Abstract
Prostate cancer is the leading type of cancer diagnosed in men. In 2010, ∼217,730 new cases of prostate cancer were reported in the United States. Prompt diagnosis of the disease can substantially improve its clinical outcome. Improving capability for early detection, as well as developing new therapeutic targets in advanced disease are research priorities that will ultimately lead to better patient survival. Eukaryotic cells secrete proteins via distinct regulated mechanisms which are either ER/Golgi dependent or microvesicle mediated. The release of microvesicles has been shown to provide a novel mechanism for intercellular communication. Exosomes are nanometer sized cup-shaped membrane vesicles which are secreted from normal and cancerous cells. They are present in various biological fluids and are rich in characteristic proteins. Exosomes may thus have potential both in facilitating early diagnosis via less invasive procedures or be candidates for novel therapeutic approaches for castration resistance prostate cancer. Because exosomes have been shown previously to have a role in cell-cell communication in the local tumor microenvironment, conferring activation of numerous survival mechanisms, we characterized constitutive lipids, cholesterol and proteins from exosomes derived from six prostate cell lines and tracked their uptake in both cancerous and benign prostate cell lines respectively. Our comprehensive proteomic and lipidomic analysis of prostate derived exosomes could provide insight for future work on both biomarker and therapeutic targets for the treatment of prostate cancer.
Prostate cancer (PCa)1 is the leading type of cancer diagnosed in men. The American Cancer Society reported 217,730 new cases of PCa in the United States last year. Death from PCa follows its incidence profile closely as the third leading cause of cancer-related death in men (1). In the early stages, the disease is locally confined to the prostate and is hormone or androgen-dependent. It can be managed at this stage by surgical intervention or radiation treatment. However, over time (varying from months to years), many prostate cancers metastasize and, even with aggressive hormone deprivation therapy, progress to castration resistant prostate cancer (CRPC), which ultimately results in death. During early metastasis, a response to androgen deprivation therapy (ADT) is usually observed. Nonetheless, despite the reduction in androgen levels after ADT, androgen receptor (AR) remains active and contributes to CRPC progression (2–4).
The routine screening test for PCa diagnosis in North America includes measurement of prostate specific antigen (PSA) in the blood, digital rectal examination and a prostate biopsy (5). PSA screening for PCa detection is controversial because certain activities can induce the production of PSA, unrelated to the presence of cancer (6). Consequently prostate biopsy, albeit an invasive procedure, remains the only definitive diagnostic test for PCa. There is an urgent current need, therefore, for the discovery of relevant biomarkers to replace the existing diagnostic tests for better and earlier detection of PCa (7).
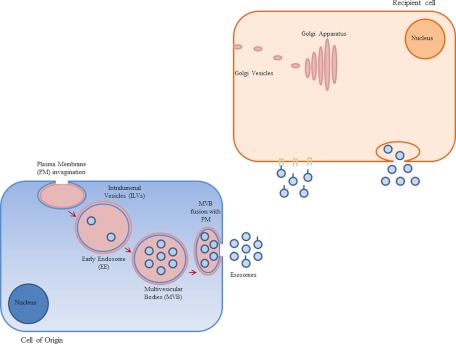
One possible source of biomarkers which could be used as part of a diagnostic test are exosomes. All cells produce and release exosomes, which are often found in different body fluids such as plasma (8), serum (9, 10) malignant ascites (11, 12) urine (13), amniotic fluid (14), bronchoalveolar lavage fluid (15, 16), and breast milk (17, 18). Recent studies suggest however that cancer cells produce exosomes, which may be differentiated from those derived from normal cells primarily based upon their cargo. Exosomes are cup-shaped (19) encapsulated by a bi-layer lipid membrane (20) with a membrane-bound compartment varying between 30–100 nm in size (19). As mentioned above, they are secreted from both normal cells and tumor cells (21) and although the underlying mechanism of exosome function is not fully understood it is known that exosomes are formed in the endosomal compartment of cells and are secreted upon fusion of multivesicular bodies (MVB) with the plasma membrane (21). The schematic cartoon in Fig. 1 depicts early endosome (EE) formation as a result of the invagination of specific regions of the plasma membrane. In addition, endocytotic cargo transported out of the cell is sorted from EE into intraluminal vesicles (ILV). Mechanisms involved in protein sorting into ILVs are still under investigation however there is evidence supporting the involvement of ubiquitin and endosomal sorting complex required for transport (ESCRT machinery) in this process. Finally, fusion of late endosome or MVB with plasma membrane releases ILVs into the extracellular matrix or the tissue microenvironment. Accumulating evidence suggests that induction of intracellular calcium (22–25), overexpression of Rab11 or citron kinase (26) as well as a reduction in membrane cholesterol, or inhibition of cholesterol biosynthesis (27), could stimulate the release of exosomes into the microenvironment.
Fig. 1.
Mechanism involved in exosome formation and trafficking in the microenvironment.
As shown in Fig. 1, once released, exosomes will interact with recipient target cells via different mechanisms such as fusion with the plasma membrane or adhesion to corresponding receptors on the plasma membrane (25).
Although, the mechanisms underlying exosome formation and secretion is still under investigation, it is well-known that factors such as cell type, cell cycle, and stage of cancer, could affect the amount and composition of exosomes formed and secreted from various cells (19). It has been shown that exosomes are secreted in a multitude of cell types and though it is postulated that they are involved in membrane trafficking as communication vesicles, their relevance in cancer initiation and specifically prostate tumor growth and progression has yet to be determined (28–30). Studies on tumor-related microvesicles suggest that exosomes play a significant role in cell communication thus potentially influencing cancer progression via different mechanisms (31). Exosomes contain and protect the integrity of various proteins and an array of lipids, mRNA and miRNA which would otherwise be hydrolytically or enzymatically broken down if they existed as free soluble molecules in the extracellular microenvironment. The presence of differential exosomal protein markers involved in cancer progression combined with the presence of exosomes in accessible biological fluids highlights a potential role of exosomes as clinical biomarkers for PCa at diagnosis and progression (32, 33). Therefore isolation, purification and characterization of exosomes derived from different body fluids is an essential first step in identifying novel biomarkers from this source.
In addition, exosomes may also present novel therapeutic strategies. If in fact implicated in cancer progression, exosomes present a new target set for development of novel therapeutics. Hence, a better understanding of the mechanisms involved in formation and secretion of exosomes for therapeutic targeting as well as investigating the relevance of the presence of different proteins in these membrane vesicles is required.
Therefore the main purpose of the present study was to observe the release of exosomes by prostate cells, and determine characteristic differences between exosomes released by parent cells of different characteristic AR phenotypes. In order to answer this question, in addition to one nonmalignant cell line, we used five different PCa cell lines which contain/lack AR and were representative of different stages of PCa.
We then confirmed the transfer of exosomes to target cells in culture using confocal microscopy of fluorescence labeled exosomes. We subsequently performed a comprehensive proteomic analysis of all six different prostate cell lines using mass spectrometry to understand differences between the protein profiles released via exosome externalization in different prostate cell lines. The final part of this study was to investigate the difference in broad classes of lipids and cholesterol as constituents of different prostate cell lines and their exosomes.
Taken together the comprehensive characterization of exosomes derived from prostate cell lines which have distinct AR ±ve phenotypes, provides a basis for evaluating transfer of identified composite exosome proteins between different PCa cells as part of a recognized cell communication phenomenon. In addition this study forms a platform for future clinical validation research using exosomes as biomarkers for PCa diagnosis as well as potential therapeutic targets which could be important in the treatment of CRPC.
EXPERIMENTAL PROCEDURES
Cell Culture
PC3, DU145 and VCaP human prostate cancer cells (ATCC) were maintained in Dulbecco's Modified Eagle's Medium (DMEM) whereas LNCaP and C4–2 cells (ATCC) were cultured in RPMI 1640, supplemented with 5% FBS (Invitrogen) and antibiotic, at 37 °C in 5% CO2. RWPE-1 (ATCC) cells also were grown in keratinocyte-SFM (KSFM) with growth supplement (GIBCO) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA). Cells were grown to 60–70% confluency were washed with sterile PBS buffer and removed from serum and incubated in culture media for 72 h for exosome purification and collection.
CLUGFP stably over-expressing LNCaP cells were maintained in 200 mg/ml G418 (Invitrogen) containing RPMI medium supplemented with 10% FBS (Invitrogen) and antibiotic, at 37 °C in 5% CO2.
Exosome Isolation
Exosomes were purified from the media following exposure to different PCa cell lines including PC3, DU145, VCaP, LNCaP, C4–2, and a benign epithelial prostate cell line, RWPE-1, as well as a CLUGFP stably overexpressing LNCaP cell line. For exosome purification, 200 ml of each cell line's conditioned medium was cleared by centrifugation at 6000 rpm at 4 °C for 10 min to remove protein aggregates and cell debris. The precleared medium was concentrated to 2 ml using a 100 kDa MWCO Centricon Plus-20 filter capsule (Millipore, Billerica, MA). Samples were transferred to ultracentrifuge tubes containing 300 μl of 30% sucrose-deuterium oxide (D2O). Sample tubes were then ultracentrifuged at 39,000 rpm for 1 h at 4 °C. Purified exosomes (350 μl) were collected off the cushion of sucrose.
Transmission Electron Microscopy (TEM)
Isolated exosomes (2.5 μl) were dried onto freshly glow discharged 300 mesh formvar/carbon-coated TEM grids (Ted Pella, Redding, CA), negatively stained with 2% aqueous uranyl acetate and observed with a Hitachi H7600 TEM (Hitachi High-Technologies Corp., Tokyo, Japan) operated at 80kV. Images were captured with a side mounted 1K AMT Advantage digital camera (Advanced Microscopy Techniques, Corp. Woburn, MA).
Western Blot Analysis
All samples of exosomes and cell lysates were analyzed for total protein concentration using the BCA protein determination kit (Sigma, Oakville, Ontario, Canada). Twenty-five μg of total protein associated with purified exosomes were loaded on 12% acrylamaide gel. Relative enzyme levels were quantified using antibodies specific for exosome markers; mouse monoclonal β-actin (1:1000 Sigma), rabbit polyclonal lysosomal associated membrane protein 2 (LAMP2) (1:1000 Abcam) rabbit monoclonal tubulin, mouse monoclonal HSP70, goat polyclonal HSP90, mouse monoclonal Rab5, mouse monoclonal CD9 and mouse polyclonal CD63, (1:1000 Santa Cruz Biotechnology, Inc., Santa Cruz, CA). In order to evaluate the purity of the exosome preparations, all exosomes samples were also blotted against GRP94 (1:1000 Cell Signaling) to demonstrate the absence of cellular contaminants from cell lysate in our exosome preparation.
Exosome Labeling
PC3, VCaP, LNCaP, C4–2 and RWPE-1 cells were seeded (10,000 cells per chamber) in each chamber slide (Lab-Tek II chamber slide with cover, Thermo Fisher Scientific) 1 day prior to exosome isolation. Fresh exosomes purified from DU145 cells (described above) were labeled with Cell TrackerTM Orange CMTMR teramethylrhodamine (0.5 μm, Invitrogen) and incubated with different prostate cell lines for 12 h at 37 °C and 5% CO2. After 12 h prostate cells were washed twice with PBS and fixed with 4% paraformaldehyde. Finally all slide chambers were mounted with Vectashield H-1200 (Vector Laboratories Inc.) containing DAPI for nuclei staining. Internalization of fluorescent DU145 exosomes by other cancerous and benign prostate cell lines was monitored under Zeiss (LSR780) confocal microscope (Carl Zeiss, Thornwood, NY). The pinhole was set at 1 Airy unit.
In an independent experiment and to confirm the uptake of a tagged protein from exosomes, fresh isolated exosomes derived from CLUGFP stably overexpressing LNCaP cell line were incubated with PC3 (AR−ve) and LNCaP (AR+ve) for 12 h at 37 °C and 5% CO2. After removal of media, cells were fixed in ice-cold MeOH/Acetone (3:1) for 10 min, and then washed in TBS buffer and permeabilized in 0.1% Triton X-100 in TBS for 15 min at room temperature (RT). Nonspecific binding was avoided by blocking in odyssey solution for 30 min at RT. Primary purified mouse anti E-Cadherin was diluted (1:250 BD Transduction Laboratories™) in blocking agent and incubated with cells for 1 h at RT. Secondary antibody Alexa Fluor 568 goat antimouse IgG (2 mg/ml, Invitrogen) was incubated with cells for 30 min at RT. Finally, as described above, all slide chambers were mounted and monitored using confocal microscopy.
Proteomic Analysis
An in solution trypsin digestion protocol was used to generate peptides for Liquid Chromatography-Quadrupole Time of Flight Mass Spectrometry (LC-QTOF MS) analysis. The isolated exosomes were initially sonicated for 5–10 min to disrupt the structures and expose proteins. An aliquot of extracted exosomes (∼2 μg/μl protein) was diluted with 8 μl 50 mm ammonium bicarbonate, 1 μl of 100 mm dithiothreitol (DTT) was added and reduction carried out at 65 °C for 35 min. This was followed by alkylation using 2 μl of 100 mm iodoacetamide and incubation for 30 min at room temperature in the dark. 1 μl of 100 ng/μl trypsin was then added and the sample incubated overnight at 37 °C. The resulting peptides were separated using a 75 μm x 100 mm 1.7 μm BEH130 C18 column using a 3–40% linear acetonitrile gradient, with 0.1% formic acid (FA) present, at 0.3 μl/min over 40 min using a NanoAcquity™ liquid chromatography (LC) system (Waters). The column was re-equilibrated for 20 min between runs. Column eluate was directed into a Synapt™ mass spectrometer through a 20 μm capillary held at 3.2 kV. Instrument calibration was carried out using glu-fibrinogen fragments and Glu-fib was also used as a lock mass to compensate for any calibration drift. The instrument was run in V-mode with a mass resolution of ∼10,000. A data dependent method was used with a 1 s scan followed by up to 3 fragment scans, using ion intensity and charge state as the main selection criteria. The accumulated data was analyzed using ProteinLynx Global Server software (PLGS2.3) using peptide and fragment mass accuracies of 25 ppm and 0.1 Da respectively. Uniform carbamido methyl C and variable N-terminal acetyl, M oxidation, N deamidation and C propionamide were selected as permitted modifications, up to one missed cleavage allowed and a maximum protein MW of 250 K. This search engine was applied to the full Uniprot 15.0 database, human species. A search of SwissProt 57.1, Homo Sapiens (human; 20401 sequences) was also carried out using Mascot (2.3) search using the pkl peak list files generated in PLGS. An ion score cutoff of 32 is specified as identical or highly homologous according to Mascot outputs. A score ≥40 is typical in many reports and we similarly considered peptides above this cutoff as positive hits. Only proteins with ≥2 positive peptide hits are considered statistically significant. As well, no proteins containing a subset of peptides within our protein hits were considered in our evaluation. Spectral counting could be used for a semiquantitative analysis however only the presence of particular proteins is reported here.
Pathway Analysis
Data generated from proteomic mass spectrometric analysis were analyzed using Ingenuity Pathway Analysis (IPA; Ingenuity System, Redwood city, CA, www.ingenuity.com) to identify potential biomarker signatures and their application in different pathological conditions, in particular cancer and specifically PCa. In addition, we randomly selected 220 proteins from 827,109 human proteins and used IPA to identify general biomarker signatures as well as PCa specific biomarkers. This was compared with exosomal proteins and biomarker data to increase confidence in conclusions drawn from the IPA data.
Ingenuity Knowledge Base tool was also used to identify biological function and canonical pathways that identified exosomal proteins are involved in. IPA is a regularly updated database which uses the current knowledge available on genes, proteins, normal cellular and pathological processes, signaling and metabolic pathways, needed for pathway construction.
Lipidomic Analysis
Isolated exosomes and cell pellets were extracted using a protocol adapted from that published by Matyash et al. (2008) which is equivalent to the Folch-Bligh and Dyer recipes (34) in terms of efficiency. Isolated exosomes (30 μl) or vortexed cell pellets (10 μl) were extracted by addition of 200 μl of methanol followed by 900 μl of MTBE (methyl-t-butyl ether), vortexing well and rotating at room temperature for 30 min. 500 μl of water was then added and the sample rotated for an additional 10 min. The phases were separated by centrifugation (10 min) using a Centrivap (∼1000g) and the upper organic layer transferred to a fresh tube. A second extraction with an additional 500 μl of MTBE was similarly carried out and pooled with the initial fraction. The pooled extracts were dried in a Centrivap, redissolved in 60 μl of 70/30 methanol/chloroform, further diluted with 140 μl methanol and centrifuged at 15,000 × g for 5 min before transferring to LC vials.
The extracts were separated with an Acquity™ LC (Waters) using a 2.1 mm × 100 mm 1.7 μm BEH C8 column with 20 mm ammonium acetate (A) and 80/20 methanol/isopropanol (B) using the following gradient: 0 min, 25%; 0.2 min, 25%; 3 min, 65%; 25 min, 95%; 40 min, 95% (%B), and a 5 min re-equilibration to initial conditions. The LC was coupled to a Synapt™ mass spectrometer run in W-mode with a mass resolution of ∼18,000. Instrument calibration was carried out using sodium formate ion clusters and leucine-enkaphalin was used as a lock mass to compensate for any calibration drift. Data was accumulated sequentially as 0.5 s low collision energy scans followed by 0.2 s higher collision scans. Masses unique to extracts, i.e. not present in blank extracts, were submitted to Lipid Maps and correlated with bulk glycerophospholipid and glycerolipid, sphingolipid and glycosphingolipid class using a 0.01 m/z cutoff. A comparative quantitative assessment of the content of each lipid class present in the samples was accomplished by obtaining extracted ion chromatograms for each m/z, retention time combination using Quanlynx. Although differences in ionization efficiency are expected, the area under curve (AUC) for each peak thus obtained still provides a reasonable quantitative estimate for the particular lipid present. The AUC data was exported to Excel and the relative amount of each bulk lipid group calculated as (sum of all AUC's for a particular bulk lipid group) ÷ (sum of AUC's for all bulk lipid groups) × 100%.
Cholesterol Analysis
The extraction for cholesterol analysis was similar to that used for lipid analysis except the samples were each spiked with 200 ng of deuterated (d6) cholesterol internal standard and were derivatized according to the method of Liebisch et al. (2006) prior to analysis. Dried extracts were dissolved in 400 μl of acetyl chloride: chloroform 1:5 and incubated at room temperature for 1 h. Samples were placed on ice, 500 μl of water added to each and then vortexed several times for 30 min. The bottom organic layer was then transferred to a fresh tube and the sample extracted with an additional 0.5 ml of chloroform for 30 min. The extracts were pooled, dried in a Centrivap, redissolved in 60 μl of 70:30 methanol/chloroform, further diluted with 140 μl methanol and centrifuged at 15,000 × g for 5 min before transferring to LC sample vials.
LC-MS analysis was carried out with a Waters Acquity UPLC coupled to a Quattro Premier XE using a 2.1 × 50 mm, BEH 1.7 μm C18 column. Mobile phase consisted of acetonitrile/0.1 M ammonium acetate 9/1 (A) and isopropanol (B) with the following gradient: 0.2 min, 25%; 5–8 min, 70%, 8.1 min, 25% (%B) with a 10 min run length. Instrument parameters were optimized for the m/z's of ammonium adducts of acetate derivatized cholesterol and the m/z 369 fragment was used for MRM quantitation. AUC's for cholesterol acetate and d6 cholesterol acetate were obtained using Quanlynx and a linear calibration curve from 0.2–10 μg/ml, R2>0.99, was generated using AUC ratios. Recoveries and conversions to derivatized species was greater than 90% for cholesterol.
Statistical Analysis
Unless indicated, analyses were performed on data generated from triplicate exosome preparations per cell line and their cell lysates. Results were expressed as mean ± standard deviation. Statistical significance for differences between exosomes derived from different prostate cell lines and their corresponding cell lysate samples were evaluated by Student's t test (p < 0.05). To compare more than two groups of cell lines or exosomes (e.g. AR+ve PCa cell line exosomes/cell lysate versus all other PCa cell lines) data were analyzed using one-way analysis of variance (ANOVA) (parametric) or Kruskal-Wallis one-way ANOVA (nonparametric), followed by Student Newman Keuls multiple comparison test (SigmaStat™ Statistical Software, Version 3.1, SPSS Inc., Chicago, IL). Biomarker analyses were statistically analyzed using Fisher's Exact Test.
RESULTS
Purification and Characterization of Exosomes Derived from Different Prostate Cells
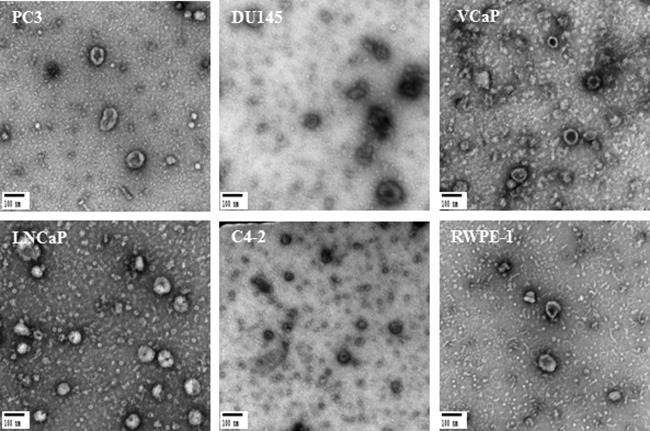
The production of exosomes from PC3, DU145, VCaP, LNCaP, C4–2 prostate cancer, and RWPE-1 benign prostate cells was examined. To validate exosome isolation and purification, transmission electron microscopy as well as Western blot analysis were used. Isolated exosomes from all six different prostate cell lines were fixed onto formvar-coated carbon EM grids for visualization by TEM. TEM data revealed that all six prostate cell lines released a homogenous mixture of cup-shaped, rounded vesicles with diameters varying between 30–100 nm (Fig. 2).
Fig. 2.
Transmission Electron Microscopy (TEM). TEM images of exosomes derived from different androgen independent and androgen sensitive prostate cancer cell lines including PC3, DU145, VCaP, LNCaP and C4–2 versus benign epithelial prostate cell line RWPE-1. Exosomes were negatively stained with 2% uracyl acetate after removing the extra moisture. Cup-shaped structures, with 30–100 nm size were identified as being exosomes.
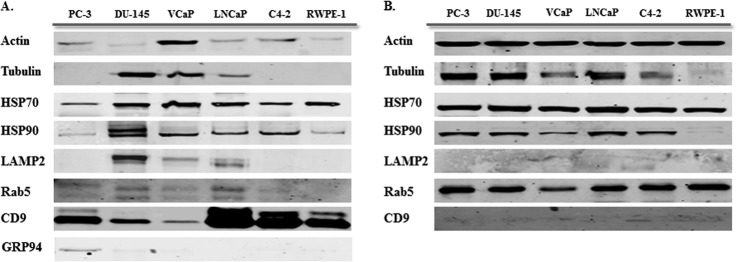
Protein concentration of vesicles was quantified using a BCA assay. Western blot analysis was performed to examine the expression of different exosomal markers in whole cell lysate as compared with derived exosomes of various prostate cell lines. Different exosomal markers were identified in exosomes derived from all six prostate cell lines. As shown in Fig. 3A, exosome samples contained at least four or more of the following markers: Actin, Tubulin (cytoskeletal protein), HSP70, HSP90 (Heat Shock Protein), CD9, CD63 (Tetraspanin) Rab5 (small GTPase), and LAMP2. Further, we investigated the presence of the above proteins in the corresponding cell lysate. As anticipated, HSP90, a stress marker often associated with cancer (35), was not enriched in either cell lysate or exosomes from the benign epithelial prostate cell line RWPE-1 however, substantial amounts of HSP90 were observed in the PCa cell lines. Interestingly, although the exosomes contained CD9 and CD63 (data not shown) we found little or no detectable band for these proteins in their parent cell lines.
Fig. 3.
Western blot analysis for exosome markers in exosomes and corresponding cell lysate samples. A, Exosomes have been purified based on their unique size and density by ultracentrifugation with 30% sucrose-deuterium. Twenty-5 μg of total protein associated with purified exosomes derived from six different prostate cell lines were analyzed by Western blotting using different exosome markers. B, Twenty-five μg of total protein associated with cell lysates of six different prostate cell lines were analyzed by Western blotting using different exosome markers.
To confirm the purification of our exosome isolate, we analyzed all exosome samples for the presence of GRP94 (ER marker). Our data indicates the absence of GRP94 in five of six exosome samples, with a very faint band seen in exosomes derived from the PC3 cell line.
Taken together, the Western blot (molecular) and TEM (biophysical) data demonstrate that both cancerous and benign prostate cells produce exosomes that have similar characteristics to those secreted from other cells. Our preparation technique, which consisted of several centrifugation steps along with filtration, and a final ultracentrifugation step using a sucrose cushion, decreases contamination with cell debris and produces high quality purified exosomes free from other membrane vesicles or protein aggregates.
Uptake of exosomes by different prostate cells
We also investigated the uptake of exosomes derived from DU145 cells by five other prostate cell lines to assess if released exosomes are taken up by surrounding cells after release. We isolated and purified exosomes from DU145 cells as this cell line is known to be one of the most aggressive PCa cell lines and also produced the highest yield of exosomes (supplemental Figure S1).
Purified DU145 exosomes were first stained with Cell TrackerTM Orange CMTMR tetramethylrhodamine. Once stained, the exosomes were used to demonstrate their transfer to surrounding cells. Stained DU145 exosomes were incubated with PC3, VCaP, LNCaP, C4–2, and RWPE-1 for 12 h (overnight). Cells were then fixed and stained with DAPI to mark nuclei prior to imaging of the cells using confocal microscopy.
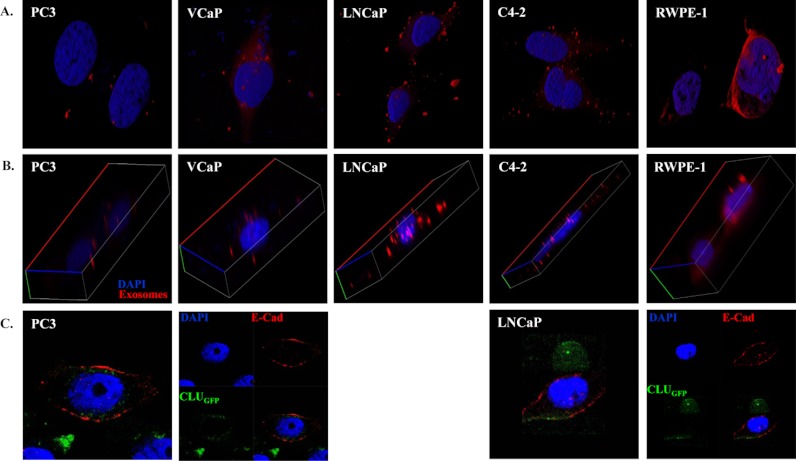
Fig. 4A clearly shows the uptake of exosomes by all five prostate cell lines as demonstrated by the presence of orange/red flecks in and around the cells. Furthermore, the z-stack confocal fluorescence imaging in Fig. 4B clearly shows cellular uptake of DU145 exosomes by other prostate cells. This image demonstrates clearly that transferred exosomes are not only attached to the cell membrane of host cells but have actually been taken up by these cells and are present in their cytoplasm. Interestingly, exosomes appear to be concentrated around the nuclei of benign RWPE-1 cells as compared with cancer cells in which they are scattered within the cytoplasm.
Fig. 4.
Confocal microscopy. A, B, Confocal microscopy was used to visualize purified DU145 derived exosomes which were stained with Cell TrackerTM Orange CMTMR teramethylrhodamine. PC3, VCaP, LNCaP, C4–2 and RWPE-1 cells (104) were cultured on each chamber slide and incubated for 12 h with purified-stained exosomes. Confocal micrograph clearly demonstrates that transferred DU145 derived exosomes are not only attached to the cell membrane of host cells but have actually been taken up by these cells and are present in their cytoplasm. C, Confocal microscopy was also used to visualize freshly isolated exosomes derived from a CLUGFP stably over-expressing LNCaP cell line, which contains CLUGFP, being taken up by PC3 (AR-ve) and LNCaP (AR+ve) PCa cell lines after overnight incubation. Both cell lines were further fixed and stained with DAPI and E-Cadherin prior to imaging of the cells by confocal microscopy.
On a separate experiment we isolated exosomes from a CLUGFP stably overexpressing LNCaP cell line. Isolated exosomes which contained CLUGFP were incubated with PC3 (AR –ve) and LNCaP (AR +ve) for 12 h (overnight). Cells were further fixed and stained with DAPI and E-Cadherin prior to imaging of the cells with confocal microscopy (Fig. 4C).
Proteomic Analysis
The main goal of our proteomic analysis was to understand the potential role of exosomes in the tumor microenvironment as mediators of cell-cell communication during PCa progression. We therefore established a comprehensive list of proteins present in the different prostate cell lines. Two biological replicates of each cell line were obtained to ensure consistent results. Exosomes were sonicated and trypsin digested to release exosomal peptides which were analyzed by LC-MS as described. Proteins were identified using ProteinLynx Global Server software (PLGS) and Mascot software. Proteomic profiles of two AR null PCa cell lines (DU145 and PC3) were compared with AR +ve cells including; VCaP, LNCaP, and C4–2 (PCa Cells), and RWPE-1 (benign epithelial prostate cell line) cells.
This process resulted in the identification of 220 proteins with more than 2 matching peptides and a Mascot score higher than 40 (Table I, supplemental Table S1) representing a broad range of functional proteins. A comparison of our proteomic data with other published articles (>25 articles reviewed on exosomes derived from a variety of sources ranging from human cell lines including human keratinocytes, human breast carcinoma cells, human mesothelioma cells, cortical neurones, dendritic cells, intestinal epithelial cells to biological fluids such as plasma and urine) confirmed the presence of more than 60 common and 150 unique proteins in exosomes derived from the different prostate cells we analyzed. These include heat shock proteins, cytoskeletal proteins, tetraspanins, multivescular bodies, and endosomal markers. A list of all the identified protein with their subcellular localization, type, Mascot score and number of peptide matches is provided in Table I (also see supplemental Table S1). This Proteomic data is also available for download at ExoCarta, a public database containing information on exosomal proteins, RNA and Lipids (36, 37).
Table I. Identification of exosome-associated proteins from six different prostate cell lines.
Proteomic profiles were compared for prostate cancer cells that are AR -ve: PC3, DU145 versus AR +ve: VCaP, LNCaP, and C4–2 in addition to the benign epithelial prostate cell line, RWPE-1. Proteins with Mascot scores ≥40 and ≥2 peptide matches were considered statistically significant (p < 0.05) and listed in this table.
| ID | Protein name | Subcellular location | Type(s) | Cell lines |
Mascot score | Peptide matches | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC3 | DU145 | VCaP | LNCaP | C4–2 | RWPE-1 | |||||||
| H2A2B_HUMAN | Histone cluster 2, H2ab | Nucleus | other | + | + | + | 79 | 3 | ||||
| H2AW_HUMAN | H2A histone family, member Y2 | Nucleus | other | + | 42 | 2 | ||||||
| H2AX_HUMAN | H2A histone family, member X | Nucleus | other | + | + | 119 | 4 | |||||
| H2AY_HUMAN | H2A histone family, member Y | Nucleus | other | + | 54 | 2 | ||||||
| H2B1B_HUMAN | Histone cluster 1, H2bb | Nucleus | other | + | + | + | + | 111 | 2 | |||
| H2B1D_HUMAN | Histone cluster 1, H2bd | Nucleus | other | + | + | 148 | 3 | |||||
| H2B1H_HUMAN | Histone cluster 1, H2bh | Nucleus | other | + | 226 | 4 | ||||||
| H2B1M_HUMAN | Histone cluster 1, H2bm | Nucleus | other | + | 106 | 2 | ||||||
| H2B3B_HUMAN | Histone cluster 3, H2bb | Nucleus | other | + | 145 | 5 | ||||||
| H12_HUMAN | Histone cluster 1, H1c | Nucleus | other | + | 41 | 3 | ||||||
| H15_HUMAN | Histone cluster 1, H1b | Nucleus | other | + | 48 | 2 | ||||||
| NPM_HUMAN | Nucleophosmin (nucleolar phosphoprotein B23, numatrin) | Nucleus | transcription regulator | + | + | + | + | 103 | 2 | |||
| RBP2_HUMAN | RAN binding protein 2 | Nucleus | enzyme | + | 48 | 3 | ||||||
| 1433E_HUMAN | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, epsilon polypeptide | Cytoplasm | other | + | + | + | + | + | 129 | 2 | (43) | |
| 1433T_HUMAN | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, theta polypeptide | Cytoplasm, nucleus | Other | + | + | 112 | 2 | |||||
| 1433Z_HUMAN | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide | Cytoplasm | enzyme | + | 119 | 2 | ||||||
| ACLY_HUMAN | ATP citrate lyase | Cytoplasm | enzyme | + | + | + | 133 | 2 | ||||
| ACPH_HUMAN | N-acylaminoacyl-peptide hydrolase | Cytoplasm | peptidase | + | 116 | 3 | ||||||
| ACTA_HUMAN | Actin, alpha 2, smooth muscle, aorta | Cytoplasm | other | + | + | + | + | + | + | 173 | 3 | |
| ACTB_HUMAN | Actin, beta | Cytoplasm | other | + | + | + | + | + | + | 132 | 2 | (13, 43, 48, 76–82) |
| ACTC_HUMAN | Actin, alpha, cardiac muscle 1 | Cytoplasm | enzyme | + | + | 393 | 9 | |||||
| ACTG_HUMAN | Actin, gamma 1 | Cytoplasm | other | + | 409 | 6 | (13, 43, 48, 76–83) | |||||
| ACTN1_HUMAN | Actinin, alpha 1 | Cytoplasm | other | + | + | + | + | 267 | 6 | (76) | ||
| ACTN2_HUMAN | Actinin, alpha 2 | Cytoplasm, nucleus | transcription regulator | + | + | 54 | 2 | |||||
| ACTN3_HUMAN | Actinin, alpha 3 | Cytoplasm | other | + | + | 107 | 2 | |||||
| ACTN4_HUMAN | Actinin, alpha 4 | Cytoplasm | other | + | + | 514 | 8 | |||||
| ALDOA_HUMAN | Aldolase A, fructose-bisphosphate | Cytoplasm | enzyme | + | + | + | + | 181 | 5 | (30, 43, 76, 84) | ||
| ALDOC_HUMAN | Aldolase C, fructose-bisphosphate | Cytoplasm | enzyme | + | 101 | 2 | ||||||
| CALR_HUMAN | Calreticulin | Cytoplasm | transcription regulator | + | 90 | 2 | ||||||
| CAND1_HUMAN | Cullin-associated and neddylation-dissociated 1 | Cytoplasm | transcription regulator | + | 56 | 2 | ||||||
| CATD_HUMAN | Cathepsin D | Cytoplasm | peptidase | + | 4 | 1, 13 (preprotein) | ||||||
| CH60_HUMAN | Heat shock 60kDa protein 1 (chaperonin) | Cytoplasm | enzyme | + | + | 295 | 5 | |||||
| COF2_HUMAN | Cofilin 2 (muscle) | Cytoplasm, nucleus | other | + | 74 | 2 | ||||||
| DPOG1_HUMAN | Polymerase (DNA directed), gamma | Cytoplasm | enzyme | + | 40 | 4 | ||||||
| DPYL2_HUMAN | dihydropyrimidinase-like 2 | Cytoplasm | enzyme | + | 204 | 4 | ||||||
| EF1A1_HUMAN | Eukaryotic translation elongation factor 1 alpha 1 | Cytoplasm | translation regulator | + | + | + | 75 | 3 | ||||
| EF1A2_HUMAN | Eukaryotic translation elongation factor 1 alpha 2 | Cytoplasm | translation regulator | + | 158 | 4 | ||||||
| EF1G_HUMAN | Eukaryotic translation elongation factor 1 gamma | Cytoplasm | translation regulator | + | + | + | 108 | 2 | ||||
| EF2_HUMAN | Eukaryotic translation elongation factor 2 | Cytoplasm | translation regulator | + | + | + | + | 244 | 7 | (43) | ||
| EIF3A_HUMAN | Eukaryotic translation initiation factor 3, subunit A | Cytoplasm | translation regulator | + | 46 | 2 | ||||||
| ENOA_HUMAN | Enolase 1, (alpha) | Cytoplasm | transcription regulator | + | + | + | 379 | 7 | (21, 30, 86) | |||
| ENOB_HUMAN | Enolase 3 (beta, muscle) | Cytoplasm | enzyme | + | 245 | 4 | ||||||
| ENOG_HUMAN | Enolase 2 (gamma, neuronal) | Cytoplasm | enzyme | + | 176 | 2 | ||||||
| ENPL_HUMAN | Heat shock protein 90kDa beta (Grp94), member 1 | Cytoplasm | other | + | + | 172 | 3 | (43) | ||||
| FAS_HUMAN | Fatty acid synthase | Cytoplasm | enzyme | + | + | + | + | 112 | 3 | |||
| FLNA_HUMAN | Flamin A, alpha | Cytoplasm | other | + | + | 102 | 2 | (43) | ||||
| FLNB_HUMAN | Filamin B, beta | Cytoplasm | other | + | + | 212 | 4 | (43, 84) | ||||
| FLNC_HUMAN | Filamin C, gamma | Cytoplasm | other | + | + | 63 | 2 | |||||
| FSCN1_HUMAN | Fascin homolog 1, actin-bundling protein (Strongylocentrotus purpuratus) | Cytoplasm | other | + | 50 | 2 | ||||||
| GLU2B_HUMAN | Protein kinase C substrate 80K-H | Cytoplasm | enzyme | + | 129 | 3 | ||||||
| G3P_HUMAN | Glyceraldehyde-3-phosphate dehydrogenase | Cytoplasm | enzyme | + | + | + | + | + | + | 403 | 6 | (30, 43, 48, 73, 76–82) |
| GANAB_HUMAN | Glucosidase, alpha; neutral AB | Cytoplasm | enzyme | + | + | 207 | 2 | |||||
| GRP78_HUMAN | Heat shock 70kDa protein 5 (glucose-regulated protein, 78kDa) | Cytoplasm | other | + | + | 121 | 3 | (13, 76) | ||||
| HS71L_HUMAN | Heat shock 70kDa protein 1-like | Cytoplasm | other | + | 126 | 4 | ||||||
| HS90A_HUMAN | Heat shock protein 90kDa alpha (cytosolic), class A member 1 | Cytoplasm | other | + | + | + | 316 | 7 | (21, 43, 86, 87) (21, 86, 87 listed as HSP90) | |||
| HS90B_HUMAN | Heat shock protein 90kDa alpha (cytosolic), class B member 1 | Cytoplasm | other | + | + | + | + | + | + | 472 | 11 | (21, 43, 86, 87) (21, 86, 87 listed as HSP90) |
| HS905_HUMAN | Putative heat shock protein HSP 90-alpha A5 OS = Homo sapiens GN = HSP90AA5P PE = 1 SV = 1 | Cytoplasm | Other | + | 106 | 4 | ||||||
| HSP7C_HUMAN | Heat shock 70kDa protein 8 | Cytoplasm | enzyme | + | + | 198 | 5 | (48, 73, 76–83, 88, 89) | ||||
| HSP71_HUMAN | Heat shock 70 kDa protein 1 OS = Homo sapiens GN = HSPA1A PE = 1 SV = 5 | Cytoplasm | Other | + | 107 | 4 | ||||||
| HSPB1_HUMAN | Heat shock 27kDa protein 1 | Cytoplasm | other | + | 124 | 2 | (43, 84) | |||||
| IMB1_HUMAN | Karyopherin (importin) beta 1 | Cytoplasm, nucleus | transporter | + | 147 | 4 | ||||||
| IPO5_HUMAN | Importin 5 | Cytoplasm, nucleus | transporter | + | + | 57 | 2 | |||||
| K1C10_HUMAN | Keratin 10 | Cytoplasm | other | + | 200 | 7 | (43) | |||||
| K1C14_HUMAN | Keratin 14 | Cytoplasm | other | + | 203 | 4 | (43) | |||||
| K1C9_HUMAN | Keratin 9 | Cytoplasm | other | + | + | 87 | 2 | (43) | ||||
| K2C1_HUMAN | Keratin 1 | Cytoplasm | other | + | 69 | 2 | ||||||
| K22E_HUMAN | Keratin 2 | Cytoplasm | other | + | 143 | 3 | ||||||
| K2C5_HUMAN | Keratin 5 | Cytoplasm | other | + | 200 | 5 | (43) | |||||
| K2C8_HUMAN | Keratin 8 | Cytoplasm | other | + | + | + | 113 | 2 | ||||
| KLHL4_HUMAN | Kelch-like 4 (Drosophila) | Cytoplasm | other | + | 41 | 3 | ||||||
| KPYM_HUMAN | Pyruvate kinase, muscle | Cytoplasm | kinase | + | + | + | + | + | + | 834 | 15 | (43) |
| KPYR_HUMAN | Pyruvate kinase, liver and RBC | Cytoplasm | kinase | + | + | 45 | 2 | |||||
| LDHA_HUMAN | Lactate dehydrogenase A | Cytoplasm | enzyme | + | + | + | + | + | 228 | 5 | (30, 43) | |
| LDH6B_HUMAN | l-lactate dehydrogenase A-like 6B OS = Homo sapiens GN = LDHAL6B PE = 1 SV = 3 | Cytoplasm | Enzyme/oxidored uctase | + | 53 | 2 | ||||||
| LDHB_HUMAN | Lactate dehydrogenase B | Cytoplasm | enzyme | + | + | + | + | 256 | 3 | (30) | ||
| MDHC_HUMAN | malate dehydrogenase 1, NAD (soluble) | Cytoplasm | enzyme | + | 49 | 2 | ||||||
| MX1_HUMAN | myxovirus (influenza virus) resistance 1, interferon-inducible protein p78 (mouse) | Cytoplasm, nucleus | enzyme | + | 119 | 3 | ||||||
| MYH9_HUMAN | myosin, heavy chain 9, non-muscle | Cytoplasm | enzyme | + | 88 | 2 | (43, 84, 90) | |||||
| NDKA_HUMAN | non-metastatic cells 1, protein (NM23A) expressed in | Cytoplasm, nucleus | kinase | + | 113 | 2 | ||||||
| NQO1_HUMAN | NAD(P)H dehydrogenase, quinone 1 | Cytoplasm | enzyme | + | 78 | 2 | ||||||
| NUCL_HUMAN | nucleolin | Cytoplasm, nucleus | other | + | 54 | 3 | ||||||
| PGAM1_HUMAN | phosphoglycerate mutase 1 (brain) | Cytoplasm | phosphatase | + | 119 | 2 | (43, 76) | |||||
| PGAM2_HUMAN | phosphoglycerate mutase 2 (muscle) | Cytoplasm | phosphatase | + | 60 | 2 | ||||||
| PGK1_HUMAN | phosphoglycerate kinase 1 | Cytoplasm | kinase | + | 138 | 3 | (43) | |||||
| PLOD1_HUMAN | procollagen-lysine 1, 2-oxoglutarate 5-dioxygenase 1 | Cytoplasm | enzyme | + | 274 | 5 | ||||||
| PRDX1_HUMAN | peroxiredoxin 1 | Cytoplasm | enzyme | + | + | 98 | 4 | (76) | ||||
| PRDX2_HUMAN | peroxiredoxin 2 | Cytoplasm | enzyme | + | 49 | 2 | ||||||
| PRGR_HUMAN | progesterone receptor | Cytoplasm, nucleus | ligand-dependent nuclear receptor | + | + | 40 | 2 | |||||
| PROF1_HUMAN | profilin 1 | Cytoplasm | other | + | + | 59 | 3 | |||||
| PSA1_HUMAN | proteasome subunit, alpha type, 1 | Cytoplasm | peptidase | + | + | + | + | + | 73 | 3 | (30, 43) | |
| PSA2_HUMAN | proteasome subunit, alpha type, 2 | Cytoplasm | peptidase | + | + | 239 | 5 | (30, 43) | ||||
| PSA3_HUMAN | proteasome (prosome, macropain) subunit, alpha type, 3 | Cytoplasm | peptidase | + | + | + | 130 | 2 | ||||
| PSA4_HUMAN | proteasomesubunit, alpha type, 4 | Cytoplasm | peptidase | + | + | + | + | + | 203 | 3 | (30, 43) | |
| PSA5_HUMAN | proteasome subunit, alpha type, 5 | Cytoplasm | peptidase | + | + | + | + | + | 110 | 3 | (43) | |
| PSA6_HUMAN | proteasome subunit, alpha type, 6 | Cytoplasm | peptidase | + | + | + | + | + | 218 | 5 | (30) | |
| PSA7_HUMAN | proteasome subunit, alpha type, 7 | Cytoplasm | peptidase | + | + | + | 206 | 3 | (30, 43) | |||
| PSA7L_HUMAN | proteasome (prosome, macropain) subunit, alpha type, 8 | Cytoplasm | peptidase | + | 109 | 2 | ||||||
| PSB1_HUMAN | proteasome subunit, beta type, 1 | Cytoplasm | peptidase | + | + | + | 191 | 4 | (30) | |||
| PSB2_HUMAN | proteasome subunit, beta type, 2 | Cytoplasm | peptidase | + | + | + | + | 81 | 3 | (30) | ||
| PSB4_HUMAN | proteasome (prosome, macropain) subunit, beta type, 4 | Cytoplasm | peptidase | + | + | 74 | 2 | |||||
| PSB5_HUMAN | proteasome subunit, beta type, 5 | Cytoplasm | peptidase | + | + | + | 165 | 3 | (30, 43) | |||
| PSB6_HUMAN | proteasome subunit, beta type, 6 | Cytoplasm | peptidase | + | + | + | + | 92 | 2 | (30, 43) | ||
| PSB7_HUMAN | proteasome (prosome, macropain) subunit, beta type, 7 | Cytoplasm | peptidase | + | 68 | 3 | ||||||
| PSB9_HUMAN | proteasome (prosome, macropain) subunit, beta type, 9 (large multifunctional peptidase 2) | Cytoplasm | peptidase | + | 123 | 3 | ||||||
| QSOX1_HUMAN | quiescin Q6 sulfhydryl oxidase 1 | Cytoplasm | enzyme | + | 155 | 3 | (43) | |||||
| RADI_HUMAN | radixin | Cytoplasm | other | + | 69 | 2 | ||||||
| RAN_HUMAN | RAN, member RAS oncogene family | Cytoplasm, nucleus | enzyme | + | + | 71 | 2 | |||||
| RLA1_HUMAN | ribosomal protein, large, P1 | Cytoplasm | other | + | 43 | 2 | ||||||
| RSSA_HUMAN | ribosomal protein SA | Cytoplasm | translation regulator | + | 118 | 2 | ||||||
| RLA2_HUMAN | ribosomal protein, large, P2 | Cytoplasm | other | + | + | 73 | 2 | |||||
| S10A6_HUMAN | S100 calcium binding protein A6 | Cytoplasm | transporter | + | 72 | 2 | ||||||
| S10AB_HUMAN | S100 calcium binding protein A11 | Cytoplasm | other | + | 95 | 2 | ||||||
| SAHH_HUMAN | adenosylhomocysteinase | Cytoplasm | enzyme | + | + | 85 | 2 | |||||
| SYTC_HUMAN | threonyl-tRNA synthetase | Cytoplasm, nucleus | enzyme | + | 44 | 2 | ||||||
| TAGL2_HUMAN | transgelin 2 | Cytoplasm | other | + | 105 | 3 | ||||||
| TBA1A_HUMAN | tubulin, alpha 1a | Cytoplasm | other | + | 294 | 5 | ||||||
| TBA1B_HUMAN | tubulin, alpha 1b | Cytoplasm | other | + | + | + | + | + | + | 263 | 5 | |
| TBA1C_HUMAN | tubulin, alpha 1c | Cytoplasm | other | + | + | + | 249 | 5 | ||||
| TBA3C_HUMAN | Tubulin alpha-3C/D chain OS = Homo sapiens GN = TUBA3C PE = 1 SV = 3 | Cytoplasm | Other | + | + | + | + | 149 | 3 | |||
| TBA4B_HUMAN | tubulin, alpha 4b (pseudogene) | Cytoplasm | other | + | + | 95 | 2 | |||||
| TBA8_HUMAN | tubulin, alpha 8 | Cytoplasm | other | + | + | + | + | 80 | 3 | |||
| TBB1_HUMAN | tubulin, beta 1 | Cytoplasm | other | + | 92 | 3 | ||||||
| TBB2A_HUMAN | tubulin, beta 2A | Cytoplasm | other | + | 401 | 6 | ||||||
| TBB2C_HUMAN | tubulin, beta 2C | Cytoplasm | other | + | + | + | + | 192 | 5 | |||
| TBB3_HUMAN | tubulin, beta 3 | Cytoplasm | other | + | + | + | + | 141 | 3 | |||
| TBB5_HUMAN | tubulin, beta | Cytoplasm | other | + | + | + | + | 220 | 6 | (13, 48, 73, 76, 77, 79–83, 89) | ||
| TBB6_HUMAN | tubulin, beta 6 | Cytoplasm | other | + | 60 | 3 | ||||||
| TCPB_HUMAN | chaperonin containing TCP1, subunit 2 (beta) | Cytoplasm | kinase | + | 123 | 4 | ||||||
| TCPE_HUMAN | chaperonin containing TCP1, subunit 5 (epsilon) | Cytoplasm | other | + | + | + | 57 | 2 | ||||
| TCPG_HUMAN | chaperonin containing TCP1, subunit 3 (gamma) | Cytoplasm | other | + | + | 58 | 2 | |||||
| TCPH_HUMAN | chaperonin containing TCP1, subunit 7 (eta) | Cytoplasm | other | + | + | + | 40 | 2 | ||||
| TCPQ_HUMAN | chaperonin containing TCP1, subunit 8 (theta) | Cytoplasm | enzyme | + | + | 103 | 2 | |||||
| TCPZ_HUMAN | chaperonin containing TCP1, subunit 6A (zeta 1) | Cytoplasm | other | + | + | 64 | 2 | |||||
| TERA_HUMAN | valosin-containing protein | Cytoplasm | enzyme | + | + | + | 756 | 17 | (84) | |||
| TITIN_HUMAN | titin | Cytoplasm | kinase | + | + | + | 42 | 10 | ||||
| TKT_HUMAN | transketolase | Cytoplasm | enzyme | + | 331 | 6 | (43) | |||||
| TPM3_HUMAN | tropomyosin 3 | Cytoplasm | other | + | 48 | 2 | ||||||
| TRAP1_HUMAN | TNF receptor-associated protein 1 | Cytoplasm | enzyme | + | 117 | 2 | ||||||
| UBA1_HUMAN | ubiquitin-like modifier activating enzyme 1 | Cytoplasm | enzyme | + | 96 | 2 | ||||||
| UGPA_HUMAN | UDP-glucose pyrophosphorylase 2 | Cytoplasm | enzyme | + | 67 | 2 | ||||||
| 1B54_HUMAN | major histocompatibility complex, class I, B | Plasma Membrane | transmembrane receptor | + | 212 | 3 | ||||||
| 1A25_HUMAN | major histocompatibility complex, class I, A | Plasma Membrane | transmembrane receptor | + | 182 | 2 | ||||||
| 1B07_HUMAN | major histocompatibility complex, class I, B | Plasma Membrane | transmembrane receptor | + | 147 | 2 | ||||||
| 1B73_HUMAN | major histocompatibility complex, class I, B | Plasma Membrane | transmembrane receptor | + | 219 | 3 | ||||||
| 4F2_HUMAN | solute carrier family 3, member 2 | Plasma Membrane | transporter | + | + | 189 | 2 | (84) | ||||
| A4_HUMAN | amyloid beta (A4) precursor protein | Plasma Membrane | other | + | 239 | 4 | ||||||
| AGRIN_HUMAN | agrin | Plasma Membrane | other | + | + | + | + | 116 | 2 | (43) (precursor) | ||
| ANK3_HUMAN | ankyrin 3, node of Ranvier (ankyrin G) | Plasma Membrane | other | + | 41 | 6 | ||||||
| ANXA1_HUMAN | annexin A1 | Plasma Membrane | other | + | 110 | 2 | ||||||
| ANXA2_HUMAN | annexin A2 | Plasma Membrane | other | + | + | + | + | 98 | 2 | (43, 48, 76, 77, 81, 83, 88, 89) | ||
| CD81_HUMAN | CD81 molecule | Plasma Membrane | other | + | + | + | 124 | 2 | (8, 87, 93) | |||
| CD9_HUMAN | CD9 molecule | Plasma Membrane | other | + | + | + | 128 | 2 | (8) | |||
| CHLE_HUMAN | butyrylcholinesterase | Plasma Membrane | enzyme | + | 147 | 3 | ||||||
| CLH1_HUMAN | clathrin, heavy chain (Hc) | Plasma Membrane | other | + | + | 111 | 4 | |||||
| CLH2_HUMAN | clathrin, heavy chain-like 1 | Plasma Membrane | other | + | 49 | 2 | ||||||
| CSTN1_HUMAN | calsyntenin 1 | Plasma Membrane | other | + | + | + | 57 | 2 | ||||
| DMD_HUMAN | dystrophin | Plasma Membrane | other | + | 46 | 7 | ||||||
| EPCAM_HUMAN | epithelial cell adhesion molecule | Plasma Membrane | other | + | 160 | 2 | ||||||
| FAT1_HUMAN | FAT tumor suppressor homolog 1 | Plasma Membrane | other | + | 71 | 3 | ||||||
| FAT2_HUMAN | FAT tumor suppressor homolog 2 | Plasma Membrane | other | + | 63 | 2 | ||||||
| FINC_HUMAN | fibronectin 1 | Plasma Membrane | enzyme | + | + | + | 588 | 15 | (43, 90) (isoform 3 preprotein) | |||
| FOLH1_HUMAN | folate hydrolase (prostate-specific membrane antigen) 1 | Plasma Membrane | peptidase | + | 54 | 2 | ||||||
| FPRP_HUMAN | prostaglandin F2 receptor negative regulator | Plasma Membrane | other | + | + | 131 | 3 | (43) | ||||
| K2C6A_HUMAN | keratin 6A | Plasma Membrane | other | + | 80 | 3 | (43) | |||||
| LG3BP_HUMAN | lectin, galactoside-binding, soluble, 3 binding protein | Plasma Membrane | transmembrane receptor | + | + | + | + | 270 | 5 | |||
| MPRI_HUMAN | insulin-like growth factor 2 receptor | Plasma Membrane | transmembrane receptor | + | 89 | 2 | ||||||
| PTPRF_HUMAN | protein tyrosine phosphatase, receptor type, F | Plasma Membrane | phosphatase | + | 81 | 3 | ||||||
| SDC4_HUMAN | syndecan 4 | Plasma Membrane | other | + | + | + | 44 | 2 | ||||
| TFR1_HUMAN | transferrin receptor | Plasma Membrane | transporter | + | + | 120 | 2 | (8) | ||||
| A2MG_HUMAN | alpha-2-macroglobulin | Extracellular Space | transporter | + | + | + | + | + | 76 | 2 | ||
| ALBU_HUMAN | albumin | Extracellular Space | transporter | + | + | + | + | + | 100 | 2 | (84) (precursor), (90) (precursor) | |
| APOA1_HUMAN | apolipoprotein A-I | Extracellular Space | transporter | + | + | 50 | 2 | |||||
| C1R_HUMAN | complement component 1, r subcomponent | Extracellular Space | peptidase | + | 99 | 2 | (90) | |||||
| CLUS_HUMAN | clusterin | Extracellular Space | other | + | + | 244 | 4 | (43, 90) (isoform) | ||||
| CO1A1_HUMAN | collagen, type I, alpha 1 | Extracellular Space | other | + | 171 | 5 | ||||||
| CO6A1_HUMAN | collagen, type VI, alpha 1 | Extracellular Space | other | + | 592 | 11 | ||||||
| CO3_HUMAN | complement component 3 | Extracellular Space | peptidase | + | 619 | 14 | ||||||
| COCA1_HUMAN | collagen, type XII, alpha 1 | Extracellular Space | other | + | + | + | + | 108 | 2 | |||
| FETUA_HUMAN | alpha-2-HS-glycoprotein | Extracellular Space | other | + | 122 | 3 | ||||||
| G6PI_HUMAN | glucose-6-phosphate isomerase | Extracellular Space | enzyme | + | 147 | 3 | (43, 84) | |||||
| GDF15_HUMAN | growth differentiation factor 15 | Extracellular Space | growth factor | + | 148 | 3 | ||||||
| GRN_HUMAN | granulin | Extracellular Space | growth factor | + | 219 | 6 | ||||||
| HEMO_HUMAN | hemopexin | Extracellular Space | transporter | + | 680 | 18 | ||||||
| ITIH2_HUMAN | inter-alpha (globulin) inhibitor H2 | Extracellular Space | other | + | 125 | 3 | ||||||
| ITIH4_HUMAN | inter-alpha (globulin) inhibitor H4 | Extracellular Space | other | + | 266 | 6 | ||||||
| LAMA5_HUMAN | laminin, alpha 5 | Extracellular Space | other | + | + | + | 245 | 9 | (43) | |||
| LAMB1_HUMAN | laminin, beta 1 | Extracellular Space | other | + | + | 119 | 3 | |||||
| LAMB2_HUMAN | laminin, beta 2 (laminin S) | Extracellular Space | enzyme | + | 105 | 4 | ||||||
| LAMC1_HUMAN | laminin, gamma 1 (formerly LAMB2) | Extracellular Space | other | + | + | + | 173 | 2 | ||||
| LAMC2_HUMAN | laminin, gamma 2 | Extracellular Space | other | + | 361 | 7 | ||||||
| MFGM_HUMAN | milk fat globule-EGF factor 8 protein | Extracellular Space | other | + | + | 69 | 2 | |||||
| MK_HUMAN | midkine (neurite growth-promoting factor 2) | Extracellular Space | growth factor | + | 102 | 3 | ||||||
| MYH14_HUMAN | myosin, heavy chain 14, non-muscle | Extracellular Space | other | + | 72 | 3 | ||||||
| PTX3_HUMAN | pentraxin 3, long | Extracellular Space | other | + | + | + | 255 | 5 | ||||
| SAP_HUMAN | prosaposin | Extracellular Space | other | + | 234 | 8 | (84) (isoform preprotein) | |||||
| SPON2_HUMAN | spondin 2, extracellular matrix protein | Extracellular Space | other | + | + | 114 | 4 | |||||
| TENA_HUMAN | tenascin C | Extracellular Space | other | + | 125 | 4 | (43) | |||||
| TFPI1_HUMAN | tissue factor pathway inhibitor (lipoprotein-associated coagulation inhibitor) | Extracellular Space | other | + | 311 | 5 | ||||||
| TRFE_HUMAN | transferrin | Extracellular Space | transporter | + | 804 | 23 | (43, 84) | |||||
| TSP1_HUMAN | thrombospondin 1 | Extracellular Space | other | + | + | 200 | 5 | |||||
| ABCAD_HUMAN | ATP-binding cassette, sub-family A (ABC1), member 13 | unknown | transporter | + | 42 | 5 | ||||||
| ACTBL_HUMAN | actin, beta-like 2 | unknown | other | + | + | + | + | + | + | 99 | 2 | |
| AHNK2_HUMAN | AHNAK nucleoprotein 2 | unknown | other | + | + | + | 49 | 3 | ||||
| AN18B_HUMAN | ankyrin repeat domain 18B | unknown | other | + | 41 | 3 | ||||||
| ARP3B_HUMAN | ARP3 actin-related protein 3 homolog B (yeast) | unknown | other | + | 40 | 2 | ||||||
| AXA2L_HUMAN | annexin A2 pseudogene 2 | unknown | other | + | + | 126 | 2 | |||||
| DYH2_HUMAN | dynein, axonemal, heavy chain 2 | unknown | other | + | 42 | 3 | ||||||
| EF1A3_HUMAN | eukaryotic translation elongation factor 1 alpha 1 pseudogene 5 | unknown | other | + | 470 | 7 | ||||||
| H2AV_HUMAN | H2A histone family, member V | unknown | other | + | 178 | 3 | ||||||
| H90B2_HUMAN | heat shock protein 90kDa alpha (cytosolic), class B member 2 (pseudogene) | unknown | other | + | + | + | 40 | 2 | ||||
| HS904_HUMAN | heat shock protein 90kDa alpha (cytosolic), class A member 4 (pseudogene) | unknown | other | + | 69 | 2 | ||||||
| HSP76_HUMAN | heat shock 70kDa protein 6 (HSP70B') | unknown | other | + | + | 98 | 2 | |||||
| HSP77_HUMAN | heat shock 70kDa protein 7 (HSP70B) | unknown | other | + | 71 | 2 | ||||||
| KATL2_HUMAN | katanin p60 subunit A-like 2 | unknown | other | + | 55 | 2 | ||||||
| MSMP_HUMAN | microseminoprotein, prostate associated | unknown | other | + | 151 | 2 | ||||||
| POTEE_HUMAN | POTE ankyrin domain family, member F | unknown | other | + | + | 361 | 6 | |||||
| PUR6_HUMAN | phosphoribosylaminoimidazole carboxylase, | unknown | enzyme | + | + | + | + | 120 | 2 | |||
| PXDN_HUMAN | peroxidasin homolog | unknown | other | + | + | 111 | 3 | |||||
| TBB8B_HUMAN | tubulin, beta polypeptide 4, member Q | unknown | other | + | 48 | 2 | ||||||
Table I has been organized based on the subcellular localization of proteins where known. As shown in Table I, 85% of nuclear proteins belong to the Histone family. The other 15% include nucleophosmins-involved in the transport of small proteins to the nucleus- and RBP2, an enzyme implicated in vitamin A uptake and intracellular metabolism.
The largest fraction of proteins identified in exosomes were enzymes that were classified as peptidases, kinases, and phosphatases as well as other enzymes such as 14-3-3, which plays a key role in ERK5 signaling, IGF-1 signaling, Myc mediated apoptosis signaling, PI3K/AKT signaling and protein kinase A signaling or ATP citrate lysate, important in citrate cycle; insulin receptor signaling, and ACTC involved in calcium and caveolar-mediated endocytosis signaling.
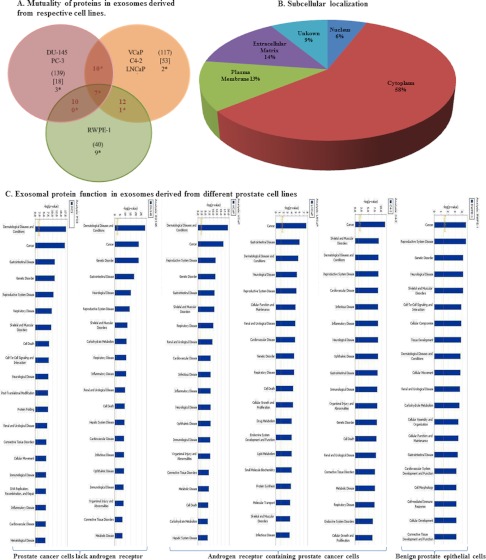
As demonstrated in Fig. 5A, 139 proteins identified from our isolated exosome samples were presented in one or the other of the AR -ve DU145 or PC3 cells. Of them, 18 proteins were present in both DU145 and PC3 cells with only 3 proteins (ENPL, GRP78, and AXA2L) being mutual only to the AR -ve DU145 or PC3 cells and not present in any of the AR +ve cell lines (VCaP, C4–2, LNCaP, and RWPE-1).
Fig. 5.
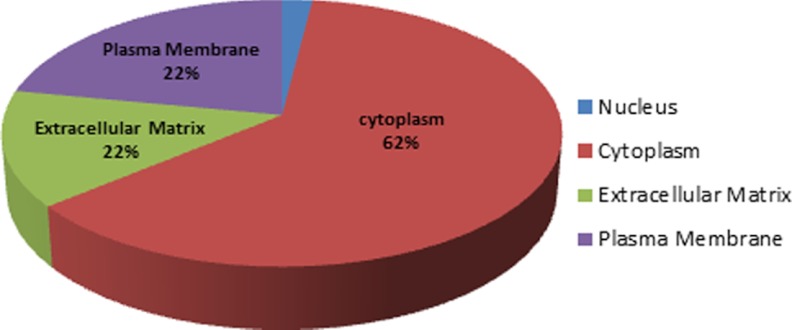
Proteomic analysis of different prostate cancer cell lines. A, Venn diagram describing the mutuality of proteins in exosomes derived from the benign epithelial prostate cell line (RWPE-1) versus five different prostate cancer cell lines categorized based on androgen sensitivity (PC3, DU145 and VCaP, LNCaP, C4–2). Numbers in ( ) are representative of the total number of proteins present in each cell lines, Numbers in () are representative of the total number of proteins present in each cell lines, Numbers in [ ] are representative of proteins present in either designated category and not present in any other undesignated category. Numbers denoted with * are the mutual proteins present in all cell lines in each category. B, Pie chart showing the subcellular localization of proteins found in exosomes derived from six different prostate cell lines. C, Bar chart indicating the cellular function of proteins found within exosomes determined using Ingenuity software. D, Predicted top canonical pathways are represented by the identified exosomal proteins.
In the case of VCaP, C4–2, and LNCaP PCa cells, which are AR +ve, 117 proteins were present in exosomes derived from at least one of the AR +ve PCa cell lines, however, 53 of these proteins were present in exosomes from all of the AR +ve PCa cell lines and at least one of the AR –ve or benign RWPE-1 cells. Interestingly, there were only two proteins identified as present in exosomes derived from all three AR +ve PCa cell lines (e.g. TCPH and H2A2B) that were not present in the AR –ve cell lines (DU145 and PC3) or RWPE-1 cells.
Comparison of the proteomic profiles of isolated exosomes from the prostate cell lines analyzed indicates the presence of 40 proteins which were present in the benign AR+ve cell line (and also present in at least one of the other PCa cell lines). The nine unique proteins that were determined to be contained in RWPE-1 exosomes only include ITIH4, LAMC2, TRFE, HEMO, K2C6A, FAT2, K2C5, K1C14, and HSPB1, none of which have been previously identified in exosomes derived from PCa cells. It would be interesting to determine the relevance of the specificity of these proteins being in exosomes derived from nonmalignant cells, however comparison of a larger panel of nonmalignant cells would be required. Other major groups of proteins present in exosomes are cytoskeletal proteins such as actin and tubulin, heat shock proteins (HSP90, HSP70, and CLU), elongation factors involved in protein synthesis and proteosomal proteins, and lipid-related proteins.
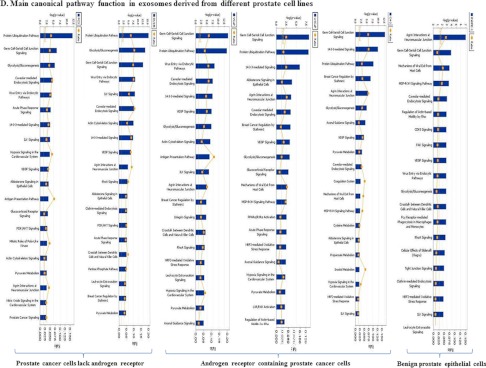
Using the Ingenuity and Mascot software all proteins were further assigned a subcellular localization and biological process. Fig. 5 displays the distribution of proteins in various prostate cell lines (Fig. 5A), their subcellular localization (Fig. 5B) their functions (Fig. 5C) as well as the main canonical pathways they are involved in (Fig. 5D).
As shown in Fig. 5B, 58% of prostate exosomal proteins (127 proteins) are localized in the cytoplasm, 14% in the extracellular matrix, 13% in plasma membrane and 6% in the nucleus. Nineteen proteins have been identified with unknown subcellular localization (9%) in this analysis.
All identified proteins could be categorized as enzymes (32%), (peptidase (9%), kinase (3%), and phosphatase (1%)), transporters (5%), transmembrane receptors (3%) translational regulators (3%), transcriptional regulators (2%), and growth factors (1%). 53% of these proteins have been categorized as protein with “other” function.
Data was also analyzed using Ingenuity Pathway Software (IPS) to gain an understanding of the function of these proteins. Our results indicate that exosomal proteins derived from prostate cells are mainly involved in cancer, dermatological disease, gastrointestinal disease, genetic disorders and neurological disease. More specifically our IPS analysis indicates the role of proteins such as ACTC1, ALDOC, ANXA2, CCT5,8, CLTC ENO1–3, GAPDH, HPX, MX1, MYH9,14, TNC, TUBA8, TUBA1B, 1C, 4B, TUBB, TUBB3, TUBB2A, 2C in cancer, reproductive system disease and cardiac inflammation, whereas exosomal proteins such as FAT2, GANAB, HIST1H2BD, KATNAL2, MX1, NPM1 and TAGLN2 tend to play a pivotal role in cell death and cell to cell signaling.
Using the IPS knowledge base tool, we also identified the canonical pathways which suggest the main possible functions of proteins identified in exosomes. All 220 proteins derived from the six prostate cell lines were included in this analysis. As indicated in Fig. 5D the protein ubiquitination pathway and germ cell-sertoli cell junction signaling are the major canonical pathways present in exosomal proteins from PCa cells.
Furthermore, IPS also was used to establish a list of novel biomarker signatures as well as their application in various pathologic conditions. Subcellular localization of the proteins in this biomarker list is summarized in Fig. 6. As reported in Table II, PCa specific biomarkers, including ANXA2 (Annexin A2), CLSTN1 (Calsyntenin 1), FASN (Fatty Acid Synthesis), FLNC (Filamin C, gamma), FOLH1 (Folate Hydrolase (prostate specific membrane antigen)-1), GDF15 (Growth Differentiation Factor 15), are present in PCa cells derived exosomes. To strengthen conclusions drawn from the IPA data, that exosomes are a rich source of biomarkers, we performed a statistical test to determine the probability of finding biomarkers by chance in a random set of proteins (expressed as a p value). Our results indicated that among 220 proteins randomly selected from the UniProt human protein database, five were identified as biomarkers, of which zero were associated with PCa diagnosis. A p value less than 0.0001 was determined for the chance of finding 50 biomarkers in the enriched exosome sample compared with the five found in the random protein group. Similarly a p value of 0.0302 was subsequently determined for the chance of finding six prostate cancer biomarkers when there were zero found in the random selection of proteins.
Fig. 6.
Biomarker proteins: Subcellular localization. Pie chart showing the subcellular localization of biomarkers found in exosomes derived from prostate cancer cell lines; PC3, DU145, VCaP, LNCaP, and C4–2.
Table II. Proteomic analysis of different biomarkers in exosomes derived from prostate cancer cell lines.
Table indicating the presence of different biomarkers in exosomes derived from five different prostate cancer cell lines including PC3, DU145, VCaP, LNCaP, and C4–2. This table also describes the pathological disorders which have been identified using Ingenuity Pathway Analysis (IPA; Ingenuity System, Redwood city, CA, www.ingenuity.com) to be associated with a claim for application of the biomarker based on IPA data base. All Biomarker applications have been assigned with specific numbers which indicate their role in different pathological disorders. Diagnosis = 1, Efficacy = 2, Prognosis = 3, Disease Progression = 4, Response to Therapy = 5, Unspecified Application = 6, Safety = 7.
| Symbol | PC3 | DU145 | VCaP | LNCaP | C4–2 | Biomarker application(s) | Pathological disorder(s) (IPA data base) |
|---|---|---|---|---|---|---|---|
| ACLY | + | + | + | Unspecified Application | Cancer6, Connective Tissue Disorders, Gastrointestinal Disease | ||
| ACTG1 | + | Unspecified Application | Auditory Disease, Cancer6, Gastrointestinal Disease | ||||
| AHCY | + | + | Unspecified Application | Cancer, Dermatological Diseases and Conditions, Gastrointestinal Disease, Neurological Disorder6 | |||
| ANXA1 | + | Diagnosis, Prognosis, Unspecified Application | Cancer1,3,6, Cardiovascular Disease, Connective Tissue Disorders | ||||
| ANXA2 | + | + | + | + | Diagnosis, Unspecified Application | Cancer1,6, Cardiovascular Disease, Dermatological Diseases and Conditions, Gastrointestinal Disease | |
| CALR | + | Unspecified Application | Cancer6, Cardiovascular Disease, Connective Tissue Disorders | ||||
| CCT6A | + | + | Diagnosis | Cancer1, Renal and Urological Disease | |||
| CD9 | + | + | + | Efficacy | Cancer2, Gastrointestinal Disease, Genetic Disorder | ||
| CLSTN1 | + | + | + | Diagnosis | Cancer1, Gastrointestinal Disease, Genetic Disorder | ||
| CLU | + | + | Diagnosis, Efficacy, Unspecified Application | Cancer1,2,6, Cardiovascular Disease, Connective Tissue Disorders, Endocrine System Disorders, Organismal Injury and Abnormalities6 | |||
| CTSD | + | Diagnosis, Unspecified Application | Cancer1,6, Cardiovascular Disease, Developmental Disorder, Neurological Disorder6 | ||||
| EEF1A2 | + | Prognosis, Unspecified Application | Cancer3,6, Cardiovascular Disease, Genetic Disorder | ||||
| ENO1 | + | + | + | Diagnosis | Cancer1, Connective Tissue Disorders, Dermatological Diseases and Conditions | ||
| ENO2 | + | Diagnosis, Efficacy, Prognosis | Cancer1,2,3, Cardiovascular Disease, Gastrointestinal Disease, Neurological Disorder1 | ||||
| EPCAM | + | Unspecified Application | Cancer6, Endocrine System Disorders, Gastrointestinal Disease | ||||
| FASN | + | + | + | + | Diagnosis, Efficacy, Prognosis | Cancer1,2,3, Connective Tissue Disorders, Dermatological Diseases and Conditions | |
| FLNA | + | Unspecified Application | Cancer6, Cardiovascular Disease, Connective Tissue Disorders | ||||
| FLNC | + | + | Diagnosis | Cancer1, Genetic Disorder, Organismal Injury and Abnormalities | |||
| FN1 | + | + | + | Diagnosis, Disease Progression, Efficacy, Prognosis, Unspecified Application | Cancer1,2,3,4, Cardiovascular Disease, Connective Tissue Disorders, Immunological Disease6, Metabolic Disorder2 | ||
| FOLH1 | + | Diagnosis, Prognosis | Cancer1,3, Cardiovascular Disease, Gastrointestinal Disease | ||||
| FSCN1 | + | Unspecified Application | Cancer6, Dermatological Diseases and Conditions, Gastrointestinal Disease | ||||
| GDF15 | + | Diagnosis | Cancer1, Cardiovascular Disease, Dermatological Diseases and Conditions | ||||
| GPI | + | Unspecified Application | Cancer, Cardiovascular Disease, Connective Tissue Disorders, Neurological Disorder6 | ||||
| GRN | + | Diagnosis, Disease Progression | Cancer1,4, Connective Tissue Disorders, Dermatological Diseases and Conditions | ||||
| HLA-A | + | Efficacy, Response to Therapy | Cancer2, Connective Tissue Disorders, Dermatological Diseases and Conditions, Immunological Disease5 | ||||
| HLA-B | + | + | Safety | Cancer, Connective Tissue Disorders, Dermatological Diseases and Conditions, Immunological Disease7 | |||
| HSP90AA1 | + | + | + | Safety, Unspecified Application | Cancer7, Dermatological Diseases and Conditions, Gastrointestinal Disease, Neurological Disorder6 | ||
| HSPA5 (GRP78) | + | + | Unspecified Application | Cancer6, Connective Tissue Disorders, Dermatological Diseases and Conditions | |||
| HSPA8 | + | + | Diagnosis | Cancer1, Connective Tissue Disorders, Dermatological Diseases and Conditions | |||
| HSPB1 | + | Diagnosis | Cancer1, Endocrine System Disorders, Gastrointestinal Disease | ||||
| IGF2R | + | Diagnosis | Cancer1, Cardiovascular Disease, Gastrointestinal Disease | ||||
| KRT5 | + | Diagnosis, Efficacy, Unspecified Application | Cancer1,2,6, Dermatological Diseases and Conditions, Genetic Disorder | ||||
| KRT8 | + | + | + | Prognosis | Cancer3, Dermatological Diseases and Conditions, Developmental Disorder | ||
| LAMA5 | + | + | + | Diagnosis | Cancer1, Cardiovascular Disease, Dermatological Diseases and Conditions | ||
| LDHA | + | + | + | + | + | Diagnosis, Disease Progression, Efficacy, Prognosis, Unspecified Application | Cancer1,2,3,4, Gastrointestinal Disease, Genetic Disorder, Neurological Disorder6 |
| MDK | + | Diagnosis | Cancer1, Cardiovascular Disease, Connective Tissue Disorders | ||||
| NPM1 | + | + | + | + | Disease Progression, Unspecified Application | Cancer4, Genetic Disorder, Hematological Disease, Neurological Disorder6 | |
| NQO1 | + | Diagnosis, Unspecified Application | Cancer1,6, Connective Tissue Disorders, Dermatological Diseases and Conditions | ||||
| PFN1 | + | + | Unspecified Application | Cancer6, Cardiovascular Disease, Dermatological Diseases and Conditions | |||
| POLG | + | Diagnosis | Cancer1, Dermatological Diseases and Conditions, Gastrointestinal Disease | ||||
| PRDX1 | + | + | Diagnosis | Cancer1, Connective Tissue Disorders, Genetic Disorder | |||
| PSMA2 | + | + | Unspecified Application | Cancer6, Dermatological Diseases and Conditions, Developmental Disorder | |||
| TFRC | + | + | Diagnosis | Cancer1, Connective Tissue Disorders, Dermatological Diseases and Conditions | |||
| TNC | + | Diagnosis, Efficacy, Response to Therapy | Cancer1,2,5, Dermatological Diseases and Conditions, Gastrointestinal Disease | ||||
| TPM3 | + | Diagnosis, Unspecified Application | Cancer1, Dermatological Diseases and Conditions, Genetic Disorder, Neurological Disorder6 | ||||
| TUBA1A | + | Unspecified Application | Cancer, Cardiovascular Disease, Connective Tissue Disorders, Neurological Disorder6 | ||||
| TUBB3 | + | + | + | + | Efficacy, Response to Therapy, Unspecified Application | Cancer2,5,6, Cardiovascular Disease, Connective Tissue Disorders | |
| YWHAE | + | + | + | + | + | Diagnosis | Cancer1, Cardiovascular Disease, Dermatological Diseases and Conditions |
| YWHAZ | + | Diagnosis | Cancer1, Genetic Disorder, Neurological Disease |
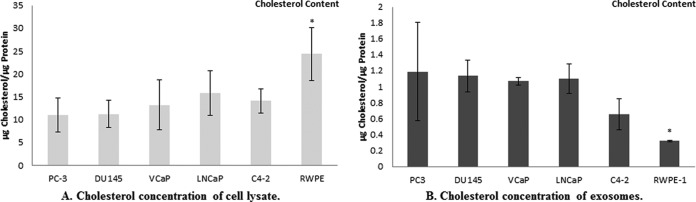
Cholesterol Content
Cholesterol content of exosomes and corresponding cells was determined using LC-MS. As shown in Fig. 7A, cholesterol content of RWPE-1 cells was significantly higher than that of the PCa cell lines. The average cholesterol concentration of PCa cell lysates was 13.1 μg cholesterol/μg protein which was approximately half that determined for RWPE-1 cells (24.4 μg cholesterol/μg proteins). Interestingly, the average cholesterol content of exosomes derived from PCa cells was three times higher than exosomes derived from RWPE-1, benign prostate cells.
Fig. 7.
Cholesterol concentration. The bar diagrams show the cholesterol concentration of A, lysates of PC3, DU145 and VCaP, LNCaP, C4–2, and RWPE-1 cells B, exosomes derived from the six different prostate cell lines. Cholesterol results were normalized to protein concentration of each sample and expressed as μg Cholesterol/μg Protein. * indicate significantly difference (p < 0.05).
Lipidomic Analysis
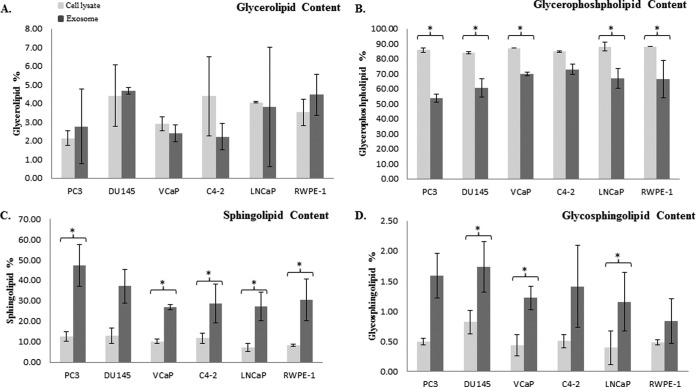
We next investigated the lipid profile (glycerolipid, glycerophospholipid, sphingolipid, glycosphingolipid) of exosomes for comparison with their parent cells. Our data indicated that glycerophospholipid was the most abundant class of lipids with an average of 86.3% and 65.1% determined in both cells and exosomes respectively for all six different prostate cell lines. Sphingolipid (9.6% in cells, 30.2% in exosomes) was the second most abundant lipid class in all different groups, followed by glycerolipid (3.6%, 3.4%) and glycosphingolipid (0.5%, 1.3%).
Data shown in Fig. 8A establishes that there is no significant difference between the glycerolipid content between different exosomes and their corresponding cells.
Fig. 8.
Exosome lipidomic data. The lipid content of four major lipid classes was measured in PC3, DU145 and VCaP, LNCaP, C4–2 and RWPE-1 cells and compared with their derived exosomes, using LC-MS. The bar diagrams are representative of A, glycerolipid, B, glycerophospholipid, C, sphingolipid, D, glycosphingolipid in cell lysates and exosomes. Relative amounts of each lipid group were calculated as (sum of all AUC's for a particular lipid group) ÷ (sum of AUC's for all lipid groups) ×100%. * denotes a significant difference (p < 0.05) between exosomes and their corresponding cells. No significant differences were seen between the cell lysates and exosomes of respective cell lines in any of lipid class.
The glycerophospholipid distribution determined in the various prostate cells and their exosomes was more balanced compared with other groups of lipids. PC3, DU145, VCaP, and LNCaP and RWPE-1 cells were shown to have a significantly higher glycerolipid content compared with their derived exosomes (Fig. 8B).
The most striking difference between the lipid profiles determined for exosomes and their corresponding cells were their sphingolipid contents. In agreement with many other published reports we show enrichment in the sphingolipid content in all of our exosome samples (20). As demonstrated in Fig. 8C, PC3, VCaP, C4–2, LNCaP, and RWPE-1 have a significantly higher sphingolipid content compared with their corresponding parent cells.
Finally, our lipid analysis showed that only DU145, VCaP, and LNCaP derived exosomes have a higher glycosphingolipid content compared with their parent cells.
No significant differences were seen between the cell lysates and exosomes of respective cell lines in any lipid class.
DISCUSSION
Exosomes are complex nanovesicles secreted from a wide range of cells including neoplastic cells (38) dendritic cells (39), reticulocytes (40), T cells (41), mastocytes (42), differentiated and un-differentiated keratinocytes (43), platelets (44), and neurons (45). They have recently been studied in different pathological conditions including cancer, atherosclerosis, vascular disease, pulmonary hypertension, and thrombosis as well as bacterial infections (46). Exosomes have been isolated and characterized from many different biological fluids such as blood components, urine, amniotic fluids, malignant effusions, breast milk, and brochoalveolar lavage fluid and contain an array of proteins and lipids as well as genetic material such as mRNA and miRNA (47). Collectively all these studies highlight membrane and cytoplasmic proteins/mRNA as exosome cargo, many of which have known functional importance in cellular function.
In this study we have characterized exosomes derived from different AR ± ve prostate cancer cell lines. The goal was not only to understand the difference between exosomes secreted from different cells as a basis for evaluating transfer of identified composite exosome proteins between cells as part of a recognized cell communication phenomenon, but also to determine potential biomarkers for different stages of PCa as well as therapeutic targets (11, 12).
The initial challenge of this work was to experimentally isolate homogenous samples of exosomes for study. In order to maintain reproducible data sets we used a protocol developed according to Lamparski HG. et al. (2002) which removes cell debris and protein aggregates using filtration and we were thus able to isolate a homogenous exosome mixture based on the unique density of exosomes in sucrose using ultracentrifugation.
Although some controversy exists regarding the exact nature of exosome biomarkers several classes of proteins have been characteristically assigned as such. These include cytoskeletal proteins, heat shock proteins, and tetraspanins (21, 47). Similarly the absence of endoplasmic reticulum, mitochondrial, and nuclear proteins are considered to vouch for “lack of significant contamination” of an exosome preparation (48, 49). This being said, our understanding of exosome biology is in its infancy, and further work is required to validate protocol in this regard. We were surprised in fact to find proteins identified by our LCMS proteomic analyses which are normally considered to be located in the nucleus and therefore not likely candidates for exosome cargo. Rather than assume that these proteins are contaminants of our exosome preparation we would rather tend to the idea that they might play a role in cell-cell communication via exosomal transfer (50). In keeping with this notion, it is thought that dysregulation of key biological mechanisms in cancer cells, such as those regulating protein synthesis and transportation, may result in altered distribution of proteins within and outside of defined intracellular compartments (51). One example, which our data supports, is the presence of a protein called nucleophosmin in exosomes. This protein is involved in a multitude of cellular functions including ribosome biogenesis, cell proliferation, regulation of tumor suppressor p53, and protein chaperoning which could imply “shuttling” between the cytoplasm and the nucleus. Although this protein is predominantly nuclear, and might be considered as a contaminant in our exosome sample, its minor role as a chaperone in the cytoplasm would allow for detection in exosomes.
We were able to generate exosomes from all six prostate cell lines. All isolated vesicles contained exosomal markers and lacked ER marker (except PC3), as validated using Western blotting, and were observed to be cup-shaped under TEM (21). Although the mechanism involved in exosome internalization by other prostate cells was not under investigation as part of this study, this has been studied by others previously using ovarian cell lines (52) human saliva, plasma, and breast milk exosomes (53). For the purpose of this study we have simply confirmed the uptake of exosomes derived from DU145 prostate cancer cells into other PCa and benign cells and furthermore, transfer of exosomal CLUGFP derived from the LNCaP cell line to PC3 and LNCaP cells. Although more research is needed to investigate the mechanism involved in the uptake of exosomes by neighboring cells in the PCa microenvironment, our finding supports the previously hypothesized role of exosomes as cell–cell communication vesicles in tumor growth (54, 55) cell migration (35, 56, 57), metastasis (58, 59), and angiogenesis (60).
Cancer is a complex disease which involves disruption of numerous cell cycle regulatory pathways (61) and several lines of evidence indicate that, for each unique cancer cell subtype, the path to malignancy is different. Over time, depending on the type of cancer, tumor cells break away from the primary site and invade surrounding tissues before migrating to distant tissue (62). Although metastasis is responsible for more than 90% of cancer related deaths, the mechanisms involved in metastasis are not fully understood. Recently there has been an increasing interest in the potential link between microvesicles and local invasion of primary cancer cells within the microenvironment, as well as metastasis to foreign microenvironments. Further, our present study attempts to understand this process by comparing the protein profiles of exosomes derived from different PCa cell lines as well as a nonmalignant epithelial prostate cell line. We used mass spectrometry to successfully identify 220 proteins in exosomes derived from six prostate cell lines. By reporting proteins with more than two peptides and a Mascot score higher than 40 we have a high level of confidence that our proteomic data accurately represents proteins found in exosome samples. To better understand the differences between exosomes derived from a variety of PCa cells as well as RWPE-1 cells we further categorized them as AR +ve and AR –ve cells.
Reinforcing the conclusions of a recent published proteomic analysis of exosomes derived from the AR null PC3 cell line (63), this proteomic study also supports the premise that exosomes provide a pool of proteins which are enriched in biomarkers for potential use in PCa diagnosis via a relatively noninvasive diagnostic test. Our list consists of 50 candidate protein biomarkers which have been previously reported to have potential in diagnosis, prognosis, disease progression, efficacy and response to therapy for a variety of pathological diseases. We also identified novel potential biomarker proteins in exosomes derived from PCa cells which may be more specific to PCa diagnosis.
Proteins which were identified mutually within exosomes derived from PCa cell lines and benign RWPE-1 cells include GAPDH, PKM2, TUBA1B, and THBS1. These were removed from the biomarker list however we recognize that comparison of a larger panel of nonmalignant cells would be required to definitively determine the relevance of the specificity of these proteins being in exosomes derived from nonmalignant cells. Several biomarker candidates in our list include ANXA2, CLSTN1, FASN, FLNC, FOLH1, and GDF15 which have all been previously reported as candidates for PCa diagnosis (http://clinicaltrials.gov/show/NCT00340717, NCT00897234, and NCT00438906). Although ANXA2 and FOLH1 have been reported in exosomes derived from hepatocyte, bladder, tracheobronchial epithelial and PCa cells, we are the first group to report the presence of CLSTN1, FASN, FLNC, and GDF15 in different PCa cell exosomes. The presence of these biomarkers, in addition to the CYP17 protein which we previously characterized in serum exosomes, and is known to play a significant role in CRPC (64), could be further explored for potential indicators of disease progression. The presence of CLU (Clusterin) in exosomes is also of significant relevance because this molecule has already been characterized as a therapeutic target for CRPC (65–69) and antisense oligonucleotide OGX-011, which was designed to target CLU, is currently in phase III clinical trial.
In our study we went on to more fully characterize exosomes by analyzing their cholesterol and lipid profiles compared with respective cells of origin. As our results demonstrate, although there is a significant difference in lipid content for some lipid classes in some of the cell lines, no significant differences have been seen between AR +ve and AR –ve cells and their exosomes or between PCa cells/exosomes and RWPE-1 cells or derived exosomes. We hypothesize that the significant differences in lipid content of exosomes and their cells of origin could imply that there is a mechanism in existence, which stabilizes exosomes in the circulation for subsequent fusion with the plasma membranes of recipient cells to which they are delivered. Interestingly our quantitative cholesterol data suggests that exosomes derived from PCa cell lines contain significantly more cholesterol than their benign counterpart cell line RWPE-1. This is in line with our previous work which indicates that cholesterol is likely to play a role in PCa progression (70, 71).
Although exosome biogenesis is still under investigation it is very well known that exosome membranes have the same topology as the cell membranes from which they are derived and are correspondingly rich in lipid rafts (72). In agreement with previously published studies our lipidomic data shows a significant enrichment of sphingolipid and glycosphingolipid in exosomes, both very well-known markers of lipid rafts which are contained within almost all exosomes derived from prostate cells (73, 74).
Lastly, it has been previously proposed that endocytic sorting behavior of lipids is exclusively depends on the chemistry of their hydrophobic tails (75). In the longer term, further study to generate a more thorough understanding of the differences in lipid content of cells and their derived exosomes would be useful to define the role of each lipid class in exosome formation as well as mechanisms involved in exosome-mediated communication within the tissue microenvironment.
Supplementary Material
Acknowledgments
The authors thank the Bio Imaging Facility at the University of British Columbia, especially Garnet Martens, for their assistance in Transmission Electron Microscopy. The authors also wish to thank Robert H. Bell for providing assistance with the statistical comparison carried out as described in this manuscript as well as Dr. Fan Zhang and Dr. Claudia Chavez-Muñoz for their valuable comments and technical assistance.
Footnotes
 This article contains supplemental Figs. S1 and Table S1.
This article contains supplemental Figs. S1 and Table S1.
1 The abbreviations used are:
- PCa
- prostate cancer
- ADT
- androgen deprivation therapy
- AR
- androgen receptor
- AUC
- area under curve
- CLU
- clusterin
- CRPC
- castration resistance prostate cancer
- DMEM
- Dulbecco's modified Eagle's medium
- DTT
- dithiothreitol
- EE
- early endosome
- ER
- endoplasmic reticulum
- ESCRT
- endosomal sorting complex required for transport
- FA
- formic acid
- HSP
- heat shock protein
- ILV
- intraluminal vesicles
- IPA
- ingenuity pathway analysis
- IPS
- ingenuity pathway software
- KSFM
- keratinocyte-SFM
- LAMP2
- lysosomal associated membrane protein 2
- LC-MS
- liquid chromatography-mass spectrometry
- LC-QTOF MS
- liquid chromatography-quadrupole time of flight mass spectrometry
- MTBE
- methyl-t-butyl ether
- MVB
- multivesicular bodies
- PLGS
- protein lynx global server software
- PSA
- prostate specific antigen
- RT
- room temperature
- TEM
- transmission electron microscopy.
REFERENCES
- 1. Jemal A., Siegel R., Ward E., Murray T., Xu J., Smigal C., Thun M. J. (2006) Cancer statistics, 2006. CA Cancer J. Clin. 56, 106–130 [DOI] [PubMed] [Google Scholar]
- 2. Chan J. M., Holick C. N., Leitzmann M. F., Rimm E. B., Willett W. C., Stampfer M. J., Giovannucci E. L. (2006) Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States). Cancer Causes Control 17, 199–208 [DOI] [PubMed] [Google Scholar]
- 3. Bruchovsky N., Klotz L. H., Sadar M., Crook J. M., Hoffart D., Godwin L., Warkentin M., Gleave M. E., Goldenberg S. L. (2000) Intermittent androgen suppression for prostate cancer: Canadian Prospective Trial and related observations. Mol Urol 4, 191–199; discussion 201 [PubMed] [Google Scholar]
- 4. Gleave M. E., Goldenberg S. L., Chin J. L., Warner J., Saad F., Klotz L. H., Jewett M., Kassabian V., Chetner M., Dupont C., Van Rensselaer S. (2001) Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 166, 500–506; discussion 506–507 [PubMed] [Google Scholar]
- 5. Lan C. Y., Huang H., Liu J. H. (2008) [Prognostic value of serum CA(125) level change during chemotherapy post-surgery in patients with advanced epithelial ovarian carcinoma]. Zhonghua Fu Chan Ke Za Zhi 43, 732–736 [PubMed] [Google Scholar]
- 6. Croswell J. M., Kramer B. S., Crawford E. D. (2011) Screening for prostate cancer with PSA testing: current status and future directions. Oncology 25, 452–460, 463 [PubMed] [Google Scholar]
- 7. Hernández J., Thompson I. M. (2004) Prostate-specific antigen: a review of the validation of the most commonly used cancer biomarker. Cancer 101, 894–904 [DOI] [PubMed] [Google Scholar]
- 8. Caby M. P., Lankar D., Vincendeau-Scherrer C., Raposo G., Bonnerot C. (2005) Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 17, 879–887 [DOI] [PubMed] [Google Scholar]
- 9. Taylor D. D., Akyol S., Gercel-Taylor C. (2006) Pregnancy-associated exosomes and their modulation of T cell signaling. J. Immunol. 176, 1534–1542 [DOI] [PubMed] [Google Scholar]
- 10. Taylor D. D., Gercel-Taylor C. (2008) MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110, 13–21 [DOI] [PubMed] [Google Scholar]
- 11. Andre F., Schartz N. E., Movassagh M., Flament C., Pautier P., Morice P., Pomel C., Lhomme C., Escudier B., Le Chevalier T., Tursz T., Amigorena S., Raposo G., Angevin E., Zitvogel L. (2002) Malignant effusions and immunogenic tumour-derived exosomes. Lancet 360, 295–305 [DOI] [PubMed] [Google Scholar]
- 12. Bard M. P., Hegmans J. P., Hemmes A., Luider T. M., Willemsen R., Severijnen L. A., van Meerbeeck J. P., Burgers S. A., Hoogsteden H. C., Lambrecht B. N. (2004) Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am. J. Respir. Cell Mol. Biol. 31, 114–121 [DOI] [PubMed] [Google Scholar]
- 13. Pisitkun T., Shen R. F., Knepper M. A. (2004) Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. U.S.A. 101, 13368–13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asea A., Jean-Pierre C., Kaur P., Rao P., Linhares I. M., Skupski D., Witkin S. S. (2008) Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J. Reprod. Immunol. 79, 12–17 [DOI] [PubMed] [Google Scholar]
- 15. Admyre C., Grunewald J., Thyberg J., Gripenbäck S., Tornling G., Eklund A., Scheynius A., Gabrielsson S. (2003) Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur. Respir. J 22, 578–583 [DOI] [PubMed] [Google Scholar]
- 16. Hawari F. I., Rouhani F. N., Cui X., Yu Z. X., Buckley C., Kaler M., Levine S. J. (2004) Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc. Natl. Acad. Sci. U.S.A. 101, 1297–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andersen M. H., Berglund L., Rasmussen J. T., Petersen T. E. (1997) Bovine PAS-6/7 binds alpha v beta 5 integrins and anionic phospholipids through two domains. Biochemistry 36, 5441–5446 [DOI] [PubMed] [Google Scholar]
- 18. Admyre C., Johansson S. M., Qazi K. R., Filén J. J., Lahesmaa R., Norman M., Neve E. P., Scheynius A., Gabrielsson S. (2007) Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 179, 1969–1978 [DOI] [PubMed] [Google Scholar]
- 19. Théry C., Ostrowski M., Segura E. (2009) Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 [DOI] [PubMed] [Google Scholar]
- 20. Laulagnier K., Motta C., Hamdi S., Roy S., Fauvelle F., Pageaux J. F., Kobayashi T., Salles J. P., Perret B., Bonnerot C., Record M. (2004) Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 380, 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Théry C., Zitvogel L., Amigorena S. (2002) Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 [DOI] [PubMed] [Google Scholar]
- 22. Krämer-Albers E. M., Bretz N., Tenzer S., Winterstein C., Möbius W., Berger H., Nave K. A., Schild H., Trotter J. (2007) Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin. Appl. 1, 1446–1461 [DOI] [PubMed] [Google Scholar]
- 23. Jaiswal J. K., Andrews N. W., Simon S. M. (2002) Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J. Cell Biol. 159, 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stoeck A., Keller S., Riedle S., Sanderson M. P., Runz S., Le Naour F., Gutwein P., Ludwig A., Rubinstein E., Altevogt P. (2006) A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem. J. 393, 609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lakkaraju A., Rodriguez-Boulan E. (2008) Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell. Biol. 18, 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Savina A., Vidal M., Colombo M. I. (2002) The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 115, 2505–2515 [DOI] [PubMed] [Google Scholar]
- 27. Llorente A., van Deurs B., Sandvig K. (2007) Cholesterol regulates prostasome release from secretory lysosomes in PC-3 human prostate cancer cells. Eur. J. Cell Biol. 86, 405–415 [DOI] [PubMed] [Google Scholar]
- 28. Aharon A., Brenner B. (2009) Microparticles, thrombosis and cancer. Best Pract. Res. Clin. Haematol. 22, 61–69 [DOI] [PubMed] [Google Scholar]
- 29. Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., Coscia C., Iessi E., Logozzi M., Molinari A., Colone M., Tatti M., Sargiacomo M., Fais S. (2009) Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284, 34211–34222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jansen F. H., Krijgsveld J., van Rijswijk A., van den Bemd G. J., van den Berg M. S., van Weerden W. M., Willemsen R., Dekker L. J., Luider T. M., Jenster G. (2009) Exosomal secretion of cytoplasmic prostate cancer xenograft-derived proteins. Mol. Cell. Proteomics 8, 1192–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abusamra A. J., Zhong Z., Zheng X., Li M., Ichim T. E., Chin J. L., Min W. P. (2005) Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 35, 169–173 [DOI] [PubMed] [Google Scholar]
- 32. Dimov I., Jankovic Velickovic L., Stefanovic V. (2009) Urinary exosomes. ScientificWorldJournal 9, 1107–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Smaele E., Ferretti E., Gulino A. (2010) MicroRNAs as biomarkers for CNS cancer and other disorders. Brain Res. 1338, 100–111 [DOI] [PubMed] [Google Scholar]
- 34. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 35. McCready J., Sims J. D., Chan D., Jay D. G. (2010) Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer 10, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mathivanan S., Fahner C. J., Reid G. E., Simpson R. J. (2012) ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 40, D1241–4 Epub 2011 Oct 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simpson R. J., Karla H., Mathivanan S. (2012) ExoCarta as a resource for exosomal research. J. Extracellular Vesicles 1 18374 - http://dx.doi.org/10.3402/jev.v1i0.18374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trams E. G., Lauter C. J., Salem N., Jr., Heine U. (1981) Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 645, 63–70 [DOI] [PubMed] [Google Scholar]
- 39. Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4, 594–600 [DOI] [PubMed] [Google Scholar]
- 40. Pan B. T., Teng K., Wu C., Adam M., Johnstone R. M. (1985) Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 101, 942–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang H., Xie Y., Li W., Chibbar R., Xiong S., Xiang J. (2011) CD4(+) T cell-released exosomes inhibit CD8(+) cytotoxic T-lymphocyte responses and antitumor immunity. Cell. Mol. Immunol. 8, 23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raposo G., Tenza D., Mecheri S., Peronet R., Bonnerot C., Desaymard C. (1997) Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol. Biol. Cell 8, 2631–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chavez-Muñoz C., Kilani R. T., Ghahary A. (2009) Profile of exosomes related proteins released by differentiated and undifferentiated human keratinocytes. J. Cell. Physiol. 221, 221–231 [DOI] [PubMed] [Google Scholar]
- 44. Heijnen H. F., Schiel A. E., Fijnheer R., Geuze H. J., Sixma J. J. (1999) Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94, 3791–3799 [PubMed] [Google Scholar]
- 45. Lachenal G., Pernet-Gallay K., Chivet M., Hemming F. J., Belly A., Bodon G., Blot B., Haase G., Goldberg Y., Sadoul R. (2011) Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell Neurosci. 46, 409–418 [DOI] [PubMed] [Google Scholar]
- 46. Anderson H. C., Mulhall D., Garimella R. (2010) Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab. Invest. 90, 1549–1557 [DOI] [PubMed] [Google Scholar]
- 47. Schorey J. S., Bhatnagar S. (2008) Exosome function: from tumor immunology to pathogen biology. Traffic 9, 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Février B., Raposo G. (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 16, 415–421 [DOI] [PubMed] [Google Scholar]
- 49. Welton J. L., Khanna S., Giles P. J., Brennan P., Brewis I. A., Staffurth J., Mason M. D., Clayton A. (2010) Proteomics analysis of bladder cancer exosomes. Mol. Cell. Proteomics 9, 1324–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Papp K., Végh P., Prechl J., Kerekes K., Kovács J., Csikós G., Bajtay Z., Erdei A. (2008) B lymphocytes and macrophages release cell membrane deposited C3-fragments on exosomes with T cell response-enhancing capacity. Mol. Immunol. 45, 2343–2351 [DOI] [PubMed] [Google Scholar]
- 51. Barboro P., Repaci E., Rubagotti A., Salvi S., Boccardo S., Spina B., Truini M., Introini C., Puppo P., Ferrari N., Carmignani G., Boccardo F., Balbi C. (2009) Heterogeneous nuclear ribonucleoprotein K: altered pattern of expression associated with diagnosis and prognosis of prostate cancer. Br. J. Cancer 100, 1608–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Escrevente C., Keller S., Altevogt P., Costa J. (2011) Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 11, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lässer C., Alikhani V. S., Ekström K., Eldh M., Paredes P. T., Bossios A., Sjöstrand M., Gabrielsson S., Lötvall J., Valadi H. (2011) Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J. Transl. Med. 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Iero M., Valenti R., Huber V., Filipazzi P., Parmiani G., Fais S., Rivoltini L. (2008) Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 15, 80–88 [DOI] [PubMed] [Google Scholar]
- 55. Liu C., Yu S., Zinn K., Wang J., Zhang L., Jia Y., Kappes J. C., Barnes S., Kimberly R. P., Grizzle W. E., Zhang H. G. (2006) Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 176, 1375–1385 [DOI] [PubMed] [Google Scholar]
- 56. Esser J., Gehrmann U., D'Alexandri F. L., Hidalgo-Estévez A. M., Wheelock C. E., Scheynius A., Gabrielsson S., Rådmark O. (2010) Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J. Allergy Clin. Immunol. 126, 1032–1040, 1040 e1–4 [DOI] [PubMed] [Google Scholar]
- 57. Vrijsen K. R., Sluijter J. P., Schuchardt M. W., van Balkom B. W., Noort W. A., Chamuleau S. A., Doevendans P. A. (2010) Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J. Cell. Mol. Med. 14, 1064–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Janowska-Wieczorek A., Wysoczynski M., Kijowski J., Marquez-Curtis L., Machalinski B., Ratajczak J., Ratajczak M. Z. (2005) Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer 113, 752–760 [DOI] [PubMed] [Google Scholar]
- 59. Hood J. L., San R. S., Wickline S. A. (2011) Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 71, 3792–3801 [DOI] [PubMed] [Google Scholar]
- 60. Skog J., Würdinger T., van Rijn S., Meijer D. H., Gainche L., Sena-Esteves M., Curry W. T., Jr., Carter B. S., Krichevsky A. M., Breakefield X. O. (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hanahan D., Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 62. Chaffer C. L., Weinberg R. A. (2011) A perspective on cancer cell metastasis. Science 331, 1559–1564 [DOI] [PubMed] [Google Scholar]
- 63. Sandvig K, Llorente A. (2012) Proteomic Analysis of microvesicles released by the human prostate cancer cell line PC-3. Mol. Cell. Proteomics 11, 10.1074/mcp.M111.012914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Locke J. A., Fazli L., Adomat H., Smyl J., Weins K., Lubik A. A., Hales D. B., Nelson C. C., Gleave M. E., Tomlinson Guns E. S. (2009) A novel communication role for CYP17A1 in the progression of castration-resistant prostate cancer. Prostate 69, 928–937 [DOI] [PubMed] [Google Scholar]
- 65. Lamoureux F., Thomas C., Yin M. J., Kuruma H., Beraldi E., Fazli L., Zoubeidi A., Gleave M. E. (2011) Clusterin inhibition using OGX-011 synergistically enhances Hsp90 inhibitor activity by suppressing the heat shock response in castrate-resistant prostate cancer. Cancer Res. 71, 5838–5849 [DOI] [PubMed] [Google Scholar]
- 66. Chi K. N., Zoubeidi A., Gleave M. E. (2008) Custirsen (OGX-011): a second-generation antisense inhibitor of clusterin for the treatment of cancer. Expert Opin. Investig. Drugs 17, 1955–1962 [DOI] [PubMed] [Google Scholar]
- 67. Sowery R. D., Hadaschik B. A., So A. I., Zoubeidi A., Fazli L., Hurtado-Coll A., Gleave M. E. (2008) Clusterin knockdown using the antisense oligonucleotide OGX-011 re-sensitizes docetaxel-refractory prostate cancer PC-3 cells to chemotherapy. BJU Int. 102, 389–397 [DOI] [PubMed] [Google Scholar]
- 68. Zoubeidi A., Chi K., Gleave M. (2010) Targeting the cytoprotective chaperone, clusterin, for treatment of advanced cancer. Clin. Cancer Res. 16, 1088–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zoubeidi A., Ettinger S., Beraldi E., Hadaschik B., Zardan A., Klomp L. W., Nelson C. C., Rennie P. S., Gleave M. E. (2010) Clusterin facilitates COMMD1 and I-kappaB degradation to enhance NF-kappaB activity in prostate cancer cells. Mol. Cancer Res. 8, 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Locke J. A., Wasan K. M., Nelson C. C., Guns E. S., Leon C. G. (2008) Androgen-mediated cholesterol metabolism in LNCaP and PC-3 cell lines is regulated through two different isoforms of acyl-coenzyme A:Cholesterol Acyltransferase (ACAT). Prostate 68, 20–33 [DOI] [PubMed] [Google Scholar]
- 71. Leon C. G., Locke J. A., Adomat H. H., Etinger S. L., Twiddy A. L., Neumann R. D., Nelson C. C., Guns E. S., Wasan K. M. (2010) Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate 70, 390–400 [DOI] [PubMed] [Google Scholar]
- 72. de Gassart A., Geminard C., Fevrier B., Raposo G., Vidal M. (2003) Lipid raft-associated protein sorting in exosomes. Blood 102, 4336–4344 [DOI] [PubMed] [Google Scholar]
- 73. Wubbolts R., Leckie R. S., Veenhuizen P. T., Schwarzmann G., Möbius W., Hoernschemeyer J., Slot J. W., Geuze H. J., Stoorvogel W. (2003) Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J. Biol. Chem. 278, 10963–10972 [DOI] [PubMed] [Google Scholar]
- 74. Izquierdo-Useros N., Naranjo-Gómez M., Archer J., Hatch S. C., Erkizia I., Blanco J., Borràs F. E., Puertas M. C., Connor J. H., Fernández-Figueras M. T., Moore L., Clotet B., Gummuluru S., Martinez-Picado J. (2009) Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 113, 2732–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mukherjee S., Soe T. T., Maxfield F. R. (1999) Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J. Cell Biol. 144, 1271–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Staubach S., Razawi H., Hanisch F. G. (2009). Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics 9, 2820–2835 [DOI] [PubMed] [Google Scholar]
- 77. Hegmans J. P., Bard M. P., Hemmes A., Luider T. M., Kleijmeer M. J., Prins J.-B., Zitvogel L., Burgers S. A., Hoogsteden H. C., Lambrecht B. N. (2004). Proteomic analysis of exosomes secreted by human mesothelioma cells. Am. J. Pathol. 164, 1807–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mears R., Craven R. A., Hanrahan S., Totty N., Upton C., Young S. L., Patel P., Selby P. J., Banks R. E. (2004). Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 4, 4019–4031 [DOI] [PubMed] [Google Scholar]
- 79. Potolicchio I., Carven G. J., Xu X., Stipp C., Riese R. J., Stern L. J., Santambrogio L. (2005). Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J. Immunol. 175, 2237–2243 [DOI] [PubMed] [Google Scholar]
- 80. Faure J., Lachenal G., Court M., Hirrlinger J., Chatellard-Causse C., Blot B., Grange J., Schoehn G., Goldberg Y., Boyer V., Kirchhoff F., Raposo G., Garin J., Sadoul R. (2006). Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 31, 642–648 [DOI] [PubMed] [Google Scholar]
- 81. Aoki N., Jin-no S., Nakagawa Y., Asai N., Arakawa E., Tamura N., Tamura T., Matsuda T. (2007). Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinol. 148, 3850–3862 [DOI] [PubMed] [Google Scholar]
- 82. Rodrigues M. L., Nakayasu E. S., Oliveira D. L., Nimrichter L., Nosanchuk J. D., Almeida I. C., Casadevall A. (2008). Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryotic Cell 7, 58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lamparski H. G., Metha-Damani A., Yao J. Y., Patel S., Hsu D. H., Ruegg C., Le Pecq J. B. (2002). Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods 270, 211–226 [DOI] [PubMed] [Google Scholar]
- 84. Gonzales P. A., Pisitkun T., Hoffert J. D., Tchapyjnikov D., Star R. A., Kleta R., Wang N. S., Knepper M. A. (2009). Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 20, 363–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gambim M. H., do Carmo Ade O., Marti L., Veríssimo-Filho S., Lopes L. R., Janiszewski M. (2007). Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit. Care 11, R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Van Niel G., Raposo G., Candalh C., Boussac M., Hershberg R., Cerf-Bensussan N., Heyman M. (2001). Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterol. 121, 337–349 [DOI] [PubMed] [Google Scholar]
- 87. Thery C., Regnault A., Garin J., Wolfers J., Zitvogel L., Ricciardi-Castagnoli P., Raposo G., Amigorena S. (1999). Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell. Biol. 147, 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Van Niel G., Mallegol J., Bevilacqua C., Candalh C., Brugière S., Tomaskovic-Crook E., Heath J. K., Cerf-Bensussan N., Heyman M. (2003). Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut 52, 1690–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Segura E., Nicco C., Lombard B., Véron P., Raposo G., Batteux F., Amigorena S., Théry C. (2005). ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 106, 216–223 [DOI] [PubMed] [Google Scholar]
- 90. Looze C., Yui D., Leung L., Ingham M., Kaler M., Yao X., Wu W. W., Shen R. F., Daniels M. P., Levine S. J. (2009). Proteomic profiling of human plasma exosomes identifies PPAR gamma as an exosome-associated protein. Biochem. Biophys. Res. Comm. 378, 433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Giri P. K., Kruh N. A., Dobos K. M., Schorey J. S. (2010). Proteomic analysis identifies highly antigenic proteins in exosomes from M. tuberculosis-infected and culture filtrate protein-treated macrophages. Proteomics 10, 3190–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kinlough C. L., Poland P. A., Bruns J. B., Harkleroad K. L., Hughey R. P. (2004). MUC1 membrane trafficking is modulated by multiple interactions. J. Biol. Chem. 279, 53071–53077 [DOI] [PubMed] [Google Scholar]
- 93. Escola J. M., Kleijmeer M. J., Stoorvogel W., Griffith J. M., Yoshie O., Geuze H. J. (1998). Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human Blymphocytes. J. Biol. Chem. 273, 20121–20127 [DOI] [PubMed] [Google Scholar]
- 94. Skokos D., Le Panse S., Villa I., Rousselle J. C., Peronet R., David B., Namane A., Mécheri S. (2001). Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J. Immunol. 166, 868–876 [DOI] [PubMed] [Google Scholar]
- 95. Clayton A., Court J., Navabi H., Adams M., Mason M. D., Hobot J. A., Newman G. R., Jasani B. (2001). Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J. Immunol. Methods 247, 163–174 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.