Abstract
Despite of the progress in identifying many Lys acetylation (Kac) proteins, Kac substrates for Kac-regulatory enzymes remain largely unknown, presenting a major knowledge gap in Kac biology. Here we identified and quantified 4623 Kac sites in 1800 Kac proteins in SIRT1+/+ and SIRT1−/− MEF cells, representing the first study to reveal an enzyme-regulated Kac subproteome and the largest Lys acetylome reported to date from a single study. Four hundred eighty-five Kac sites were enhanced by more than 100% after SIRT1 knockout. Our results indicate that SIRT1 regulates the Kac states of diverse cellular pathways. Interestingly, we found that a number of acetyltransferases and major acetyltransferase complexes are targeted by SIRT1. Moreover, we showed that the activities of the acetyltransferases are regulated by SIRT1-mediated deacetylation. Taken together, our results reveal the Lys acetylome in response to SIRT1, provide new insights into mechanisms of SIRT1 function, and offer biomarker candidates for the clinical evaluation of SIRT1-activator compounds.
Lysine (Lys)1 acetylation is a dynamic, reversible, and evolutionarily conserved protein post-translational modification (PTM). After the discovery of Lys acetylation in histones more than forty years ago (1), early studies mainly focused on histones and transcription factors, establishing the modification's fundamental role in DNA-templated biological processes (2, 3). The discovery of Lys acetylation in tubulin and the presence of sirtuins in mitochondria argued that Lys acetylation may not be restricted to nuclei (4–6). The complexity of Lys acetylomes outside the nuclei and the high abundance of the PTM in mitochondria revealed by proteomics studies suggested that the regulatory functions of this PTM may mirror those of protein phosphorylation (7–9).
Lys acetylation is regulated by two groups of enzymes with opposing activities, lysine acetyltransferases and deacetylases (10). Sirtuins are a family of highly conserved NAD+-dependent deacetylases (11, 12). Numerous studies, mainly focusing on the family's founding member, SIRT1 in mammals or Sir2, the enzyme's homolog in yeast, show that sirtuins regulate diverse cellular functions and appear to affect a variety of aging-related diseases, such as cancer, metabolic diseases, and inflammation and are involved in pathways such as metabolisms, oxidative stress, DNA damage, cell cycle, and signaling (12–15). A number of protein substrates for SIRT1 have been identified including p53, DNA methyltransferase 1 (DNMT1), NF-κB, forkhead transcription factors, PGC-1α, and histones (16–22).
Despite significant progress in the past decade, the molecular mechanisms by which SIRT1 regulates cellular physiology are not well understood. A major hurdle in our understanding of SIRT1 biology is our incomplete knowledge of Lys acetylation proteins that mediate SIRT1 functions. Because SIRT1 is a lysine deacetylase, a major mechanism for SIRT1 to exert its functions should be its deacetylation activity. However, SIRT1-induced lysine acetylation proteins are far from complete. This knowledge limitation also exists with other deacetylases as well as with lysine acetyltransferases, representing a major challenge in Lys acetylation biology and in evaluating clinical compounds that target dysregulated Lys-acetylation regulatory enzymes.
Here we report the first proteomics quantification of Lys acetylation in response to a regulatory enzyme. Our study identified 4623 Lys acetylation sites from SIRT1+/+ and SIRT1−/− MEF cells, among which 4130 Lys acetylation sites were quantified. The data identified multiple pathways that are affected by SIRT1. From this wealth of new information on the SIRT1-modulated Lys acetylome, we discovered consensus motifs for SIRT1-mediated Lys acetylation sites and identified extensive correlation between Lys acetylation and phosphorylation, SUMOylation, and mutations associated with disease, suggesting potential cross-talks between Lys acetylation with other modification and mutations. We further demonstrated that SIRT1 regulates diverse lysine acetyltransferases and their associated protein complexes through deacetylation, which further impacts their enzymatic activities. Overall, the SIRT1-response Lys acetylome generated in this study provides a rich resource for the future functional dissection of SIRT1-dependent cellular pathways.
EXPERIMENTAL PROCEDURES
Stable Isotope Labeling in Cultured Cell Lines
The SIRT1−/− MEF cells derived from SIRT1−/− mouse (23) (a kind gift from Dr. Fred W. Alt and Hwei-Ling Cheng). Proteins in SIRT1+/+ and SIRT1−/− MEF cells were labeled with either “heavy” or “light” isotopic lysine using a SILAC Protein Quantitation Kit (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. Briefly, two cell lines were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and either the “heavy” form of [U-13C6]-l-lysine or “light” [U-12C6]-l-lysine for more than six generations before being harvested, to achieve more than 99% labeling efficiency.
Preparation of Cell Lysates
Cells were harvested and washed twice with cold phosphate-buffered saline. The cells were lysed in 2× NETN buffer (40 mm Tris, pH 8.0, 200 mm NaCl, 2 mm EDTA, 1% Nonidet P-40) on ice for 20 min. Cell lysates were centrifuged at 16,000 × g for 15 min at 4 °C. The supernatant was collected as an NETN-soluble fraction and the pellet was further lysed by SDS buffer (containing 62.5 mm Tris-HCl, pH 6.8, and 2% SDS) with sonication to generate an NETN-pellet fraction. Protein concentrations were measured using Bradford assay. Equal amounts of protein from the NETN-soluble fractions of SIRT1+/+ and SIRT1−/− cells were mixed and separated on preparative high-performance liquid chromatography (HPLC) into 20 fractions using a polyCAT/WAX ion exchange column (Shimadzu, Kyoto, Japan). Proteins from each HPLC fraction were precipitated using trichloroacetic acid/acetone. The resulting protein precipitate was washed twice with ice-cold acetone and stored at −20 °C before in-solution tryptic digestion. Equal amounts of protein from the NETN-pellet fractions of SIRT1+/+ and SIRT1−/− cells were mixed and dialyzed overnight against phosphate-buffered saline to remove SDS. Proteins were then precipitated by the TCA/acetone method and washed with ice-cold acetone prior to in-solution tryptic digestion.
In-solution Digestion of Proteins and Enrichment of Lysine Acetylated Peptides
The precipitated proteins were digested with trypsin using a procedure previously described (24). To enrich Lys-acetylated peptides, the trypic digest in NETN buffer (100 mm NaCl, 1 mm EDTA, 50 mm Tris-HCl, 0.5% Nonidet P-40, pH 8.0) was incubated with anti-acetyllysine agarose beads (PTM Biolabs Inc., Chicago, IL) at 4 °C for 4 h with gentle shaking. The beads were washed four times with 1 ml of NETN buffer and twice with ddH2O. The bound peptides were eluted from the beads with 1% trifluoroacetic acid. The eluted fractions were combined and dried in a SpeedVac (ThermoFisher Scientific, Waltham, MA). The peptides from the NETN-soluble fractions were analyzed by HPLC-MS/MS, whereas the peptides from the NETN-pellet fractions were resolved into 12 fractions in a 3100 OFFGEL Fractionator (Agilent Technologies, Inc., Santa Clara, CA) according to the manufacturer's instructions. Each fraction was cleaned in a C18 ZipTip (Millipore Corp., Billerica, MA) before HPLC/MS/MS analysis.
HPLC-MS/MS Analysis of Tryptic Peptides
HPLC/MS/MS analysis was performed on an Eksigent nanoLC-1D plus pump (Eksigent Technologies, Dublin, CA) coupled online to an LTQ Orbitrap Velos mass spectrometer (ThermoFisher Scientific, Waltham, MA). Peptides were dissolved in HPLC solvent A (0.1% formic acid in water), directly loaded onto a reversed-phase column (360 μm OD × 75 μm ID) packed in-house with Jupiter Proteo resin (4 μm particle, 90 Å pore size, Phenomenex, Torrance, CA), and eluted with a linear gradient of 5–30% HPLC solvent B (0.1% formic acid in acetonitrile) at a constant flow rate of 200 nL/min. Eluted peptides were electrosprayed into LTQ Velos dual-pressure linear ion trap mass spectrometers (ThermoFisher Scientific, Waltham, MA) operating in a data-dependent mode that acquired MS/MS spectra for the top 20 most intense ions. Full MS was acquired using FTMS in the Orbitrap at 60,000 resolution at 400 m/z, and MS/MS was acquired using collision-induced dissociation (CID) in the Velos at 35% normalized collision energy. A lock-mass ion from ambient air (m/z 445.120024) was used for internal calibration of all full-scan measurements with the Orbitrap detector as described (25). Dynamic exclusion was enabled with repeat count of 2, repeat duration of 8 s, exclusion duration of 60 s, and exclusion mass width including ±0.01% relative to the reference mass. Details for data analysis are described in supplemental Information.
Public Access to Mass Spectrometry Data
All mass spectrometry data published in this study are publically accessible. Please refer to Supplementary method for detail instructions.
RESULTS
Experimental Strategy for Quantification of the Lys Acetylome
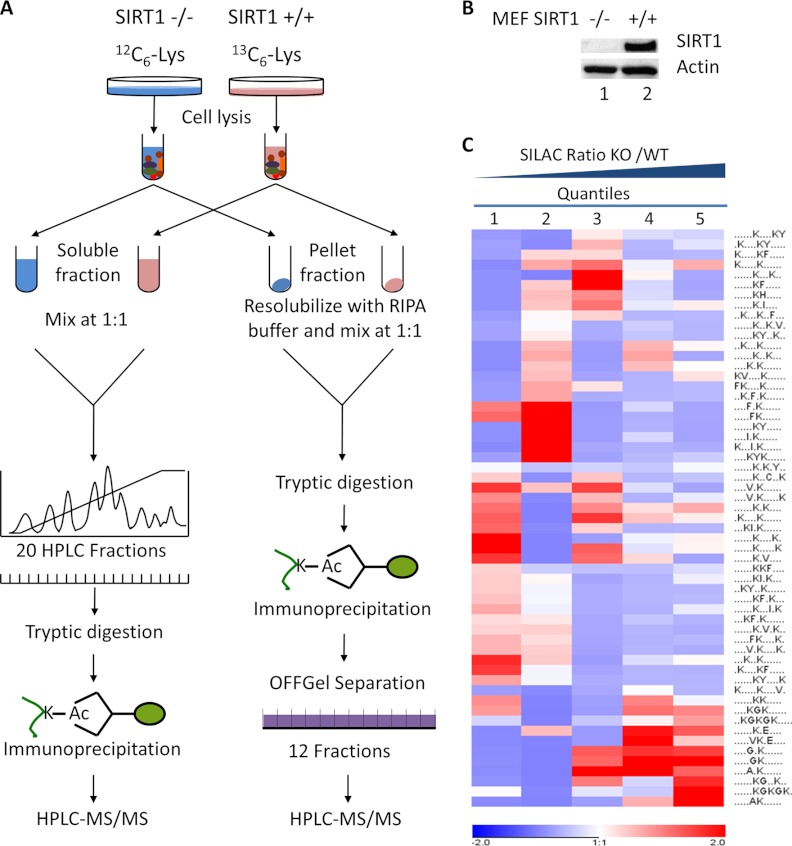
To quantify the changes of Lys acetylation in response to SIRT1, SIRT1+/+ and SIRT1−/− MEF cells were grown using stable-isotope labeling with amino acids in cell culture (SILAC), containing “heavy” (Lys6) or “light” (Lys0) isotopic forms of lysine (26). From each type of culture, we generated two protein fractions, an NETN-soluble fraction and an NETN-pellet fraction, using two sequential steps of cell lysis with NETN buffer and SDS-lysis buffer. Proteins in NETN-soluble fractions generated from the “light” and “heavy” cells were mixed in equal amounts and resolved into 20 HPLC fractions using ion-exchange chromatography. The proteins in each fraction were precipitated and digested with trypsin (Fig. 1A). The lysine-acetylated peptides were enriched using immobilized anti-acetyllysine antibodies. In a parallel experiment, proteins in NETN-pellet fractions from “light” and “heavy” cells were combined in equal amounts and proteolytically digested. Subsequently, Lys-acetylated peptides were enriched and resolved into 12 fractions using isoelectric fractionation (Fig. 1A).
Fig. 1.
The experimental strategy for identification and quantification of Lys acetylation sites in mouse embryonic fibroblast (MEF) cells. A, MEF SIRT1+/+ (WT) and SIRT1−/− (KO) cells were grown in SILAC media with U-13C6-Lys and U-12C6-Lys respectively. Cells were sequentially lysed, generating two fractions: an NETN-soluble fraction and an NETN-pellet fraction. For each fraction, equal amounts of proteins from the two pools of cells were mixed. Proteins from NETN-soluble fractions were further resolved by ion-exchange chromatography, followed by tryptic digestion and immunoprecipitation using anti-Kac pan-antibodies. The combined proteins from NETN-pellet fractions were digested by trypsin. The resulting peptides were immunoprecipitated by anti-Kac pan-antibodies followed by OFFGel fractionation. The enriched Kac-containing peptides in each fraction were subjected to HPLC/MS/MS analysis. B, Western blot analysis of protein whole-cell lysates from MEF SIRT1+/+ and SIRT1−/− cells. C, Clustering analysis of Lysine acetylation motifs among different quantiles. Lys acetylation motifs were identified using Motif-x for all Lys acetylation sites and for sites in each quintile with Bonferroni corrected p < 0.05. HyperG test was performed for the enrichment test of each motif in all quantiles and p value was transformed into Z-score for hierarchical clustering analysis.
The enriched peptides were analyzed using a nano-HPLC/LTQ Orbitrap mass spectrometer. The resulting MS/MS data were processed using the Mascot search engine (v2.2) (27) and Maxquant software (v1.0.13.13) (28) with an overall false discovery rate for peptides of less than 1%. We identified 5832 Lys acetylation sites using this criterion. We then applied the highest possible stringency to improve the reliability of our proteomics analysis. We removed 886 Lys acetylation sites with low Mascot scores (using 20.0 as a cutoff threshold) (supplemental Table S1A, S1B), including 167 sites with Mascot scores below 10.0 and 719 sites between 10.0 and 20.0 (supplemental Table S1C). We then eliminated site-redundant peptides.
Using this procedure, we identified 4623 nonredundant Lys acetylation sites in 1800 proteins. Over 94% of these sites are conserved between mouse and human. The average mass error for acetylated peptides was 0.8 ppm with a standard deviation of only 0.7 ppm. The largest Lys acetylation data set previously revealed by a proteomics study on a single cell line contains 2455 Lys acetylation sites from the human Jurkat cell line after treatment of HDAC inhibitors (1% false discovery rate, and removing hits with Mascot score lower than 10.0) (8). Our study therefore represents a roughly 100% increase in the number of Lys acetylation sites collected in a single experiment. In addition, by including Ser, Thr, and Tyr phosphorylation as variable modifications during database searching, we also identified and manually verified five phosphorylation sites that colocalized with Lys acetylation in same tryptic peptides.
The Proteome of Lysine Acetylation in MEF Cells
Similar to previous findings (7, 8), our data suggest that Lys-acetylated proteins are significantly enriched in cellular processes such as metabolism (p < 10−150), gene expression (p < 10−80), and translation (p < 10−60) (supplemental Table S2A). Using the CORUM database, a manually curated database of mammalian proteins, we identified 57 complexes that were significantly enriched in our data set (p < 0.05), including the 20S proteosome (p < 10−13), the chaperonin complex (p < 10−8), the Ksr1-MEK-MAPK complex (p < 10−5), the SWI/SNF-related (PYR) chromatin remodeling complex (p < 10−5), the Dorsha complex (p < 10−5), the MCM complex (p < 10−4), and the SWAP complex (p < 10−4) (supplemental Table S2B). Using a more comprehensive protein-protein interaction network database, STRING, and a complex detection algorithm, MCODE, we detected 87 protein-protein interaction networks enriched in our data set (p < 0.05), including a ribosomal protein network (p < 10−20), an mRNA splicing network (p < 10−20), EGF stimulation networks (p < 10−20) and a tRNA synthetase network (p < 10−6) (supplemental Table S2C).
Quantification Analysis of Lys Acetylated Peptides
Of the 4623 Lys acetylation sites identified in this study, 4130 sites were quantifiable. More than 85% of acetylation sites (3529 sites) exhibited less than a twofold change between SIRT1 KO and WT cells. Acetylation at 485 sites was at least twofold or higher in SIRT1 knockout cells (supplemental Table S1). We also performed a quantitative comparison of protein expression between SIRT1 KO, and WT cells. Our results showed that only 3.3% of total quantifiable proteins exhibited SILAC ratio changes of more than twofold, suggesting that SIRT1 knockout does not significantly impact global protein expression levels (supplemental Table S3). To demonstrate the reproducibility of the quantification analysis, we performed a biological replicate analysis with reverse labeling of the two cell lines and carried out a one-time immunoprecipitation followed by HPLC/MS/MS analysis. The correlation of Log2 SILAC quantification ratios between the two studies showed a Pearson correlation coefficient of 0.82, suggesting good reproducibility of our quantification data (supplemental Fig. S1A). Moreover, the acetylation level of several known targets of SIRT1 the K383 site on the tumor repressor gene p53 (Trp53) and the repeating glycine-lysine dipeptide region (the GK linker) in DNA methyltransferase 1 (DNMT1) increased by more than fivefold and twofold respectively, in SIRT1 KO cells (supplemental Table S1B), further demonstrating the reliability of the quantification results. Consistent with the previous observations, Kac in histones are not changed (29, 30).
To examine the nature of the SIRT1-induced Lys acetylome, we divided the acetylation data set into five quantiles, four of which were based on the cumulative distribution of SIRT1 KO/WT Log2 SILAC ratios (less than 15%, 15–50%, 50–85%, more than 85%), and one quantile for the sites identified only in SIRT1 knockout cells. An enrichment analysis was performed separately in each quantile for diverse categories and the overrepresented annotations were clustered through one-way hierarchical clustering for comparative analysis.
Motif Analysis for Acetylation Sites Regulated by SIRT1
Previous studies have shown preferences for particular amino acids at positions surrounding Lys acetylation sites (7, 8, 31). The reliability and accuracy of these studies depend on the size of the input data set. The large data sets collected in this study enabled us to perform a high-powered motif analysis. Using the Motif-x program (32), we extracted a total of 57 motifs with Bonferroni-corrected p values of less than 0.05 (Fig. 1C and supplemental Fig. S1B). The motif set covers all the previously known residue preferences for Lys acetylation, including the GK* motif and the K*F/K*Y motif (K* refers to the modified Lys) (7). No KK* motif, with K at the –1 position, was identified, consistent with previous findings (8). Interestingly, we identified 20 paired motifs containing two lysine residues in which either lysine could be modified, such as FK*xxxxK and FKxxxxK*.
To discover the potential consensus motif for SIRT1-mediated Lys acetylation sites, we extracted acetylation data for each of the 57 motifs and calculated the enrichment of the motifs in each of the five quantiles described above. The data were clustered using a one-way hierarchical clustering method. This analysis identified multiple motifs regulated by SIRT1 (Fig. 1C). For example, motifs with a small nonpolar amino acid at position –1 or –2 relative to the acetylated Lys (e.g. GK*, AK*, GxK*, where K* indicates the acetylated Lys and x any amino acid), and the motif with E at the +2 position (i.e. K*xE) are significantly enriched in quantiles with increased acetylation levels in SIRT1 knockout cells (supplemental Fig. S1B). Among the motifs, only the GK* motif has been previously associated with SIRT1 (31). On the other hand, motifs with bulky amino acids in the vicinity of the acetylated Lys, such as K*F, K*Y, IxK*, or FK*, are largely enriched in quantiles with little change in acetylation level upon SIRT1 knockout. Our results therefore uncover potential sequence preferences for the acetylation sites that are regulated by SIRT1.
Biological Functions Regulated by SIRT1
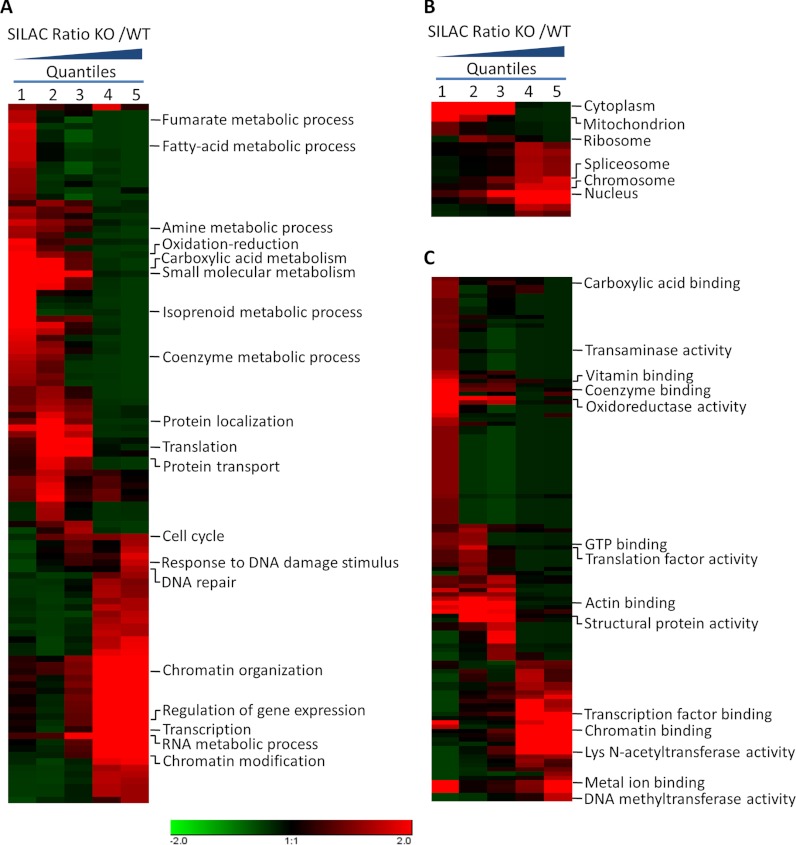
To elucidate the cellular functions regulated by SIRT1, we tested the data set for enrichment in three Gene Ontology (GO) categories: molecular function, cellular compartment, and biological process (Fig. 2A–2C and supplemental Fig. S2A–S2C). In the biological process category, processes related to transcription, gene expression, and mRNA splicing are significantly enriched in quantiles with high KO/WT SILAC ratios. This pattern suggests that the SIRT1 knockout significantly affected the acetylation level in these processes and that the enzyme is involved in diverse transcription-related cellular functions. In agreement with this observation, the analysis by cellular compartment showed that the SIRT1 knockout produced a more profound impact on the acetylation level among nuclear proteins. Moreover, the analysis of molecular functions showed that proteins involved in the binding of transcription activators, DNA binding, and chromatin binding, as well as lysine N-acetyltransferases, were enriched in quantiles with increased acetylation levels in SIRT1 knockout cells.
Fig. 2.
Enrichment and clustering analysis of the Lys acetylation data sets based on Gene Ontology annotations. Genes were classified by Gene Ontology annotation based on three categories: A, biological process, B, cellular compartment, and C, molecular function. In each category, quantification ratios of all Kac sites for each gene were divided into five quantiles, four of which were based on accumulative normal distribution (0–15%, 15–50%, 50–85%, 85–100%). The fifth quantile consisted of genes with Kac sites identified only in SIRT1−/− cells. An enrichment analysis was performed using the HyperG test with Benjamini-Hochberg adjustment. The p values were transformed into z-scores prior to hierarchical clustering analysis.
Analysis of Dynamics in Protein Domains by SIRT1 Knockout
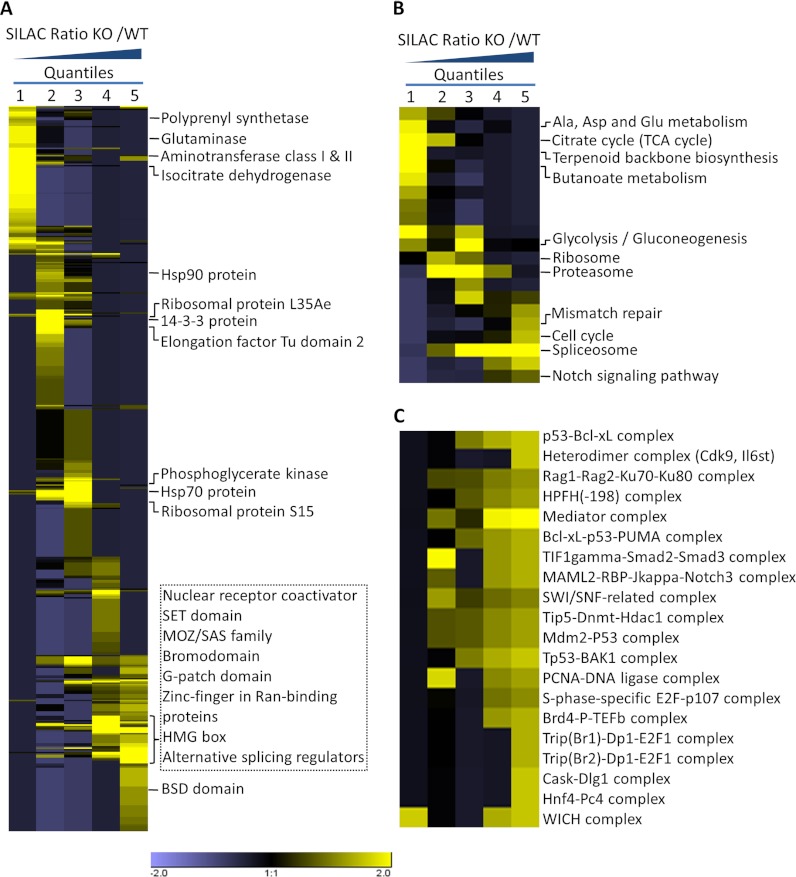
Protein functions are largely determined by specific domain structures in the sequence. To reveal the domain structures most regulated by SIRT1 knockout, we performed PFAM domain enrichment analysis on the five SILAC ratio quantiles (Fig. 3A and supplemental Fig. S3A). In agreement with our findings for molecular function and biological processes, our data showed that protein domains involved in transcription, including the nuclear receptor coactivator domain, zinc finger domains, and domains involved in RNA processing (including the G-patch domain and alternative splicing regulators), are enriched in quantiles with increased acetylation. Interestingly, we observed a significant enrichment of bromo domain and MOZ/SAS families in quantiles exhibiting increased acetylation in SIRT1 knockout cells. This finding, together with the observation of increased acetylation of lysine N-acetyltransferases in the molecular function enrichment analysis, suggests that SIRT1 may exert its cellular function by regulating acetyltransferases and further modulating the functions of proteins containing Lys acetylation recognition domains. We extracted all of the lysine N-acetyltransferases identified in our data set (Table I) and discovered that acetylation levels of the MYST acetyltransferase family were most affected by SIRT1 knockout. This result is in agreement with previous findings that Kat5 (Tip60) and Kat8 (Myst1), two of the MYST family proteins, are SIRT1 substrates that are negatively regulated by SIRT1 (33–36). Moreover, we showed that Ing3, a known member of Kat7 (Myst2) protein complex, demonstrates an over 10-fold increase in acetylation level at two sites (K181, K264) in SIRT1 knockout cells.
Fig. 3.
Enrichment and clustering analysis of Kac substrate proteins based on protein domains, cellular pathways, and protein complexes. Genes were annotated based on (A) the PFAM domain database, (B) the KEGG pathway database, and (C) the CORUM protein complex database. The analysis was carried out as described in Fig. 2. In addition, k-means clustering was used to identify 20 SIRT1-regulated mouse protein complexes.
Table I. Quantification of SIRT1-modulated Lys acetylation in known acetyltransferases, including Ep300, Crebbp, Ncoa3, Hat1, Kat14 (Csrp2bp), Kat5, Kat8 (Myst1), Kat7 (Myst2), Kat6a (Myst3), and Kat6b (Myst4).
“Known site” refers to Kac sites previously identified in either human or mouse based on the Uniprot (http://www.uniprot.org) and PhosphoSite (http://www.phosphosite.org) databases. Kat# for acetyltransferases are based on published nomenclature for chromatin-modifying enzymes (66). * Kac sites with Mascot scores below 20.0.
| Acetyltransferases | Gene name/Kat# | Acetylation position | Ratio KO/WT | Known site? |
|---|---|---|---|---|
| Nuclear receptor coactivator 3 | Ncoa3/Ncoa3 | 608 | 8.42 | Yes |
| 611 | 8.06 | Yes | ||
| 612 | 2.62 | Yes | ||
| Histone acetyltransferase type B catalytic subunit | Hat1/Hat1 | 6 | 2.48 | |
| Cysteine-rich protein 2-binding protein | Csrp2bp/Kat14 | 275 | KO only | |
| Histone acetyltransferase KAT5 | Kat5/Kat5 | 85 | 8.38 | Yes |
| MYST histone acetyltransferase 1 | Myst1/Kat8 | 113* | 15.61 | Yes |
| 154 | 17.7 | |||
| 168 | 8.16 | |||
| 351 | 0.94 | |||
| 410* | 2.31 | |||
| MYST histone acetyltransferase 2 | Myst2/Kat7 | 157 | 1.88 | Yes |
| 201 | 13.76 | |||
| 223 | 10.17 | |||
| 279* | KO only | |||
| 282* | KO only | |||
| MYST histone acetyltransferase 3 | Myst3/Kat6a | 94 | 2.47 | |
| 355 | 0.75 | Yes | ||
| 406 | KO only | |||
| 414 | KO only | Yes | ||
| 813 | 1.23 | |||
| 816 | 1.23 | Yes | ||
| 840 | 8.85 | |||
| MYST histone acetyltransferase 4 | Myst4/Kat6b | 380 | 2.91 | |
| 394 | 2.91 | |||
| 433 | KO only | |||
| 441 | KO only | Yes | ||
| E1A binding protein p300 | Ep300/Ep300 | 351* | 5.39 | Yes |
| 637* | 1.98 | Yes | ||
| 1179 | 0.96 | Yes | ||
| 1541 | 1.43 | Yes | ||
| 1545 | 1.55 | Yes | ||
| 1553 | 1.57 | Yes | ||
| 1554 | 1.57 | Yes | ||
| 1557 | 1.44 | Yes | ||
| 1559 | 1.57 | Yes | ||
| 1589 | 1.01 | Yes | ||
| 1673 | 1.55 | Yes | ||
| CREB binding protein | Crebbp/Crebbp | 365* | 5.39 | |
| 1217 | 1.19 | Yes | ||
| 1584 | 1.9 | Yes | ||
| 1587 | 1.9 | Yes | ||
| 1592 | 2.54 | Yes | ||
| 1593 | 2.48 | Yes | ||
| 1596 | 2.48 | Yes | ||
| 1598 | 2.48 | Yes | ||
| 1621 | 1.01 | |||
| 1628 | 1.01 | Yes | ||
| 1712 | 1.55 | Yes | ||
| 1742 | 2.52 | Yes | ||
| 1745 | 0.79 | Yes | ||
| 1938 | 1.18 | |||
| 2092 | KO only |
The impact of SIRT1 Knockout on Cellular Pathways
To identify cellular pathways regulated by SIRT1, we performed a pathway clustering analysis of the SIRT1-reponse Lys acetylome for pathways from the Kyoto Encyclopedia of Genes and Genomes (KEGG). Our data showed that DNA repair, the cell cycle, Notch signaling, and RNA splicing are the most prominent pathways enriched in quantiles with increased acetylation levels in SIRT1 knockout cells, suggesting a role of SIRT1 in these pathways (Fig. 3B and supplemental Fig. S3B). In ribosome, proteasome, and glycolysis pathways, acetylation levels were not significantly affected by SIRT1 knockout.
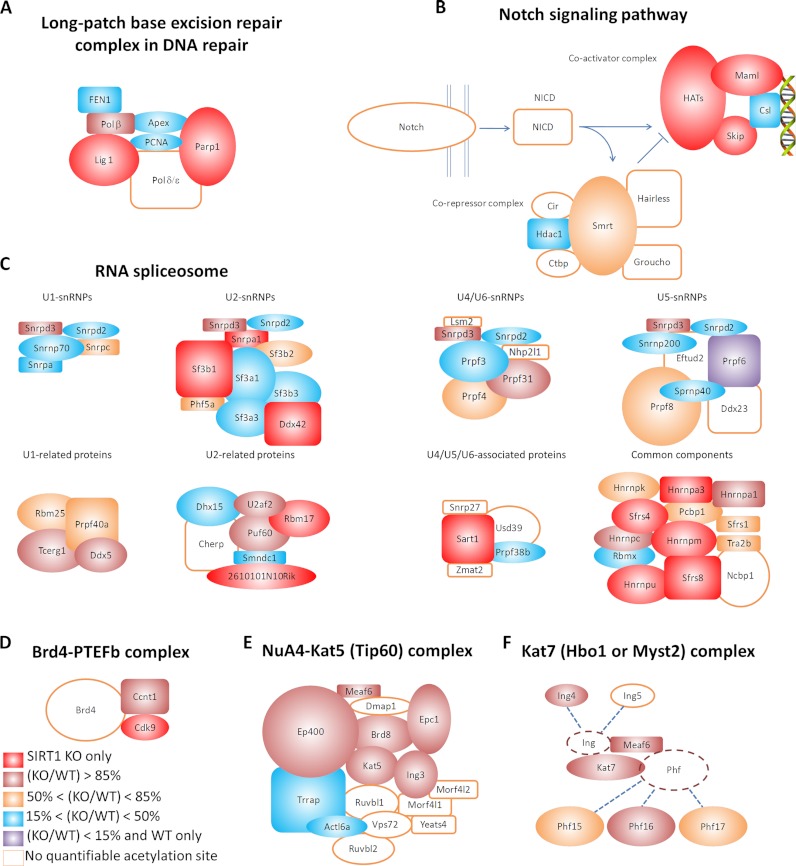
DNA repair is a crucial cellular pathway that autonomously maintains genome integrity and previously known to be regulated by SIRT1 (37, 38). Among various types of repair mechanisms, our study showed that SIRT1 may play a profound role in regulating base excision repair (BER) and mismatch repair (MR), two types of single-strand DNA repair mechanisms. Six out of eight subunits in the long patch BER repair complex are Lys-acetylated and the abundance of Lys acetylation increased by more than 9-fold on Polβ and Lig1 after SIRT1 knockout (Fig. 4A). Three acetylation sites on Lig1 and Parp1 were identified only in SIRT1 knockout cells. Three out of six major active components in the mismatch repair system are Lys-acetylated, whereas K377 on Mlh1 and K431 on Pms2 were identified to be Lys-acetylated only in SIRT1 knockout cells.
Fig. 4.
Impact of SIRT1 knockout on selected cellular pathways and protein complexes. SIRT1 modulates acetylation levels on (A) the subunits of the long-patch base excision repair (BER) complex in the DNA repair pathway, (B) Notch co-activator and corepressor complexes in the Notch signaling pathway, (C) the RNA spliceosome in the RNA splicing pathway, (D) the Brd4-PTEFb complex, (E) the NuA4-Kat5 (Tip60) complex, and (F) the Kat7 (Hbo1) protein complex. Each gene is color-coded based on the highest SILAC KO/WT ratios for the gene.
In mammalian cells, cell cycle progression are controlled by a set of proteins including cyclins, cyclin-dependant kinases (CDKs), origin-recognition complex, and mini-chromosome maintenance complex. Our data showed that acetylation of K67 on cyclin A2, a ubiquitously expressed cyclin, is nearly doubled upon SIRT1 knockout. Five out of six subunits in the mini-chromosome maintenance complex are Lys-acetylated. Acetylation of K857 and K818 on Mcm4 increased by at least 40% upon SIRT1 knockout, and one acetylation site (K173) on Mcm6 was identified only in SIRT1 knockout cells.
The Notch signaling pathway is essential for cellular communication and differentiation, recently shown to be regulated by SIRT1 (39). Our data showed that SIRT1 regulates Lys acetylation on multiple components of the Notch co-activator and corepressor complexes (Fig. 4B). All subunits in the Notch co-activator complex are Lys-acetylated. Acetylation of five sites on Maml2 and five sites on Snw1 were nearly tripled upon SIRT1 knockout. Three subunits in the Notch corepressor complex are Lys-acetylated. Although Lys acetylation on Hdac1 and Hdac2 were not affected by SIRT1 knockout, the abundance of acetylation on Ncor2 increased by 10–60% upon SIRT1 knockout. Recent studies have shown that SIRT1 plays an inhibitory role in Notch signaling through deacetylation of NICD (39). Therefore, our data provides evidence for additional mechanisms by which SIRT1 may be involved in the regulation of the Notch signaling pathway.
RNA spliceosome proteins are known to be acetylated in human cells (7, 8). In this study, we found that acetylation of 30 proteins in the RNA spliceosome increased upon SIRT1 knockout (Fig. 4C). Acetylation of 19 proteins was significantly increased in SIRT1 knockout cells, including U2af2, Sf3b1, Snw1, Snrpa1, Rbm17, Sart1, Hnrnpu, Hnrnpa1, and Hnrnpm. Our data therefore reveal a potentially important role of SIRT1 in the RNA splicing pathway.
SIRT1 Regulation of Protein Complexes
Lys acetylation is known to target large protein complexes and to potentially regulate their cellular functions (8). Using a manually curated CORUM database, we first performed enrichment analysis on protein complexes and then used k-means clustering to identify the specific subgroups that are most impacted by SIRT1 knockout. We obtained 20 complexes with significant enrichment in quantiles with increased acetylation levels in SIRT1 knockout cells (Fig. 3C). These complexes can be considered to be SIRT1-regulated core complexes, three of which are described below.
First, the mediator coactivator complex is an essential transcription coactivation complex bridging gene activator proteins and the pre-initiation complex. It has been known to interact with acetyltransferases, such as Ep300 (p300) (40, 41). We found that three subunits of this protein complex (Med1, Med6, and Thrap3) exhibit significantly higher acetylation levels upon SIRT1 knockout. Seven Lys acetylation sites were identified on Thrap3, among which acetylation of four sites (K252, K524, K555, and K706) was more than threefold higher, and two sites (K417 and K468) were identified only in SIRT1 KO cells.
Second, the SWI/SNF-related complex plays important roles during chromatin-remodeling process and SIRT1 has been shown to regulate known chromatin-remodeling complexes such as Clock-Bmal1 (42). The interaction between the SWI/SNF and NuA4 HAT complexes has been shown to stabilize the SWI/SNF complex during chromatin remodeling at promoters (43). Bona fide subunits of the SWI/SNF complex include Smarce1 (Baf57) and Smarcc1 (Baf155), and it has been reported that the complex associates with Mecp2, Hdac2, and Sin3a (44). Our data showed that Smarce1, Smarcc1, Mecp2, and Hdac2 were lysine acetylated. Upon SIRT1 knockout, acetylation of Smarce1 on K146 increased by more than fourfold, and acetylation of Smarcc1 on K358 was identified only in SIRT1 knockout cells.
Third, the Brd4-PTEFb complex is a key kinase complex involved in RNA-polymerase II-mediated mRNA transcription elongation (45). PTEFb is usually composed of two subunits, Cdk9 and one of the three cyclins (T1, T2, or K). Our data showed that Cdk9 and cyclin T1 are Lys-acetylated on K3 and K491, respectively. K3 acetylation of Cdk9 was identified only in SIRT1 knockout cells, whereas K491 acetylation of cyclin T1 increased by nearly 50-fold upon SIRT1 knockout (Fig. 4D). Previous studies have shown that the viral transactivator Tat from HIV-1 can inhibit SIRT1 through binding to the SIRT1 deacetylase domain (46). Interestingly, cyclin T1 is an essential cofactor in Tat-mediated viral transcription, and its acetylation leads to the formation of an active form of the PTEFb complex and initiation of viral transcription (47). Biological relevance of cyclin T1 acetylation and its regulation by Sirt1 in HIV-1 life cycle remain to be investigated in the future.
Together, these results suggest that key components in diverse cellular networks, such as transcription, DNA damage response, chromatin remodeling, and cellular signaling, can be regulated by SIRT1.
Major Acetyltransferase Complexes Regulated by SIRT1
To examine whether major acetyltransferase complexes are regulated by SIRT1, we extracted all annotated acetyltransferase complexes in the CORUM database and performed enrichment analysis upon these candidates. For complexes identified in species other than mouse, the corresponding mouse ortholog genes were used. The results showed that the acetylation levels of 23 highly conserved acetyltransferase complexes were significantly higher upon SIRT1 knockout with enrichment in either quantile 4 or quantile 5 (p < 0.01). Affected complexes included the NuA4-Kat5 (Tip60) HAT complex, the Kat7 (Hbo1 or Myst2) complex, the ING4 complex, and the ING5-Brpf complex (supplemental Table S4, Fig. 4E–4F).
The NuA4-Kat5 (Tip60) HAT complex is critical for DNA damage repair, cell cycle control, and apoptosis (48). Acetylation of six out of 14 proteins in this complex, including Ing3, Brd8, Eaf6, Ep400, Epc1, and Kat5 (Tip60), was significantly higher on SIRT1 knockout (supplemental Table S4, Fig. 4E). Ing3, a member of the ING tumor suppressor family, contains a PHD finger domain and is a component required for the complex to acetylate chromatin substrates and initiate p53-dependant pathways (49). Our study found Ing3 to be acetylated on K403, a residue located in a PHD finger domain; acetylation of K403 was increased by more than twofold upon SIRT1 knockout.
The Kat7 (Hbo1 or Myst2) acetyltransferase complex plays an essential role in DNA replication and the cell cycle (49). It is usually composed of four subunits: Kat7 (Hbo1 or Myst2), Meaf6, one of Ing4 or Ing5, and one of Phf15, Phf16, or Phf17. Among the seven proteins involved in the complex, six were found to be acetylated in our study (supplemental Table S4, Fig. 4F) (49). Our data showed that acetylation of four proteins was significantly higher in SIRT1 knockout cells, including Meaf6, Ing4, Kat7 (Hbo1 or Myst2), and Phf16. Ing4, another member of the ING tumor suppressor family, was acetylated on five lysines. We found that the acetylation level on K235, a residue located in a PHD finger domain, was nearly doubled in SIRT1 knockout cells. Kat7 (Hbo1 or Myst2) protein, a histone acetyltransferase, was found to be acetylated on three sites, among which K201 locates to a C2-HC zinc finger domain. Our data showed that acetylation of K201 was increased by more than 10-fold in SIRT1 knockout cells, indicating the potential impact of the SIRT1 knockout upon the DNA-binding properties of the Kat7 (Hbo1 or Myst2) acetyltransferase complex.
SIRT1 Regulates Activities of Key Acetyltransferases In Vivo
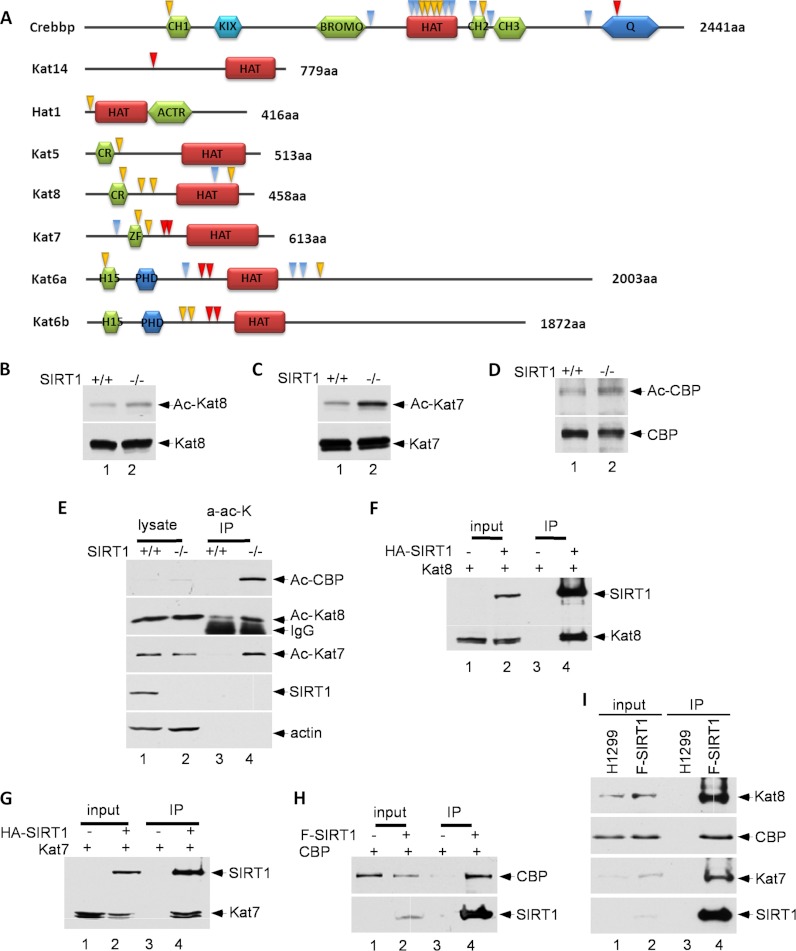
In this study, we have identified a number of lysine acetyl-transferases (HATs) as potential substrates of SIRT1, including Crebbp, Kat14 (Csrp2bp), Hat1, Kat5 (Tip60), Kat8 (Myst1), Kat7 (Myst2), Kat6a (Myst3), and Kat6b (Myst4) (Fig. 5A). To confirm these HATs as bona fide substrates of SIRT1, we examined whether the acetylation levels of these HATs are indeed regulated by SIRT1. To this end, the endogenous Kat8 (Myst1) proteins were immunoprecipitated by Kat8 (Myst1)-specific antibodies from wild type and SIRT1-null MEF cell extracts. Western blot analysis revealed that Kat8 (Myst1) acetylation was indeed enhanced in SIRT1-null cells (Fig. 5B). Similar assays were also performed on endogenous Kat7 (Myst2) and Crebbp (CBP) proteins, and acetylation of Kat7 (Myst2) and Crebbp (CBP) were also significantly increased in SIRT1-depleted cells (Fig. 5C–5D). To corroborate these findings, we also performed the reciprocal experiments, in which the cellular extracts from both wild type and SIRT1-null MEFs were immunoprecipitated with the anti-acetylated lysine antibody and the immunoprecipitates were analyzed for acetylation of these HATs. We found that acetylated Kat8 (Myst1), Kat7 (Myst2) and Crebbp (CBP) were indeed enriched in SIRT1-null cells (Fig. 5E).
Fig. 5.
SIRT1 deacetylates histone acetyltransferases (HATs). A, Schematic representation of HATs. The acetylated lysine residues identified through SILAC are indicated by arrows. Yellow arrows indicate a site quantification ratio (SIRT1 KO/WT) greater than 2, red arrows indicate sites only identified in SIRT1 knockout cells, and blue arrows indicate all other sites. B, C, and D, MEF cells (SIRT1 KO and WT) were treated with 1 μm trichostatin A (TSA) for 16 h to prevent the interferences from class I and II HDACs. The protein lysate containing 1 μm TSA and 10 mm nicotinamide was immunoprecipitated using corresponding antibodies, and immunoblotted with an anti-acetyllysine antibody. E, MEF cells were treated with 1 μm TSA for 16 h. The protein lysate was immunoprecipitated using anti-acetyllysine antibody and immunoblotted with antibodies of interest. F, G, and H, Protein extract from whole transfected 293 cells was immunoprecipitated and immunoblotted with antibodies of interest. I, Protein extracts from whole Flag-SIRT1/H1299 cells and H1299 parental cells were immunoprecipitated with M2 beads and immunoblotted.
To validate whether these decreasing acetylation levels are directly caused by SIRT1, we tested whether these HATs interact with SIRT1 in vivo. To this end, 293 cells were transfected with vectors expressing HA-tagged SIRT1 and Kat8 (Myst1). On Western blots, Kat8 (Myst1) is readily detectable in the immunoprecipitates of SIRT1 proteins (Fig. 5F). Similar results were also obtained for Kat7 (Myst2) and Crebbp (CBP) (Fig. 5G–5H). To test endogenous protein-protein interactions, we established Flag-SIRT1 H1299 stable cell line. Immunoprecipitation confirmed the endogenous interactions of SIRT1 with Crebbp (CBP), Kat7 (Myst2), and Kat8 (Myst1) (Fig. 5I).
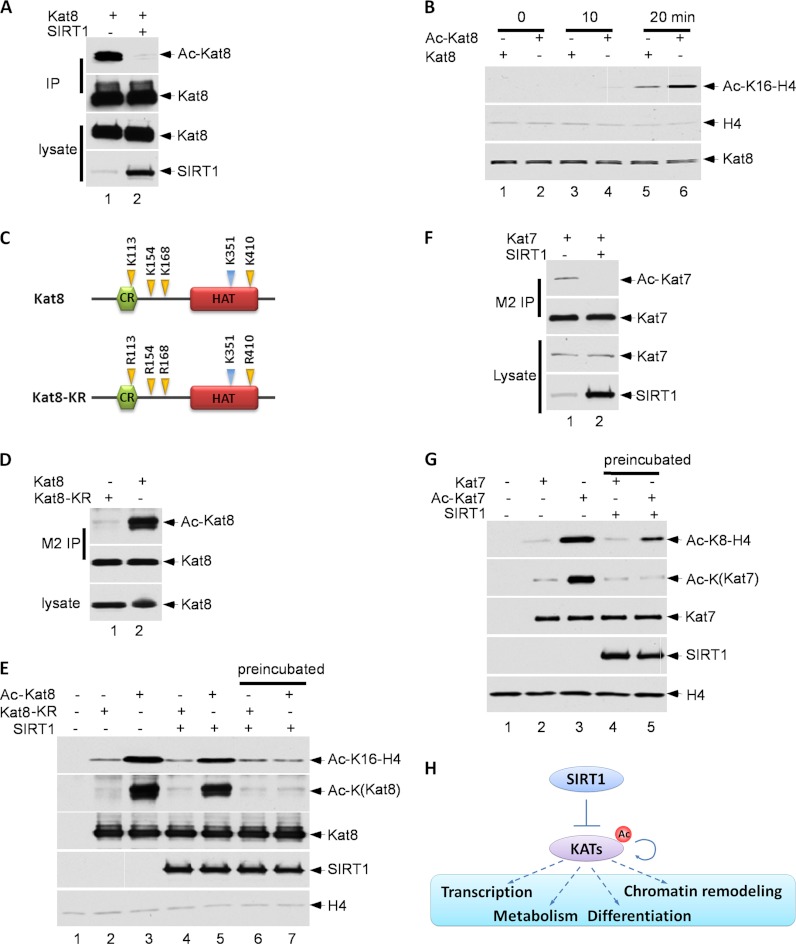
To elucidate the functional consequence of these interactions, we tested whether SIRT1 is able to deacetylate these histone acetyltransferases in vivo. We found that Kat8 (Myst1) was normally highly acetylated, but its acetylation level was dramatically reduced in the presence of SIRT1 expression (Fig. 6A). Notably, using an in vitro acetylation assay of purified Kat8 (Myst1) protein with lysine 16 of histone H4 as the substrate, we found that acetylated Kat8 (Myst1) was much more active than the unacetylated form (Fig. 6B). Moreover, our acetylated peptide analysis identified K113, K154, K168, and K410 as the major acetylation sites of Kat8 (Myst1) that are regulated by SIRT1 (Fig. 6C). Indeed, lysine to arginine mutations at these sites (Kat8 (Myst1)-KR) nearly abolished acetylation of Kat8 (Myst1) (Fig. 6D). More importantly, the acetylation-defective mutant Myst1-KR has dramatically decreased capability in acetylating histone H4 (Fig. 6E). These data suggest that SIRT1 can inhibit the activity of Kat8 (Myst1) by deacetylating the enzyme. We performed similar experiments on Kat7 (Myst2) and obtained the same conclusion (Fig. 6F–6G). Taken together, these data demonstrate that SIRT1 can induce deacetylation of diverse lysine acetyltransferases both in vitro and in vivo, directly modulate KATs' enzymatic activities and further impact cellular functions (Fig. 6H).
Fig. 6.
SIRT1 deacetylates histone acetyltransferases (HATs) and impacts their acetylation activity. A, 293 cells were transfected with Flag-Kat8 (Myst1) and SIRT1, and treated with 1 μm Trichostatin A (TSA) for 8 h. Kat8 (Myst1) proteins were purified from cell lysates. Protein levels and their acetylation status were evaluated by Western blot. B, Flag-Kat8 (Myst1) was purified from transfected 293 cell lysates, untreated or treated with 1 μm TSA and 5 mm nicotinamide for 16 h. Kat8 (Myst1) (purified from nontreated cells) and Ac-Kat8 (Myst1) (purified from treated cells) were incubated with mono-nucleosomes for the indicated times. Protein levels and their acetylation status were evaluated by Western blot. C, Schematic representation of Kat8 (Myst1) wild type and KR mutant. D, 293 cells were transfected with Flag-Kat8 (Myst1) or Flag-Kat8 (Myst1) KR mutant, and treated with 1 μm TSA and 5 mm nicotinamide for 16 h. The tagged proteins were purified from cell lysates, and protein levels and acetylation status were evaluated by Western blot. E, 293 cells were transfected with a Flag-tagged protein of interest, and treated with 1 μm TSA and 5 mm nicotinamide for 16 h. The protein of interest was purified from cell lysates. Reactions similar to those described in b were carried out for 20 min; besides in lane 6 and lane 7, Flag- Kat8 (Myst1) were preincubated with Sirt1 with NAD for 30 min. F, The 293 cells were transfected with Flag-Kat7 (Myst2), with or without SIRT1, and treated with 1 μm TSA for 8 h. The Flag-Kat7 (Myst2) protein was purified from cell lysates. The protein levels and their acetylation status were evaluated by Western blot. G, The Flag-Kat7 (Myst2) was purified from transfected 293 cell lysates, untreated or treated with 1 μm TSA and 5 mm nicotinamide for 16 h. Kat7 (Myst2) (purified from nontreated cells) and Ac-Kat7 (Myst2) (purified from treated cells) were incubated with mono-nucleosomes for 20 min; besides in lane 4 and lane 5, Flag- Kat7 (Myst2) were preincubated with Sirt1 with NAD for 30 min. Protein levels and their acetylation status were evaluated by Western blot. H, Schematic representation of SIRT1 regulation of KATs through deacetylation and potentially further regulate cellular functions.
DISCUSSION
The quantitative proteomics studies presented here represent the first systematic analysis of Lys-acetylated proteins in response to knockout of a Lys deacetylase. Our study identified 4623 Lys acetylation sites in 1800 proteins, which nearly doubles the largest number of Kac sites previously found in the proteomics study of a single cell line and represents the unprecedented deep coverage of Lys acetylome. Among 4623 sites, 4425 sites have not been reported in mouse before, thus dramatically expanding the depth of the Lys acetylation proteome. More importantly, we show that Lys acetylation levels increase by more than 100% at 485 sites in SIRT1 knockout cells. These proteins are likely important mediators responsible for the cellular functions of SIRT1. The quantitative analysis of this study showed that a number of previously identified SIRT1 substrates, including p53, DNA methyltransferase, Rel A subunit of NF-kB, and Kat8, have apparent increase of Lys acetylation in response to SIRT1 KO. Interestingly, we also found a few known SIRT1 substrates whose acetylation levels were not increased in this study, including Stat3, Apex1 and Acss2, which is likely caused by two reasons. First, the Kac sites may be modulated by the deacetylation activities of other HDACs. Alternatively, the Kac sites regulated by SIRT1 were not identified in this study. This wealth of information about the Lys acetylation proteome and SIRT1-response acetylome produced several interesting observations about the interactions of Lys acetylation with other PTMs and with diseases.
Correlation Between Lys Acetylation and Phosphorylation
Cross-influence between Lys acetylation and protein phosphorylation has been known to tune the function of proteins such as histones, p53, and NF-κB (50–52). Our study revealed the coexistence of protein phosphorylation and Lys acetylation on tryptic peptides from five proteins: nuclear receptor coactivator 2 and 6 (Ncoa2 and Ncoa6), Smad nuclear-interacting protein1 (Snip1), E1A-binding protein p400 (Ep400), and Hnpru protein (Hnrnpu) (supplemental Table S5). Phosphorylation sites in Ncoa2, Snip1, and Hnrnpu are located in the Cdk5/Cdk2 kinase motif, Akt kinase motif, and PKA motif, respectively. Acetylation of the three sites in Ncoa2, Ep400, and Hnrnpu was increased more than threefold in SIRT1 knockout cells, whereas the two acetylation sites in Snip1 and Ncoa6 were identified only in SIRT1 knockout cells. We also examined the SILAC ratios of the corresponding unphosphorylated but Lys-acetylated peptides. Surprisingly, the unphosphorylated but Lys-acetylated peptides on Ncoa2, Ncoa6, and Hnrnpu were not identified, suggesting a synergistic effect of the two PTMs in the proteins.
Our results identified 127 Lys acetylation sites on 70 protein kinases (supplemental Table S6A) and 58 sites on 31 phosphatases (supplemental Table S6B). Quantification analysis showed that acetylation of 10 sites on the protein kinases and five sites on the phosphatases was increased by more than 50% in SIRT1 knockout cells. These data further corroborate the notion of cross-talk between cellular phosphorylation and Lys acetylation networks.
Possible Interactions Between Lys Acetylation and SUMOylation
The SIRT1-response Lys acetylome motif K*xE, identified in this study, correlates with the well-known Lys SUMOylation consensus motif ψK*xE (where ψ refers to any of the nonpolar hydrophobic amino acids “VMILF”). We identified 451 Lys acetylation sites bearing K*xE motifs, among which 164 sites (or 36%) satisfied the Lys SUMOylation motif ψK*xE. One Lys acetylation site identified in this study was previously known to be SUMOylated—K15 on protein C-ets-1 (Ets1)—and six Lys acetylation sites were previously identified as SUMOylation sites by similarity to their human homolog proteins: K237 on transcription factor jun-B (Junb), K383 on p53 (Trp53), K28 on Smad nuclear-interacting protein1 (Snip1), K11 on small ubiquitin-related modifier 3 (Sumo3), K731 on Nuclear receptor coactivator 2 (Ncoa2), and K14 on ubiquitin-conjugating enzyme E2 K (Ube2k) (53, 54) (data source: http://www.phosphosite.org). Acetylation of all sites, except for K14 on Ube2k, was increased two- to 16-fold upon SIRT1 knockout (supplemental Table S7). More interestingly, we found that the acetylation and phosphorylation sites on the Ncoa2 peptide ELSQESSSTAPGSEVTVK(ac)QEPAS(ph)PK satisfied the phosphorylation-dependent SUMOylation motif ψK*xExxSP (55). The corresponding SUMOylation site on the orthologous human gene, K731, is known to be SUMOylated (56). Our data therefore suggest possible interplay among phosphorylation, Lys acetylation, and SUMOylation in Ncoa2-dependant transcription activation. As Lys acetylation and SUMOylation are mutually exclusive, our results expand our knowledge on the cross-talk between SIRT1-regulated Lys acetylation and SUMOylation.
Possible Interactions of Lys Acetylation with Diseases and SNPs
To examine the possible role of Lys acetylation sites in disease, we compared our Lys acetylation data set with the Human Gene Mutation Database (57). We identified nine Lys acetylation sites where the conserved sites in human homolog proteins were mutated in various diseases (Supplemental Table S8). Acetylation of three of these sites was up-regulated upon SIRT1 knockout. A mutation of K302 on p53 is known to be linked with glioma, and acetylation of this residue increased nearly fivefold in SIRT1 knockout cells. A mutation of K347 on Glb1 is found in patients with gangliosidosis GM1, and acetylation at this site increased by more than 40% in SIRT1 knockout cells. Residue K260 of Lmna, mutation of which is found in patients with dilated cardiomyopathy, also exhibited an over 40% increase in acetylation in SIRT1 knockout cells (58). Taken together, our data provide insight into potential links between these Lys acetylation sites and disease-related genetic mutations.
The regulatory role of SIRT1 in lysine acetylation has historically been focused on its deacetylation activity. SIRT1 is well known to regulate the status of Lys acetylation among histones and multiple transcriptional factors, including p53 and NF-κB. Recently, it was reported that notch SIRT1 can modulate the activity of acetyltransferases, Kat5 (Tip60) and Kat8 (Myst1) in HeLa and 293T cells (33–36). In this study, we report that SIRT1 regulates Lys acetylation in 10 histone Lys acetyltransferases and 23 protein complexes containing HATs, indicating that a portion of the in vivo deacetylation effects of SIRT1 could be caused by reducing activities of closely related Lys acetyltransferases. Such a mechanism is reminiscent of the in vivo regulation of kinase activity by protein phosphatases (59, 60). Thus, our results provide an alternative explanation of SIRT1 functions.
The interaction between SIRT1 and KATs discovered in our study raises an intriguing question that some up-regulated Kac sites upon SIRT1 knockout might be caused by indirect effect. This issue is shared with any proteomics experiment that aims to identify an enzyme's substrates, such as those for kinases and ubiquitination enzymes. It is daunting to carry out a comprehensive experiment to distinguish direct or indirect SIRT1 in vivo substrates. However, a few lines of evidence suggest that a significant portion of up-regulated Kac sites from our study are likely the direct SIRT1 substrates. For example, it has been reported that histone H4K16 acetylation site is a major target of Kat8 (Myst1) (61), whereas H3K9 and H3K27 acetylation sites are those of Crebbp (CBP)/Ep300 (p300) (62). In addition, HDAC1 is a deacetylase known to be acetylated by Ep300 efficiently with limited auto-deacetylation capability (63), Interestingly, our data set showed dramatic increase in acetylation levels of Kat8, Crebbp, and Ep300, but not their substrates, histone H3, H4, or HDAC1, upon SIRT1 knockout.
The ability to identify enzymes that regulate Lys acetylation sites has important clinical implications. HDAC inhibitors and activators are currently being tested in various clinical trials for the treatment of cancers and metabolic diseases. They also hold potential as therapeutics for neurodegenerative and inflammatory disorders (64, 65). An atlas of the HDAC-response Lys-acetylome will provide an opportunity to correlate the dynamics of Lys acetylation induced by an HDAC modulator with its inhibitory activity and therapeutic efficacy. Such an analysis will be valuable for evaluating the clinical relevance of an HDAC modulator, and possibly for stratifying patients for the purpose of personalized medicine. Thus, the catalog of the SIRT1-responsive Lys acetylome identified in this study will provide not only insights into the enzyme's diverse functions, but also a new setting for the examination of clinical compounds.
Supplementary Material
Acknowledgments
We thank Fred W. Alt and Hwei-Ling Cheng for providing SIRT1−/− mice.
Footnotes
* Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers CA126832 and RR020839 to Y. Zhao, and R01CA098821 and CA085533 to W. Gu. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Y. Zhao is also supported by the Nancy and Leonard Florsheim Family Fund. Y. Chen is supported by NIH Fellowship (T32 HL094282).
 This article contains supplemental Figs. S1 to S4 and Tables S1 to S8.
This article contains supplemental Figs. S1 to S4 and Tables S1 to S8.
1 The abbreviations used are:
- Lys
- lysine
- PTM
- post-translational modification
- SILAC
- stable-isotope labeling with amino acids in cell culture.
REFERENCES
- 1. Gershey E. L., Vidali G., Allfrey V. G. (1968) Chemical studies of histone acetylation. The occurrence of epsilon-N-acetyllysine in the f2a1 histone. J. Biol. Chem. 243, 5018–5022 [PubMed] [Google Scholar]
- 2. Roth S. Y., Denu J. M., Allis C. D. (2001) Histone acetyltransferases. Annu. Rev. Biochem. 70, 81–120 [DOI] [PubMed] [Google Scholar]
- 3. Gu W., Roeder R. G. (1997) Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90, 595–606 [DOI] [PubMed] [Google Scholar]
- 4. Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X. F., Yao T. P. (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458 [DOI] [PubMed] [Google Scholar]
- 5. Onyango P., Celic I., McCaffery J. M., Boeke J. D., Feinberg A. P. (2002) SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc. Natl. Acad. Sci. U.S.A. 99, 13653–13658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwer B., North B. J., Frye R. A., Ott M., Verdin E. (2002) The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J. Cell Biol. 158, 647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim S. C., Sprung R., Chen Y., Xu Y., Ball H., Pei J., Cheng T., Kho Y., Xiao H., Xiao L., Grishin N. V., White M., Yang X. J., Zhao Y. (2006) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 23, 607–618 [DOI] [PubMed] [Google Scholar]
- 8. Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 [DOI] [PubMed] [Google Scholar]
- 9. Zhao S., Xu W., Jiang W., Yu W., Lin Y., Zhang T., Yao J., Zhou L., Zeng Y., Li H., Li Y., Shi J., An W., Hancock S. M., He F., Qin L., Chin J., Yang P., Chen X., Lei Q., Xiong Y., Guan K. L. (2010) Regulation of cellular metabolism by protein lysine acetylation. Science 327, 1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang X. J., Seto E. (2007) HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26, 5310–5318 [DOI] [PubMed] [Google Scholar]
- 11. Smith B. C., Hallows W. C., Denu J. M. (2008) Mechanisms and molecular probes of sirtuins. Chem. Biol. 15, 1002–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imai S., Guarente L. (2010) Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol. Sci. 31, 212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donmez G., Guarente L. (2010) Aging and disease: connections to sirtuins. Aging Cell 9, 285–290 [DOI] [PubMed] [Google Scholar]
- 14. Haigis M. C., Sinclair D. A. (2010) Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 5, 253–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verdin E., Hirschey M. D., Finley L. W., Haigis M. C. (2010) Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem. Sci. 35, 669–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luo J., Nikolaev A. Y., Imai S., Chen D., Su F., Shiloh A., Guarente L., Gu W. (2001) Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107, 137–148 [DOI] [PubMed] [Google Scholar]
- 17. Vaziri H., Dessain S. K., Ng Eaton E., Imai S. I., Frye R. A., Pandita T. K., Guarente L., Weinberg R. A. (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 [DOI] [PubMed] [Google Scholar]
- 18. Peng L., Yuan Z., Ling H., Fukasawa K., Robertson K., Olashaw N., Koomen J., Chen J., Lane W. S., Seto E. (2011) SIRT1 deacetylates the DNA methyltransferase 1 (DNMT1) protein and alters its activities. Mol. Cell Biol. 31, 4720–4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yeung F., Hoberg J. E., Ramsey C. S., Keller M. D., Jones D. R., Frye R. A., Mayo M. W. (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., Hu L. S., Cheng H. L., Jedrychowski M. P., Gygi S. P., Sinclair D. A., Alt F. W., Greenberg M. E. (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 21. Nemoto S., Fergusson M. M., Finkel T. (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J. Biol. Chem. 280, 16456–16460 [DOI] [PubMed] [Google Scholar]
- 22. Imai S., Armstrong C. M., Kaeberlein M., Guarente L. (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 [DOI] [PubMed] [Google Scholar]
- 23. Cheng H. L., Mostoslavsky R., Saito S., Manis J. P., Gu Y., Patel P., Bronson R., Appella E., Alt F. W., Chua K. F. (2003) Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 100, 10794–10799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim S. C., Chen Y., Mirza S., Xu Y., Lee J., Liu P., Zhao Y. (2006) A clean, more efficient method for in-solution digestion of protein mixtures without detergent or urea. J Proteome Res. 5, 3446–3452 [DOI] [PubMed] [Google Scholar]
- 25. Olsen J. V., de Godoy L. M., Li G., Macek B., Mortensen P., Pesch R., Makarov A., Lange O., Horning S., Mann M. (2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell Proteomics 4, 2010–2021 [DOI] [PubMed] [Google Scholar]
- 26. Ong S. E., Blagoev B., Kratchmarova I., Kristensen D. B., Steen H., Pandey A., Mann M. (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 [DOI] [PubMed] [Google Scholar]
- 27. Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 28. Cox J., Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 [DOI] [PubMed] [Google Scholar]
- 29. McBurney M. W., Yang X., Jardine K., Bieman M., Th'ng J., Lemieux M. (2003) The absence of SIR2alpha protein has no effect on global gene silencing in mouse embryonic stem cells. Mol. Cancer Res. 1, 402–409 [PubMed] [Google Scholar]
- 30. Vaquero A., Scher M. B., Lee D. H., Sutton A., Cheng H. L., Alt F. W., Serrano L., Sternglanz R., Reinberg D. (2006) SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 20, 1256–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blander G., Guarente L. (2004) The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 73, 417–435 [DOI] [PubMed] [Google Scholar]
- 32. Schwartz D., Gygi S. P. (2005) An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat. Biotechnol. 23, 1391–1398 [DOI] [PubMed] [Google Scholar]
- 33. Yamagata K., Kitabayashi I. (2009) Sirt1 physically interacts with Tip60 and negatively regulates Tip60-mediated acetylation of H2AX. Biochem. Biophys. Res. Commun. 390, 1355–1360 [DOI] [PubMed] [Google Scholar]
- 34. Wang J., Chen J. (2010) SIRT1 regulates autoacetylation and histone acetyltransferase activity of TIP60. J. Biol. Chem. 285, 11458–11464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun B., Guo S., Tang Q., Li C., Zeng R., Xiong Z., Zhong C., Ding J. (2011) Regulation of the histone acetyltransferase activity of hMOF via autoacetylation of Lys274. Cell Res. 21, 1262–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu L., Li L., Lv X., Wu X. S., Liu D. P., Liang C. C. (2011) Modulations of hMOF autoacetylation by SIRT1 regulate hMOF recruitment and activities on the chromatin. Cell Res 21, 1182–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang R. H., Sengupta K., Li C., Kim H. S., Cao L., Xiao C., Kim S., Xu X., Zheng Y., Chilton B., Jia R., Zheng Z. M., Appella E., Wang X. W., Ried T., Deng C. X. (2008) Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 14, 312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ming M., Shea C. R., Guo X., Li X., Soltani K., Han W., He Y. Y. (2010) Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc. Natl. Acad. Sci. U.S.A. 107, 22623–22628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guarani V., Deflorian G., Franco C. A., Krüger M., Phng L. K., Bentley K., Toussaint L., Dequiedt F., Mostoslavsky R., Schmidt M. H., Zimmermann B., Brandes R. P., Mione M., Westphal C. H., Braun T., Zeiher A. M., Gerhardt H., Dimmeler S., Potente M. (2011) Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature 473, 234–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malik S., Roeder R. G. (2010) The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 11, 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Black J. C., Choi J. E., Lombardo S. R., Carey M. (2006) A mechanism for coordinating chromatin modification and preinitiation complex assembly. Mol. Cell 23, 809–818 [DOI] [PubMed] [Google Scholar]
- 42. Nakahata Y., Kaluzova M., Grimaldi B., Sahar S., Hirayama J., Chen D., Guarente L. P., Sassone-Corsi P. (2008) The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hassan A. H., Neely K. E., Workman J. L. (2001) Histone acetyltransferase complexes stabilize swi/snf binding to promoter nucleosomes. Cell 104, 817–827 [DOI] [PubMed] [Google Scholar]
- 44. Harikrishnan K. N., Chow M. Z., Baker E. K., Pal S., Bassal S., Brasacchio D., Wang L., Craig J. M., Jones P. L., Sif S., El-Osta A. (2005) Brahma links the SWI/SNF chromatin-remodeling complex with MeCP2-dependent transcriptional silencing. Nat. Genet. 37, 254–264 [DOI] [PubMed] [Google Scholar]
- 45. Jang M. K., Mochizuki K., Zhou M., Jeong H. S., Brady J. N., Ozato K. (2005) The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19, 523–534 [DOI] [PubMed] [Google Scholar]
- 46. Kwon H. S., Brent M. M., Getachew R., Jayakumar P., Chen L. F., Schnolzer M., McBurney M. W., Marmorstein R., Greene W. C., Ott M. (2008) Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe 3, 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cho S., Schroeder S., Kaehlcke K., Kwon H. S., Pedal A., Herker E., Schnoelzer M., Ott M. (2009) Acetylation of cyclin T1 regulates the equilibrium between active and inactive P-TEFb in cells. EMBO J. 28, 1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Doyon Y., Selleck W., Lane W. S., Tan S., Côté J. (2004) Structural and functional conservation of the NuA4 histone acetyltransferase complex from yeast to humans. Mol. Cell Biol. 24, 1884–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Doyon Y., Cayrou C., Ullah M., Landry A. J., Côté V., Selleck W., Lane W. S., Tan S., Yang X. J., Côté J. (2006) ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol. Cell 21, 51–64 [DOI] [PubMed] [Google Scholar]
- 50. Sakaguchi K., Herrera J. E., Saito S., Miki T., Bustin M., Vassilev A., Anderson C. W., Appella E. (1998) DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 12, 2831–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen L. F., Williams S. A., Mu Y., Nakano H., Duerr J. M., Buckbinder L., Greene W. C. (2005) NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol. Cell Biol. 25, 7966–7975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheung P., Tanner K. G., Cheung W. L., Sassone-Corsi P., Denu J. M., Allis C. D. (2000) Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol. Cell 5, 905–915 [DOI] [PubMed] [Google Scholar]
- 53. Ji Z., Degerny C., Vintonenko N., Deheuninck J., Foveau B., Leroy C., Coll J., Tulasne D., Baert J. L., Fafeur V. (2007) Regulation of the Ets-1 transcription factor by sumoylation and ubiquitinylation. Oncogene 26, 395–406 [DOI] [PubMed] [Google Scholar]
- 54. Garaude J., Farrás R., Bossis G., Charni S., Piechaczyk M., Hipskind R. A., Villalba M. (2008) SUMOylation regulates the transcriptional activity of JunB in T lymphocytes. J. Immunol. 180, 5983–5990 [DOI] [PubMed] [Google Scholar]
- 55. Hietakangas V., Anckar J., Blomster H. A., Fujimoto M., Palvimo J. J., Nakai A., Sistonen L. (2006) PDSM, a motif for phosphorylation-dependent SUMO modification. Proc. Natl. Acad. Sci. U.S.A. 103, 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kotaja N., Karvonen U., Jänne O. A., Palvimo J. J. (2002) The nuclear receptor interaction domain of GRIP1 is modulated by covalent attachment of SUMO-1. J. Biol. Chem. 277, 30283–30288 [DOI] [PubMed] [Google Scholar]
- 57. Stenson P. D., Mort M., Ball E. V., Howells K., Phillips A. D., Thomas N. S., Cooper D. N. (2009) The Human Gene Mutation Database: 2008 update. Genome Med. 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arbustini Eloisa A. E., Pilotto A., Pasotti M., Grasso M., Diegoli M., Campana C., Gavazzi A., Alessandra R., Tavazzi L. (2005) Gene symbol: LMNA. Disease: Cardiomyopathy, dilated, with conduction defect 1. Hum. Genet. 117, 298. [PubMed] [Google Scholar]
- 59. Garcia-Morales P., Minami Y., Luong E., Klausner R. D., Samelson L. E. (1990) Tyrosine phosphorylation in T cells is regulated by phosphatase activity: studies with phenylarsine oxide. Proc. Natl. Acad. Sci. U.S.A. 87, 9255–9259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Douglas P., Moorhead G. B., Ye R., Lees-Miller S. P. (2001) Protein phosphatases regulate DNA-dependent protein kinase activity. J. Biol. Chem. 276, 18992–18998 [DOI] [PubMed] [Google Scholar]
- 61. Akhtar A., Becker P. B. (2000) Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell 5, 367–375 [DOI] [PubMed] [Google Scholar]
- 62. Jin Q., Yu L. R., Wang L., Zhang Z., Kasper L. H., Lee J. E., Wang C., Brindle P. K., Dent S. Y., Ge K. (2011) Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 30, 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Qiu Y., Zhao Y., Becker M., John S., Parekh B. S., Huang S., Hendarwanto A., Martinez E. D., Chen Y., Lu H., Adkins N. L., Stavreva D. A., Wiench M., Georgel P. T., Schiltz R. L., Hager G. L. (2006) HDAC1 acetylation is linked to progressive modulation of steroid receptor-induced gene transcription. Mol. Cell 22, 669–679 [DOI] [PubMed] [Google Scholar]
- 64. Halili M. A., Andrews M. R., Sweet M. J., Fairlie D. P. (2009) Histone deacetylase inhibitors in inflammatory disease. Curr. Top. Med. Chem. 9, 309–319 [DOI] [PubMed] [Google Scholar]
- 65. Kazantsev A. G., Thompson L. M. (2008) Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat. Rev. Drug Discov. 7, 854–868 [DOI] [PubMed] [Google Scholar]
- 66. Allis C. D., Berger S. L., Cote J., Dent S., Jenuwien T., Kouzarides T., Pillus L., Reinberg D., Shi Y., Shiekhattar R., Shilatifard A., Workman J., Zhang Y. (2007) New nomenclature for chromatin-modifying enzymes. Cell 131, 633–636 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.