Abstract
The aim of this study was to assess the genetic status of cagA, vacA subtype and iceA genotypes of Helicobacter pylori and the relationship with upper gastrointestinal diseases in Northeast China. Gastric biopsies were obtained from 378 patients with upper gastrointestinal diseases and 197 samples were used. The cagA, vacA alleles and iceA genotypes were determined by polymerase chain reaction. CagA was present in 176 (89.3%) of 197 patients. Of the 197 cases, 186 (94.4%) had vacA signal sequence s1c allele, 6 (3%) had s1a and 5 (2.5%) had s1b. The vacA s2 genotype was not detected in our study. VacA middle region sequences, m1 and m2, were found in 20 (10.2%) and 150 (76.1%), respectively. The allelic variant iceA1 (70.1%) was more prevalent than iceA2 (23.4%). The vacA allele s1am2 had a significant relationship with the presence of gastric cancer (p<0.05) and the iceA1 genotype was also associated with gastric cancer (p<0.05). These may be useful risk factors for upper gastrointestinal diseases.
Keywords: Helicobacter pylori, prevalence, genotype, Northeast China
Introduction
Helicobacter pylori (H. pylori) is a gram-negative microaerophilic bacterium which is one of the most common pathogens in humans and has a worldwide distribution. It is associated with the development of chronic gastritis, peptic ulcer and even gastric cancer (1). On the basis of abundant epidemiological research, H. pylori was classified as a class I carcinogen in humans by the World Health Organization International Agency for Research on Cancer (2).
Several H. pylori virulence genes that may be associated with the risk of developing diseases have been identified. The cagA is a marker of genomic pathogenicity island (cagPAI) encoding the gene product which causes upregulation of interleukin-8 (IL-8) (3). It is considered that H. pylori strains possessing cagA are related to a more severe clinical outcome such as atrophic gastritis or gastric cancer (4,5). The vacA exists in all H. pylori strains and encodes vacuolating cell toxins which cause vacuole degeneration of epithelial cells. It includes two different parts: the signal (s) region encoding the signal peptide and the middle (m) region. The s-region is situated at the 5′ end of the gene and exists as s1 and s2 alleles. The s1 exists as an s1a, s1b and s1c. The m-region occurs as m1 or m2 alleles (6). The mosaic combination of s- and m-region allelic types produces cytotoxin and is associated with the pathogenicity of the bacterium. In general, type s1m1 and s1m2 strains produce high and moderate levels of toxin, respectively, whereas s2m2 strains produce little or no toxin. (7). VacA m1 strains are associated with greater gastric epithelial damage than m2 strains (8). Another virulence gene designated iceA has two main allelic variants iceA1 and iceA2 but the function of these variants is unknown. IceA1 is upregulated upon contact of H. pylori with the gastric epithelium and has been considered as a marker for peptic ulcer disease (9).
In Northeast China, there are no data regarding the pattern of H. pylori genotypes in patients. This study aimed to investigate the prevalence of the vacA, cagA and iceA genotypes of H. pylori from patients with upper gastrointestinal diseases and the relationship with clinical outcome in Northeast China.
Materials and methods
Study subjects
We evaluated 378 patients with upper gastrointestinal diseases referred for endoscopy at the Second Affiliated Hospital of Harbin Medical University in 2007 and 2008. Gastric mucosal biopsy specimens were obtained from each patient: one for pathological diagnosis, another for histological detection of H. pylori and the last for genomic DNA extraction and polymerase chain reaction (PCR).
The study was approved by the Ethics Committee of Harbin Medical University. Written informed consent was obtained from each patient prior to enrolling in the study.
Histological assessment
The biopsy samples were fixated in 10% formalin, then sliced into 4- to 6-mm pieces, dehydrated in ethanol, embedded in paraffin wax, sectioned (5-μm thick), and stained with hematoxylin and eosin (H&E). The presence of H. pylori in the sections was determined using a modified Gram staining protocol and taking into consideration its morphological characteristics which included a curved and spiral form and intense purple coloring (10). Pathological diagnoses were evaluated in a blinded manner by two independent pathologists and were defined as gastritis (active chronic gastritis or closed-type atrophic gastritis), gastric ulcer and gastric cancer.
Genomic DNA extraction
DNA was extracted from the biopsy specimens using the Genomic DNA purification system (Promega, USA) according to the manufacturer’s instructions and stored at −20°C until analysis.
Diagnosis of H. pylori infection
H. pylori-positive status was defined as positive histology and positive 16S-rRNA PCR. A 500-bp region of 16S-rRNA was amplified by PCR using primers CP-1/CP-2 (Table I). Five microlitres of DNA was added to 50 μl of reaction mixture containing 1X PCR buffer, 0.2 mM dNTPs and 0.3 μM primers as well as 1.25U Taq polymerase (Takara Bio, Inc., Japan). The incubation conditions were as follows: a 5-min preincubation at 95°C, followed by 30 cycles of 1 min at 94°C, 1 min at 58°C, 1 min at 72°C, and a final 5-min incubation at 72°C. Positive results were indicative of a diagnosis of H. pylori infection.
Table I.
Primer sequences for human HP 16S rRNA, cagA, vacA and iceA.
| Gene | Primer | Primer sequence (5′→3′)a | Product size (bp) | Reference |
|---|---|---|---|---|
| 16S rRNA | cp-1 | GCGCAATCAGCGTCAGGTAATG | 500 | (37) |
| cp-2 | GCTAAGAGATCAGCCTATGTCC | |||
| cagA | cagA-F | GATAACAGGCAAGCTTTTGAGG | 349 | (18) |
| cagA-R | CTGCAAAAGATTGTTTGGCAGA | |||
| s1a | S1a-F | TCTYGCTTTAGTAGGAGC | 212 | (18) |
| VA1-R | CTGCTTGAATGCGCCAAAC | |||
| s1b | SS3-R | AGCGCCATACCGCAAGAG | 187 | (18) |
| VA1-R | CTGCTTGAATGCGCCAAAC | |||
| s1c | S1c-F | CTYGCTTTAGTRGGGYTA | 213 | (18) |
| VA1-R | CTGCTTGAATGCGCCAAAC | |||
| s2 | SS2-F | GCTAACACGCCAAATGATCC | 199 | (8) |
| VA1-R | CTGCTTGAATGCGCCAAAC | |||
| m1 | VA3-F | GGTCAAAATGCGGTCATGG | 290 | (38) |
| VA3-R | CCATTGGTACCTGTAGAAAC | |||
| m2 | VA4-F | GGAGCCCCAGGAAACATTG | 352 | (38) |
| VA4-R | CATAACTAGCGCCTTGCAC | |||
| iceA1 | iceA1-F | GTGTTTTTAACCAAAGTATC | 247 | (35) |
| iceA1-R | CTATAGCCASTYTCTTTGCA | |||
| iceA2 | iceA2-F | GTTGGGTATATCACAATTTAT | 229/334 | (35) |
| iceA2-R | TTRCCCTATTTTCTAGTAGGT |
Y is C or T, R is A or G and S is C or G.
Genotyping of H. pylori
The systems of PCR were the same as mentioned above except for the primers. The amplification cycles consisted of an initial denaturation at 94°C for 5 min and then denaturation at 94°C for 30 sec, primer annealing at 60, 56, 58 and 48°C for cagA, vacA (s1a, s1b, s1c and s2), vacA (m1, m2) and iceA, respectively, for one-half minute and extension at 72°C for 45 sec. All reactions were performed through 35 cycles. The final cycle included an extension step for 5 min. Primers used for genotyping cagA, vacA and iceA genes are listed in Table I. PCR products were analyzed on 1.5% agarose gel electrophoresis with ethidium bromide. Images were quantified via the Gene Genius system (Syngene, England, UK). For strains that were cagA-negative as determined by PCR, Southern blotting was performed according to the method described by Pan et al (11).
Statistical analyses
Statistical tests were performed with SPSS software version 11.5 (SPSS Inc., Chicago, IL, USA). A Chi-square test and Fisher’s exact test were used to assess the association amongst the genotypes and between specific genotypes and upper gastrointestinal diseases. P-values <0.05 were considered to indicate a statistically significant result.
Results
DNA was successfully extracted from 378 gastric mucosa tissues of patients with gastrointestinal diseases and 197 were confirmed as H. pylori infection-positive by histology and PCR amplification. H. pylori-infected patients were evaluated for the relationship of age and gender with disease as shown in Table II.
Table II.
Distribution of 197 patients with different clinical outcomes, according to age and gender.
| Clinical status
|
||||
|---|---|---|---|---|
| Classification | GUa n=86 (%) | GSb n=58 (%) | GCc n=53 (%) | Total n=197 (%) |
| Age (years) | ||||
| 21–30 | 7 (8.1) | 2 (3.5) | 0 (0.0) | 9 (4.6) |
| 31–40 | 12 (14.0) | 11 (19.0) | 4 (7.5) | 27 (13.7) |
| 41–50 | 33 (38.4) | 17 (29.3) | 17 (32.1) | 67 (34.0) |
| 51–60 | 24 (27.9) | 17 (29.3) | 16 (30.2) | 57 (29.0) |
| >60 | 10 (11.6) | 11 (18.9) | 16 (30.2) | 37 (18.7) |
| Gender | ||||
| Male (M) | 52 (60.5) | 37 (63.8) | 34 (64.2) | 123 (62.4) |
| Female (F) | 34 (39.5) | 21 (36.2) | 19 (35.8) | 74 (37.6) |
| M:F | 1:0.7 | 1:0.6 | 1:0.6 | 1:0.6 |
Gastric ulcer;
gastritis;
gastric cancer.
Detection of H. pylori genotypes
Overall, the presence of the cagA gene was detected in 176 cases (89.3%). A negative status for the other 21 (10.7%) cases was confirmed by Southern blotting, and the results were negative as before. All of the samples were positive for vacA (both the s-region and the m-region). Of the 197 cases, 186 (94.4%) had vacA signal sequence s1c allele, 6 (3%) had s1a and 5 (2.5%) had s1b. The vacA s2 genotype was not detected in our study. In the m-region, 27 cases contained both m1 and m2. In these cases the m1 allele was found in 20 (10.2%) isolates and m2 (76.1%) in 150 cases, which indicating the presence of mixed infection. The vacA s1am2 genotype was identified in 6 (3.0%) participants, the vacA s1bm2 was identified in 5 (2.5%) participants, s1cm1 was identified in 20 participants, and s1cm2 gene was identified in 139 ones. IceA1 was found in 138 (70.1%) and iceA2 was detected in 46 (23.4%) cases. The iceA2 amplification yielded both the 229-and 334-bp bands due to the presence of a 105-bp in-frame amplicon present in the 334-bp band that was absent in the 229-bp band. Mixed iceA (iceA1 + iceA2) genotypes were found in 13 (6.7%) of our isolates (Fig. 1).
Figure 1.
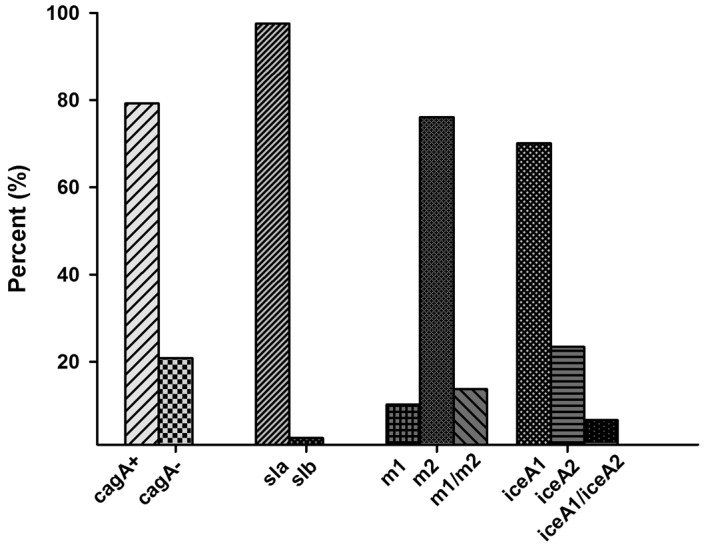
Distribution of cagA, vacA and iceA alleles of H. pylori from 197 patients with upper gastrointestinal diseases. M1m2, multiple vacA genotypes with m1 and m2. IceA1/iceA2, mixed iceA genotypes with iceA1 and iceA2.
Association among the genotypes
CagA was present in 124 out of 138 iceA1 cases (91.1%) and 44 out of 46 iceA2 cases (95.5%) (p>0.05) where 29 patients with mixed infection were excluded. Due to the lack of vacA s2, we could not analyse the association between cagA status and vacA genotypes and between iceA and vacA genotypes (Table III).
Table III.
Association of vacA with cagA and iceA genotypes.
| vacA | cagA+ n (%) | cagA− n (%) | iceA1 n (%) | iceA2 n (%) | iceA1/iceA2 n (%) |
|---|---|---|---|---|---|
| s-region | |||||
| s1a | 4 (66.7) | 2 (33.3) | 5 (83.3) | 1 (16.7) | 0 (0.0) |
| s1b | 3 (60.0) | 2 (40.0) | 5 (100.0) | 0 (0.0) | 0 (0.0) |
| s1c | 169 (90.9) | 17 (9.1) | 128 (68.8) | 45 (24.2) | 13 (7.0) |
| m-region | |||||
| m1 | 18 (90.0) | 2 (10.0) | 14 (70.0) | 6 (30.0) | 0 (0.0) |
| m2 | 139 (93.0) | 11 (7.0) | 110 (73.4) | 38 (25.3) | 2 (1.3) |
| m1m2 | 19 (84.0) | 8 (16.0) | 14 (70.4) | 2 (22.2) | 11 (7.4) |
| s/m region | |||||
| s1am2 | 4 (66.7) | 2 (33.3) | 5 (83.3) | 1 (16.7) | 0 (0.0) |
| s1bm2 | 3 (60.0) | 2 (40.0) | 5 (100.0) | 0 (0.0) | 0 (0.0) |
| s1cm1 | 18 (90.0) | 2 (10.0) | 14 (70.0) | 6 (30.0) | 0 (0.0) |
| s1cm2 | 132 (95.0) | 7 (5.0) | 100 (71.9) | 37 (26.6) | 2 (1.5) |
| s1cm1m2 | 19 (70.4) | 8 (29.6) | 14 (51.9) | 2 (7.4) | 11 (40.7) |
Relationship between genotypes and gastric diseases
Of the 197 strains studied, 86 were diagnosed with gastric ulcer, 58 with gastritis and 53 with gastric cancer. VacA s1cm2 was detected in all the disease conditions, and it was more significantly associated with the presence of gastric cancer (p<0.05). S1am2 and s1cm1 were detected in all the disease except gastric cancer, while s1bm2 was found in gastric cancer alone (Table IV). Surprisingly, iceA1 had a statistically significant association with gastric cancer (p<0.05). Neither cagA nor iceA2 was associated with various diseases. The most prevalent combination cagA/s1cm2/iceA1 was present in 56.6% (95 of 168) including 58.0% (40 of 69) of gastric ulcer, 47.0% (23 of 49) of gastritis and 64.0% (32 of 50) of gastric cancer (Table V). However, no significant association was found between the combination genotypes and diseases (p>0.05).
Table IV.
vacA, cagA and iceA status of H. pylori from 197 patients.
| Clinical status
|
||||
|---|---|---|---|---|
| Genotype status | GUa n=86 (%) | GSb n=58 (%) | GCc n=53 (%) | Total n=197 (%) |
| vacA | ||||
| s1am2 | 4 (4.6) | 2 (3.4) | 0 (0.0) | 6 (3.0) |
| s1bm2 | 0 (0.0) | 0 (0.0) | 5 (9.4) | 5 (2.5) |
| s1cm1 | 16 (18.6) | 4 (6.9) | 0 (0.0) | 20 (10.2) |
| s1cm2 | 51 (59.4) | 43 (74.2) | 45 (84.9)d | 139 (70.6) |
| s1cm1m2 | 15 (17.4) | 9 (15.5) | 3 (5.7) | 27 (13.7) |
| cagA | ||||
| cagA+ | 78 (90.7) | 53 (91.4) | 45 (84.9) | 176 (89.3) |
| cagA− | 8 (9.3) | 5 (8.6) | 8 (15.1) | 21 (10.7) |
| iceA | ||||
| iceA1 | 63 (73.3) | 34 (58.6) | 41 (77.4)d | 138 (70.0) |
| iceA2 | 14 (16.3) | 21 (36.2) | 11 (20.8) | 46 (23.4) |
| iceA1/iceA2 | 9 (10.4) | 3 (5.2) | 1 (1.9) | 13 (6.6) |
Gastric ulcer;
gastritis;
gastric cancer.
P<0.05.
Table V.
Combined vacA, cagA, iceA genotypes.
| Clinical status
|
||||
|---|---|---|---|---|
| Combination | GUa n (%) | GSb n (%) | GCc n (%) | Total n (%) |
| s1am2/cagA+/iceA1 | 1 (1.5) | 2 (4.1) | 0 (0.0) | 3 (1.8) |
| s1am2/cagA−/iceA1 | 2 (2.9) | 0 (0.0) | 0 (0.0) | 2 (1.2) |
| s1am2/cagA+/iceA2 | 1 (1.5) | 0 (0.0) | 0 (0.0) | 1 (0.6) |
| s1bm2/cagA+/iceA1 | 0 (0.0) | 0 (0.0) | 3 (6.0) | 3 (1.8) |
| s1bm2/cagA−/iceA1 | 0 (0.0) | 0 (0.0) | 2 (4.0) | 2 (1.2) |
| s1cm1/cagA+/iceA1 | 10 (14.3) | 2 (4.1) | 0 (0.0) | 12 (7.1) |
| s1cm1/cagA−/iceA1 | 2 (2.9) | 0 (0.0) | 0 (0.0) | 2 (1.2) |
| s1cm1/cagA+/iceA2 | 4 (5.8) | 2 (4.1) | 0 (0.0) | 6 (3.6) |
| s1cm2/cagA+/iceA1 | 40 (58.0) | 23 (47.0) | 32 (64.0) | 95 (56.5) |
| s1cm2/cagA−/iceA1 | 2 (2.9) | 1 (2.0) | 2 (4.0) | 5 (3.0) |
| s1cm2/cagA+/iceA2 | 6 (8.7) | 18 (36.7) | 11 (22.0) | 35 (20.8) |
| s1cm2/cagA−/iceA2 | 1 (1.5) | 1 (2.0) | 0 (0.0) | 2 (1.2) |
| Total | 69 (100) | 49 (100) | 50 (100) | 168 (100) |
gastric ulcer;
gastritis;
gastric cancer. Twenty-nine patients with mixed infection were excluded.
Discussion
This study was designed to characterize the genotype of H. pylori from gastric biopsy specimens from patients with upper gastrointestinal diseases and the relationship with clinical outcome in Northeast China. H. pylori was analysed for the presence of the genes for cagA, vacA and iceA. To our knowledge, this was the first study to analyse the different proposed virulence genes characterized in H. pylori and the relationship between the genes and upper gastrointestinal diseases in Northeast China.
CagA gene, as a major H. pylori virulence factor, was reported to be strongly associated with atrophic gastritis and gastric cancer as previously described. This is probably the main cause of a high incidence of gastric cancer in the region of East Asia, where the percentage of cagA-positive strains is above 90% (12). Worldwide, the presence of the cagA gene varies from 50% in some Middle Eastern countries to 99% in East Asian countries (13–15). In this study, cagA was found in 89.3% of H. pylori-infected patients. The result is similar to data reported from other districts of China (11,16). However, we did not find an association between cagA and clinical results. Notably, of the 29 mixed infection cases, 8 had cagA-negative and strains with an absence of cagA appeared to be associated with mixed infection (p=0.004).
The present study demonstrated that all strains of H. pylori carried the vacA s1 allele. Previous studies noted that s1c was present exclusively in isolates from East Asia (16–18). Our report also demonstrated a high prevalence of type s1c strains in this region, up to 94.4%. The result was similar to the report of Wang et al (19) and slightly higher than the prevalence in Beijing and Shanghai, which may result from the fact that more foreigners from America and Europe live in the two cities above, as either the s1a or s1b subtype was present in almost all strains in Central and South America, and in the majority of strains in Spain and Portugal (20,21), nevertheless rarely in East Asia (12,16). The vacA s2 genotype was prominently prevalent in Africa (9), and consistent to the outcome reported from China and Korea, s2 failed to be detected in this study (19,22).
Worldwide prevalence of vacA strains varies geographically. S1m1 strains were predominant in Japan and Korea (18,23) while s1m2 was found in Turkey and Northern and Eastern Europe (20,24). In Alaskans, H. pylori had either the vacA s1m1 (44.6%) or s2m2 (38.3%) (15). In China, prevalence of strains documented a greatly distinct pattern, with s1m1 and s1m2 sharing the same proportion in the Province of Xi’an (25) and s1m2 strains in Beijing, Taiwan and Hong Kong (16,19,26). The latter condition was similar to our study.
Generally, s1m2 forms of vacA bind to and vacuolate a narrower range of cells than s1m1 forms and induce less damage, yet they also act as efficient membrane pores and increase paracellular (27) permeability. The alleles of s1m1 and s1m2 encode to produce toxin which are common in patients with gastrosis (27). In Latin America and Germany, s1m1 was found to have a high correlation with gastric ulcer and gastric carcinoma (21,28). The strains of vacA s2m1 and s2m2 engender low toxic toxin which rarely correlates with gastric ulcer and gastric carcinoma (29). In our study, the vacA gene encoding the s1cm2 was associated with gastic cancer. Therefore, the s-region should be responsible for gastrosis other than the m-region.
Another virulent factor is the iceA gene, with two allelic variants iceA1 and iceA2 having been identified. The prevalence of the iceA1 genotype is 70.1% in this study, basically consistent with data reported from China, Thailand, Korea and Tunisia (9,23,30,31). Meanwhile, iceA2 is predominant in Brazil, the US, Europe and South Africa (6,18,32,33). It was demonstrated that iceA1 was significantly associated with peptic ulcer disease in Holland (34) and the US (35). However, studies from other countries such as in Korea, Colombia and India could not confirm the result (18,36). Some researchers found that the iceA2 genotype was most frequently found in patients with duodenal ulcer or gastric carcinoma (18,36). However, it is difficult to admit that iceA2, a gene that is considered as a protective factor in some regions and that is associated with more severe diseases in other places, could be considered a molecular marker of more virulent H. pylori strains (33). It was well worth mentioning that the iceA1 strains, based on this study, have a significant association with gastric cancer.
In common with other studies, there exists a strong indication that the presence of multiple H. pylori strains are detectable in clinical samples. Some studies have claimed multiple genotypes have a link with duodenal ulcers (9). It may be speculated that multiple strains contribute to increasing the potential chances of infecting pathogen. By colonizing a variety of receptors expressed on gastric epithelial cells, m1 and m2 strains probably tend to bring about pathological changes. Multi-colonization arising from the co-existence of more than one strain exert burden to patients under eradication treatment and furthermore dramatically enhance the risk of malignant tumors of the digestive tract among adult patients. However, our data did not indicate that multiple strain infection increases the risk of developing diseases (p>0.05).
In conclusion, the present study identified the prevalence of main virulence factor genes cagA, s1cm2 and iceA1 in Northeast China. The vacA gene encoding s1cm2 was found to predominate in gastic cancer patients, and the iceA1 genotype was also associated with gastric cancer. It may be insufficient to analyse gastrointestinal diseases simply by genotyping H. pylori, and therefore, we must evaluate the pathogenesis of diseases by a combination of the analysis of bacterial factors, genetic factors of the host and environmental factors.
Acknowledgments
This study was supported by a grant from the Natural Science Foundation of Heilongjiang Province (grant no. D2007-72).
References
- 1.Blaser MJ. Ecology of Helicobacter pylori in the human stomach. J Clin Invest. 1997;100:759–762. doi: 10.1172/JCI119588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamazaki S, Yamakawa A, Okuda T, et al. Distinct diversity of vacA, cagA, and cagE genes of Helicobacter pylori associated with peptic ulcer in Japan. J Clin Microbiol. 2005;43:3906–3916. doi: 10.1128/JCM.43.8.3906-3916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenks PJ, Megraud F, Labigne A. Clinical outcome after infection with Helicobacter pylori does not appear to be reliably predicted by the presence of any of the genes of the cag pathogenicity island. Gut. 1998;43:752–758. doi: 10.1136/gut.43.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636–1644. doi: 10.1053/j.gastro.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Rathbone M, Rathbone B. Helicobacter pylori and gastric cancer. Recent Results Cancer Res. 2011;185:83–97. doi: 10.1007/978-3-642-03503-6_5. [DOI] [PubMed] [Google Scholar]
- 6.Tanih NF, McMillan M, Naidoo N, Ndip LM, Weaver LT, Ndip RN. Prevalence of Helicobacter pylori vacA, cagA and iceA genotypes in South African patients with upper gastrointestinal diseases. Acta Trop. 2010;116:68–73. doi: 10.1016/j.actatropica.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Tan HJ, Rizal AM, Rosmadi MY, Goh KL. Distribution of Helicobacter pylori cagA, cagE and vacA in different ethnic groups in Kuala Lumpur, Malaysia. J Gastroenterol Hepatol. 2005;20:589–594. doi: 10.1111/j.1440-1746.2005.03783.x. [DOI] [PubMed] [Google Scholar]
- 8.Atherton JC, Cao P, Peek RM, Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 9.Ben Mansour K, Fendri C, Zribi M, et al. Prevalence of Helicobacter pylori vacA, cagA, iceA and oipA genotypes in Tunisian patients. Ann Clin Microbiol Antimicrob. 2010;9:10. doi: 10.1186/1476-0711-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assumpcao MB, Martins LC, Melo Barbosa HP, et al. Helicobacter pylori in dental plaque and stomach of patients from Northern Brazil. World J Gastroenterol. 2010;16:3033–3039. doi: 10.3748/wjg.v16.i24.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan ZJ, van der Hulst RW, Feller M, et al. Equally high prevalences of infection with cagA-positive Helicobacter pylori in Chinese patients with peptic ulcer disease and those with chronic gastritis-associated dyspepsia. J Clin Microbiol. 1997;35:1344–1347. doi: 10.1128/jcm.35.6.1344-1347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda S, Ogura K, Yoshida H, et al. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut. 1998;42:338–343. doi: 10.1136/gut.42.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Qabandi A, Mustafa AS, Siddique I, Khajah AK, Madda JP, Junaid TA. Distribution of vacA and cagA genotypes of Helicobacter pylori in Kuwait. Acta Trop. 2005;93:283–288. doi: 10.1016/j.actatropica.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Lai CH, Kuo CH, Chen YC, et al. High prevalence of cagA- and babA2-positive Helicobacter pylori clinical isolates in Taiwan. J Clin Microbiol. 2002;40:3860–3862. doi: 10.1128/JCM.40.10.3860-3862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miernyk K, Morris J, Bruden D, et al. Characterization of Helicobacter pylori cagA and vacA genotypes among Alaskans and their correlation with clinical disease. J Clin Microbiol. 2011;49:3114–3121. doi: 10.1128/JCM.00469-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong BC, Yin Y, Berg DE, et al. Distribution of distinct vacA, cagA and iceA alleles in Helicobacter pylori in Hong Kong. Helicobacter. 2001;6:317–324. doi: 10.1046/j.1523-5378.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 17.van Doorn LJ, Figueiredo C, Sanna R, et al. Expanding allelic diversity of Helicobacter pylori vacA. J Clin Microbiol. 1998;36:2597–2603. doi: 10.1128/jcm.36.9.2597-2603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, van Doorn LJ, Robinson PA, et al. Regional variation among vacA alleles of Helicobacter pylori in China. J Clin Microbiol. 2003;41:1942–1945. doi: 10.1128/JCM.41.5.1942-1945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Doorn LJ, Figueiredo C, Megraud F, et al. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology. 1999;116:823–830. doi: 10.1016/s0016-5085(99)70065-x. [DOI] [PubMed] [Google Scholar]
- 21.Sugimoto M, Yamaoka Y. The association of vacA genotype and Helicobacter pylori-related disease in Latin American and African populations. Clin Microbiol Infect. 2009;15:835–842. doi: 10.1111/j.1469-0691.2009.02769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choe YH, Kim PS, Lee DH, et al. Diverse vacA allelic types of Helicobacter pylori in Korea and clinical correlation. Yonsei Med J. 2002;43:351–356. doi: 10.3349/ymj.2002.43.3.351. [DOI] [PubMed] [Google Scholar]
- 23.Kim SY, Woo CW, Lee YM, et al. Genotyping CagA, VacA subtype, IceA1, and BabA of Helicobacter pylori isolates from Korean patients, and their association with gastroduodenal diseases. J Korean Med Sci. 2001;16:579–584. doi: 10.3346/jkms.2001.16.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erzin Y, Koksal V, Altun S, et al. Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA, babA2 genotypes and correlation with clinical outcome in Turkish patients with dyspepsia. Helicobacter. 2006;11:574–580. doi: 10.1111/j.1523-5378.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 25.Qiao W, Hu JL, Xiao B, et al. cagA and vacA genotype of Helicobacter pylori associated with gastric diseases in Xi’an area. World J Gastroenterol. 2003;9:1762–1766. doi: 10.3748/wjg.v9.i8.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perng CL, Lin HJ, Sun IC, Tseng GY, Facg Helicobacter pylori cagA, iceA and vacA status in Taiwanese patients with peptic ulcer and gastritis. J Gastroenterol Hepatol. 2003;18:1244–1249. doi: 10.1046/j.1440-1746.2003.03214.x. [DOI] [PubMed] [Google Scholar]
- 27.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miehlke S, Kirsch C, Agha-Amiri K, et al. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. Int J Cancer. 2000;87:322–327. [PubMed] [Google Scholar]
- 29.Bindayna KM, Al Mahmeed A. vacA genotypes in Helicobacter pylori strains isolated from patients with and without duodenal ulcer in Bahrain. Indian J Gastroenterol. 2009;28:175–179. doi: 10.1007/s12664-009-0069-1. [DOI] [PubMed] [Google Scholar]
- 30.Han YH, Liu WZ, Zhu HY, Xiao SD. Clinical relevance of iceA and babA2 genotypes of Helicobacter pylori in a Shanghai population. Chin J Dig Dis. 2004;5:181–185. doi: 10.1111/j.1443-9573.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 31.Chomvarin C, Namwat W, Chaicumpar K, et al. Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA and babA2 genotypes in Thai dyspeptic patients. Int J Infect Dis. 2008;12:30–36. doi: 10.1016/j.ijid.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Podzorski RP, Podzorski DS, Wuerth A, Tolia V. Analysis of the vacA, cagA, cagE, iceA, and babA2 genes in Helicobacter pylori from sixty-one pediatric patients from the Midwestern United States. Diagn Microbiol Infect Dis. 2003;46:83–88. doi: 10.1016/s0732-8893(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 33.Ashour AA, Collares GB, Mendes EN, et al. iceA genotypes of Helicobacter pylori strains isolated from Brazilian children and adults. J Clin Microbiol. 2001;39:1746–1750. doi: 10.1128/JCM.39.5.1746-1750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Doorn LJ, Figueiredo C, Sanna R, et al. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 35.Peek RM, Jr, Thompson SA, Donahue JP, et al. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc Assoc Am Physicians. 1998;110:531–544. [PubMed] [Google Scholar]
- 36.Mukhopadhyay AK, Kersulyte D, Jeong JY, et al. Distinctiveness of genotypes of Helicobacter pylori in Calcutta, India. J Bacteriol. 2000;182:3219–3227. doi: 10.1128/jb.182.11.3219-3227.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clayton CL, Kleanthous H, Coates PJ, Morgan DD, Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 1992;30:192–200. doi: 10.1128/jcm.30.1.192-200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


