Abstract
Background
With widespread use of antiretroviral therapy (ART) and prolonged survival of HIV-infected children, toxicities like lipodystrophy are becoming more evident. Little is known about lipodystrophy in children in Uganda yet there is increased use of ART. The aim of this study was to determine the prevalence and factors associated with fat redistribution and metabolic abnormalities among HIV-infected children on highly active antiretroviral therapy (HAART) in Uganda.
Methods
A cross-sectional study of 364 HIV positive children aged between 2 and 18 years on ART were enrolled after consent and assent as appropriate. Sociodemographic, clinical and immunological data were collected and recorded in a questionnaire. Fat redistribution was assessed clinically for physical findings of lipohypertrophy and lipoatrophy. A fasting blood sample was taken for lipid profile and blood glucose analysis. Lipodystrophy was defined as presence of abnormal fat redistribution or metabolic abnormalities or both. The proportion of children with fat redistribution and metabolic abnormalities was calculated. We conducted multivariate analysis for factors associated with lipodystrophy among children with lipodystrophic features and those without.
Results
The median age of the participants was eight years (range 2 to 18), with 43% of these aged ≥10 years and a male to female ratio of 1.1:1. Majority (65%) had advanced HIV (WHO Stage III/IV) at ART initiation with a mean duration on ART of 3.8 years (±1.2). The prevalence of fat redistribution and hyperlipidemia was 27.0% and 34.0%, respectively. None of the children had hyperglycaemia. Among the children with hyperlipidemia, 16.8% exhibited hypercholesterolemia and 83% had hypertriglyceridemia. Only 29% of children with fat redistribution had hyperlipidemia. We found significant association between fat redistribution and Tanner stages 2 to 5 OR=2.3 (95%CI 1.3 to 3.8), age≥5 years OR=3.9 (95%CI 1.5 to 9.9) and d4T exposure OR=3.4 (95%CI 2.0 to 5.8). A Tanner stage 2 to 5 was independently associated with hyperlipidemia. No significant association was observed with HIV clinical stage and any of the anthropometric measurements.
Conclusion
The prevalence of lipodystrophy is high among HIV-infected children on ART with a likelihood of developing fat redistribution and metabolic abnormalities increased during puberty.
Keywords: human immunodeficiency virus, children, lipodystrophy, fat redistribution, hyperlipidemia, metabolic abnormalities, highly active antiretroviral therapy
Introduction
Antiretroviral therapy (ART) has markedly improved the prognosis of persons living with HIV. Meanwhile, with the widespread use of and increased duration of ART, toxicities are becoming more evident [1, 2]. Frequent long term side effects include metabolic derangements and abnormal fat redistribution [3–7]. These side effects of metabolic abnormalities and fat redistribution have been defined as lipodystrophy syndrome although there is no consensus on proper definition of lipodystrophy syndrome.
Results from paediatric studies have estimated the prevalence of fat redistribution to be between 18% and 33% [4–7]. Fat redistribution is significantly associated with duration of antiretroviral drugs, older age [7, 8] and stavudine use [8]. However, metabolic changes in the absence of fat redistribution are not related to the duration of ART nor to the number of drugs received [8]. Abnormal physical changes have also been linked to use of zidovudine in comparison to non-thymidine NRTIs like abacavir, tenofovir, and lamivudine with minimal effect [9]. Protease inhibitors have been linked to dyslipidaemia and less strongly to morphological changes [3].
In Uganda availability of ART for children has been increasing since 2004. By 2009, approximately 17,000 children were on ART throughout the country. Stavudine (d4T) is still used as first line ART therapy for HIV-infected children aged five years and below [10] although d4T has been associated with lipodystrophy in many paediatric studies [3, 6, 8]. There is increased access to ART in sub-Saharan Africa [11] which has led to prolonged survival and hence a prolonged exposure to ART in children.
Prolonged exposure to ART contributes to metabolic changes thus increasing the cardiovascular risk in adulthood [12, 13], while the abnormal body fat changes may affect the self-image and ultimately affect adherence to treatment [14]. These abnormalities could therefore affect the long-term prognosis of HIV-infected children on ART.
Furthermore, the assessment and monitoring for abnormal fat redistribution and metabolic changes in children on ART is not routinely done in HIV care centres in our setting. The cost of a lipid profile is prohibitive in many of the centres and therefore not performed routinely and although the physical body changes may be identified early with good clinical care without laboratory tests [15], the clinical assessment of physical changes is still a challenge in resource limited centres because of limited skilled personnel to care for the children.
The aim of this study was to determine the prevalence of fat redistribution and metabolic abnormalities and the factors associated with each of the individual features of lipodystrophy among HIV-infected children on first line ART regimens.
Patients and methods
Study site
The study was carried out at the Baylor-Bristol Myers Children's Clinical Centre of Excellence, a large HIV paediatric clinic (PIDC) at a national referral and teaching hospital for Makerere University, College of Health Sciences in Uganda. As of March 2009, 3971 children were active in HIV care at the clinic with an average of 120 children seen per clinic day. Sixty-three percent of the children in the clinic were on a first line regimen containing a non-nucleoside reverse transcriptase inhibitor together with AZT/d4T, with about 28% and 72% on d4T and AZT-based regimen, respectively. Protease inhibitors are used as first line regimens in infants previously exposed to Nevirapine during the prevention of mother to child transmission but otherwise are reserved for second line. The dosage of the ART drugs is based on the children's body weight. The clinic ART policies are based on the Uganda Ministry of Health (MOH) ART treatment guidelines [9]. At initiation of this study, a number of children aged five years and older had been recently switched to zidovudine (AZT) from d4T based regimen in line with the new guidelines.
Study subjects and procedures
We conducted a cross-sectional study of HIV-infected children on first line ART regimens between February 2009 and March 2009. Children were eligible for this study if: (1) aged between 2 and 18 years, (2) on first line ART regimen for at least six months and (3) those whose caretakers provided written informed consent and children ≥8 years of age who assented to the study. We excluded children on: (1) protease inhibitors, (2) corticosteroids for at least one month of starting the study, (3) confirmed or known patients of diabetes mellitus, renal disease, cardiac disease and severe malnutrition, (4) on hormonal contraceptives, (5) with severe clinical illness at the time of the study.
Medical charts were screened at the reception and every fourth patient who met the eligibility criteria was approached by the study nurse and provided information about the study. Parents of all children provided written informed consent and children over eight years also provided assent.
The study was approved by the Makerere University Faculty of Medicine Research and Ethics committee, the National Council for Science and Technology, Baylor College of Medicine, Houston, TX and Case Western Reserve University, Cleveland, OHio.
Procedures
A standardized data collection form was completed for each participant. Data recorded included clinical history through chart review and interviews, physical exam and laboratory results. Information extracted from the charts included WHO staging at initiation of ART, previous laboratory data and ART treatment history. The physical assessment for fat redistribution and pubertal maturity was done by the principal investigator or a trained research assistant who was an experienced clinician. A trained nurse measured the weight, height, mid upper arm circumference, waist and hip circumference.
A fasting blood sample (at least eight hours fast) was obtained for blood glucose and lipid profile.
Laboratory methods
Blood glucose was measured by an automated analyzer (Accu-Chek Active Roche diagnostics Gmbh, D-68298 Mannheim, Germany). Lipid profile (triglycerides (TG), total cholesterol (TC), high density lipoprotein and low density lipoprotein) measured by Konelab 30 system chemistry analyzer.
Definitions
Fat redistribution was defined clinically by the physical findings of any fat wasting of extremities, face or buttocks; with presence of prominent vessels or fat accumulation in the abdomen and dorsocervical spine (buffalo hump). The fat redistribution was scored separately for limbs, face, buttocks, abdomen, thorax and neck on a scale of 0 to 3 based on an adult scoring system [16]. Where 0 represented the absence of fat changes, 1=minor changes (changes noticeable only when specifically inspected), 2=moderate changes (changes readily obvious to even caretakers/patients) and 3=major changes (changes noticeable by other people, such as family or classmates). Presence of a score ≥1 in any part of the body was considered as abnormal fat redistribution.
Hyperglycaemia was defined as blood glucose ≥126 mg/dl. Hyperlipidemia was defined as the presence of either hypercholesterolemia TC>200 mg/dl or hypertriglyceridemia TG>150mg/dl using the National Cholesterol Education Program (NCEP) criteria [15]. Metabolic abnormalities were defined as presence of hyperlipidemia and/or hyperglycaemia. Lipodystrophy was defined as presence of abnormal fat redistribution and metabolic abnormalities or both.
Statistical analysis
Primary outcomes of study were the proportion of children with fat redistribution and metabolic abnormalities. We evaluated patient characteristics including age, gender, BMI, blood pressure, Tanner stage, WHO stage, ART regimen and duration. The children who had been switched to an AZT based regimen but were previously exposed to d4T for at least six months were analyzed as children on d4T.
Categorical variables were compared using the chi-square test. The CDC 2000 BMI reference centiles were used in assessing BMI.
Multivariable logistic regression was done to determine factors independently associated with fat redistribution and metabolic abnormalities. All the variables with p-value ≤0.2 at univariate analysis were included in the model. A two-sided p-value <0.05 were considered statistically significant. Statistical analysis was performed using the Stata 9.2 (StataCorp, College Station, TX).
Results
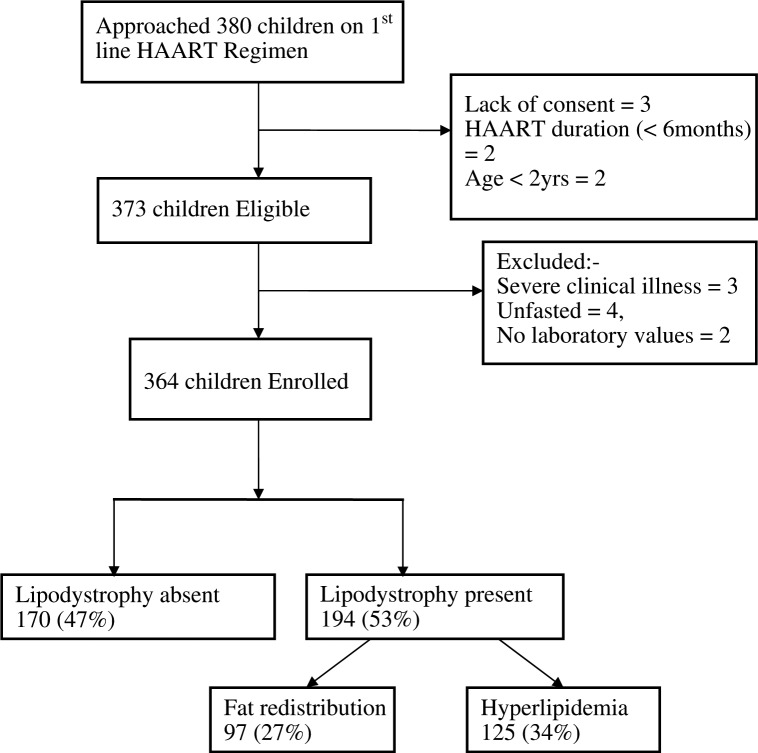
As Figure 1 shows, we studied 364 HIV-infected children aged between 2 and 18 years (median age eight years). All the children were on first line ART regimens taking 3TC and a non-nucleoside reverse transcriptase inhibitor (Efavirenz/Nevirapine) in addition to either AZT or d4T. The majority, 291 (80%) out of 364, were on an AZT based regimen but 30 (10.3%) of the 291 children on AZT were previously exposed to d4T containing regimen for at least six months. The mean duration for ARV treatment was 3.8 years. Approximately 56% of the participants were WHO Stage III at initiation of ART. The rest of the characteristics are shown in Table 1.
Figure 1.
Study profile.
Table 1.
Characteristics of the study children (n=364)
| General characteristics | Frequency | Percent (%) |
|---|---|---|
| Age group | ||
| <5 years | 76 | 20.88 |
| 5 to 9 years | 130 | 35.71 |
| ≥10 years | 158 | 43.41 |
| Sex | ||
| Male | 193 | 53.02 |
| Female | 171 | 46.98 |
| Regimen | ||
| d4T based | 73 | 20.05 |
| AZT based | 291 | 79.95 |
| HAART duration | ||
| ≤24months | 72 | 19.78 |
| 25 to 48 months | 194 | 53.30 |
| >48 months | 98 | 26.92 |
| WHO Stage at ART initiation | ||
| I | 23 | 6.32 |
| II | 104 | 28.57 |
| III | 203 | 55.77 |
| IV | 34 | 9.34 |
| Nutrition status (BMI) | ||
| ≤85 centile | 300 | 82.42 |
| >85 centile | 64 | 17.58 |
| Wasted | ||
| ≤–2 Weight for age Z-score | 67 | 18.41 |
| Not wasted | ||
| >–2 Weight for age Z-score | 297 | 81.59 |
| Waist circumference | ||
| >90 centile | 16 | 4.4 |
| ≤90 centile | 348 | 95.6 |
| Tanner stage | ||
| 1 | 215 | 59.07 |
| 2 | 68 | 18.68 |
| 3 | 40 | 10.99 |
| 4 | 34 | 9.34 |
| 5 | 7 | 1.92 |
ART, antiretroviral therapy; AZT, zidovudine; d4T, stavudine; BMI, body mass index; HAART, highly active antiretroviral therapy.
Prevalence of metabolic abnormalities
Metabolic abnormalities were observed in 125 (34%) of the 364 children. All the 125 children with metabolic abnormalities had hyperlipidemia and none had hyperglycaemia. Among the children with hyperlipidemia, 21 (16.8%) of the 125 exhibited hypercholesterolemia and 104 (83.2%) had hypertriglyceridemia. Hyperlipidemia was detected in 28 (29%) of 97 children with fat redistribution.
Prevalence of fat redistribution
Abnormal fat redistribution was observed in 97 (27%) of 364 children. The prevalence increased with age and it was uncommon in children younger than five years as shown in Table 2. Only six out of the 76 children younger than five years exhibited abnormal fat redistribution. Children with fat redistribution in the study had a higher mean duration on ART of 3.5 years (±1.3) as compared to 3.1 years (±1.2) of children without fat redistribution.
Table 2.
Factors associated with fat redistribution at univariate analysis
| Fat redistribution | ||||
|---|---|---|---|---|
|
| ||||
| Characteristics | Yes | No | Odds ratio | p-Value |
| Sex | ||||
| Female | 44 (45.36) | 127 (47.57) | 0.70 (0.60 to 1.50) | 0.709 |
| Male | 53 (54.69) | 140 (52.43) | ||
| Age group | ||||
| ≥5 years | 91 (93.81) | 197 (73.78) | 5.39 (2.26 to 12.9) | 0.000 |
| <5 years | 6 (6.19) | 70 (26.22) | ||
| Nutritional status (BMI) | ||||
| >85th centile | 15 (15.46) | 49 (18.35) | 0.81 (0.43 to 1.53) | 0.523 |
| ≤85th centile | 82 (84.54) | 218 (81.65) | ||
| Waist circumference | ||||
| ≤90th centile | 95 (97.94) | 253 (94.76) | 2.63 (0.59 to 11.78) | 0.207 |
| >90th centile | 2 (2.06) | 14 (5.24) | ||
| HAART duration | ||||
| >36 months | 64 (65.98) | 133 (49.81) | 1.95 (1.20 to 3.17) | 0.007 |
| ≤36 months | 33 (30.93) | 134 (50.19) | ||
| d4T exposure** | ||||
| Yes | 45 (46.39) | 58 (21.72) | 3.12 (1.90 to 5.11) | 0.000 |
| No | 52 (53.61) | 209 (78.28) | ||
| AZT exposure | ||||
| Yes | 81 (83.51) | 219 (82.02) | 1.10 (0.60 to 2.06) | 0.743 |
| No | 16 (16.49) | 48 (17.98) | ||
| WHO stage at ART initiation | ||||
| III/IV | 67 (69.07) | 170 (63.67) | 1.27 (0.77 to 2.10) | 0.340 |
| I/II | 30 (30.93) | 97 (36.33) | ||
| Tanner stage | ||||
| Tanners 2 to 5 | 59 (60.82) | 90 (33.71) | 3.05 (1.89 to 4.94) | 0.000 |
| Tanner 1 | 38 (39.18) | 177 (66.29) | ||
| Cholesterol | ||||
| High | 2 (0.5) | 19 (5.2) | 0.3 (0.1 to 1.2) | 0.086 |
| Normal | 95 (26.1) | 248 (68.1) | ||
| Triglycerides | ||||
| High | 27 (7.4) | 85 (23.3) | 0.8 (0.5 to 1.4) | 0.465 |
| Normal | 70 (19.2) | 182 (50.0) | ||
d4T exposure=yes if child ever been exposed to d4T for duration greater than six months. ART, antiretroviral therapy; AZT, zidovudine; d4T, stavudine; BMI, body mass index; OR, odds ratio; HAART, highly active antiretroviral therapy.
Bold terms represent the statistically significant values.
The commonest body shape change in children with fat redistribution was of lipoatrophy with facial wasting in 80 (82.5%) of 97 children with fat redistribution. However, lipohypertrophy was the commonest body shape change in children younger than five years with abdominal fat accumulation in four of these children.
Multivariate logistic regression
The fat redistribution model included age, ART duration, and exposure to d4T, WHO clinical stage at ART initiation, waist circumference and Tanner stage. Hyperlipidemia model included gender, age group, exposure to d4T and AZT, Tanner stage and the BMI percentiles. Fat redistribution was independently associated with Tanner stages 2 to 5 (sexual maturity), age ≥5 years and d4T exposure as shown in Table 3. A Tanner stage 2 to 5 was independently associated with hyperlipidemia. No significant association was found between lipodystrophic features and patient gender, HIV clinical stage and any of the anthropometric measurements the data for factors that were not statistically significant is not shown in the model.
Table 3.
Multivariate analysis of independent variables for abnormal fat redistribution and hyperlipidemia
| Characteristic | Odds ratio (95%CI) | p-Value |
|---|---|---|
| Independent variables for fat redistribution | ||
| Tanner stage | ||
| Tanners 2 to 5 versus Tanner 1 | 2.26 (1.33 to 3.84) | 0.003 |
| Age group | ||
| ≥5 years versus <5years | 3.87 (1.51 to 9.88) | 0.005 |
| d4T exposure** | ||
| Yes versus No | 3.43 (2.03 to 5.80) | <0.001 |
| Independent variable for hyperlipidemia | ||
| Tanner stage | ||
| Tanner 2 to 5 versus Tanner 1 | 1.72 (1.11 to 2.67) | 0.015 |
d4T exposure=yes if child ever been exposed to d4T for duration greater than six months. Table indicates only the factors that were significant with p-value <0.05 at logistic regression. d4T, stavudine; OR, odds ratio.
Discussion
There are few published studies that have been conducted among HIV-infected children in Africa which assess both clinical and biochemical features of lipodystrophy, thus the findings in this study are compared mainly to studies in developed settings. Due to the absence of a proper definition of lipodystrophy in children, we defined lipodystrophy as presence of any or both of the features of lipodystrophy: fat redistribution and metabolic abnormalities.
Fat redistribution was observed in 27% of children on non-PI based ART regimen. This is consistent with results from other studies which have reported a prevalence of between 18% and 33% [4, 6–8].
As in the other studies, this study considered presence of fat redistribution on the basis of physical evaluation by the study doctors (physicians) which may be subjective. No significant association was found between lipodystrophic features and any of the anthropometric measurements in this study, yet these would be more applicable in this setting because they are more objective, simple and inexpensive. Hartman et al. [17] found that anthropometric measurements were associated with lipoatrophy but they included only children with moderate to severe lipoatrophy on scoring, our study included all children with lipoatrophy including the mild lipoatrophy.
The objective methods for assessing fat redistribution are not performed routinely in our environment and in many instances are unavailable. Therefore this prevalence may also be an underestimate since it reports the cases that are physically obvious to the physician and may miss those with subtle physical changes. However physician's evaluation for fat redistribution has been reported to agree well with objective measures like Dual-energy X-ray absorpiometry (DEXA) scans [16].
The prevalence of hyperlipidemia was 34% which is inconsistent with other paediatric studies which found higher prevalence of between 40% and 78% [4, 6–8, 18]. The discrepancy in the findings may be due to the difference in the study setting as regards dietary composition between these published studies and our study. Secondly, most of the published studies used for comparison were using both NRTIS and PIs for treatment of the children [6, 18]. Protease inhibitors have been independently associated with gross metabolic derangements in many studies [3]; therefore there is a higher prevalence of hyperlipidemia in those studies.
Fat redistribution and hyperlipidemia was found to be significantly associated with sexual maturity (Tanner stages 2 to 5). Fat redistribution is a common physiological process during puberty, which may make diagnosis of abnormal fat redistribution difficult. However, Vigano et al. [19] found that puberty appears to worsen peripheral fat loss using an objective method of DEXA-scan. Many studies using physical detection of fat redistribution have reported sexual maturation to be significantly associated with abnormal fat redistribution [7, 17, 20]. This finding underscores the association of puberty and abnormal physical changes and suggests a hormonal role in the development of the changes, but could also just be a cumulative effect.
Physiologically, total cholesterol levels increase from birth, stabilize at about two years, peak before puberty and then decline during adolescence [21]. Therefore the normal physiological process cannot explain the association between puberty and hyperlipidemia. It is plausible that there might be interplay between ART and hormonal changes at puberty that may lead to increased risk of hyperlipidemia in adolescent children.
Older age ≥5 years was independently associated with fat redistribution which is consistent with many studies carried out in children [6, 7, 22]. Literature shows that the risk of abnormal fat redistribution increases with advanced age with the risk much higher in pubertal ages, greater than 10 years. In this study 55% of children with abnormal fat redistribution were 10 years of age and older. This reflects a cumulative exposure to drugs that may be a progressive process and with increasing survival of children on ART, fat redistribution will be a more prevalent problem in children in older age groups.
As in other studies, [6, 8, 20] d4T use was independently associated with physical body changes. Several studies have found NRTIs particularly d4T significantly associated with lipoatrophy rather than lipohypertrophy [23, 24]. The risk of developing lipodystrophy with d4T is strongly related to the dosage [4] and duration of exposure to antiretroviral agents [6, 7]. Amaya et al. [4] found that low concentration of the drugs using paediatric dosing reduces risk of fat redistribution, however, in this study paediatric dosing based on body weight was used for all children and we still saw a high prevalence of physical changes. Furthermore, this study found no association between duration of exposure to ART and physical changes. A study in Malawi [25] with a large retrospective cohort found no children switched from d4T due to d4T induced lipodystrophy; however, they studied a younger population with a shorter mean duration on ART. Other paediatric studies on lipodystrophy have also shown a lack of association between duration of therapy and lipodystrophy [4, 22].
Stavudine is still used in our setting among children in cases where AZT is contraindicated. The alternative non-thymidine NRTI drugs like abacavir are expensive and not readily available. AZT may also cause physical changes as reported in studies and a number of children on AZT based regimen in this study had physical changes although it was not statistically significant. Therefore we should continue to look actively for these abnormalities despite change of guidelines from d4T to AZT- based regimens.
Only 29% of children with fat redistribution had hyperlipidemia, however, this is inconsistent with findings in other studies that found a higher prevalence of metabolic disturbances (50% to 71%) among children with fat redistribution [6, 7, 26]. This may be due to the already low prevalence of hyperlipidemia in this study as compared to many other studies [4, 6, 20]. Therefore hyperlipidemia and fat redistribution may occur independently and lipid profile should not be restricted to only those with fat redistribution. Although a lipid profile test is costly in this setting, a test should at least be performed on those children who are at the highest risk especially the adolescents.
This study had some limitations that may have influenced the study results. We lacked an objective tool for dietary assessment for our setting, we were unable to objectively assess fat redistribution in this study due to cost and access to DEXA scans and MRI and unavailability of lipid reference values for the general paediatric population in Uganda. This study had few patients on d4T with most having been switched to AZT based regimen; this may have affected the study outcomes.
In conclusion, the prevalence of hyperlipidemia and fat redistribution among children on zidovudine or stavudine based ART regimens is high. With increased survival of children on ART and therefore prolonged exposure to drugs, the prevalence of lipodystrophy may even increase. Therefore active screening for fat redistribution through good clinical assessment and training of health workers to look for these changes using simple scoring systems should be instituted and reinforced in paediatric HIV care centres, especially among pubertal children.
Acknowledgements
We are grateful to Edward Were for assisting with the data analysis and Sebastian Wanless for revising the manuscript. We thank the clinical study team of Dennis Kintu, Carol Nerima, Rosemary Mbabazi, Victoria Sebudde, Dorothy Mugabi, and Alice Asiimwe for her assistance with the study proposal. We also thank the laboratory staff of Baylor College of Medicine-Bristol Myers Squibb Children's Clinical Centre of Excellence for their support in caring for the children. We are indeed grateful to the study participants and their parents/guardians.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
TP participated in the conception, design and implementation of the study, interpretation and writing of the manuscript. SBK participated in the conception and design of the study, interpretation and writing of the manuscript. AK and MRK participated in design of the study, interpretation and writing of the manuscript. All authors have read and approved the final manuscript.
Abbreviations
ART, antiretroviral therapy; AZT, zidovudine; d4T, stavudine; HAART, highly active antiretroviral therapy; TG, triglycerides; TC, total cholesterol.
Disclaimer
The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official views of FIC or NIH.
Funding sources
This publication was made possible by grant number TW00011 from the Fogarty International Center (FIC) at the National Institutes of Health (NIH). The authors thank the AIDS International Training Research Programme (AITRP) for funding the study.
References
- 1.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–30. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 2.Fellay J, Boubaker K, Ledergerber B, Bernasconi E, Furrer H, Battegay M, et al. Prevalence of adverse events associated with potent antiretroviral treatment: Swiss HIV cohort study. Lancet. 2001;358:1322–7. doi: 10.1016/s0140-6736(01)06413-3. [DOI] [PubMed] [Google Scholar]
- 3.Leonard EG, McComsey AG. Metabolic complications of antiretroviral therapy in children. Pediatr Infect Dis J. 2003;22:77–84. doi: 10.1097/00006454-200301000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Amaya RA, Kozinetz CA, McMeans A, Schwarzwald H, Kline MW. Lipodystrophy syndrome in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2002;21:405–10. doi: 10.1097/00006454-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Babl FE, Regan AM, Pelton SI. Abnormal body-fat distribution in HIV-1-infected children on antiretrovirals. Lancet. 1999;353:1243–4. doi: 10.1016/S0140-6736(98)05754-7. [DOI] [PubMed] [Google Scholar]
- 6.Viganò A, Thorne C, Brambilla P, Newell ML, European Paediatric Lipodystrophy Group Antiretroviral therapy, fat redistribution and hyperlipidaemia in HIV-infected children in Europe. AIDS. 2004;18:1443–51. doi: 10.1097/01.aids.0000131334.38172.01. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez Torres AM, Munoz Muniz R, Madero R, Borque C, Garcia-Miguel MJ, De Jose Gomez MI. Prevalence of fat redistribution and metabolic disorders in human immunodeficiency virus-infected children. Eur J Pediatr. 2005;164:271–6. doi: 10.1007/s00431-004-1610-y. [DOI] [PubMed] [Google Scholar]
- 8.Ene L, Goetghebuer T, Hainaut M, Peltier A, Toppet V, Levy J. Prevalence of lipodystrophy in HIV-infected children: a cross-sectional study. Eur J Pediatr. 2007;166:13–21. doi: 10.1007/s00431-006-0193-1. [DOI] [PubMed] [Google Scholar]
- 9.Innes S, Leon L, Cotton M. Lipodystrophy syndrome in HIV infected children on HAART. South Afr J HIV Med. 2009:76–80. doi: 10.4102/sajhivmed.v10i4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ministry of Health, Uganda . National antiretroviral treatment and care guidelines for adults and children. Kampala: Ministry of health, Uganda; 2008. [Google Scholar]
- 11.UNAIDS/WHO. Geneva; 2006. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach, 2006 revision. [cited 2008 August 15]. Available from: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed] [Google Scholar]
- 12.Friis-Møller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 13.El-Sadr W, Reiss P, De Wit S. Relationship between prolonged exposure to combination ART and myocardial infarction: effect of sex, age and lipid changes; USA. (Abstract 42): Boston; 2005. Feb, 12th Conference on Retroviruses and Opportunistic Infections. [Google Scholar]
- 14.Ammassari A, Antinori A, Cozzi-Lepri A, Trotta MP, Nasti G, Ridolfo AL. Relationship between HAART adherence and adipose tissue alterations. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):140–4. doi: 10.1097/00126334-200212153-00011. [DOI] [PubMed] [Google Scholar]
- 15.Dart Trial Team. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375:123–31. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–9. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 17.Hartman K, Verweel G, de Groot R, Hartwig NG. Detection of lipoatrophy in human immunodeficiency virus-1-infected children treated with highly active antiretroviral therapy. Pediatr Infect Dis J. 2006;25(5):427–31. doi: 10.1097/01.inf.0000215003.32256.aa. [DOI] [PubMed] [Google Scholar]
- 18.María RM, Noris P. Fat redistribution and metabolic disturbances in HIV-infected children and adolescents under highly active antiretroviral therapy (HAART) Bol Med Hosp Infant Mex. 2009 Jul-Aug;66:41–6. [Google Scholar]
- 19.Vigano A, Thorne C. European Collaborative Study. 2003 Feb; Boston. (Abstract 774) Italian register on HIV in children. Fat redistribution and metabolic abnormalities in HIV-infected children and adolescents in Europe. 10th Conference on Retroviruses and Opportunistic infections. [Google Scholar]
- 20.Beregszaszi M, Dollfus C, Levine M, Faye A, Deghmoun S, Bellal N, et al. Longitudinal evaluation and risk factors of lipodystrophy and associated metabolic changes in HIV-infected Children. J Acquir Immune Defic Syndr. 2005;40:161–8. doi: 10.1097/01.qai.0000178930.93033.f2. [DOI] [PubMed] [Google Scholar]
- 21.US Preventive Services Task Force. Screening for lipid disorders in children, recommendation statement. Pediatrics. 2007;120:e215–9. doi: 10.1542/peds.2006-1812. [DOI] [PubMed] [Google Scholar]
- 22.Jaquet D, Levine M, Ortega-Rodriguez E, Faye A, Polak M, Vilmer E, et al. Clinical and metabolic presentation of the lipodystrophic syndrome in HIV-infected children. AIDS. 2000;14:2123–8. doi: 10.1097/00002030-200009290-00008. [DOI] [PubMed] [Google Scholar]
- 23.Carr A, Miller J, Law M, Cooper D. A syndrome of lipodystrophy (LD), lactic acidemia and liver dysfunction associated with HIV nucleoside analogue reverse transcriptase inhibitor therapy: a contribution to PI-related LD syndrome. Antiviral Ther. 1999;4(2):19–20. [Google Scholar]
- 24.Joly V, Flandre P, Meiffredy V, Leturque N, Harel M, Aboulker JP, et al. Increased risk of lipoatrophy under stavudine in HIV-1-infected patients: results of a substudy from a comparative trial. AIDS. 2000;16:2447–54. doi: 10.1097/00002030-200212060-00010. [DOI] [PubMed] [Google Scholar]
- 25.Buck WC, Kabue MM, Kazembe PN, Kline MW. Discontinuation of standard first-line antiretroviral therapy in a cohort of 1434 Malawian children. Journal of the International AIDS Society. 2010;13(31) doi: 10.1186/1758-2652-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spoulou V, Kanaka-Gantenbein C, Bathrellou I, Mora S, Mostrou G, Sidossis L, et al. Monitoring of lipodystrophic and metabolic abnormalities in HIV-1 infected children on antiretroviral therapy. Hormones. 2011;10(2):149–55. doi: 10.14310/horm.2002.1305. [DOI] [PubMed] [Google Scholar]