Abstract
Background
HIV screening in a labour ward is the last opportunity to initiate an antiretroviral prophylaxis among pregnant women living with HIV to prevent mother-to-child HIV transmission. Little is known about the feasibility and acceptability of HIV screening during labour in West Africa.
Findings
A cross-sectional survey was conducted in the labour ward at the Tokoin Teaching Hospital in Lomé (Togo) between May and August 2010. Pregnant women admitted for labour were randomly selected to enter the study and were interviewed on the knowledge of their HIV status. Clinical and biological data were collected from the individual maternal health chart. HIV testing or re-testing was systematically proposed to all pregnant women. Among 1530 pregnant women admitted for labour, 508 (32.2%) were included in the study. Information on HIV screening was available in the charts of 359 women (71%). Overall, 467 women accepted HIV testing in the labour ward (92%). The HIV prevalence was 8.8% (95% confidence interval: 6.4 to 11.7%). Among the 41 women diagnosed as living with HIV during labour, 34% had not been tested for HIV during pregnancy and were missed opportunities. Antiretroviral prophylaxis had been initiated antenatally for 24 women living with HIV and 17 in the labour room.
Conclusions
This study is the first to show in West Africa that HIV testing in a labour room is feasible and well accepted by pregnant women. HIV screening in labour rooms needs to be routinely implemented to reduce missed opportunities for intervention aimed at HIV care and prevention, especially PMTCT.
Keywords: HIV, mother-to-child transmission, acceptance, labour, Africa
Background
Over the past two decades, considerable biomedical successes have been achieved in the science of prevention of mother-to-child transmission of HIV (PMTCT) in sub-Saharan Africa. Effective interventions that can significantly reduce in utero, intra-partum and postnatal HIV transmission are now available [1–3]. Despite the increasing availability of antiretroviral drugs for PMTCT and the international mobilization for the implementation of these services, the escalation of PMTCT interventions in many countries in Africa has proved elusive and paediatric HIV remains an uncontrolled epidemic. In 2009, only 35% of the 125 million pregnant women in low-and middle-income countries received an HIV test [4].
In 2010, United Nations agencies prioritized the reduction of mother-to-child transmission of HIV, and called for its effective elimination by 2015 [5,6]. In order to reach this ambitious target, strategies for increasing HIV testing among pregnant women, which is the entry point to subsequent PMTCT interventions, are urgently needed. One such strategy consists of HIV screening in labour wards as recommended by the World Health Organization (WHO), and is viewed as the last opportunity to identify pregnant women living with HIV and to initiate an antiretroviral prophylaxis aimed at PMTCT [7].
The experience of HIV screening in labour wards is rarely reported and its acceptance varies between 67 and 98% [8–12]. In West Africa, the region of the world where HIV testing coverage is the lowest (21%), there has been no report to date on the use of this strategy.
The objectives of the study are to describe missed opportunities for effective PMTCT interventions and to assess the feasibility and acceptability of HIV screening in a labour ward in a West-African setting.
Methods
A cross-sectional survey was conducted between May and August 2010 in the labour ward of the Gynaecology Obstetrics unit of the Tokoin Teaching Hospital in Lomé, Togo. It was not logistically possible to include all pregnant women admitted for labour in this unit during the study period. All pregnant women admitted for delivery on randomly selected days and periods of time were systematically included in the study (Monday from 8am to 2pm, Wednesday from 2pm to 8pm, Saturday from 8pm to Sunday 8am).
Standardized forms were used to collect socio-demographic characteristics (age, level of education, marital status, and number of previous pregnancies) and pregnancy history (number of antenatal visits, knowledge of HIV status, PMTCT prophylaxis received), as well as mode of delivery, birth outcome, and anthropometric data of the newborn. This information was collected from maternal health records, the delivery registry and direct interviews with the women, which were conducted before or after delivery according to the time of delivery.
On admission to the labour room 5 ml of venous blood was collected from each pregnant woman. The recommended national algorithm used for HIV diagnosis was: Determine HIV-1/2 (Abbott Diagnostics) and First Response HIV Card test 1-2.0 (PMC Medical). A woman was considered as living with HIV when both tests were positive. In case of no concordance of the two tests, the blood sample was sent to the national reference laboratory for an ELISA test.
The outcomes were:
Acceptance of HIV testing in a labour room.
Missed opportunity for HIV testing, defined as the proportion of women who did not know their HIV status before delivery.
Missed opportunity for an effective PMTCT intervention defined as the proportion of women living with HIV who did not initiate any PMTCT intervention before delivery.
The distribution of continuous variables were compared using the Wilcoxon rank-sum test, and comparisons between categorical variables were performed using the Chi-square test or Fisher's exact test when appropriate. We estimated the prevalence of HIV infection with its 95% confidence interval (95% CI). Univariable and multivariable logistic regressions were used. All statistical analyses were performed with STATA®, version 9.0 (StataCorp, College Station, Texas, USA).
The study's protocol was approved by the National Ethic Committee of the Ministry of Health of Togo in May 2010. All pregnant women signed an informed consent document before their participation in the study.
Results
Between May and August 2010, 1530 pregnant women were admitted for delivery at the Tokoin Teaching Hospital, and among them 508 (33.2%) were enrolled in this study. There was no significant statistical difference between the women included and those not included in the study (Table 1). Median age was 27 years [inter-quartile range (IQR), 23 to 31 years], 87.8% were married or co-habiting with a partner. Overall, 502 women (98.8%) had attended at least one antenatal consultation before delivery: 106 (21.1%) at the Tokoin Teaching Hospital and 396 (78.9%) at health facilities in the periphery of Lomé.
Table 1.
Characteristics of pregnant women delivering at the Tokoin teaching hospital. May to August 2010, Lomé, Togo
| Included in the study, n (%) | ||||
|---|---|---|---|---|
| Total, n (%) | Yes | No | ||
| N = 1530 | n = 508 | n = 1022 | p value | |
| Age (years) | ||||
| Median (IQR) | 27 (23 to 31) | 26.5 (23 to 31) | 27 (23 to 31) | 0.819 |
| ≥30 | 516 (33.7) | 165 (32.5) | 351 (34.3) | 0.468 |
| Number of pregnancies | ||||
| Median (IQR) | 2 (1 to 3) | 2 (1 to 3) | 2 (1 to 3) | 0.449 |
| 1 | 559 (36.5) | 181 (35.6) | 378 (37.0) | 0.650 |
| 2 to 3 | 660 (43.2) | 217 (42.7) | 443 (43.3) | |
| ≥4 | 311 (20.3) | 110 (21.7) | 201 (19.7) | |
| Mode of delivery | 0.188 | |||
| Vaginal | 1014 (66.3) | 333 (55.5) | 681 (66.6) | |
| C-section | 516 (33.7) | 175 (34.5) | 341 (33.4) | |
| Infant vital status | 0.468 | |||
| Live born | 1453 (95.0) | 481 (94.7) | 972 (95.1) | |
| Dead | 76 (5.0) | 27 (5.3) | 49 (4.9) | |
| Gender* | 0.964 | |||
| Boy | 835 (54.5) | 279 (54.5) | 556 (54.4) | |
| Girl | 695 (45.4) | 229 (45.5) | 466 (45.6) | |
| Birth weight (grams)* | ||||
| Median (IQR) | 3000 (2800 to 3300) | 3000 (2750 to 3350) | 3000 (2800 to 3300) | |
| <2500g | 179 (12.6) | 66 (14.7) | 113 (11.6) | 0.101 |
IQR: Interquartile range.
Only for live birth (n=1420).
A total of 502 (98.8%) attended at least one antenatal visit and among them 359 (71.5%) had information on their HIV status reported in their individual maternal health chart.
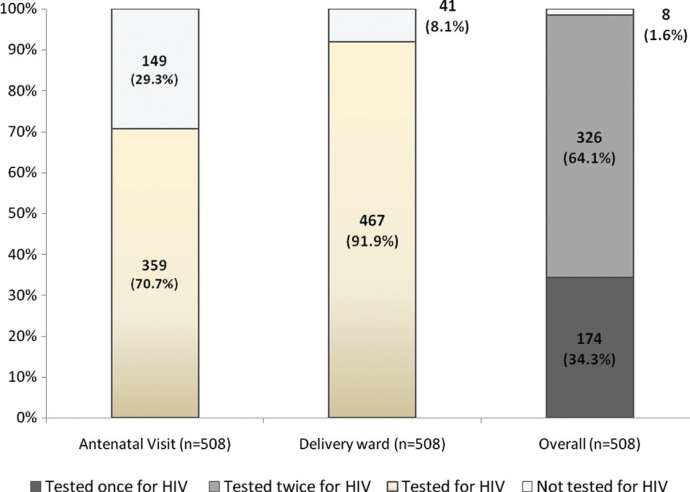
Figure 1 shows the proportion of women diagnosed for HIV infection during antenatal visits and in the labour ward. Acceptance of HIV testing in the labour ward was 91.9% (n=467); 90.8% (326/359) among those tested previously according to their antenatal record, and 94.6% (141/149) among those tested the first time in labour ward (p=0.15). Acceptance of HIV testing in the labour ward was 91.0% (323/355) among women who declared knowing their HIV status and 94.1% (144/153) among those who did not (p=0.234). In univariate and multivariate analysis, none of studied variables were associated with the acceptance of HIV testing in the labour ward.
Figure 1.
Proportion of women screened for HIV infection during antenatal visits and in delivery ward. May to August 2010, Lomé, Togo.
Overall, 467 women (91.9%) accepted HIV testing in the labour ward and among them, 41 were living with HIV (8.8%, 95% CI: 6.4 to 11.2%). Of these 41 women living with HIV, 27 (65.9%) knew their HIV positive status already reported in the antenatal record and 14 (34.1%) were newly diagnosed during labour ward screening. Prevalence of HIV infection was 8.3% (27/326) among women for whom the HIV testing result was reported in their antenatal record, and 9.9% (14/141) among those who were tested for the first time (p=0.56). Concordance of HIV testing between antenatal consultation and the labour ward is reported in Table 2. In addition to the 27 cases of HIV infection identified during pregnancy and confirmed during labour, 14 new cases were identified by testing in the labour ward.
Table 2.
Concordance of HIV testing between antenatal consultation and delivery ward, n (%). May to August 2010, Lomé, Togo
| HIV testing in delivery ward | ||||
|---|---|---|---|---|
| Positive | Negative | Refusal | ||
| N (%) | N (%) | N (%) | Total | |
| HIV testing in antenatal consultation* | ||||
| Positive | 27 (100.0) | 0 | 0 | 27 (100.0) |
| Negative | 0 | 299 (90.1) | 33 (9.9) | 332 (100.0) |
| Not done or not reported | 14 (9.4) | 127 (85.2) | 8 (5.4) | 149 (100.0) |
| Total | 41 | 426 | 41 | 508 (100.0) |
Based on HIV testing information reported in the maternal health chart.
Percentages represent the proportion of women on line.
Overall, 41.5% (n=17) of the 41 women living with HIV had not initiated any PMTCT intervention antenatally. In multivariate analysis, the missed opportunities for antenatal PMTCT interventions were associated with gravidity ≥2 (adjusted Odds Ratio [aOR], 2.07; 95% CI: 1.29 to 3.33), not being married (aOR, 1.82; 95% CI: 1.00 to 3.23), and having made antenatal visits at health facilities in the periphery of Lomé (aOR, 3.57; 95% CI: 1.96 to 6.67). Among the 27 pregnant women who knew their status HIV antenatally, two had initiated antiretroviral therapy for their own health, 22 received zidovudine and a single-dose of nevirapine and three did not initiate any antiretroviral intervention before their admission to the labour ward. Among the 14 women living with HIV diagnosed in labour room, all of them received an antiretroviral prophylaxis during labour.
Discussion
In this study carried out in the obstetrical ward at the Tokoin Teaching Hospital, which is the biggest referral health centre for delivery in Togo, we found that 92% of pregnant women agreed to be tested for HIV in the labour room. HIV prevalence in the labour room in this referral centre was 8.8% and the 14 newly diagnosed women (34% of the total) initiated antiretroviral prophylaxis.
In 2004, HIV testing in labour rooms was recommended by the US Centers Diseases for Control and Prevention [13] and in 2010, WHO recommended that in the countries where HIV epidemic is considered generalized (prevalence >1% in pregnant women) there should be HIV retesting in all HIV-negative or HIV status unknown women at the time of delivery [7]. The rationale to focus on HIV testing in the labour ward in a country such as Togo, which has an HIV testing coverage in pregnant women estimated at 18%, is the fact that this strategy is the last opportunity to introduce an antiretroviral prophylaxis for PMTCT in order to reduce the many missed opportunities for intervention [5,7,14,15].
The acceptance of HIV testing in the labour room was relatively higher than the percentage reported in a study in Zambia where 67% of 217 women accepted to undergo HIV testing [10]. Elsewhere, the acceptance rate was 98% in a University hospital located in rural India [12] and 85% in a US trial [9]. Overall, these studies, including ours, show that HIV testing is highly feasible and acceptable even in busy delivery wards. The key factor that may influence the acceptance of HIV testing in labour wards is the type of HIV test used. Oral fluid-based rapid HIV tests seem more convenient and do not elicit patients’ fear of blood draw (as reported in India). The lack of dedicated staff for this procedure is another concern. Indeed, in the US study, low acceptance of HIV testing during the night shift was reported because of limited staff availability [9]. In our study, 29% of pregnant women, despite having had antenatal care visits did not know their HIV status before entering the labour ward. This figure decreased to 1.6% after introducing HIV testing in the labour room. Therefore, as already supported by international guidelines, it is of the utmost importance to place standing orders in all labour rooms in sub-Sahara Africa to inform every woman that she will be tested unless she states otherwise [13].
During this study, we faced some challenges. Firstly, the duration of pre-test counselling was short, on average 3 minutes, which was close to the duration of the individual pre-test counselling in African settings [14]. Secondly, the understanding of the message about HIV testing during labour was a concern, but we were unable to document it in this study. Finally, the team discussed case by case whether they would postpone HIV testing until after delivery in order to ensure that patients were not coerced into HIV testing during labour.
The prevalence of HIV among pregnant women admitted for delivery was estimated at 8.8%, more than twice the HIV prevalence reported in pregnant women in Togo in 2009 (3.9%) and very close to the HIV prevalence reported in Lomé, the capital of Togo (6.3%). The site selected for this study which is the reference centre located at the teaching hospital could explain the higher prevalence figure observed.
Among the 41 women identified as living with HIV in the labour ward, 17 (34%) did not know their HIV status before being admitted to the labour ward and the testing procedure allowed the immediate initiation of ARV prophylaxis to all of them. In rural India in 2007, 11 out of 15 women living with HIV (73%) learnt of their HIV status in the delivery room [12]. In the absence of ARV intervention in breastfeeding populations between 30 and 40% of pregnant women living with HIV transmit HIV to their infant [3]. According to the study reported in Malawi, which included HIV-exposed infants whose mothers did not receive antiretroviral prophylaxis, the rate of HIV transmission was 15.5% at eight weeks of life in children who received zidovudine + single-dose nevirapine, a 50% reduction compared to no intervention [15].
This study has some limitations. First, it was conducted in a single labour ward and specifically in a referral centre. Therefore, our sample is not representative of all pregnant women in Togo, limiting the generalization of the findings to the rest of the country. Second, the choice to propose an antiretroviral prophylaxis to newly diagnosed pregnant women living with HIV was part of the project intervention and an anonymous cord blood-surveillance strategy was not used to measure PMTCT coverage as done previously in the PEARL survey [14]. Despite these limitations, this is to our knowledge the first study conducted in the largest delivery ward in Togo and the first one evaluating the feasibility and acceptance of HIV testing during labour in West Africa. Since only 1.2% women refused HIV testing, the HIV prevalence estimate is very close to the true prevalence in pregnant women attending this hospital.
Conclusions
In conclusion, HIV testing in the labour room is highly feasible and acceptable and can significantly reduce missed opportunities for effective PMTCT interventions. The implementation of this strategy should be put in place in all maternity hospitals and the dedicated staff should be trained for this procedure as well. However, involuntary testing is a potential challenge to implementing provider initiating testing strategy in the labour ward. This salvage intervention for PMTCT will increase the PMTCT coverage which remains low in sub-Saharan Africa and will help to will reinforce the linkage for early infant diagnosis. In the context of virtual elimination of MTCT by 2015, there is no doubt that this strategy is extremely relevant.
Acknowledgments
We are grateful to the women who accepted to participate in the study. The authors would like to acknowledge the medical staff of the Gynaecology Obstetrics unit of the Tokoin Teaching Hospital in Lomé.
Competing interests
The authors have no conflict of interest to declare.
Authors’ contributions
DKE, BGK designed the study. BGK collected the data and DKE and PC analyzed the data. DKE, BGK, PC, RB interpreted the data. DKE, PC and RB wrote the first draft of the manuscript.
All the authors contributed to the writing of the manuscript and all the authors agree with the manuscript's results and conclusions.
Abbreviations
PMTCT, prevention of mother-to-child transmission of HIV; MTCT, mother-to-child transmission of HIV.
Funding sources
This study was performed thanks to funding from the Institut de Santé Publique Epidémiologie Développement (ISPED) of the Bordeaux Segalen University in Bordeaux (France) and the French National AIDS Research Agency (ANRS) through the PAC-CI research program in Abidjan (Cote d'Ivoire).
References
- 1.Becquet R, Ekouevi DK, Arrive E, Stringer JS, Meda N, Chaix ML, et al. Universal antiretroviral therapy for pregnant and breast-feeding HIV-1-infected women: towards the elimination of mother-to-child transmission of HIV-1 in resource-limited settings. Clin Infect Dis. 2009;49(12):1936–45. doi: 10.1086/648446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mofenson LM. Antiretroviral drugs to prevent breastfeeding HIV transmission. Antivir Ther. 2010;5(4):537–53. doi: 10.3851/IMP1574. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Recommendations for use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Guidelines on care, treatment and support for women living with HIV/AIDS and their children in resource-constrained settings. 2010. [cited 2012 Feb 22]. Available from: http://www.who.int/hiv/pub/mtct/guidelines/en/
- 4.WHO/UNAIDS/UNICEF. Towards universal access. Scaling up priority HIV/AIDS interventions in the health sector. 2010. [cited 2012 February 22]. Available from: http://www.who.int/hiv/pub/2010progressreport/report/en/index.html Progress Report.
- 5.WHO. Technical consultation on the Elimination of mother-to-child transmission of HIV. 2010. [cited 2012 February 22]. Available from: http://www.who.int/hiv/events/mtct/en/index.html.
- 6.Moszynski P. Global elimination of mother to child HIV transmission is now achievable, say agencies. BMJ. 2010;341:c5152. doi: 10.1136/bmj.c5152. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Delivering HIV test results and messages for re-testing and counselling in adults. 2010. [cited 2012 February 22]. Available from: http://www.who.int/hiv/pub/vct/hiv_re_testing/en/index.html. [PubMed]
- 8.Beltman JJ, Fitzgerald M, Buhendwa L, Moens M, Massaquoi M, Kazima J, et al. Accelerated HIV testing for PMTCT in maternity and labour wards is vital to capture mothers at a critical point in the programme at district level in Malawi. AIDS Care. 2010;22(11):1367–72. doi: 10.1080/09540121003758473. [DOI] [PubMed] [Google Scholar]
- 9.Bulterys M, Jamieson DJ, O'Sullivan MJ, Cohen MH, Maupin R, Nesheim S, et al. Rapid HIV-1 testing during labor: a multicenter study. JAMA. 2004;292(2):219–23. doi: 10.1001/jama.292.2.219. [DOI] [PubMed] [Google Scholar]
- 10.Megazzini KM, Chintu N, Vermund SH, Redden DT, Krebs DW, Simwenda M, et al. Predictors of rapid HIV testing acceptance and successful nevirapine administration in Zambian labor wards. J Acquir Immune Defic Syndr. 2009;52(2):273–9. doi: 10.1097/QAI.0b013e3181ab6e7c. [DOI] [PubMed] [Google Scholar]
- 11.Nogueira SA, Lambert JS, Albuquerque AL, Rodrigues R, Reis S, Bornia R, et al. Assessment of a rapid HIV test strategy during labor: a pilot study from Rio de Janeiro, Brazil. J Hum Virol. 2001;4(5):278–82. [PubMed] [Google Scholar]
- 12.Pai NP, Barick R, Tulsky JP, Shivkumar PV, Cohan D, Kalantri S, et al. Impact of round-the-clock, rapid oral fluid HIV testing of women in labor in rural India. PLoS Med. 2008;5(5):e92. doi: 10.1371/journal.pmed.0050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Rapid HIV-1 antibody testing during labor and delivery for women of unknown HIV status: a practical guide and model protocol. 2012. [cited 2012 February 22]. Available from: http://www.cdc.gov/hiv/topics/testing/ressources/guidelines/rt-labor&delivery.htm.
- 14.Ekouevi DK, Stringer E, Coetzee D, Tih P, Creek T, Stinson K, et al. Health facility characteristics and their relationship to coverage of PMTCT of HIV services across four African countries: the PEARL study. PLoS One. 2012;7(1):e29823. doi: 10.1371/journal.pone.0029823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taha TE, Kumwenda NI, Hoover DR, Fiscus SA, Kafulafula G, Nkhoma C, et al. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA. 2004;292(2):202–9. doi: 10.1001/jama.292.2.202. [DOI] [PubMed] [Google Scholar]