (B) Mean data of infarct size (expressed as percentage of total ventricular area). *P<0.05, compared with control (CON) group; #P<0.05, compared with IPO 2’ group. n=5 or 6.
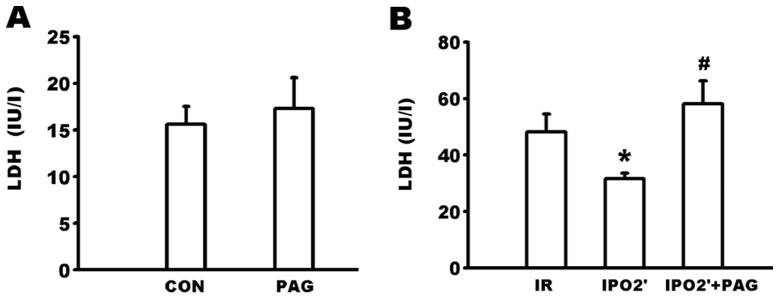
Changes in LDH levels in the coronary effluent
As shown in Fig. 6A, there were no significant differences in LDH levels in the coronary efflux between the CON and PAG groups. The LDH content in the coronary effluent at 120 min of reperfusion (Fig. 6B) was significantly lower in the IPO 2’ group compared to the IR group (31.60±1.95 vs. 48.20±6.32 IU/l, P<0.05). However, the LDH level was significantly increased in the IPO 2’ + PAG group (58.20±8.06 IU/l) compared to the IPO 2’ group (P<0.05).
Figure 6.
Change in lactate dehydrogenase (LDH) content in the coronary effluent (mean ± SD). (A) DL-propargylglycine (PAG) alone had no significant effect on LDH content. (B) Effect of ischemic postconditioning (IPO) 2’ on LDH content in the presence and absence of PAG. *P<0.05, compared with ischemia-reperfusion (IR) group; #P<0.05, compared with IPO 2’ group. n=5.
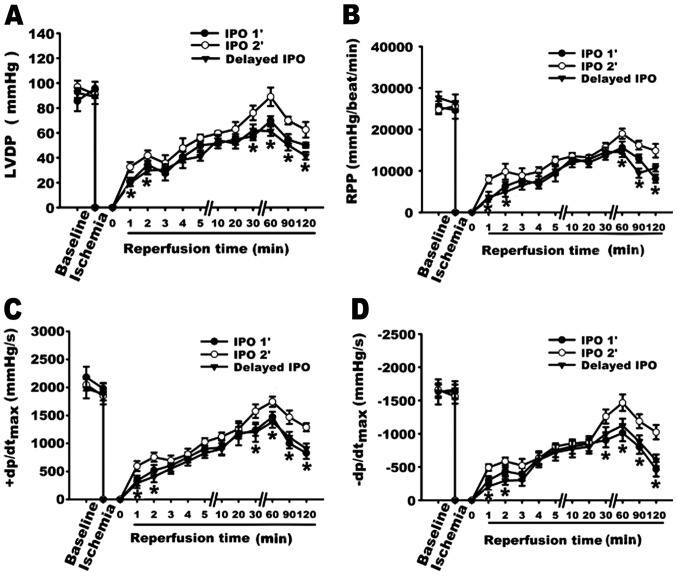
Effect of IPO 1’ and Delayed IPO
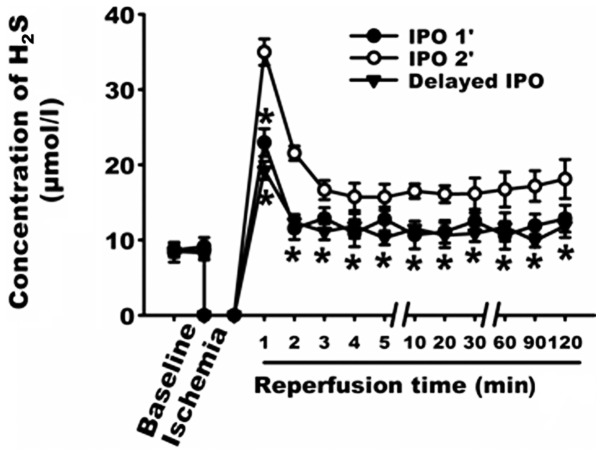
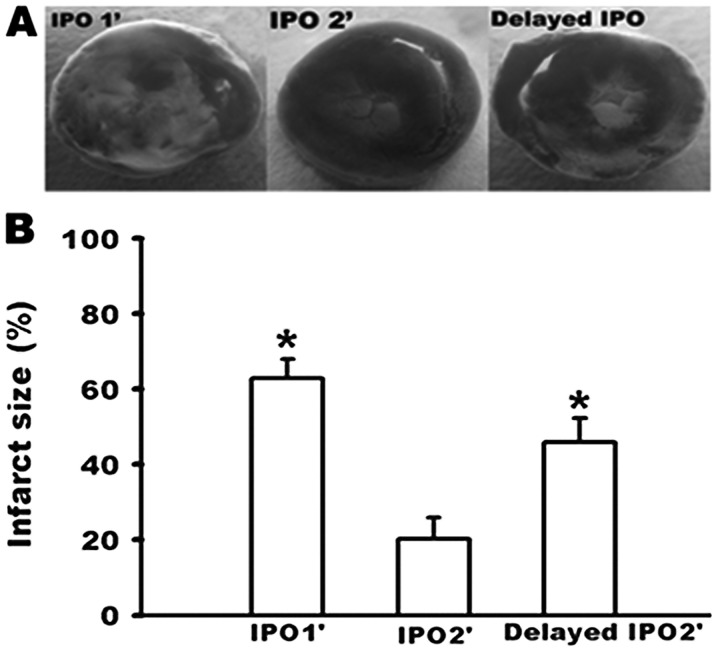
As shown in Fig. 7, the concentrations of H2S in the IPO 1’ and the delayed IPO groups were decreased when compared to the IPO 2’ group. The cardio-protective effects were reduced in the IPO 1’ and the delayed IPO groups as represented by greater infarct size (Fig. 8) and lower recovery of cardiodynamic function (Fig. 9).
Figure 7.
Effect of ischemic postconditioning (IPO) 1’ and delayed IPO on the concentration of hydrogen sulfide (H2S) (mean ± SD). *P<0.05, compared with IPO 2’ group. n=5.
Figure 8.
Effect of ischemic postconditioning (IPO) 1’ and delayed IPO on the infart size (mean ± SD). (A) Representative photographs of infarcted heart slices stained with triphenyltetrazolium chloride. (B) Mean data of infarct size (expressed as percentage of total ventricular area). *P<0.05, compared with IPO 2’ group. n=4 or 5.
Figure 9.
Effect of ischemic postconditioning (IPO) 1’ and delayed IPO on cardiodynamics (mean ± SD). (A) Left ventricular developed pressure (LVDP); (B) rate pressure product (RPP); (C) maximum gradient during systoles (+dP/dtmax); (D) minimum gradient during diastoles (−dP/dtmax). *P<0.05, compared with IPO 2’ group. n=5.
Discussion
The main objective of this study was to explore the role of endogenous H2S in the cardioprotection induced by IPO during early reperfusion. We found that IPO 2’ (IPO lasting for 2 min) significantly improved the heart contractile function and reduced multiple manifestations of IR injury, including infarct size and LDH levels. This is consistent with previous findings that IPO protects the heart from lethal IR injury when assessed by infarct size (3,21,22), cardiodynamic performance (23–26) and cellular injury biomarkers (6,27).
The myocardium generates a considerable amount of H2S under physiological conditions (8,9); however, H2S production is decreased upon ischemia treatment (19). In the present study, we demonstrated that the concentration of H2S was stable throughout the entire experiment in the CON group, and its concentration reached a peak during the first minute of reperfusion in the IR group. This indicates that H2S accumulates in the myocardium during the 30 min of global ischemia and is then washed out immediately during early reperfusion. In addition, during the first minute of reperfusion the concentration of H2S in the IPO 2’ group was higher than that in the IR group, suggesting that intermittent reperfusion delayed the washout of H2S during early reperfusion. On the other hand, H2S in aqueous solution is dissociated into HS− and H+, which exist in equilibrium with each other. The proportion of undissociated H2S under standard temperature conditions at physiological pH (7.4) is 30–33% (20,28), and the proportion increases as pH decreases. As shown in a previous study, acidotic status induced by ischemia is prolonged in IPO 2’ (4), thus the lower pH status induced by IPO 2’ can further increase the concentration of H2S at the onset of reperfusion.
It has been previously reported that the early phase of reperfusion may be the key to cardioprotection induced by IPO (3,4). Therefore, we designed other forms of IPO, IPO starting 1 min after reperfusion (delayed IPO) or lasting only 1 min (IPO 1’), to examine the important time frame of IPO 2’. Our data demonstrated that IPO 1’ and delayed IPO failed to exert a cardioprotective effect, and with both treatments the peak of the H2S concentration decreased simultaneously during the first minute of reperfusion. There may be 2 explanations for the lower concentration of H2S during early reperfusion in both groups. Firstly, there was not enough time for H2S accumulation during early reperfusion since IPO 1’ lasted only 1 min. H2S was washed out immediately as the reperfusion was initiated, which resulted in reduced retention of H2S at the onset of the reperfusion in delayed IPO. Secondly, during early reperfusion in both IPO 1’ and delayed IPO, pH quickly returned to a neutral level. A lower pH is responsible for the generation of increased amounts of H2S (4). Indeed, the time-relative changes of H2S level are consistent with the critical time frame of the IPO effect. Therefore, our data suggest that IPO lasting only 2 min and commencing at the onset of reperfusion could preserve H2S in order to mediate the cardioprotection invoked by IPO.
The accumulation of endogenous material during early reperfusion is one of the important mechanisms of IPO-mediated effects. The observations of Zhao et al (2) suggested that endogenous mechanisms are put into action within the first few minutes of reperfusion that attenuate reperfusion injury specifically. Kin et al (25) reported that IPO delays the washout of endogenous adenosine during the critical early moments of reperfusion, thereby increasing intravascular adenosine concentrations, which can activate adenosine receptors to elicit protection against myocardial infarction. In addition, previous studies (29,30) have shown that with IPO maneuvers the heart releases autacoids that accumulate in an intermittent manner during early reperfusion and trigger pathways leading to a protected state. In the present study, we noted that the higher concentration of H2S during early reperfusion in IPO 2’ was associated with improved recovery of contractile function, decreased infarct size and LDH level. However, the inhibition of endogenous H2S synthesis by PAG (the specific and irreversible inhibitor of CSE) used 20 min prior to ischemic treatment notably inhibited the generation of H2S and abolished the cardioprotective effects exerted by IPO 2’. Our data indicated that the increase in endogenous H2S during early reperfusion contributes to the cardioprotective effects of IPO 2’.
There is a significant burst of oxygen-derived free radicals generated within the first minute of reperfusion peaking 4–7 min following the onset of reperfusion (6), which contribute to the IR injury. H2S is a thiol that can interact with and ‘scavenge’ free radicals, including ONOO (31), H2O2 (12) and HOCl (32). Therefore, the potential cellular mechanisms for H2S-mediated early cardioprotection invoked by IPO 2’ may involve the reduction of the peak generation of ROS occurring during the first minutes of reperfusion. In addition, a previous study has shown that H2S, the KATP channel opener, was involved in cardiac protection (33). For this reason we presumed that H2S may protect the ischemic myocardum by opening KATP channels. However, the exact mechanisms remain uncertain in the present study. It is not known whether deleterious mechanisms are attenuated, or whether beneficial mechanisms are triggered by H2S. This is a limitation of our present study and warrants further investigation.
In conclusion, this study demonstrates that endogenous H2S mediates the cardioprotection induced by IPO during early reperfusion. H2S may be considered to protect by ‘pharmacological IPO’ thus offering greater opportunity for protection clinically, such as at the time of thrombolysis, percutaneous transluminal coronary angioplasty (PTCA) and coronary artery bypass grafting (CABG). This study provides an impetus for further investigation into the synthesis of a drug that can release H2S. If the certain protection of H2S were proved clinically, then ischemia-reperfusion injury could be attenuated by simply administering an oral medication that released H2S.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30570766, 30971169, 81170277; ZS Jiang) and Aid Program for Science and Techology Innovative Research Team in Higher Educational Institutions (2008-244; ZS Jiang) of Hunan Province.
References
- 1.Na HS, Kim YI, Yoon YW, Han HC, Nahm SH, Hong SK. Ventricular premature beat-driven intermittent restoration of coronary blood flow reduces the incidence of reperfusion-induced ventricular fibrillation in a cat model of regional ischemia. Am Heart J. 1996;132:78–83. doi: 10.1016/s0002-8703(96)90393-2. [DOI] [PubMed] [Google Scholar]
- 2.Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 3.Kin H, Zhao ZQ, Sun HY, et al. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Sun HY, Wang NP, Kerendi F, et al. Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+ overload. Am J Physiol Heart Circ Physiol. 2005;288:H1900–1908. doi: 10.1152/ajpheart.01244.2003. [DOI] [PubMed] [Google Scholar]
- 7.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? Faseb J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 8.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 9.Geng B, Yang J, Qi Y, et al. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun. 2004;313:362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- 10.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. Embo J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yong QC, Pan TT, Hu LF, Bian JS. Negative regulation of beta-adrenergic function by hydrogen sulphide in the rat hearts. J Mol Cell Cardiol. 2008;44:701–710. doi: 10.1016/j.yjmcc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Geng B, Chang L, Pan C, et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 13.Elrod JW, Calvert JW, Morrison J, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemiareperfusion injury - evidence for a role of K ATP channels. Basic Res Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 15.Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 16.Zhu YZ, Wang ZJ, Ho P, et al. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol. 2007;102:261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 17.Yong QC, Lee SW, Foo CS, Neo KL, Chen X, Bian JS. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol Heart Circ Physiol. 2008;295:H1330–H1340. doi: 10.1152/ajpheart.00244.2008. [DOI] [PubMed] [Google Scholar]
- 18.Morrison RR, Talukder MA, Ledent C, Mustafa SJ. Cardiac effects of adenosine in A(2A) receptor knockout hearts: uncovering A(2B) receptors. Am J Physiol Heart Circ Physiol. 2002;282:H437–H444. doi: 10.1152/ajpheart.00723.2001. [DOI] [PubMed] [Google Scholar]
- 19.Bian JS, Yong QC, Pan TT, et al. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 20.Zhu YZ, Chong CL, Chuah SC, et al. Cardioprotective effects of nitroparacetamol and paracetamol in acute phase of myocardial infarction in experimental rats. Am J Physiol Heart Circ Physiol. 2006;290:H517–H524. doi: 10.1152/ajpheart.00572.2005. [DOI] [PubMed] [Google Scholar]
- 21.Darling CE, Jiang R, Maynard M, Whittaker P, Vinten-Johansen J, Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am J Physiol Heart Circ Physiol. 2005;289:H1618–1626. doi: 10.1152/ajpheart.00055.2005. [DOI] [PubMed] [Google Scholar]
- 22.Yang XM, Philipp S, Downey JM, Cohen MV. Postconditioning’s protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol. 2005;100:57–63. doi: 10.1007/s00395-004-0498-4. [DOI] [PubMed] [Google Scholar]
- 23.Crisostomo PR, Wang M, Wairiuko GM, Terrell AM, Meldrum DR. Postconditioning in females depends on injury severity. J Surg Res. 2006;134:342–347. doi: 10.1016/j.jss.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Fantinelli JC, Mosca SM. Comparative effects of ischemic pre and postconditioning on ischemia-reperfusion injury in spontaneously hypertensive rats (SHR) Mol Cell Biochem. 2007;296:45–51. doi: 10.1007/s11010-006-9296-2. [DOI] [PubMed] [Google Scholar]
- 25.Kin H, Zatta AJ, Lofye MT, et al. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–133. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Zhu M, Feng J, Lucchinetti E, et al. Ischemic postconditioning protects remodeled myocardium via the PI3K-PKB/Akt reperfusion injury salvage kinase pathway. Cardiovasc Res. 2006;72:152–162. doi: 10.1016/j.cardiores.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Wang HC, Zhang HF, Guo WY, et al. Hypoxic postconditioning enhances the survival and inhibits apoptosis of cardiomyocytes following reoxygenation: role of peroxynitrite formation. Apoptosis. 2006;11:1453–1460. doi: 10.1007/s10495-006-7786-z. [DOI] [PubMed] [Google Scholar]
- 28.Dombkowski RA, Russell MJ, Olson KR. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol Regul Integr Comp Physiol. 2004;286:R678–R685. doi: 10.1152/ajpregu.00419.2003. [DOI] [PubMed] [Google Scholar]
- 29.Penna C, Mancardi D, Rastaldo R, Losano G, Pagliaro P. Intermittent activation of bradykinin B2 receptors and mitochondrial KATP channels trigger cardiac postconditioning through redox signaling. Cardiovasc Res. 2007;75:168–177. doi: 10.1016/j.cardiores.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Weihrauch D, Krolikowski JG, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Morphine enhances isoflurane-induced postconditioning against myocardial infarction: the role of phosphatidylinositol-3-kinase and opioid receptors in rabbits. Anesth Analg. 2005;101:942–949. doi: 10.1213/01.ane.0000171931.08371.a2. [DOI] [PubMed] [Google Scholar]
- 31.Whiteman M, Armstrong JS, Chu SH, et al. The novel neuro-modulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 32.Whiteman M, Cheung NS, Zhu YZ, et al. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun. 2005;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- 33.Hassouna A, Matata BM, Galinanes M. PKC-epsilon is upstream and PKC-alpha is downstream of mitoKATP channels in the signal transduction pathway of ischemic preconditioning of human myocardium. Am J Physiol Cell Physiol. 2004;287:C1418–C1425. doi: 10.1152/ajpcell.00144.2004. [DOI] [PubMed] [Google Scholar]