Abstract
Menstruation is the expulsion of the endometrial lining of the uterus following a nearly month long preparation for embryo implantation and pregnancy. Increasingly, the health of the endometrium is being recognized as a critical factor in female fertility, and proteomes and transcriptomes from endometrial biopsies at different stages of the menstrual cycle have been studied for both diagnostic and therapeutic purposes (1 Kao, L. C., et al. 2003 Endocrinology 144, 2870–2881; Strowitzki, Tet al. 2006 Hum. Reprod. Update 12, 617–630; DeSouza, L., et al. 2005 Proteomics 5, 270–281). Disorders of the uterus ranging from benign to malignant tumors, as well as endometriosis, can cause abnormal menstrual bleeding and are frequently diagnosed through endometrial biopsy (Strowitzki, Tet al. 2006 Hum. Reprod. Update 12, 617–630; Ferenczy, A. 2003 Maturitas 45, 1–14). Yet the proteome of menstrual blood, an easily available noninvasive source of endometrial tissue, has yet to be examined for possible causes or diagnoses of infertility or endometrial pathology. This study employed five different methods to define the menstrual blood proteome. A total of 1061 proteins were identified, 361 were found by at least two methods and 678 were identified by at least two peptides. When the menstrual blood proteome was compared with those of circulating blood (1774 proteins) and vaginal fluid (823 proteins), 385 proteins were found unique to menstrual blood. Gene ontology analysis and evaluation of these specific menstrual blood proteins identified pathways consistent with the processes of the normal endometrial cycle. Several of the proteins unique to menstrual blood suggest that extramedullary uterine hematopoiesis or parenchymal hemoglobin synthesis may be occurring in late endometrial tissue. The establishment of a normal menstrual blood proteome is necessary for the evaluation of its usefulness as a diagnostic tool for infertility and uterine pathologies. Identification of unique menstrual blood proteins should aid the forensic community in distinguishing menstrual blood from circulating blood.
Menstrual blood is a complex biological fluid composed of blood, vaginal secretions, and the endometrial cells of the uterine wall as they exist immediately prior to menses. These cells are the end product of a dynamic cyclical process focused on pregnancy and reproduction. Consequently, many of the proteins in these cells are expressed in preparation for blastocyst implantation and nurturing (2, 5). Other proteins in the menstrual blood proteome are a consequence of no implantation and include proteolytic enzymes, cytokines, members of apoptotic pathways, and a host of proteins from the diverse types of immune cells that are an integral part of menstruation (6–9). Protein expression in all organs is a consequence of function. In the uterus, however, function changes on a near daily basis. Consequently evaluation of the menstrual blood proteome depends on an understanding of the complex uterine cycle.
The uterus is composed of three main layers: 1) the luminal facing endometrium, 2) the visceral muscle myometrium immediately beneath it, and 3) the perimetrium, a serous membrane facing the abdominal cavity. The endometrium, which undergoes the greatest changes in response to the monthly endocrine cycle and is shed during menstruation, is further divided into a functional layer, the stratum functionalis, which faces the lumen, and a basal layer beneath it, the stratum basalis. By convention the menstrual cycle begins on the first day of menses and has an idealized duration of 28 days. Ovulation occurs at ∼day 14, with the prior 10 days denoted as the proliferative phase of the cycle and the following 2 weeks the secretory phase. At the beginning of the menstrual cycle estrogen and progesterone concentrations are at their lowest levels. Menstruation typically lasts ∼4 days after which the uterus is denuded of the stratum functionalis and much of the stratum basalis (2, 8). However, even as the endometrium is being shed, proliferation from cells of the adjacent cervix and fallopian uterotubal junction, as well as glandular cells deep in the stratum basalis, begin the process of re-establishing the endometrial lining (6, 8). Starting at about the sixth day of the cycle and continuing for about a week, estrogen levels rise. This is a time of increased proliferation and extensive angiogenesis and gland formation. Under the influence of estradiol there are also increases in the formation of intracellular organelles (ribosomes, mitochondria, Golgi apparatus, etc.), as well as the formation of microvilli and cilia with concomitant increases in cytokeratins and other cytoskeletal elements.
On approximately day 14 ovulation occurs and estrogen levels decline as progesterone levels rise. Significant changes in gene expression are observed as proliferation gives way to differentiation (2, 3). Dramatic morphological changes also occur (6, 8) including the hallmark formation of spiral arteries in the functional layer and conspicuous appearance of glycogen vacuoles at the base of glandular cells. Giant mitochondria also appear, and the nuclear envelop forms long channels that penetrate the nucleolus forming the nucleolar canal system (NCS), an organelle found only in the mid-secretory stage of the female endometrium (6, 8, 10). The NCS is believed necessary for the significant amount of protein synthesis required by the maturing endometrium in preparation for blastocyst implantation including the production of glycoproteins and extensive amounts of glycogen (6, 8). Vascular remodeling continues in this phase regulated by a variety of cytokines and matrix metalloproteases (2). Finally, as the late endometrial tissue transitions to predecidua (in preparation of implantation) cytokines recruit large numbers of lymphocytes including uterine natural killer cells, T-cells and macrophages (2, 8). The endometrial lining more than doubles in thickness between the proliferative and secretory phases.
The absence of implantation (e.g. no fertilized egg) results in declining levels of progesterone and leads to menses. Vasoconstriction of basal arteries results in ischemia, apoptosis and necrosis (8, 9). Blood pools beneath the epithelial layer and fills with cell debris and inflammatory exudates. Plasmin, activated by released proteases, prevents the blood from clotting, and matrix metalloproteases aid in the digestion of the extracellular matrix (8). The stratum functionalis cleaved from the basalis is shed over the following 4–5 days even as renewal from basal glandular cells and the edges of the uterus begins.
The health of the endometrium is a key to successful pregnancy. Implantation failure or the inability to maintain and nourish a developing blastocyst can lead to infertility. Numerous studies have focused on understanding the causes of endometrial receptivity failure and the search for proteins that may be used as diagnostic markers as well as therapeutic agents is ongoing (1–3). In addition, abnormal uterine bleeding can be a sign of significant endometrial disorders ranging from benign fibroids to endometriosis to malignancies (4). Here too, identification of reliable and easily obtainable markers from menstrual blood could aid in both early detection and follow up monitoring.
The menstrual blood proteome offers a snapshot of the processes occurring in the endometrium lining immediately prior to menses. Establishing a normal menstrual blood proteome sets a baseline against which pathologies can be evaluated. Identification of unique menstrual blood markers will enable the forensic community to distinguish menstrual blood from circulating blood.
MATERIALS AND METHODS
Materials
Chemical reagents including salts, chaotropic salts, buffers, detergents, organics, alpha-cyano-4-hydroxycinnamic acid (CHCA), Bradford protein assay kit and albumin and IgG immunodepletion ProteoPrep Immunoaffinity kit were purchased from Sigma (St. Louis, MO). RIPA buffer was from Fisher (Pittsburgh, PA). Ni-nitrilotriacetic acid (NTA) agarose beads were purchased from Qiagen (Valencia, CA). Sequencing grade modified trypsin was from Promega (Madison, WI). Precast 15% PAGE gels and immobilized pH gradient (IPG) gel strips were purchased from Bio-Rad (Hercules, CA). Lysing Matrix D 1.4 mm ceramic beads were purchased from MP Biomedicals (Cleveland, OH).
Sample Collection
Menstrual blood was collected from ten healthy volunteers (35–45 years of age). Women were asked to collect menstrual blood by Tampon (Tampax Pearl, Procter and Gamble, Cincinnati, OH) and label it as “First Day,” “Last Day,” or “Middle” if in between the first and last days of their period. One “middle” liquid sample was collected. Samples were stored at −80 °C until processed. Women were not screened for pregnancy. This study was approved by the New York City Department of Health and Mental Hygiene IRB.
Sample Preparation
The “middle” liquid menstrual blood sample (S1) and one “middle” Tampon sample (S2) were used in this study. Two different solubilization buffers were employed depending on downstream processing. For in-solution digestion RIPA buffer was used (50 mm Tris-HCl pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS). For samples to be depleted of hemoglobin (Hb), albumin (Alb)1 and IgG immunoglobulins (IgG) lysis buffer was used (50 mm sodium phosphate buffer pH 8.0, 300 mm NaCl, 10 mm imidazole, 1% Nonidet P-40, 5 mm DTT).
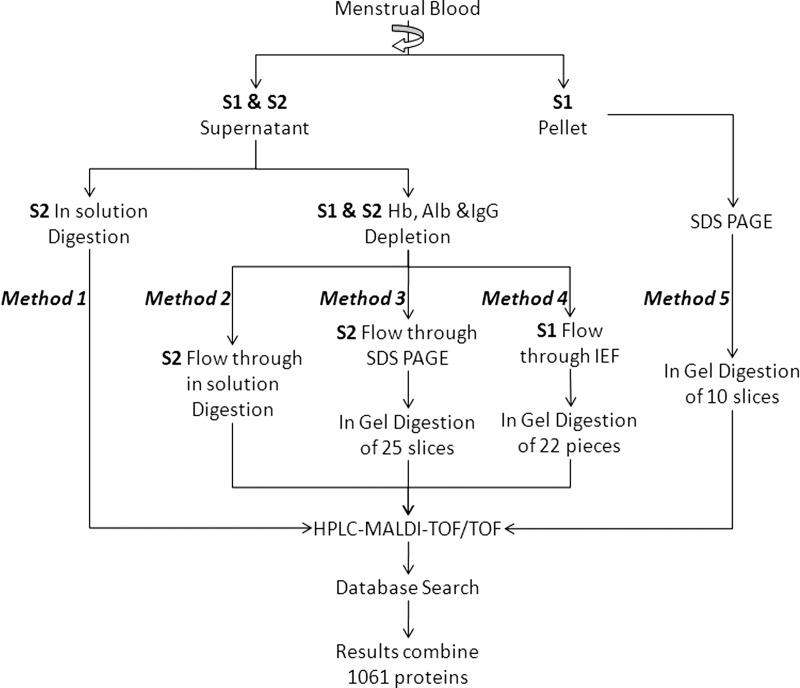
As shown in Fig. 1, five different methods were used to process samples in order to identify the largest number of menstrual blood proteins. For liquid menstrual blood (S1), Methods 4 and 5 were used; for tampon collected menstrual blood (S2) Methods 1, 2, and 3 were used. The Bradford method was used to determine protein content at each step, bovine serum album (BSA) was used as standard.
Fig. 1.
Flowchart of methods used to process menstrual blood samples for MS proteome study. S1 = liquid menstrual blood sample; S2 = Tampon extracted menstrual blood.
Liquid Menstrual Blood (S1)
For liquid menstrual blood the sample was vigorously vortexed and an aliquot taken and solubilized in ten volumes of lysis buffer using a FastPrep-24 reciprocating homogenizer (MP Biomedicals, Cleveland, OH) set to a maximum speed of 6 m/sec for 40 s at room temperature using Lysing Matrix D (1.4 mm ceramic beads). After solubilizing, the sample was transferred to a second microfuge tube and spun at 15,000 × g for 30 min. at 4 °C. The S1 supernatant (∼300 μg protein/μl) was subsequently depleted of Hb, Alb, and IgG (see below) and 600 μg of the depleted sample subjected to isoelectric focusing (IEF, Method 4, see below). The entire IPG strip was divided into 22 equal parts for in-gel reduction, alkylation, and digestion (see below). Digested peptides were extracted, dried, resuspended in 50 μl 2% acetonitrile/0.1% TFA made in HPLC grade water and 10 μl used for nano-HPLC (see below) followed by MALDI TOF/TOF analysis. The S1 sample pellet was resuspended in 2× SDS-PAGE loading buffer heated to 95 °C for 5 min. and ∼ 50 μg protein separated on a 15% polyacrylamide gel and stained with Coomassie brilliant blue (Method 5). The gel was cut into 10 pieces of approximately equal staining and subjected to in-gel reduction, alkylation and digestion followed by nano-HPLC and MALDI TOF/TOF analysis.
Tampon Menstrual Blood (S2)
Protein analysis was performed on both whole menstrual blood extracted from tampons (Method 1, in-solution digestion using RIPA buffer), as well as Hb, Alb, and IgG depleted tampon extracted blood (Methods 2 and 3 using lysis buffer). In both cases the initial extraction procedures were the same. Approximately 100 mg of heavily soaked tampon was mixed with 500 μl of either RIPA or lysis buffers in MP Biomedicals 2 ml screw cap tubes containing matrix D beads and were disrupted as described above. After solubilization, the 2 ml tubes were spun at 15,000 × g for 30 min. at 4 °C to remove tampon material. Supernatants were carefully removed for downstream processing (Fig. 1). Protein content was determined by Bradford with BSA as standard.
Depletion of Hemoglobin, Albumin and IgG
Hemoglobin was depleted from samples using NTA agarose beads according to the method of Ringrose (11) and following the manufacturer's instructions. Approximately 800 μl Ni-NTA agarose beads were added to a volume of menstrual blood containing 20 mg protein in lysis buffer. The suspension was gently mixed end-over-end for 1 h at room temperature and beads separated from the depleted sample by centrifugation. The beads were washed twice with lysis buffer, centrifuged and the washes combined with the original supernatant. The combined Hb-depleted fraction typically contained about 10% (2 mg) of the original sample's total protein. Hb was eluted from the Ni-NTA column using lysis buffer containing 250 mm imidazole. Following Hb depletion, the sample was depleted of albumin and IgG using Sigma's ProteoPrep Immunoaffinity Albumin and IgG Depletion kit following the manufacturer's instructions. Bound proteins were eluted using manufacturer's elution buffer (40 mm Trizma Base, 7.0 m urea, 2.0 m thiourea, and 1% C7BzO detergent, pH 10.4).
IEF Analysis of Depleted Menstrual Blood Protein [Method 4]
Approximately 600 μg of the Hb, Alb and IgG depleted menstrual blood sample was precipitated with four volumes of cold acetone (−20 °C), incubated at −20 °C for 2 h and then spun at 15,000 × g × 5 min. at 4 °C. The pellet was re-suspended in 200 μl of IEF rehydration buffer (7 m urea, 2 m thiourea, 2% CHAPS, 50 mm DTT, 0.2% Bio-Lyte 3/10 ampholyte) and loaded on an inverted 11 cm pH 3–10 IPG strip in a focusing tray. Isoelectric focusing was performed on a Bio-Rad PROTEAN IEF system (Hercules, CA). Conditions for performing IEF were as follows: after rehydration of the IPG strip for one hour at room temperature, the run was started at 250 V for 15 min. followed by rapid voltage ramping to 8000 V without exceeding 50 μA/strip. The IEF run was finished when the voltage reached 35,000 Vhr. The voltage was held at 500 V until the run was stopped. The IEF run was performed at 20 °C.
In-solution Protein Digestion [Methods 1 and 2]
Approximately 50 μg protein of whole menstrual blood [Method 1] or 50 μg protein of Hb, Alb, and IgG depleted/precipitated menstrual blood [Method 2] were resuspended in RIPA buffer for in-solution digestion. Five mm DTT and 15 mm iodoacetamide (IAA) were used to reduce and alkylate proteins. After alkylation 2.5 μg trypsin were added to digest protein at 37 °C overnight. Following digestion, samples were dried under vacuum and resuspended in 2% acetonitrile (ACN), 0.1% trifluoroacetic acid (TFA) made in water. Two μg of peptides were then separated by nano-HPLC and analyzed by MALDI (see below).
In-gel Digestion of IEF and SDS-PAGE Resolved Proteins
Menstrual blood proteins separated by IEF (Method 4) and SDS-PAGE (Methods 3 and 5) were digested in situ as follows. The 11 cm IPG strips (Method 4) was cut into 22 equal parts (0.5 cm each). For the S1 pellet SDS-PAGE gel (Method 5), the gel lane was cut into ten pieces. For SDS-PAGE separated Hb, Alb and IgG depleted menstrual blood (Method 3) 25 gel slices of major bands in lanes 2–5 (Fig. 2) were cut. Bands were destained 3 × 15 min in 50/50 (v/v) 50 mm ammonium bicarbonate/ACN followed by 1 × 15 Min 50 mm ammonium bicarbonate solution. Next, 10 mm DTT and 55 mm IAA were added to reduce and alkylate the protein respectively. Proteins were digested with trypsin (12.5 ng/μl) in 25 mm ammonium bicarbonate at 37 °C overnight. The resulting tryptic peptides were extracted from gel pieces with 5% formic acid in 50% ACN and then evaporated in a vacuum centrifuge and re-suspend in 2% ACN in 0.1% TFA made in water.
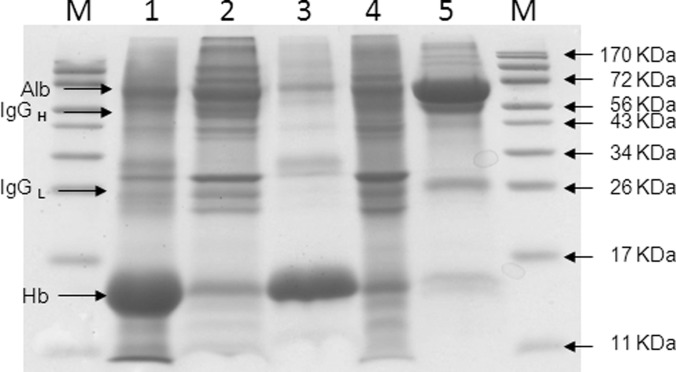
Fig. 2.
SDS-PAGE analysis of menstrual blood following Hb, Alb and IgG depletion. Coomassie-stained 15% SDS-PAGE gel of S2 menstrual blood as well as samples depleted of Hb, Alb and IgG. Lane 1, whole menstrual blood; Lane 2, Hb-depleted menstrual blood (Ni-agarose flow through); Lane 3, Hb fraction eluted from Ni-agarose beads; Lane 4, Hb and IgG/Alb depleted menstrual blood (ProteoPrep Immunoaffinity column flow through); Lane 5, IgG and Alb ProteoPrep Immunoaffinity column elute. M = molecular weight markers. 50 μg protein loaded/lane. Hb ∼17 kDa, Alb ∼66 kDa, IgGH heavy chain ∼50 kDa, IgGL light chain ∼25 kDa.
Nano-HPLC
All nano-HPLC runs were reverse phase and performed on an Ultimate 3000 LC System (Dionex, Sunnyvale, CA). Tryptic peptides were first desalted using an inline Acclaim PepMap100 μ-Precolumn trap (C18, 5 μm, 100 Å beads in a 300 μm i.d. × 5 mm column, Dionex) and then separated by a linear 5–40% ACN gradient on an Acclaim PepMap100 column (C18, 3 μm, 100 Å, 75 μm i.d. × 15 cm, Dionex) at a flow rate of 300 nl/min. ACN gradients were prepared by mixing solvent A (98% water, 2% acetonitrile, 0.1% TFA) with solvent B (98% acetonitrile, 2% water, 0.1% TFA). For separation of in-solution digested peptides (Methods 1 and 2) a 160 min. gradient program was used. For in-gel digestions (Methods 3, 4, and 5) a 40 min. gradient program was used. Sample spotting on MALDI plates included inline (1:1) mixing of sample with α-cyano-4-hydroxycinnamic acid matrix (7 mg HCCA/ml in 75% ACN, 25% water with 0.1% TFA). For the 160 min. program 384 spots were collected, for the 40 min. separation 96 spots were collected.
MALDI-TOF/TOF Analysis
MS and MS/MS data were collected on an AB Sciex 4800 MALDI TOF/TOF Analyzer (Foster City, CA). MS data was acquired at a laser repetition rate of 200 Hz with 1500 laser shots/spectrum (50 laser shots/sub-spectrum). MS/MS data were acquired at 200 Hz in 1kV MS/MS mode with 50 laser shots/subspectrum under the following TOF/TOF Series Explorer Stop Conditions: maximum shots per spectrum 3,500; minimum shots per spectrum 1000; number of MS/MS fragments 8; signal to noise ratio for each fragment was set at 75. Top 30 strongest peaks were selected for MS/MS analysis.
Database Search
Peptides were identified via automated database searching of raw data using ProteinPilot 3.0 software (AB SCIEX, Foster City, CA) against the Swiss-Prot Homo sapiens database (40,670 Protein Entries, April 2009). Paragon was selected as processing method. Identification was selected as sample type. Iodoacetamide and trypsin were selected as alkylation reagent and enzyme respectively. In order to achieve a more comprehensive search, “Through ID” was selected in the ProteinPilot software. For each method (Fig. 1) the individual MS data of each run were combined prior to search submissions to reduce redundancy. A value of 1.0 (>90% confidence) was initially selected as detected protein threshold. The results obtained from ProteinPilot were exported to Microsoft Excel for further combination analysis. For protein identification, at least one peptide at 95% confidence level and global false positive ratio <5% or two or more peptides at 90% level were required.
Gene Ontology Analysis
The NIH DAVID Bioinformatics Resource Tool (version 6.7, http://david.abcc.ncifcrf.gov/) was used to perform Gene Ontology (GO) analysis of biological processes of menstrual blood, venous blood (supplemental Table S2) and vaginal fluid (supplemental Tables S3 and S5) proteins as well as those proteins uniquely identified in menstrual blood (i.e. those not present in either venous blood or vaginal fluid) (supplemental Table S4). David default parameter settings were used; only biological processes with p values ≤ 0.05 were considered. For comparison of menstrual blood, venous blood and vaginal fluid biological processes category 1 was used. Because of the smaller size of its proteome, category 2 was used to analyze unique menstrual blood proteins.
RESULTS
Depletion of Hb, Alb and IgGs
Hemoglobin was removed from menstrual blood by immobilization to Ni-NTA agarose beads (11). SDS-PAGE analysis of starting and depleted menstrual blood samples (Fig. 2) shows that the bulk of Hb was removed, Lanes 1 and 2. Lane 3 shows Hb eluted from the Ni-NTA beads. Following Hb removal, menstrual blood samples were passed through an Alb and IgG ProteoPrep Immunoaffinity column (see Methods). Lane 4 shows the menstrual blood sample following Hb, Alb, and IgG depletion. Lane 5 shows Alb and IgGs eluted from the ProteoPrep Immunoaffinity matrix. As may be seen in both Lanes 3 and 5, several nonspecifically bound proteins coeluted with Hb as well as with Alb and IgGs, a not uncommon finding in affinity chromatography (11). Subsequent excision and in-gel digestion of co-eluted bands followed by LC-MALDI TOF/TOF revealed that these were typically abundant proteins and that no unique proteins were identified, i.e. proteins not found in Lane 4.
Identification of Menstrual Blood Proteins
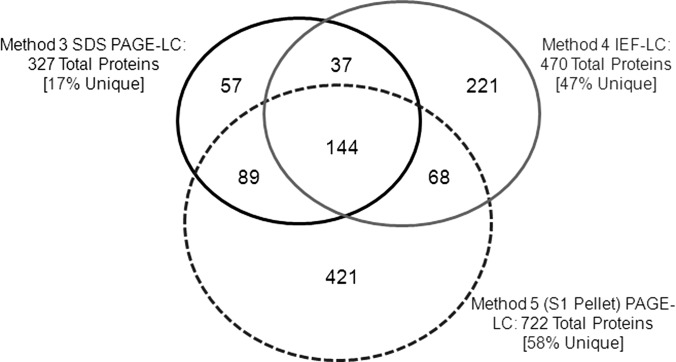
As shown in Fig. 1, five methods were employed to identify menstrual blood proteins. In Method 1 whole, undepleted menstrual blood was directly analyzed by LC-MALDI. In Methods 2–4 menstrual blood depleted of Hb, Alb, and IgGs was analyzed using three different procedures: LC-MALDI, SDS-PAGE-LC-MALDI, and IEF-LC-MALD. Finally, in Method 5, the pellet from the S1 menstrual blood sample was processed by SDS-PAGE-LC-MALDI. The total number of proteins identified by each method is listed in Table I (see supplemental Table S1 for complete list). A comparison of Methods 1 (84 protein entries) and 2 (168 protein entries) demonstrates that depletion of abundant proteins doubles the number of proteins that can be identified. Subsequent protein separation, either by SDS-PAGE (327 protein entries) or IEF (471 protein entries), approximately doubles that number again. The greatest number of proteins identified, however, was found in the S1 pellet separated by SDS-PAGE (722 protein entries). A Venn diagram (Fig. 3) of samples subjected to protein purification prior to LC-MS (Methods 3–5) reveals the number of unique proteins found by each method: Method 3 (supernatant SDS-PAGE) 57 unique proteins, Method 4 (supernatant IEF) 221 unique proteins, and Method 5 (pellet SDS-PAGE) 421 unique proteins.
Table I. Methods used to identify menstrual blood proteins and the number of protein groups and entries identified at >95% confidence.
| Method (see Figure 1) | Sample | Depletion (Hb, Alb, IgG) | μg Protein processed (after treatment) | Protein groupsa identified (>95% Confidence) | Protein entriesa (>95% Confidence) | |
|---|---|---|---|---|---|---|
| 1 | LC MALDI TOF/TOF | S2 | No | 50 μg | 67 | 84 |
| 2 | LC MALDI TOF/TOF | S2 | Yes | 50 μg | 129 | 168 |
| 3 | SDS PAGE + 1C MALDI TOF/TOF | S2 | Yes | 200 μg | 243 | 327 |
| 4 | IEF + LC MALDI TOT/TOF | S1 Supernatant | Yes | 600 μg | 275 | 470 |
| 5 | SDS PAGE + LC MALDI TOF/TOF | S1 Pellet | No | 50 μg | 626 | 722 |
a Protein entries represent all possible proteins identified in a database search that possess one or more of the peptides found in the sample. Protein groups combine proteins that share common peptides, for example splice variants.
Fig. 3.
Comparison of proteins identified from depleted menstrual blood by methods 3 (SDS-PAGE), 4 (IEF) and 5 (SDS-PAGE of S1 Pellet) followed by LC MALDI.
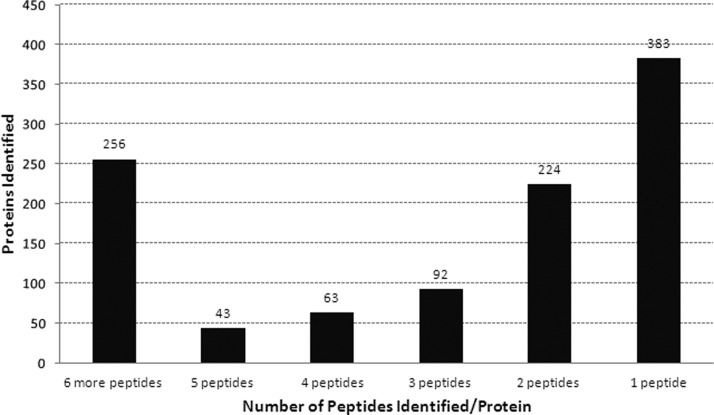
The combined methods identified a total of 1061 menstrual blood proteins by at least one peptide with a confidence score >95%, or two or more peptides with confidence scores >90% (supplemental Table S1). Fig. 4 shows that a total of 256 proteins were identified by 6 or more peptides and 422 with between 2 and 5 peptides, 383 proteins were identified by one peptide.
Fig. 4.
Distribution of the 1061 proteins identified in menstrual blood by the number of peptides/protein.
Comparison of Menstrual Blood, Vaginal Fluid, and Venous Blood Proteomes
Menstrual blood is composed of three distinct body fluids: blood, vaginal fluid, and the cells and fluid of the late secretory phase of the uterine endometrial lining which is shed during menstruation. One way to evaluate the menstrual blood proteome is to subtract out the circulating venous blood and vaginal fluid proteomes. For venous blood the proteome published by Haudek et al. (12) was used. These authors employed 2-D and 1-D PAGE gel shotgun approaches to identify the proteins of both plasma and the cellular constituents of circulating blood, including: T cells, monocytes, neutrophils, erythrocytes, and platelets to identify 1774 unique proteins (supplemental Table S2). For vaginal fluid the proteins identified in nine separate studies (13–21) were combined to make a single vaginal fluid proteome of 823 unique proteins (supplemental Table S3).
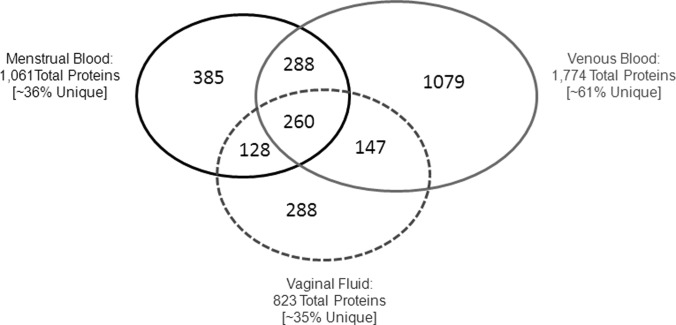
As may be seen in Fig. 5, 548 proteins are shared between venous and menstrual blood, while 513 proteins (48%) are unique to menstrual blood. A comparison of menstrual blood to vaginal fluid shows 388 shared proteins, while 673 (63%) are unique to menstrual blood. When all three body fluids are compared menstrual blood contained a total of 385 proteins (36% of total) not found in vaginal fluid or venous blood (supplemental Table S4). In addition to venous blood and vaginal fluid, the 1061 menstrual blood proteins identified here were also compared with 722 proteins identified by Casado-Vela et al. (22) in endometrial fluid aspirate, and 95 human proteins identified by DeSouza et al. (3) in proliferative and secretory endometrial tissue. Although 167 proteins were shared between the endometrial fluid and menstrual blood proteomes, 151 of these were also common to venous blood and/or vaginal fluid, leaving only 16 common to menstrual blood alone. Similarly, of 48 endometrial tissue proteins shared with the menstrual blood proteome, 47 were common with venous blood and/or vaginal fluid and only one shared with menstrual blood alone (supplemental Table S5).
Fig. 5.
Venn diagram showing the number of shared and unique proteins in menstrual blood, venous blood, and vaginal fluid.
Gene Ontology (GO) Analysis of Menstrual Blood Proteins
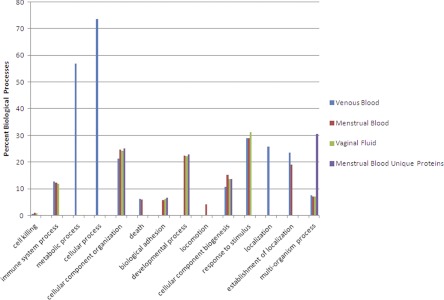
One way to evaluate complex proteomes is the use of bioinformatics tools that can group proteins into common biological pathways or processes. The NIH Bioinformatics Resource DAVID (23) was used here to analyze biological processes of the menstrual blood, venous blood and vaginal fluid proteomes, as well as the 385 unique proteins found in menstrual blood alone. As may be seen in Fig. 6, blood and vaginal fluid share six processes that are also found in menstrual blood: cell killing, immune system process, cellular component organization, cellular component biogenesis, response to stimulus and multiorganism process. Blood and menstrual blood share two processes: death and establishment of localization, while vaginal fluid and menstrual blood also share two common processes: biological adhesion and developmental process. It should be noted that neither metabolic nor cellular processes, which involve the greatest percentage of proteins in blood, were identified in menstrual blood, and that locomotion is the sole biological process identified only in menstrual blood.
Fig. 6.
GO biological processes identified in venous blood, menstrual blood, vaginal fluid and the 385 unique menstrual blood proteins. Data represent the percentage of proteins used in each biological process from total proteins identified from each body fluid. Proteins may be used in more than one biological process.
Because of the mixed nature of menstrual blood it is difficult to know if the biological processes common to blood and menstrual blood, and vaginal fluid and menstrual blood, are also natural processes of the late secretory shedding endometrium. One way to evaluate this is to examine the 385 proteins found only in the menstrual blood sample. Fig. 6 shows that the menstrual blood unique proteins share only three processes with blood and vaginal fluid (cellular component organization, cellular component biogenesis and multiorganism process), but continue to share two processes with vaginal fluid (biological adhesion and developmental process), see Discussion below.
Cellular Component Ontology
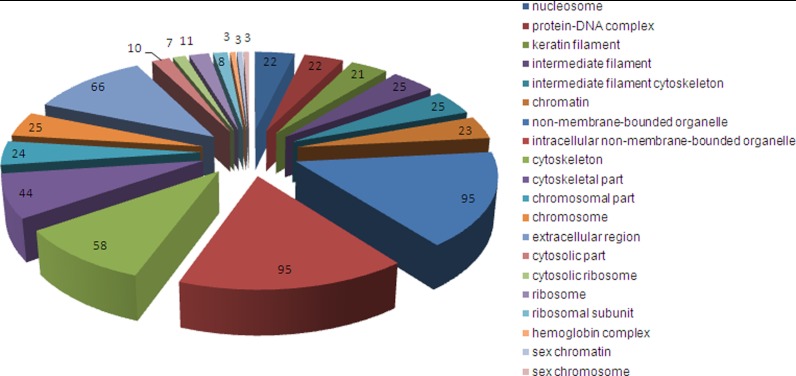
Another way to assess the proteome is cellular component ontology where proteins are classified by their subcellular location, whether as part of large organelles (e.g. the nucleus), or macromolecular complexes (e.g. two or more proteins that form a functional unit such as an enzyme complex, or α and β Hb subunits). Using the NIH David Bioinformatics Resource tool, the 385 unique menstrual blood proteins were divided into 20 cellular component groups (Fig. 7). The bulk of these proteins fall into three major categories: nuclear/chromosomal, cytoskeletal, and ribosomal, reflecting the large number of histones, cytoskeletal elements, and ribosomal proteins identified (see supplemental Table S4). Other cellular components identified include the “extracellular region” (i.e. proteins involved in processes outside of the cell such as signaling, adhesion and release), and “sex chromatin/chromosome” which identified histones reported to function in X-chromosome inactivation (24, 25). The “hemoglobin complex” is of particular interest and will be discussed below.
Fig. 7.
GO cellular components identified in 385 unique menstrual blood proteins. (GOTERM_CC_FAT) ranked by p value from nucleosome (p = 1.62 × 10−19) clockwise through sex chromosome (p = 4.43 × 10−2). Numbers indicate the number of proteins identified within each group.
Analysis of Protein Function
Excluding structural, ribosomal, and defensive proteins, an examination of other proteins in the unique menstrual blood proteome reveals that many are part of biological processes consistent with the functions of the pre-decidua and menstruation. Table II identifies 24 such proteins involved in cell proliferation and migration, apoptosis, reproduction, and of particular interest, hematopoiesis, including hemoglobins ζ, θ, and μ which are normally found in developing fetus (26–28). The potential significance of these proteins is discussed below.
Table II. Function, tissue distribution, and method of identification of selected proteins from the 385 unique menstrual blood proteins identified.
| Category | Protein | Function | Tissue distributiona | Method | Disease associatedb |
|---|---|---|---|---|---|
| Cell proliferation and migration | A disintegrin and metalloprotease 33 (ADAM33) | Protease involved in respiratory mucosal remodeling and angiogenesis (40) | Respiratory, smooth muscle, placenta (41) | IEF-LC-MALDI | |
| Epithelial discoidin domain-containing receptor 1 (DDR1) | Mediates cell growth, migration, differentiation and MMP-2 and 9 (42) | Placenta (43) | IEF LC-MALDI | Breast carcinoma (43) | |
| Fascin (FSCN1) | Actin-bundling protein provides rigidity, cytoskeleton remodeling and cell migration (44) | Ovary (44) | SDS PAGE LC-MALDI | Cervical, ovarian cancer (44) | |
| Orexin-A (OREX) | Neovascularization, increases migration and tube formation in human umbilical vein endothelial cells (45) | Syncytiotrophoblasts and decidual cells of the placenta, endothelial cells (46) | IEF LC-MALDI | ||
| Plexin-A1, D1 (PLXA1, D1) | Plexins receptor for semaphorins, blocks extracellular matrix adhesion, induces cell rounding (47), apoptosis (48) | A1 placenta, uterus, ovary, D1 placenta, uterus, ovary (49) | IEF LC-MALDI | ||
| Podocan (PODN) | Mediates cell growth and migration (50) | Uterus, prostate, cervix (51) | IEF LC-MALDI | ||
| Semaphorin-4B, 6B, 6C (SEM4B, 6B, 6C) | Semaphorins binds plexin receptors (see above) | 4B placenta, uterus 6B uterus, 6C uterus (52) | IEF LC-MALDI | ||
| Thrombospondin type I domain containing 7A (THSD7A) | Mediates endothelial cell migration and tube formation in angiogenesis (53) | Placenta vasculature, umbilical vein endothelium (53) | IEF LC-MALDI | ||
| Wnt (WNT) | Regulates proliferation and differentiation in endometrium (54, 55) | Endometrium and endometrial hyperplasia and carcinogenesis (54, 55) | IEF LC-MALDI | Endometrial cancer (55) | |
| Apoptosis | ANKRD26-like family C-1A (POTE-E) | Apoptosis (56) | Ovary, testes, placenta (57) | LC-MALDI | |
| Death-inducer obliterator 1 (DIDO1) | Apoptosis (58) | Placenta, uterus, ovary (59) mRNA | PAGE LC-MALDI, IEF LC-MALDI | ||
| MAP kinase-activating death domain protein (MADD) | Apoptosis, interacts with tumor necrosis factor (60) | Uterus, placenta, decidua, cervix, ovary (60) Ab staining | LC-MALDI | Endometrial cancer (61) | |
| Protein shisa-5 (SHSA5) | Apoptosis (62) | Placenta (63) | IEF LC-MALDI | ||
| Tumor necrosis factor receptor superfamily member 16 (TNFRII, p75) | Apoptosis (64) | Endometrium (64) | IEF LC-MALDI | Endometriosis (64) | |
| Urocortin 3 (UCN3) | Inflammation, vascularization and apoptosis (65) | Late secretory endometrium (65) mRNA | IEF LC-MALDI | Endometriosis also part of inflammatory response (65) | |
| Hematopoiesis | Ephrin-A4 (EFNA4) | Erythropoiesis (66) | Uterus (67) mRNA | PAGE LC-MALDI, IEF LC-MALDI | |
| F-box only protein 7 (FBXO7) | Regulates erythropoiesis (68) | Placenta, uterus, umbilical cord (69) mRNA | IEF LC-MALDI | ||
| Hemoglobin μ (HBM) | Oxygen transport | Umbilical cord, fetal liver (26) | Pellet | ||
| Hemoglobin θ-1 (HBAT) | Oxygen transport | Fetal erythroid tissue mRNvA (27) | Pellet | ||
| Hemoglobin ζ (HBAZ) | Oxygen transport | Fetal blood (70) | Pellet | ||
| SL Cytokine (FLT3L) | Regulates hematopoiesis (71) | Uterus, decidua (72) antibody IHC | IEF LC-MALDI | ||
| Uroporphyrinogen-III synthase (HEM4) | Porphyrin biosynthesis (Hb constituent) (73) | Early erythroid, fetal liver (73) | Pellet | ||
| Reproduction | Choriogonadotropin subunit beta (CGB) and variants 1 and 2 | Embryo implantation, maintain pregnancy (74) | Placenta, late secretory endometrium (74) | IEF-LC-MALDI | |
| Zona pellucida sperm-binding protein 4 (ZP4) | Sperm-binding protein (75, 76) | Oocyte (75, 76) | Pellet |
a Tissue Distribution: As determined by mRNA or antibody recognition via immuohistochemistry or ELISA. Note: Other tissues may also show gene/protein expression.
b Only diseases associated with the female reproductive system are noted.
DISCUSSION
Menstrual blood is a complex body fluid composed of endometrial and immune cells shed from the uterus, vaginal fluid, and of course, blood. To identify the maximum possible number of proteins present in menstrual blood, five different methods were employed, three using samples depleted of Hb, Alb, and IgGs to reduce dynamic range and consequently unmask low abundant proteins. Although together these methods identified 1061 proteins, only 40 were common to all five methods while 700 were unique to one of the methods. These data demonstrate the importance of multiple separation techniques for identifying the maximum number proteins in a complex sample (Table I, Fig. 3) and are in agreement with other proteomes described in the literature. Schenk (29) for example, employed eight different methods to evaluate the human plasma proteome and found only 56 of 697 proteins by all eight methods.
Of particular interest were results from the 15,000 × g pellet (Method 5) - a sample not infrequently discarded in proteome analyses. The pellet contained the largest number of proteins identified (722) of which 421 were not found in the supernatants from Methods 3 and 4 (Fig. 3). Of the 385 unique menstrual blood proteins identified in this study, 126 (∼33%) were found only in the pellet, e.g. zona pellucida sperm-binding protein 4. These results are not surprising as most solubilizing buffers contain detergents and/or chaotropic salts inadequate to completely disrupt and solubilize nuclei, mitochondria, the plasmalemma, as well as other internal membranes, all of which contain both integral and associated proteins. Many soluble proteins, perhaps trapped within membranous structures (e.g. histones, enzymes, cytokines, immunoglobulins, and cytoskeletal elements from cilia and microvilli), were also identified in the pellet, some found in greater amounts than in the supernatants, whereas some were not found in the supernatants at all, e.g. fetal hemoglobins ζ, θ and μ (Table II). These data suggest that pelleted materials should be routinely analyzed for protein content.
A comparison of the 1061 proteins identified in menstrual blood with the proteomes of circulating blood (1774 proteins, supplemental Table S2) and vaginal fluid (823 proteins, supplemental Table S3) identified 385 unique menstrual blood proteins (supplemental Table S4). Discerning which proteins from the complete (1,061) menstrual blood proteome are specifically expressed in the shedding endometrium, however, is not as simple as subtracting common proteins found in blood and vaginal fluid. Certainly many of these proteins are also expressed in shedding endometrial cells whether they function in routine housekeeping or specific cellular pathways. Ultimately, absolute quantitation of all proteins in each of these body fluids will be required in order to determine the relative contribution of common proteins in the shedding endometrium. However, despite likely co-expression of some proteins, it is still worth assessing the 385 unique menstrual blood proteins in order to evaluate biological processes occurring in the endometrium immediately prior to and during menstruation. Certainly, the large number of histones, ribosomal proteins, cytoskeletal elements, cytokines, and immunoglobulins identified in the unique menstrual blood proteins (Fig. 7, supplemental Table S4) are consistent with the biological processes known to occur in the late secretory phase of the menstrual cycle. For as the secretory endometrium progresses toward predecidua and prepares for blastocyst implantation, nuclei becomes euchromatic, the number of ribosomes increases and cell cytoplasms fills with additional cytoskeletal elements (6, 8, 30). At this time endometrial granulocytes appear in order to suppress other immune cells and thereby protect a genetically distinct implanting embryo from its mother's immune defense system (6, 8).
Lack of implantation leads to menstruation which today is regarded as an inflammatory response preceded by leukocyte infiltration (eosinophils, mast cells, T-cells, and uterine specific natural killer cells) and mediated through numerous cytokines (2, 6–8, 31). These latter changes in the endometrium are observed in the relatively high percentage of unique menstrual blood proteins found to participate in “multi-organism processes” (Fig. 6), a GO category that includes both inter- and intraspecies defense (the uterus, like all mucosa exposed to the external environment secrets mucus with defensive proteins to block pathogen invasion), as well as pregnancy, parturition, and mating (23). It is interesting to note that the one biological process found in whole menstrual blood that is not shared by either whole blood or vaginal fluid is locomotion (Fig. 6) which also includes subcategories consistent with the functional processes of the predecidua and menstruation including: “morphogenesis of an epithelium, inductive cell migration, cytokinesis, secretion by cells, germ cell development, reproduction and oogenesis” (23).
Complementary to and expanding on the biological processes identified by the gene ontology analysis discussed above (Figs. 6 and 7) are the specific proteins described in Table II. These proteins are involved in the dynamic processes of apoptosis and cell shedding, (e.g. death-inducer obliterator 1, MAP kinase-activating death domain protein and plexin-A1, D1 which blocks extracellular matrix adhesion and induces cell rounding), as well as endometrial renewal and angiogenesis (e.g. epithelial discoidin domain-containing receptor 1, thrombospondin type I domain containing 7A Wnt and ADAM33 an extracellular protease that acts in concert with plexins and semaphorins in the release of cells from the extracellular matrix, and also function in mucosal remodeling and angiogenesis). Two proteins were also identified that function in female reproduction, zona pellucida sperm-binding protein 4 (ZP4) which is found in the oocyte plasmalemma and human choriogonadotropin (hCG) subunit β. Although hCG is produced by the developing embryo, Zimmermann et al. (74) have demonstrated in tissue biopsies that “Epithelial human chorionic gonadotropin is expressed and produced in human secretory endometrium during the normal menstrual cycle.”
Of particular interest, however, are the fetal hemoglobins and additional proteins known to function in hematopoiesis. Evidence from the literature documents the rare occurrence of extramedullary uterine hematopoiesis, but it is typically associated with pathology—endometriosis, endometrial polyps, and leiomyomas—or the result of retained products of conception following spontaneous abortion or terminated pregnancy (32, 33). Some patients in which extramedullary uterine hematopoiesis is diagnosed are initially referred with symptoms of chronic anemia and are subsequently diagnosed with a myeloproliferative disease. Currently, there is debate as to whether identified uterine hematopoiesis is pathognomonic of an underlying myeloid disorder, and consequently, if it may be used for diagnostic purposes (32, 33). Recently Dassen et al. (34) have identified hemoglobins (immunohistochemically by a pan anti-human Hb antibody and molecularly for Hbs α, β, δ, and γ by RT-PCR), as well the heme metabolizing enzyme heme oxygenase 1 within the epithelia and stromal cells of the endometrium. They have postulated that hemoglobin biosynthesis within these nonerythroid cells functions to protect the endometrium against oxidative and nitrosative stress. It should be noted, however, that while these investigators found mRNAs for hemoglobins α, β, δ, and γ in their endometrial samples, neither they, nor other, have reported finding fetal hemoglobins ζ, θ, and μ in endometrial tissue (although Hb γ is a fetal hemoglobin, it has been reported to make up ∼0.4% of adult hemoglobins (35).
Although it is possible that hemoglobins ζ, θ, and μ were from a volunteer who was unknowing pregnant (pregnancy tests were not performed on volunteers, and erythropoiesis and hemoglobin production can occur in the yolk sac at 18 days post conception (36–38), it seems unlikely that a “late period” (unrecognized spontaneous abortion) was the source of these fetal hemoglobins for several reasons. First, the sample in which hemoglobins ζ, θ and μ (as well as all the other proteins involved in hematopoiesis and hCG) were identified was the same sample in which zona pellucida sperm-binding protein 4 was found. It seems unlikely that ZP4, an oocyte specific protein, was still present 18 days post conception. Second, Dassen et al. (34) identified individual hemoglobins by RT-PCR and chose primers only for α, β, δ, and γ; consequently they may have found ζ, θ, and μ had they looked. Finally, the menstrual blood donor did not report a late period. (It should be noted that hemoglobins α, β, δ, γ, as well as ε were all identified in the 1061 menstrual blood proteome (supplemental Table S1). However, as they were also found in venous blood and/or vaginal fluid they were not included in the 385 unique menstrual blood proteins evaluated in Table II.)
It is not possible to say whether the hematopoietic markers identified here are the result of pathology or are part of the normal menstrual cycle, although volunteers self-reported as healthy. Only by testing numerous individuals can a basic menstrual blood proteome be established with confidence. Testing of 50 additional women of various ages, ethnicities and with or without use of oral contraceptives is currently underway.
The menstrual cycle is a complex, finely orchestrated series of cellular, physiological, and biochemical changes under precise endocrine control that routinely builds a rich endometrial lining in constant anticipation of blastocyst implantation. Lack of implantation results in the controlled elimination of the endometrial lining only to be immediately replenished as the monthly cycle begins again. Each phase of the cycle—proliferative, secretory, and menstrual—has a specific function, and the proteins expressed during these phases reflect those functions (2, 3, 8). It has long been recognized that changes in the biochemistry or cellular composition of the endometrium can be diagnostic of uterine pathology and is often accompanied by abnormal menses (2, 8). Table II, for example, identifies several proteins have been associated with endometrioses as well as endometrial cancer. In recent years it has become equally as evident that such changes can influence fertility, or more precisely infertility (2). In the late secretory phase, as the uterus prepares for implantation, the stratum functionalis transitions to predecidua with concomitant expression of new or different levels of existing proteins, many of which have been proposed as markers of reduced uterine receptivity, including: integrins, matrix metalloproteases (MMPs), galectins (GALs), glucose transporter proteins (GLUT), interleukins (ILs), immune cell regulators, insulin-like growth factor-binding protein 1 (IGFBP-1), and glycodelin A (2, 39). Many of these proteins were detected in the menstrual blood proteome (supplemental Table S1, e.g. IGFBP-1, MMP-9, galectin-3, glycodelin A, GLUT1, IL1, and others) and may prove useful for diagnosis.
To our knowledge this is the first proteome analysis of menstrual blood. The two samples were chosen from mid-menstrual cycle for consistency as it seems likely that protein content will change during the course of a woman's period. Indeed, only by testing numerous women throughout their periods can a thorough menstrual blood proteome be established with confidence. Toward that end, testing of fifty additional women of various ages, ethnicities and with or without use of oral contraceptives is currently underway.
Menstrual blood is an easily obtainable body fluid and collection is noninvasive. The data presented here demonstrate that many proposed markers of uterine infertility are present in the menstrual blood proteome which could, consequently, be used for diagnostic purposes. Abnormal menstruation can be a sign of underlying uterine pathology, and here too a comparison against the normal menstrual blood proteome could prove a useful diagnostic tool. Finally, the detection of a distinct subset of 385 menstrual blood proteins not identified in venous blood or vaginal fluid constitutes an important list of candidate markers for the identification menstrual blood in forensic investigations.
Acknowledgments
We would like to thank Elisa Wurmbach for careful reading of the manuscript.
Footnotes
* This work was supported in part by award 2008-DN-BX-K011 and 2010-DN-BX-K192 funded by the National Institute of Justice, Office of Justice Programs, United States Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the Department of Justice.
 This article contains supplemental Tables S1 to S6.
This article contains supplemental Tables S1 to S6.
1 The abbreviations used are:
- Alb
- albumin
- CHCA
- α-cyano-4-hydroxycinnamic acid
- GALs
- galectins
- GLUT
- glucose transporter proteins
- GO
- gene ontology
- Hb
- hemoglobin
- IAA
- Iodoacetamide
- IGFBP-1
- insulin-like growth factor-binding protein 1
- IgG
- immunoglobulin G
- MMPs
- matrix metalloproteases
- NCS
- nucleolar canal system
- NTA
- Ni-nitrilotriacetic acid agarose beads
- RT-PCR
- reverse transcription polymerase chain reaction
- S1
- liquid menstrual blood sample
- S2
- Tampon extracted menstrual blood
- ZP4
- zona pellucida sperm-binding protein 4.
REFERENCES
- 1. Kao L. C., Germeyer A., Tulac S., Lobo S., Yang J. P., Taylor R. N., Osteen K., Lessey B. A., Giudice L. C. (2003) Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 144, 2870–2881 [DOI] [PubMed] [Google Scholar]
- 2. Strowitzki T., Germeyer A., Popovici R., von Wolff M. (2006) The human endometrium as a fertility-determining factor. Hum. Reprod. Update 12, 617–630 [DOI] [PubMed] [Google Scholar]
- 3. DeSouza L., Diehl G., Yang E. C., Guo J., Rodrigues M. J., Romaschin A. D., Colgan T. J., Siu K. W. (2005) Proteomic analysis of the proliferative and secretory phases of the human endometrium: protein identification and differential protein expression. Proteomics 5, 270–281 [DOI] [PubMed] [Google Scholar]
- 4. Ferenczy A. (2003) Pathophysiology of endometrial bleeding. Maturitas 45, 1–14 [DOI] [PubMed] [Google Scholar]
- 5. Garrido N., Navarro J., Garcia-Velasco J., Remoh J., Pellice A., Simón C. (2002) The endometrium versus embryonic quality in endometriosis-related infertility. Hum. Reprod. Update 8, 95–103 [DOI] [PubMed] [Google Scholar]
- 6. Lommel A. T. L. V. (2003) From Cells to Organs. Kluwer Academic Publishers, Boston [Google Scholar]
- 7. King A. E., Critchley H. O. (2010) Oestrogen and progesterone regulation of inflammatory processes in the human endometrium. J. Steroid Biochem. Mol. Biol. 120, 116–126 [DOI] [PubMed] [Google Scholar]
- 8. Ferenczy A., Mutter G. (2008) The endometrial cycle. Glob. Libr. Women's Med. 10.3843/GLOWM.10293 [DOI] [Google Scholar]
- 9. Li A., Felix J. C., Hao J., Minoo P., Jain J. K. (2005) Menstrual-like breakdown and apoptosis in human endometrial explants. Hum. Reprod. 20, 1709–1719 [DOI] [PubMed] [Google Scholar]
- 10. Wang T., Schneider J. (1992) Origin and fate of the nucleolar channel system of normal human endometrium. Cell Res. 2, 97–102 [Google Scholar]
- 11. Ringrose J. H., van Solinge W. W., Mohammed S., O'Flaherty M. C., van Wijk R., Heck A. J., Slijper M. (2008) Highly efficient depletion strategy for the two most abundant erythrocyte soluble proteins improves proteome coverage dramatically. J. Proteome Res. 7, 3060–3063 [DOI] [PubMed] [Google Scholar]
- 12. Haudek V. J., Slany A., Gundacker N. C., Wimmer H., Drach J., Gerner C. (2009) Proteome maps of the main human peripheral blood constituents. J. Proteome Res. 8, 3834–3843 [DOI] [PubMed] [Google Scholar]
- 13. Venkataraman N., Cole A. L., Svoboda P., Pohl J., Cole A. M. (2005) Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J. Immunol. 175, 7560–7567 [DOI] [PubMed] [Google Scholar]
- 14. Dasari S., Pereira L., Reddy A. P., Michaels J. E., Lu X., Jacob T., Thomas A., Rodland M., Roberts C. T., Jr., Gravett M. G., Nagalla S. R. (2007) Comprehensive proteomic analysis of human cervical-vaginal fluid. J. Proteome Res. 6, 1258–1268 [DOI] [PubMed] [Google Scholar]
- 15. Di Quinzio M. K., Oliva K., Holdsworth S. J., Ayhan M., Walker S. P., Rice G. E., Georgiou H. M., Permezel M. (2007) Proteomic analysis and characterisation of human cervico-vaginal fluid proteins. Aust. N. Z. J. Obstet. Gynaecol. 47, 9–15 [DOI] [PubMed] [Google Scholar]
- 16. Klein L. L., Jonscher K. R., Heerwagen M. J., Gibbs R. S., McManaman J. L. (2008) Shotgun proteomic analysis of vaginal fluid from women in late pregnancy. Reprod. Sci. 15, 263–273 [DOI] [PubMed] [Google Scholar]
- 17. Pereira L., Reddy A. P., Jacob T., Thomas A., Schneider K. A., Dasari S., Lapidus J. A., Lu X., Rodland M., Roberts C. T., Jr., Gravett M. G., Nagalla S. R. (2007) Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J. Proteome Res. 6, 1269–1276 [DOI] [PubMed] [Google Scholar]
- 18. Tang L. J., De Seta F., Odreman F., Venge P., Piva C., Guaschino S., Garcia R. C. (2007) Proteomic analysis of human cervical-vaginal fluids. J. Proteome Res. 6, 2874–2883 [DOI] [PubMed] [Google Scholar]
- 19. Zegels G., Van Raemdonck G. A., Coen E. P., Tjalma W. A., Van Ostade X. W. (2009) Comprehensive proteomic analysis of human cervical-vaginal fluid using colposcopy samples. Proteome Sci. 7, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaw J. L., Smith C. R., Diamandis E. P. (2007) Proteomic analysis of human cervico-vaginal fluid. J. Proteome Res. 6, 2859–2865 [DOI] [PubMed] [Google Scholar]
- 21. Gravett M. G., Thomas A., Schneider K. A., Reddy A. P., Dasari S., Jacob T., Lu X., Rodland M., Pereira L., Sadowsky D. W., Roberts C. T., Jr., Novy M. J., Nagalla S. R. (2007) Proteomic analysis of cervical-vaginal fluid: identification of novel biomarkers for detection of intra-amniotic infection. J. Proteome Res. 6, 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Casado-Vela J., Rodriguez-Suarez E., Iloro I., Ametzazurra A., Alkorta N., Garcia-Velasco J. A., Matorras R., Prieto B., González S., Nagore D., Simón L., Elortza F. (2009) Comprehensive proteomic analysis of human endometrial fluid aspirate. J. Proteome Res. 8, 4622–4632 [DOI] [PubMed] [Google Scholar]
- 23.DAVID. (Version 6.7) NIH Bioinformatics Resources. http://david.abcc.ncifcrf.gov.
- 24. Chadwick B. P., Willard H. F. (2001) Histone H2A variants and the inactive X chromosome: identification of a second macroH2A variant. Human Mol. Gen. 10, 1101–1113 [DOI] [PubMed] [Google Scholar]
- 25. Rougeulle C., Chaumeil J., Sarma K., Allis C. D., Reinberg D., Avner P., Heard E. (2004) Differential histone H3 Lys-9 and Lys-27 methylation profiles on the X chromosome. Mol. Cell. Biol. 24, 5475–5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goh S. H., Lee Y. T., Bhanu N. V., Cam M. C., Desper R., Martin B. M., Moharram R., Gherman R. B., Miller J. L. (2005) A newly discovered human alpha-globin gene. Blood 106, 1466–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leung S., Proudfoot N. J., Whitelaw E. (1987) The gene for theta-globin is transcribed in human fetal erythroid tissues. Nature 329, 551–554 [DOI] [PubMed] [Google Scholar]
- 28. Peschle C., Mavilio F., Carè A., Migliaccio G., Migliaccio A. R., Salvo G., Samoggia P., Petti S., Guerriero R., Marinucci M., et al. (1985) Haemoglobin switching in human embryos: asynchrony of zeta→alpha and epsilon→gamma-globin switches in primitive and definite erythropoietic lineage. Nature 313, 235–238 [DOI] [PubMed] [Google Scholar]
- 29. Schenk S., Schoenhals G. J., de Souza G., Mann M. (2008) A high confidence, manually validated human blood plasma protein reference set. BMC Med. Genomics 1, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paule S. G., Airey L. M., Li Y., Stephens A. N., Nie G. (2010) Proteomic approach identifies alterations in cytoskeletal remodelling proteins during decidualization of human endometrial stromal cells. J. Proteome Res. 9, 5739–5747 [DOI] [PubMed] [Google Scholar]
- 31. Weiss G., Goldsmith L. T., Taylor R. N., Bellet D., Taylor H. S. (2009) Inflammation in reproductive disorders. Reprod. Sci. 16, 216–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gru A. A., Hassan A., Pfeifer J. D., Huettner P. C. (2010) Uterine extramedullary hematopoiesis: what is the clinical significance?. Int. J. Gynecol. Pathol. 29, 366–373 [DOI] [PubMed] [Google Scholar]
- 33. Valeri R. M., Ibrahim N., Sheaff M. T. (2002) Extramedullary hematopoiesis in the endometrium. Int. J. Gynecol. Pathol. 21, 178–181 [DOI] [PubMed] [Google Scholar]
- 34. Dassen H., Kamps R., Punyadeera C., Dijcks F., de Goeij A., Ederveen A., Dunselman G., Groothuis P. (2008) Haemoglobin expression in human endometrium. Hum Reprod. 23, 635–641 [DOI] [PubMed] [Google Scholar]
- 35. Dover G. J., Boyer S. H. (1980) Quantitation of hemoglobins within individual red cells: asynchronous biosynthesis of fetal and adult hemoglobin during erythroid maturation in normal subjects. Blood 56, 1082–1091 [PubMed] [Google Scholar]
- 36. Tavian M., Péault B. (2005) Embryonic development of the human hematopoietic system. Int. J. Dev. Biol. 49, 243–250 [DOI] [PubMed] [Google Scholar]
- 37. Pereda J., Niimi G. (2008) Embryonic erythropoiesis in human yolk sac: two different compartments for two different processes. Microsc. Res. Tech. 71, 856–862 [DOI] [PubMed] [Google Scholar]
- 38. Palis J., Yoder M. C. (2001) Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp. Hematol. 29, 927–936 [DOI] [PubMed] [Google Scholar]
- 39. Kliman H. J., Honig S., Walls D., Luna M., McSweet J. C., Copperman A. B. (2006) Optimization of endometrial preparation results in a normal endometrial function test (EFT) and good reproductive outcome in donor ovum recipients. J. Assist. Reprod. Genet. 23, 299–303 [DOI] [PubMed] [Google Scholar]
- 40. van Goor H., Melenhorst W. B., Turner A. J., Holgate S. T. (2009) Adamalysins in biology and disease. J. Pathol. 219, 277–286 [DOI] [PubMed] [Google Scholar]
- 41. Yoshinaka T., Nishii K., Yamada K., Sawada H., Nishiwaki E., Smith K., Yoshino K., Ishiguro H., Higashiyama S. (2002) Identification and characterization of novel mouse and human ADAM33s with potential metalloprotease activity. Gene 282, 227–236 [DOI] [PubMed] [Google Scholar]
- 42. Castro-Sanchez L., Soto-Guzman A., Guaderrama-Diaz M., Cortes-Reynosa P., Salazar E. P. (2011) Role of DDR1 in the gelatinases secretion induced by native type IV collagen in MDA-MB-231 breast cancer cells. Clin. Exp. Metastasis 28, 463–477 [DOI] [PubMed] [Google Scholar]
- 43. Epithelial discoidin domain-containing receptor 1 (DDR1), UniProtKB, Q08345 (DDR1_HUMAN) Reviewed, UniProtKB/Swiss-Prot, http://www.uniprot.org/uniprot/Q08345
- 44. Fascin (FSCN1), Atlas of Genetics and Cytogenetics in Oncology and Haematology, http://atlasgeneticsoncology.org/Genes/FSCN1ID44342ch7p22.html
- 45. Kim M. K., Park H. J., Kim S. R., Choi Y. K., Shin H. K., Jeon J. H., Jang H. O., Yun I., Bae S. K., Bae M. K. (2010) Angiogenic role of orexin-A via the activation of extracellular signal-regulated kinase in endothelial cells. Biochem. Biophys. Res. Commun. 403, 59–65 [DOI] [PubMed] [Google Scholar]
- 46. Nakabayashi M., Suzuki T., Takahashi K., Totsune K., Muramatsu Y., Kaneko C., Date F., Takeyama J., Darnel A. D., Moriya T., Sasano H. (2003) Orexin-A expression in human peripheral tissues. Mol. Cell. Endocrinol. 205, 43–50 [DOI] [PubMed] [Google Scholar]
- 47. Barberis D., Artigiani S., Casazza A., Corso S., Giordano S., Love C. A., Jones E. Y., Comoglio P. M., Tamagnone L. (2004) Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J. 18, 592–594 [DOI] [PubMed] [Google Scholar]
- 48. Moretti S., Procopio A., Lazzarini R., Rippo M. R., Testa R., Marra M., Tamagnone L., Catalano A. (2008) Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by promoting Fas translocation into lipid rafts. Blood 111, 2290–2299 [DOI] [PubMed] [Google Scholar]
- 49. Plexin-A1, D1, UGID:228145, UniGene Hs. 432329, Homo sapiens (human) PLXNA1, UGID:181263, UniGene Hs, 301685. Homo sapiens (human), PLXND1 http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?UGID=228145
- 50. Shimizu-Hirota R., Sasamura H., Kuroda M., Kobayashi E., Saruta T. (2004) Functional characterization of podocan, a member of a new class in the small leucine-rich repeat protein family. FEBS Lett. 563, 69–74 [DOI] [PubMed] [Google Scholar]
- 51. Podocan (PODN), NCBI UniGene, UGID:1954930, UniGene Hs. 586141, http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=HsandCID=586141
- 52. Semaphorin-4B, 6B, 6C UGID:690094, UniGene Hs. 474935, Homo sapiens (human) SEMA4B, UGID:680801 UniGene Hs. 465642 Homo sapiens (human) SEMA6B, UGID:907063 UniGene Hs. 516316 Homo sapiens (human) SEMA6C http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=5912
- 53. Wang C. H., Su P. T., Du X. Y., Kuo M. W., Lin C. Y., Yang C. C., Chan H. S., Chang S. J., Kuo C., Seo K., Leung L. L., Chuang Y. J. (2010) Thrombospondin type I domain containing 7A (THSD7A) mediates endothelial cell migration and tube formation. J. Cell. Physiol. 222, 685–694 [DOI] [PubMed] [Google Scholar]
- 54. Sonderegger S., Pollheimer J., Knöfler M. (2010) Wnt signalling in implantation, decidualisation and placental differentiation—review. Placenta 31, 839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Y., Hanifi-Moghaddam P., Hanekamp E. E., Kloosterboer H. J., Franken P., Veldscholte J., van Doorn H. C., Ewing P. C., Kim J. J., Grootegoed J. A., Burger C. W., Fodde R., Blok L. J. (2009) Progesterone inhibition of Wnt/beta-catenin signaling in normal endometrium and endometrial cancer. Clin. Cancer Res. 15, 5784–5793 [DOI] [PubMed] [Google Scholar]
- 56. Liu X. F., Bera T. K., Liu L. J., Pastan I. (2009) A primate-specific POTE-actin fusion protein plays a role in apoptosis. Apoptosis 14, 1237–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bera T. K., Huynh N., Maeda H., Sathyanarayana B. K., Lee B., Pastan I. (2004) Five POTE paralogs and their splice variants are expressed in human prostate and encode proteins of different lengths. Gene 337, 45–53 [DOI] [PubMed] [Google Scholar]
- 58. Garcia-Domingo D., Ramirez D., González de Buitrago G., Martinez A. C. (2003) Death inducer-obliterator 1 triggers apoptosis after nuclear translocation and caspase upregulation. Mol. Cell. Biol. 23, 3216–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Death inducer-obliterator 1 (DIDO1), NCBI UniGene, UGID:907919, UniGene Hs. 517172, Homo sapiens cDNA http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=517172
- 60. MAP kinase-activating death domain protein (MADD), The Human Protein Atlas, http://www.proteinatlas.org/ENSG00000110514
- 61. MAP-kinase activating death domain, MADD by antibody CAB012647, The Human Protein Atlas, http://www.proteinatlas.org/ENSG00000177595/cancer/endometrial+cancer
- 62. Bourdon J. C., Renzing J., Robertson P. L., Fernandes K. N., Lane D. P. (2002) Scotin, a novel p53-inducible proapoptotic protein located in the ER and the nuclear membrane. J. Cell Biol. 158, 235–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Protein shisa-5, Genatlas:Gene Database, http://genatlas.medecine.univ-paris5.fr/fiche.php?symbol=SHISA5
- 64. Kharfi A., Labelle Y., Mailloux J., Akoum A. (2003) Deficient expression of tumor necrosis factor receptor type 2 in the endometrium of women with endometriosis. Am. J. Reprod. Immunol. 50, 33–40 [DOI] [PubMed] [Google Scholar]
- 65. Novembri R., Carrarelli P., Toti P., Rocha A. L., Borges L. E., Reis F. M., Piomboni P., Florio P., Petraglia F. (2011) Urocortin 2 and urocortin 3 in endometriosis: evidence for a possible role in inflammatory response. Mol. Hum. Reprod. 17, 587–593 [DOI] [PubMed] [Google Scholar]
- 66. Ting M. J., Boyd A. W. (2008) The role of eph receptor tyrosine kinases and ephrin ligands in hematopoietic cell development and function. Open Hematol. J. 2, 103–110 [Google Scholar]
- 67. Hafner C., Becker B., Landthaler M., Vogt T. (2006) Expression profile of Eph receptors and ephrin ligands in human skin and downregulation of EphA1 in nonmelanoma skin cancer. Mod. Pathol. 19, 1369–1377 [DOI] [PubMed] [Google Scholar]
- 68. Meziane el K., Randle S. J., Nelson D. E., Lomonosov M., Laman H. (2011) Knockdown of Fbxo7 reveals its regulatory role in proliferation and differentiation of haematopoietic precursor cells. J. Cell Sci. 124, 2175–2186 [DOI] [PubMed] [Google Scholar]
- 69. F-box only protein 7 (Fbxo7) UGID:131757, UniGene Hs. 5912, Homo sapiens (human), NCBI UniGene, http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=5912
- 70. Al-Mufti R., Hambley H., Farzaneh F., Nicolaides K. H. (2000) Fetal and embryonic hemoglobins in erythroblasts of chromosomally normal and abnormal fetuses at 10–40 weeks of gestation. Haematologica 85, 690–693 [PubMed] [Google Scholar]
- 71. McKenna H. J., Stocking K. L., Miller R. E., Brasel K., De Smedt T., Maraskovsky E., Maliszewski C. R., Lynch D. H., Smith J., Pulendran B., Roux E. R., Teepe M., Lyman S. D., Peschon J. J. (2000) Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 95, 3489–3497 [PubMed] [Google Scholar]
- 72. SL-Cytokine (FLT3), The Human Protein Atlas, http://www.proteinatlas.org/ENSG00000122025/normal/uterus,+pre-menopause
- 73. Aizencang G., Solis C., Bishop D. F., Warner C., Desnick R. J. (2000) Human uroporphyrinogen-III synthase: genomic organization, alternative promoters, and erythroid-specific expression. Genomics 70, 223–231 [DOI] [PubMed] [Google Scholar]
- 74. Zimmermann G., Ackermann W., Alexander H. (2009) Epithelial human chorionic gonadotropin is expressed and produced in human secretory endometrium during the normal menstrual cycle. Biol. Reprod. 80, 1053–1065 [DOI] [PubMed] [Google Scholar]
- 75. Prasad S. V., Skinner S. M., Carino C., Wang N., Cartwright J., Dunbar B. S. (2000) Structure and function of the proteins of the mammalian Zona pellucid. Cells Tissues Organs 166, 148–164 [DOI] [PubMed] [Google Scholar]
- 76. Zona pellucida sperm-binding protein 4 (ZP4), Q12836 (ZP4_HUMAN), UniProtKB/Swiss-Prot Reviewed, http://www.uniprot.org/uniprot/Q12836