Abstract
Renal glomerular endothelial cells are specialized cells with an important role in physiological filtration and glomerular disease. However, maintenance of human primary endothelial cells requires stimulation with serum and growth factors that often results in modification of the cells properties. Previously, expression of early adenovirus region E4 was shown to help maintaining long-term survival of human endothelial cells in serum free media without addition of growth factors. In the current study, we showed that media conditioned with human epithelial cells stably transfected with Ad E4 region also supported survival of human glomerulus-derived endothelial cells in serum-free media. Mass-spectrometry analysis of the conditioned media identified pigmental epithelium derived factor (PEDF) as a major component of the conditioned media. PEDF expression in 293-E4 cells was validated by RT-PCR, Western blot and ELISA analysis. PEDF expression was detected in mouse glomeruli. Supplementation with recombinant PEDF supported survival of primary endothelial cells and the cells transformed with SV40 large T antigen in serum-free media, and extended the life-span of both cell cultures. PEDF did not inhibit FGF-2 stimulated growth and tubulogenesis of endothelial cells. Thus we demonstrated that adenoviral E4 region stimulated expression and secretion of PEDF by human renal epithelial cells that acted as a survival factor for glomerulus-derived endothelial cells.
Renal glomerular endothelial cells are specialized cells with an important role in physiological filtration and glomerular disease (1). Despite their unique features and their importance, it was a challenge to study these cells comparing to other endothelial cells because of difficulty in maintaining them in culture (2, 3). Primary cells have limited capacity to divide in culture and quickly reach a nonproliferative state known as senescence. Senescence or mortality checkpoint (M1)1 is characterized by the absence of cell division because of the inhibition of the cell cycle (4). Cells can overcome M1 when they lose the cell cycle inhibitory signals, such as functional Rb or p53 proteins. These cells extend their replicative life span but eventually reach the second mortality checkpoint (M2) also known as crisis (4) which is associated with shortening telomere sequences (5). Culturing primary endothelial cells requires supplementation with serum and pro-angiogenic factors, such as VEGF-A, FGF-2, EGF and IGF. Deprivation of growth factors or serum results in a rapid cell death (6). Thus the limited life span and dependence on serum and growth factors put a considerable limitation to studies that can be conducted with primary endothelial cells. Human primary glomerulus-derived endothelial cells (PrGECs) either transformed with a simian virus 40 (SV40) (7) or conditionally immortalized with temperature-sensitive SV40 large T antigen and telomerase using retroviral vectors (8) retain the ability to grow up to 60 passages and maintain endothelial cell markers. However, culturing these transformed or immortalized cell lines still requires supplementation with serum and growth factors and their withdrawal leads to quick cell death (9). Previously, expression of adenoviral early region 4 (Ad E4) or E4ORF1 gene product was shown to support long-term survival of different types of primary endothelial cells including human umbilical vein endothelial cells and human testicular endothelial cells, while preserving these cell's potential for tubulogenesis and sprouting (6, 10, 11). Introduction of AdE4ORF1 into primary endothelial cells increases their survival in serum and growth factor -free media without stimulation of cell proliferation. The mechanism by which Ad E4ORF1 induces cell survival is not yet known.
In the present study, we showed that media conditioned with human epithelial embryonic kidney cells stably transfected with AdE4 (293-E4 cells) supported survival of primary glomerulus-derived endothelial cells (PrGECs) in serum-free and growth factor-free media without the need to transform the primary cells with AdE4 gene expression vector. Supplementation with the media conditioned with 293-E4 cells (293-E4 media) prolonged the life-span of the PrGECs and also human primary glomerulus-derived endothelial cells transformed with SV40 large T antigen (HGECs). The mass-spectrometry analysis of the 293-E4 media identified pigmental epithelium derived factor (PEDF) that was absent in the control media conditioned with the parental 293 cells (293 media). PEDF expression in 293-E4 cells was validated by RT- PCR, Western blot, and ELISA analysis. PEDF was also observed in mouse glomeruli by immunostaining. Supplementation with recombinant PEDF increased PrGECs and HGECs survival in serum-free media, protecting the cells from necrosis and apoptosis while preserving their ability for FGF-2 stimulated growth and tubulogenesis.
MATERIALS AND METHODS
Reagents
Dulbecco's modified Eagle's medium (DMEM) culture medium, fetal bovine serum (FBS) and penicillin/streptomycin were obtained from Invitrogen. Complete CSC endothelial cell medium was purchased from Cell Systems Corporation (WA). Culture plates were purchased from Fisher Scientific. Unless indicated, all other chemicals and enzymes were obtained from Sigma-Aldrich. Mouse monoclonal antibody against human PEDF was from Millipore, against SV-40 T antigen from Oncogen Research Products (MA) and against human vWF from Dako North America Inc. The corresponding horseradish peroxidase-conjugated secondary antibody was from Invitrogen. Human recombinant FGF-2 and VEGF were purchased from R&D Systems. Human recombinant PEDF was obtained from Millipore.
Cell Culture
Human renal epithelial cell line 293 was obtained from ATCC. 293-E4 cell line was a gift from Dr. X. Ye (Children's National Medical Center, Washington DC). Human primary glomerulus-derived endothelial cells (PrGEC) were obtained from Cell Systems Corporation (WA). PrGECs were maintained in the complete CSC medium containing 10% FBS and growth factors (Cell Systems Corporation). A human glomerulus-derived endothelial cell line (HGEC) was generated by transformation of PrGEC with pRSV-T plasmid containing RSV-LTR promoter and sequence encoding the simian virus large T antigen gene (33). Briefly, PrGEC were transfected with 1 mg of plasmid DNA using Lipofectamine plus reagent (Invitrogen, Carlsbad, CA). Transfected cells formed colonies 4–6 weeks after transfection. Individual colonies were maintained separately in DMEM media supplemented with 10% FBS and penicillin/streptomycin (all from Invitrogen). HGEC grew exponentially for 10–35 generations and then detached and died. HGEC, human embryonic kidney cells 293 and 293-E4 were maintained in DMEM media with 10% fetal bovine serum (FBS) and penicillin/streptomycin at 37 °C and 5%CO2 (all from Invitrogen).
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde (Sigma) for 10 min, block with 10% goat serum (Sigma) for 10 min and incubated with primary antibody for 1h. Cells were labeled with mouse anti-vWF (Dako North America Inc, 1:200 dilution), and mouse anti-SV40 large T antigen antibodies (Santa Cruz Biotechnology, Sznta Cruz, CA; 1:400 dilution) following goat anti- mouse- biotin conjugate and streptavidin- horse radish peroxidase (both from Invitrogen). AEC kit (Invitrogen) was used for visualization.
RT-PCR of PEDF
Total RNA was extracted from 293 and 293-E4 cells using TRIzol reagent according to the manufacturer's protocol (Invitrogen). Total RNA (100 ng) was reverse transcribed to cDNA using Superscript™ RT-PCR kit (Invitrogen), hexamers and oligo-dT were used as primers. For Real-Time PCR analysis, cDNA was amplified using Roche Light Cycler 480 SYBR Green1 Master single-color real-time PCR detection system (Roche Diagnostics) using the program denaturation at 95 °C for 10 s, annealing at 60 °C for 10 s, and extension at 72 °C for 10 s for 45 cycles on a Roche 480 real-time PCR detection system and used to quantify the mRNA levels of 18S rRNA and PEDF. 18S rRNA was used as the internal control. Primer sequences for 18SrRNA forward-GCTGTTGCTACATCGACCTTT, reverse-CTCCAGGTTTTGCAACCAGT, amplicon size-168; PEDF forward-ACAGCAGCTGCCAGAACC, reverse-GCACCCGGTACAGGTCATAG, amplicon size-159. Mean cp values for 18S rRNA and PEDF were determined and expression levels determined taking 18S rRNA expression as 1. Unpaired t test was used to test statistical significance.
LC-MS/MS Analysis of Cell Secretome
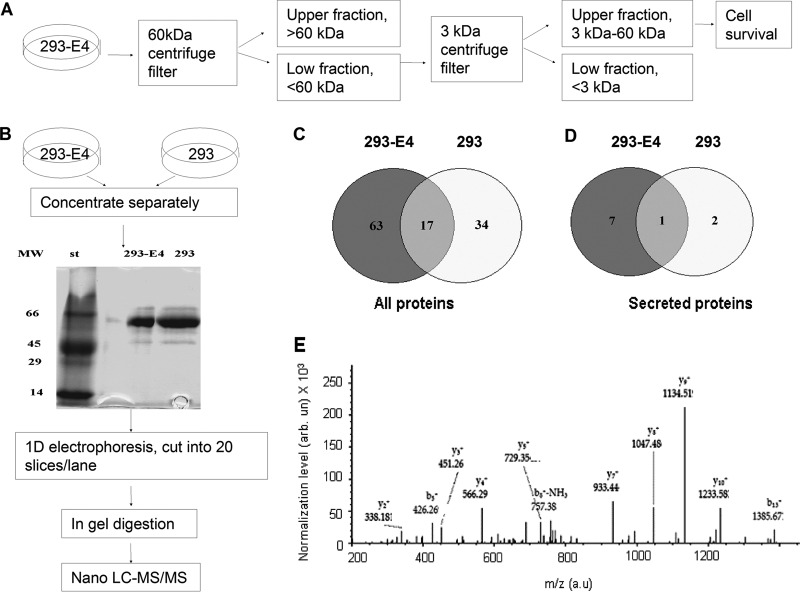
293 and 293-E4 cells were grown in 100 mm cell culture dish until confluence, than they were washed three times with PBS and media was replaced for serum-free high glucose containing DMEM. After 18 h of incubation at 37 °C media was collected, filtered through 0.22 μm filter (Fisher Scientific) and 0.6 ml of conditioned media was concentrated to 30 μl using Nanosep Centrifugal filter, 3 kDa cut (PALL Life Sciences). Twenty five microliters of concentrated media was separated on 10% SDS-PAGE and stained with Coomassie Blue. Each lane from the gel was cut into twenty 5 mm sections and sections that corresponds to proteins with molecular weight less than 67 kDa were used for nano LC-MS/MS. Samples selected for analysis were in-gel reduced, alkylated and digested with trypsin. The resulting peptides in each sample were separated by reversed-phase liquid chromatography (HPLC), using micro-capillary column C18, coupled in line with tandem mass spectrometer Thermo LTQ Orbitrap XL. The mass spectrometer was operated in a data-dependent MS/MS mode using normalized collision-induced dissociation (CID) energy of 35%. Peptide identifications were made using SEQUEST (Thermo Finnigan). Sequential database searches were performed using human FASTA database (released 08/18/2003). This protein database has a total of 199808 entries and 60322667 residues. Only peptides having cross-correlation (XCorr) cutoffs of 2.6 for [M + 2H]2+, 3.0 for [M + 3H]3+ and higher charge state were considered. These SEQUEST criteria thresholds resulted in a 1–2% of False Discovery Rate. FDR is determined by search on a decoy database. The proteome analysis of the spectra was made by Proteome Discoverer 1.2 software (Thermo Fisher Scientific). The Venn diagrams were constructed using genevenn software (http://genevenn.sourceforge.net/). Raw data files and the Proteome Discoverer analyzed files can be downloaded from ftp://ftp.pride.ebi.ac.uk/2012/08/PXD000014 or http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=14.
Western Blot Analysis
293 and 293-E4 cells were lyzed in SDS-loading buffer, resolved by 8% Tris-Tricine gel SDS-PAGE (34) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore). As control, 100 ng of recombinant PEDF was used. Western blot analysis was performed with mouse anti-PEDF (Millipore).
PEDF ELISA
Self-made ELISA was prepared by immobilizing proteins from either 293 or 293-E4 cells conditioned media (300 μl per well) on a high binding 96-wells plate (Thermo Fisher Scientific) for 18 h at +4 °C. Human recombinant PEDF (Millipore) was diluted with PBS and used as a standard. Wells were blocked with 20% bovine albumin (Sigma) for 1 h and incubated with mouse anti-PEDF antibodies (1:300 dilution, Millipore) for 2h, following incubation with goat-anti-mouse-HRP antibodies (Invitrogen). Sure Blue TMB microwell peroxidase substrate (KPL, MD) was used as a substrate solution. The sensitivity of assay was less than 100 pg/ml of recombinant PEDF. The assay was linear in the range from 200 pg/ml to 20 ng/ml of recombinant PEDF.
Immunohystochemistry Analysis of PEDF in Tissues
Fresh-frozen sections (4 μm) of one month old mouse were fixed in cold methanol for 10 min, and incubated in cold acetone for 3 min. M.O.M. kit (Vector Laboratories, Inc) for detecting mouse primary antibodies on mouse tissue were used according to manufacture instructions. Sections were incubated with primary mouse anti-PEDF antibodies (1:50 dilution, Millipore) for 1 h. DAB substrate kit (Vector Laboratories) was used for visualization. Hematoxylin (Invitrogen) was used for nuclei staining.
Senescence-associated Beta-galactosidase (SA-β-gal) Assay
Cells were fixed in EtOH for 10 min, wash in PBS and incubate in SA-β-gal buffer at 37 °C overnight. Buffer contained 40 mM citric acid/sodium phosphate pH 6.0, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide,150 mM NaCl, 2 mM MgCl2 (all from Sigma-Aldridge) and 1 mg/ml X-gal (Gold Bio Technology).
Detection of Apoptotic and Necrotic Cells
Detection of apoptosis and necrosis in the HGEC was carried out by the ApoDETECT annexin V-FITC kit (Zymed Laboratories Inc., South San Francisco, CA) according to manufacture instructions. Briefly, the media in HGEC was changed to serum-free media supplemented with either 1/100 dilution of media from 293-E4 cells or 200 pg/ml of PEDF (Millipore). After 18 h of incubation at 37 °C, cells were incubated with anti-annexinV-FITC for 30 min at +4 °C without fixation. Propidium iodide (PI) was used for nuclei staining.
HGEC Tube Formation Assay
For studying the effect of cell condition media and PEDF on HGEC tube formation 5 × 104 endothelial cells were plated in 50 μl of DMEM medium onto 24 well plates coated with Matrigel (R&D Systems, Minneapolis, MN). 200 μl of complete CSC medium were added to the endothelial cells with addition of 20 ng/ml of hrFGF-2. 1/100 dilution of either 293 or 293-E4 condition media was added. PEDF was added at concentration 200 pg/ml. Tube- formation was monitored at 24 h using an inverted microscope Nikon Eclipse TE300 with Optronics digital camera. Two independent experiments with duplications were performed.
RESULTS
293-E4 media increases long-term survival of primary glomerulus-derived endothelial cells (PrGECs). Adenoviral E4 vectors expressing all seven E4 genes or E4ORF1 gene alone promote survival of primary endothelial cells in the absence of angiogenic factors and serum (6, 10, 11). We collected media from a culture of human epithelial embryonic kidney cells that were stably transfected with all adenoviral E4 genes (293-E4 cells). Supplementation with this conditioned media (293-E4 media) increased survival of PrGECs in serum-free media in the absence of additional angiogenic factors as compared with control media conditioned with parental 293 cells (293 media) (Fig. 1A). Addition of 293-E4 media also increased survival of PrGECs in complete media (Cell Systems Corporation media supplemented with 10% FBS and endothelial cell growth factors) (Figs. 1B and 1C). PrGECs (obtained from Cell Systems Corporation at passage 2) showed signs of senescence after ∼7–10 days in culture with the complete media (Fig. 1B, control media, arrows, day 10). In contrast, PrGECs cultured with the addition of 293-E4 media continued to grow for 15–20 days before they showed signs of senescence (Fig. 1B, 293-E4 media, cells shown at day 10; and Fig. 1C). To achieve this prolongation of the continuous cell growth, a very small amount of 293-E4 media was needed (about 1/100 of the total media volume or 1/100 dilution). The control 293 media did not have this effect and did not prolong the survival of PrGECs (not shown). Cells treated with 293-E4 media were not immortalized as evidenced by a delayed senescence. Thus, supplementation with 293-E4 media allowed PrGECs to survive in serum-free media without the addition of growth factors and also significantly increased the duration of continuous cell growth until senescence or M1 checkpoint.
Fig. 1.
Media conditioned with 293-E4 cells supported survival of primary glomerulus-derived endothelial cells (PrGEC) in the absence of serum and growth factors and increased their life span in complete media. A, Viability of PrGECs cultured for 24 h in complete media or in serum-free media without growth factors with addition of 293 or 293-E4 condition media (1/100 dilution). B, Phase contrast microscopy of PrGECs cultured in complete media with and without 293-E4 condition media for 10 days. Arrows show senescent cells. (Magnification: 200×). C, Growth curve of PrGEC in complete CSC media supplemented with 10% FBS and growth factors with and without the addition of 293-E4 conditioned media. Data are shown as mean ± S.E. from three independent experiments.
To investigate whether 293-E4 media also increases the time of cell growth before the M2 checkpoint, a human glomerulus-derived endothelial cell line (HGEC) was generated by transformation of PrGECs with pRSV-T plasmid containing RSV-LTR promoter and sequence encoding the simian virus large T antigen gene. The SV40 large T antigen binds to and interferes with the activity of the Rb and p53 proteins and allows a bypass of M1 but not the M2 checkpoint (12). Transfection of PrGECs with pRSV-T resulted in the establishment of several cell clones with extended life span. Expression of T-antigen was verified by immunohistochemistry (Fig. 2A). Although the primary human endothelial cells become senescent after 3–5 population doubling (PDL), HGECs survived an average 24 PDLs in a complete media containing 10% FBS and lacking additional angiogenic growth factors (Fig. 2B, mean ± S.E. = 24 ± 13). Approximately at 24 PDLs, a cellular crisis with massive cell death occurred (Fig. 2C, control media). Supplementation with 293-E4 media (1/100 dilution) significantly increased the number of HGECs PDLs without the immortalization (Fig. 2B, 293-E4 media mean ± S.E. = 72 ± 25; and Fig. 2C, PDL 25). HGECs, cultured with the addition of 293-E4 media, did not become immortal and finally reached a crisis after a period of a prolonged growth. Prolonged survival of HGECs depended on the addition of 293-E4 media and its withdrawal rapidly led to a crisis (Fig. 2D, the arrow shows the day of 293-E4 media withdrawal). Addition of 293-E4 media but not the control 293 media also prevented the death of HGECs in serum-free media (Fig. 2E).
Fig. 2.
293-E4 media supported survival and increased life span of human glomerulus-derived endothelial cells transformed with SV40 large T antigen (HGEC) in the absence of serum and growth factors. A, Expression of SV40 large T antigen in HGEC is shown (black color). Mouse nonspecific antibodies were used as control (Magnification 200×). B, Cell population doubling (PDL) in HGEC with and without 293-E4 condition media. Data are shown as a mean ± STD from 5 independent experiments. C, HGEC at PDL = 25 in the complete media with and without 293-E4 media. (Magnification 200×). D, Cell survival after withdrawal of 293-E4 condition media from HGEC. Arrow shows the day of 293-E4 media withdrawal. Data are shown as a mean ± S.E. from three independent experiments. E, Viability of HGEC cultured for 24 h in complete media or in serum-free media without growth factors with addition of 293 or 293-E4 condition media (1/100 dilution). Data are shown as a mean ± S.E. from three independent experiments.
Taken together, media conditioned with 293 cells expressing adenoviral E4 genes prolonged survival of both primary and SV40 large T antigen transformed human glomerulus-derived endothelial cells in the serum-supplemented media and increased cell survival in the serum-free media.
Mass-spectroscopy Analysis of 293-E4 Media
To analyze the protein composition of 293-E4 media, we first analyzed whether its effect was because of small or large molecular weight components of the media. The 293-E4 media was fractionated by centrifugation on Amicon Ultra-4 filter with 60 kDa cut-off (see a workflow diagram on Fig. 3A). Flow-through media that contained proteins with molecular weight less than 60 kDa but not the media retained by the Amicon filters stimulated survival of HGECs in serum-free media without supplementation with growth factors (not shown). The flow through media was further fractionated on Nanosep Centrifugal Device with 3 kDa cut-off (Pall Life Science, East Hills, NY). The flow through that contained molecules with molecular weight of less than 3 kDa did not stimulate survival of HGECs whereas the retained fraction stimulated it (not shown). Thus the survival factor(s) in the conditioned media were proteins with the molecular weight in the range of 3–60 kDa. Human VEGF and FGF-2, two major angiogenic growth factors that promote growth of endothelial cells were not detected by ELISA in the 293-E4 media or control 293 media (not shown). Thus we conducted a comparative analysis 293-E4 media versus 293 media using high resolution mass-spectrometry on LTQ XL Orbitrap to identify a novel angiogenic factor(s) in the 293-E4 media. The 293-E4 media was collected, concentrated, resolved on 10% SDS-PAGE and stained with colloidal Coomassie Blue as described in Materials and Methods (see also a workflow diagram in Fig. 3B). Coomassie Blue staining showed a major band with mobility close to bovine albumin (molecular weight 67 kDa) and several additional minor bands (Fig. 3B). The gel strips containing resolved 293-E4 and 293 media samples were cut into 5 mm sections and the sections corresponding to proteins with molecular weight of less than 67 kDa were subjected to in-gel reduction, alkylation and then digestion with trypsin. Tryptic peptides obtained from each slice were applied to nano-LC C18 column connected in line to LTQ-XL Orbitrap mass spectrometer and MS/MS analysis was conducted.
Fig. 3.
Workflow for 293-E4 media fractionation and the comparative secretome analysis of 293 and 293-E4 cells. A, Diagram of 293-E4 conditioned media fractionation using centrifuge filters (60 kDa and 3 kDa cutoff). Only fractions containing proteins with molecular weight between 3 kDa and 60 kDa stimulated survival of HGEC in serum-free media without growth factors. B, Diagram of the label-free approach to identify the differential proteins in the 293-E4 and 293 cell secretomes. C, Venn diagram of all proteins found in the media conditioned with 293 and 293-E4 cells. D, Venn diagram of secreted proteins found in the 293-E4 or 293 media. E, SEQUEST MS/MS analysis for PEDF peptide LAAAVSNFGYDLYR found in 293-E4 secretome. The marked peaks indicate matched MS/MS fragments. SEQUEST match with XCorr = 3.52 for peptide and overall protein Score = 19.58 shows a high confidence of PEDF protein detection.
The mass-spectrometry analysis showed a match to 80 proteins in the 293 E4 media and 51 proteins in the control 293 media, with 17 proteins found in both media (Fig. 3C, see also Materials and Methods for access of raw and SEQUEST analyzed spectra). Only 8 secreted proteins were found in 293-E4 media and only 3-in the control 293 media with 1 protein found in both media (Fig. 3D and Table I). PEDF also known as serpin F1 (SERPINF1) was matched with the highest score among other secreted protein candidates in 293-E4 media and was not present in 293 media (Table I). SEQUEST search that uses the correlation value (XCorr) and Score parameters (13) detected PEDF with high confidence by the presence of LAAAVSNFGYDLYR peptide (Fig. 3E) with XCorr = 3.52 and overall Score = 19.58. Detailed definition of XCorr and Score could be found in Ref. 13. Briefly, XCorr is a parameter, which reflects a number of experimental MS/MS spectrum peaks that match to database peaks for specific peptide. The XCorr value of 3.52 corresponded to ∼50 matches of MS/MS fragments and neutral loss peptides to database values. The Score parameter is a sum of XCorr values for all detected peptides of specific protein. For PEDF protein, six MS/MS spectra were matched by SEQUEST to two peptides LAAAVSNFGYDLYR (XCorr = 3.91; 3.51; 3.34) and DTDTGALLFIGK (XCorr = 3.01; 2.93; 2.87). As described in Materials and Methods, this XCorr value gives a False Discovery Rate (FDR) of less than 1–2%. FDR is determined by the search on a decoy database. Because of the high detection confidence, PEDF was a good candidate for a novel growth factor that might be present in 293-E4 media.
Table I. Secreted proteins in 293-E4 and 293 cells conditioned media.
| Description | GenBank accession | MW | Scorea | #Peptides | Coverage, % |
|---|---|---|---|---|---|
| 293-E4 conditioned media | |||||
| Pigment epithelium-derived factor (PEDF) | P36955 | 46.3 | 19.58 | 2 | 6.22 |
| Sarcolectin | CAB41416 | 51.3 | 8.24 | 3 | 5.54 |
| Cationic trypsinogen | AAG30949 | 9.1 | 5.08 | 1 | 23.81 |
| Alpha 2 macroglobulin | EAW88590 | 151.0 | 2.08 | 1 | 0.60 |
| Apolipoprotein B | AAA51759 | 8.4 | 2.04 | 1 | 26.39 |
| Antithrombin | CAA48690 | 13.8 | 1.66 | 1 | 5.74 |
| sICAM-1 | AAB46863 | 4.3 | 0 | 1 | 50.00 |
| Fibroblast growth factor 17 precursor | 060258 | 10.42 | 0 | 2 | 7.41 |
| 293 conditioned media | |||||
| Alpha 2 macroglobulin | EAW88590 | 151.0 | 5.24 | 1 | 0.61 |
| Inter-alpha-trypsin inhibitor heavy chain | AAA59195 | 103.3 | 2.0 | 1 | 1.08 |
| Suppressin | AAB62704 | 52.7 | 0 | 1 | 2.21 |
a Proteins are shown based on Score. Definition of Score is presented in section Mass-spectroscopy analysis of 293-E4 media.
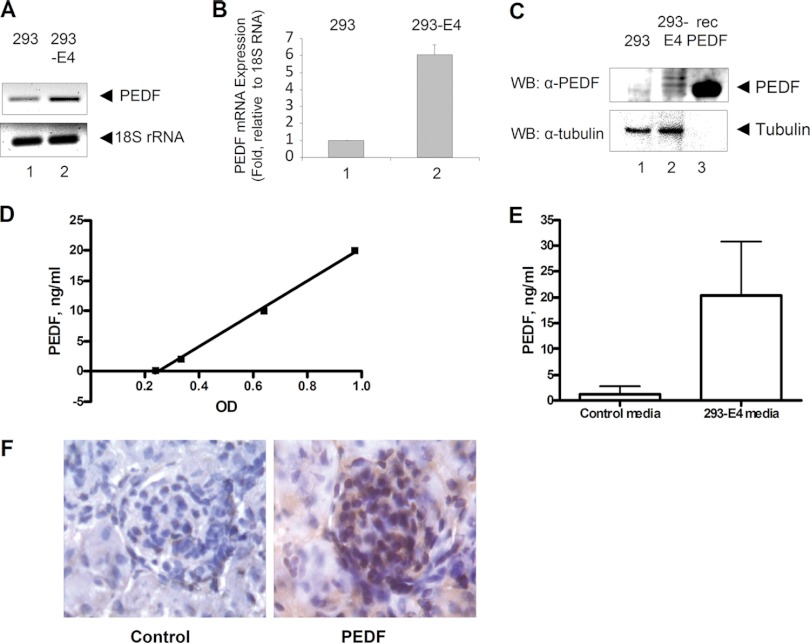
To validate the expression of PEDF in 293-E4 cells, total RNA was isolated from 293 and 293-E4 cells and PEDF mRNA was detected by RT-PCR that showed its increased expression in 293-E4 cells (Fig. 4A, lane 2). Quantification by real-time PCR showed about 6-fold increase in PEDF mRNA expression in 293-E4 cells in comparison to 293 cells (Fig. 4B, lane 2). Protein expression analysis by Western blot showed PEDF expression in 293-E4 but not in 293 cells (Fig. 4C, lane 2).
Fig. 4.
Validation of PEDF expression in 293-E4 cells. A, PCR amplification with PEDF or 18S rRNA specific primers using total mRNA isolated from 293 (lane 1) or 293-E4 (lane 2) cells that was reverse transcribed and amplified with the indicated primers. B, Quantitative RT-PCR of PEDF expression. 18S rRNA was used as a control. Data are shown as a mean ± STD. C, Immunoblot of 293 and 293-E4 cell lysates using mouse monoclonal anti-PEDF antibodies. Anti-tubulin antibodies were used as a loading control and recombinant PEDF (recPEDF) - as positive control. (D and E) Concentration of PEDF in the condition media from 293 and 293-E4 cells was determined by PEDF ELISA. Recombinant PEDF was used for a standard in panel D. Data are shown as mean ± S.E. from 5 independent experiments in panel E. p < 0.05. (F) Immunohistochemistry of PEDF in mouse kidney (brown color). Hematoxylin was used for blue nuclei staining. Nonspecific mouse antibodies were used as a control. (Magnification: 400×).
To analyze PEDF in the media conditioned with 293-E4 cells, an ELISA was performed using recombinant PEDF as control that had a linear detection range of 0.2–20 ng/ml (Fig. 4D). Concentration of PEDF in 293-E4 conditioned media was 20.4 ± 10.5 ng/ml as compared with 1.2 ± 1.6 ng/ml in the control media conditioned with the parental 293 cells. Because 293-E4 media was used at 1/100 dilution, the final concentration of PEDF that was added to PrGECs and HGECs in the experiments described above was about 200 pg/ml.
Immunostaining of mouse kidney demonstrated PEDF expression in glomeruli (Fig. 4F), supporting the hypothesis that PEDF can be a renal survival factor for glomerular endothelial cells.
Taken together, we detected PEDF in 293-E4 media by mass spectrometry, and validated PEDF expression in 293-E4 cells by RT-PCR and Western blot analysis. PEDF was detected in 293-E4 conditioned media by ELISA, and PEDF expression was shown in murine glomerulus.
PEDF Promotes Survival of both PrGEC and HGEC
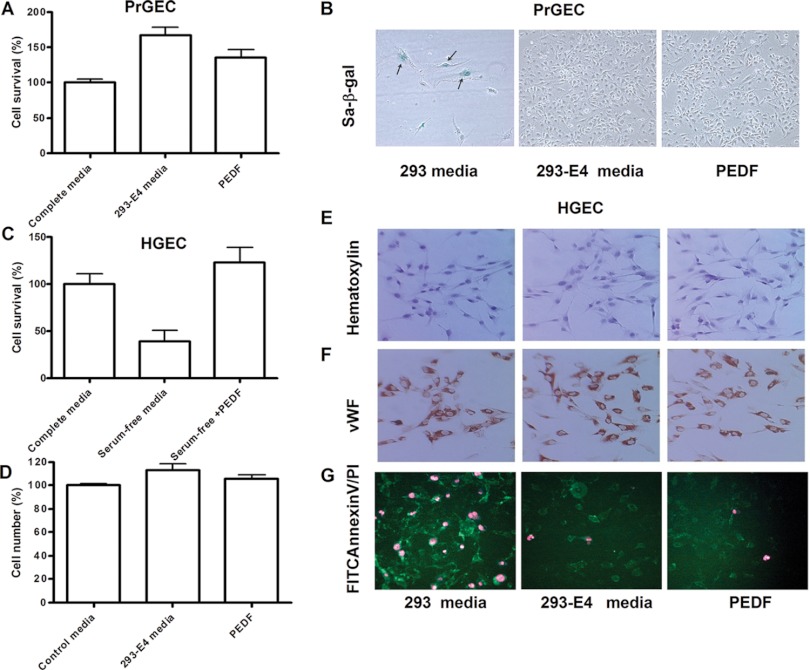
PEDF is a 50-kDa secreted glycoprotein with a wide spectrum of biological functions including pro-survival function in cerebella granule cells in culture (14). PEDF was shown to act as a growth inducer or growth inhibitor of endothelial cells depending on the cells phenotype (15). The effect of PEDF on human PrGECs was not previously examined. We showed above that 293-E4 media (1) increased survival of PrGECs and HGECs in serum and growth factor free media; (2) prolonged life-span of PrGECs before senescence; and (3) prolonged life-span of HGECs before M2 crisis. Thus we analyzed if recombinant PEDF can recapitulate these three activities.
Treatment of primary PrGECs with PEDF at the concentration corresponding to that in the 1/100 diluted 293-E4 conditioned media (200 pg/ml of PEDF) significantly increased cell survival in complete media after 7 days of culture (Figs. 5A and 5B). Treatment with both 293-E4 media and PEDF inhibited expression of senescence marker protein senescence-associated beta-galactosidase (SA-β-gal) (Fig. 5B). An increase of HGEC survival in serum-free and growth factors free media was observed in the cells treated with the same PEDF concentration for 24 h (Fig. 5C). In contrast, addition of either PEDF (200 pg/ml) or 293-E4 conditioned media to DMEM media containing 10% FBS did not significantly stimulate the growth of HGECs (Fig. 5D). Treatment with both PEDF and 293-E4 media did not change cell morphology (Fig. 5E, Hematoxilin staining, PDL 23) and did not have an effect on the expression of endothelial markers (Fig. 5F, vWF staining). However, treatment with 293-E4 conditioned media or PEDF reduced necrosis and apoptosis of HGECs in serum-free media (Fig. 5G). Only few FITC annexin V positive cells (green color) corresponding to an early apoptosis were detected in both 293-E4 conditioned media and PEDF treated culture after 18 h incubation in serum-free growth factor free media (Fig. 5G). In contrast, HGEC maintained in 293 condition media demonstrated both FITC-Annexin V positive and PI- positive staining that corresponds to latest stages of cell death from either apoptosis or necrosis (Fig. 5G).
Fig. 5.
PEDF promotes survival of both PrGEC and HGEC. A, Survival of PrGEC in complete media after 7 days in culture with or without 200 pg/ml of PEDF. 293-E4 media was used as positive control. B, PEDF inhibits the senescence-associated β-galactosidase expression (sa-β-gal, blue color, arrows) in PrGEC cells after 7 days in culture. 293-E4 media was used as a positive control. C, Survival of HGEC in serum-free and growth factors free media supplemented with 200 pg/ml of PEDF for 24 h. Survival of cells in the complete media was used as a positive control. D, PEDF did not stimulate HGEC growth in the complete media. HGEC incubated with 200 pg/ml of PEDF or 293-E4 conditioned media (1/100 dilution) for 3 days before cell number analysis. E, Treatment with both PEDF and 293-E4 conditioned media did not change HGEC morphology. Hematoxilin staining of HGEC is shown at PDL = 23 (Magnification 200×). F, Treatment with both PEDF and 293-E4 conditioned media did not change expression of vWF (cytoplasmic red color) (Magnification 200×). G, PEDF protects HGEC from apoptosis and necrosis in serum-free media. Annexin V staining for an early apoptosis shown in green, nuclear PI staining for late apoptosis shown in red (Magnification: 200×). Data are shown as a mean ± S.E. from three independent experiments.
Because PEDF has been shown to inhibit the growth of cells stimulated by VEGF (15) we investigated the effect of PEDF on the growth of HGECs cells stimulated by angiogenic growth factors. As shown above in Fig. 5D, PEDF did not inhibit serum factors - stimulated growth of HGEC. Also PEDF did not inhibit HGECs growth stimulated by human recombinant Fibroblast Growth factor 2 (FGF-2, Fig. 6A). In contrast, 293-E4 media and PEDF inhibited VEGF-stimulated growth of HGECs in dose-dependent manner (Fig. 6B). Interestingly, lower amounts of 293-E4 condition media (1/100 dilution) did not inhibit VEGF- stimulated growth of HGECs whereas higher amounts (1/10 dilution) inhibited the growth (Fig. 6B). Treatment of HGECs with 293-E4 or PEDF did not affect the ability of HGECs to form tubes on Matrigel stimulated with FGF-2 (Fig. 6C).
Fig. 6.
PEDF preserves angiogenic functions of HGEC. A, Addition of PEDF (200 pg/ml) or 293-E4 condition media (1/100 dilution) did not inhibit FGF-2 stimulated growth of HGEC. HGEC were cultured for 3 days in complete media with the addition of 20 ng/ml of human recombinant FGF-2 before the cells were counted. B, PEDF and 293-E4 media inhibited VEGF-stimulated growth of HGEC in the dose-dependent manner. HGECs were cultured for 3 days in complete media with addition of 5 ng/ml of human recombinant VEGF before the cells were counted. Two dilutions of 293-E4 condition media (1/10 and 1/100) and two PEDF concentration (200 pg/ml and 2 ng/ml) are shown. Data are shown as a mean ± S.E. from three independent experiments. C, Addition of 293-E4 condition media (1/100 dilution) and PEDF (200 pg/ml) did not inhibit tubulogenesis of HGEC on Matrigel. 293 condition media was used as a control. About 5 × 104 HGEC were plated onto 24 well plates coated with Matrigel with the addition of 20 ng/ml of FGF-2. Tube formation was monitored at 24 h. Two independent experiments with duplications were performed (Magnification 200×).
Taken together we demonstrated that 293-E4 media contained PEDF and PEDF prolonged survival of PrGECs before senescence and increased survival of HGEC in serum-free media. PEDF preserved some but not all functions of human glomerulus-derived endothelial cells in culture.
DISCUSSION
Here we demonstrated that renal epithelial cells with stable expression of Ad E4 genes secreted PEDF that maintained survival of PrGECs and HGEC transformed with SV40 large T antigen in serum free media without growth factors. PEDF also increased life span of both cell types in complete media.
Endothelial cells provide a permissive niche for maintenance of organ-specific cells via paracrine communications by soluble mediators (16). Primary endothelial cells maintained in culture have a shortened life-span and require supplementation of growth media with cytokines and serum that result in the loss of their unique endothelial cell features (6). Different methods were previously used to transform endothelial cells to increase their life-span including transformation with human telomerase (17, 18), polyoma middle T antigen (19), SV40 large T antigen, adenoviral E4 genes (10) or combination of transforming agents (8).
Glomerulus-derived endothelial cells are specialized renal endothelial cells with important role in physiological filtration and maintenance of podocyte-permissive niche (1). In renal glomeruli, endothelial cells form a continuous inner layer of the glomerular capillaries. The cytoplasm of these cells has numerous fenestrae, trans-cellular pores that have high permeability for water and low molecular weight plasma components. Despite their unique features they have been little studied because of a short, ∼7 days, life span in culture. To extend the life span of these cells we transformed them with SV40 large T antigen. The resulting HGECs were not immortal and reached M2 crisis after ∼26 generations. Still, HGECs culture required supplementation with growth factor and serum which removal led to a quick cell death.
Previously, stable expression of Ad E4 genes in human primary endothelial cells increased cell survival in serum-free media without change of endothelial cells functions (6, 20). The mechanism of this survival was not determined. Unexpectedly, we found that media conditioned with renal epithelial cells stably transformed with Ad E4 gene increased survival of both PrGEC and HGEC in serum-free media, increased life-time of PrGEC before senescence M1 and increased life-time of HGEC before crisis M2. These observations led us to hypothesize that 293-E4 cells secreted factors that supported long term survival of renal glomerular endothelial cells.
To study secreted proteins, we first cultured 293-E4 cells in complete media until confluence and transfer the cells into serum-free media. In most cells, serum-free media changes cell metabolism and induces cell death (21, 22). We found that DMEM media with high glucose content preserved viability of both 293 and 293-E4 cells. This preservation of viability significantly reduced the amount of intracellular and membrane proteins that otherwise would be released from dying cells compromising secretome analysis. Further, we only analyzed proteins with molecular weight less than 67 kDa thus eliminating contaminants such as bovine serum albumin and IgGs. Surprisingly, only 80 proteins were found in 293 E4 media and 51 proteins in the 293 media. Among these proteins only eight secreted proteins were found in 293 E4 media and only 3 secreted proteins in 293 media. Comparing to previously studied cultured cells secretome, we found significantly less proteins, both total and secreted (21). One possible explanation is that we conducted high resolution MS/MS analysis on Orbitrap Fourier Transform (FT) detector, which significantly reduced the number of protein candidates. The measurement sequence contained FT MS scan followed by three dependent FT MS/MS scans made on three major multiply charged MS peaks. High resolution scans dramatically decreased the amount of false positives and reduced the time of Sequest analysis. Nevertheless this also increased the duration of single measurement cycle, thus increasing the chance to miss the fragmentation of some ions during LC/MS run for complex samples. PEDF (SERPINF1) had the highest detection score among the secreted proteins, detected in the in the 293-E4 media and was not presented in 293 condition media. PEDF is a 50-kDa secreted glycoprotein and a noninhibitory member of the serine protease inhibitor (SERPIN) gene family with a wide spectrum of biological functions (15). PEDF was shown to have a neuronal differentiating activity, anti-oxidative, anti-inflammatory, anti-thrombogenic, and vasculoprotective properties in various cell types (23). PEDF exerts opposite effects on endothelial cells depending on their phenotype (15). In some experimental models, PEDF inhibited migration and proliferation of various endothelial cells induced by VEGF and FGF-2 (24, 25). In other models, PEDF stimulated cell growth synergistically with VEGF and inhibited vascular permeability (15, 26). The effect of PEDF on human glomerular endothelial cell growth was not previously described. In the rat model of adriamycin-induced nephritic syndrome, administration of PEDF significantly reduced proteinuria (27). Moreover, PEDF was shown to be expressed in glomerular areas and particular in podocytes and mesanglial cells in normal rat kidneys (27). We also demonstrated PEDF expression in mouse renal glomeruli supporting the hypothesis that PEDF can be a renal survival factor for glomerular endothelial cells. It is interesting that tubular epithelial cells in mice and rats as well as human embryonic tubular epithelial cells (293) did not express PEDF. In our experiments PEDF expression was stimulated in 293 cells by Ad E4 genes.
Increased vascular permeability in kidneys plays a key role in the development of nephritic syndrome (28), and PEDF administration ameliorated development of proteinuria in rat model (27) but the direct role of PEDF in endothelial cells survival or permeability was not investigated at those studies. Interestingly, in our experiments PEDF inhibited VEGF-stimulated growth of HGEC but did not block the angiogenic and tubulogenic activity of FGF-2. The further investigation is necessary to define PEDF signaling in HGEC and its crosstalk with different pro-angiogenic signals.
We demonstrated here that PEDF inhibited both the fast developing senescence in PrGECs and serum-starvation induced apoptosis in HGEC. The role of PEDF as a pro-survival factor may be associated with its anti-oxidative properties. It has been demonstrated that PEDF reduced ROS generation in cultured porcine retinal endothelial cells by suppressing the NADPH oxidase activity via down regulating the phosphorylation of p22 (PHOx) (26). The role of NADPH oxidases (Nox) in the apoptosis in endothelial cells has been demonstrated previously, and the accumulation of nuclear NOX2 and perinuclear NOX4 was a potential source of ROS production in homocysteine-induced apoptosis of HUVEC (29). On the other hand, inhibition of NADPH oxidases (Nox1 and Nox4) by siRNA significantly extended the replicative lifespan of HUVEC (30). PEDF was also shown to rescue serum starvation-induced apoptosis, which is mediated by apoptosis-inducing factor (AIF) but not caspases, in the cells derived from the rat retina by preventing translocation of AIF into the nucleus (31). The future investigation would elucidate a role of PEDF in the prevention of senescence and apoptosis in human endothelial glomerular cells.
It is not known if PEDF also increases the lifespan of other types of endothelial cells or other types of renal cells. We also cannot exclude the possibility that additional secreted proteins in 293-E4 conditioned media act as pro-survival factors. For example, sarcolectin that was also found in 293-E4 conditioned media was shown to stimulate T cells growth (32). A future study will investigate if sarcolectin stimulates endothelial cells growth or survival.
Taken together we demonstrated that Ad E4 expression in renal epithelial cells stimulated expression and secretion of PEDF, and that PEDF can function as pro-survival factor for human glomerulus-derived endothelial cells. Future studies will determine if PEDF also functions as a pro-survival factor for other types of endothelial cells.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
* This work was in part supported by National Kidney Foundation grant (to M.J.) and National Institutes of Health Research Grants SC1GM082325 (S.N.), R25 HL003679 (S.N.), 2G12RR003048 (S.N.), 8G12MD007597 (S.N.), K25GM097501 (Y.O.) and 1P30HL107253 (S.N.).
1 The abbreviations used are:
- Ad E4
- adenoviral early region 4
- 293-E4 cells
- cells stably transfected with Ad E4
- PEDF
- pigmental epithelium derived factor
- FGF-2
- fibroblast growth factor-2
- M1
- mortality checkpoint 1
- M2
- mortality checkpoint 2
- Rb
- retinoblastoma protein
- VEGF-A
- vascular endothelial growth factor A
- EGF
- epidermal growth factor
- IGF
- insulin-like growth factor
- PrGECs
- human primary glomerulus-derived endothelial cells
- HGECs
- primary glomerulus-derived endothelial cells transformed with SV40 large T antigen
- PDL
- population doubling
- vWF
- von Willebrand factor
- PI
- propidium iodide
- SA-β-gal
- senescence-associated beta-galactosidase.
REFERENCES
- 1. Fogo A. B., Kon V. (2010) The glomerulus–a view from the inside–the endothelial cell. Int. J. Biochem. 42, 1388–1397 [DOI] [PubMed] [Google Scholar]
- 2. Satchell S. C., Anderson K. L., Mathieson P. W. (2004) Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J. Am. Soc. Nephrol. 15, 566–574 [DOI] [PubMed] [Google Scholar]
- 3. van Setten P. A., van Hinsbergh V. W., van der Velden T. J., van de Kar N. C., Vermeer M., Mahan J. D., Assmann K. J., van den Heuvel L. P., Monnens L. A. (1997) Effects of TNF alpha on verocytotoxin cytotoxicity in purified human glomerular microvascular endothelial cells. Kidney Int. 51, 1245–1256 [DOI] [PubMed] [Google Scholar]
- 4. Velicescu M., Yu J., Herbert B. S., Shay J. W., Granada E., Dubeau L. (2003) Aneuploidy and telomere attrition are independent determinants of crisis in SV40-transformed epithelial cells. Cancer Res. 63, 5813–5820 [PubMed] [Google Scholar]
- 5. Lustig A. J. (1999) Crisis intervention: the role of telomerase. Proc. Natl. Acad. Sci. U.S.A. 96, 3339–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seandel M., Butler J. M., Kobayashi H., Hooper A. T., White I. A., Zhang F., Vertes E. L., Kobayashi M., Zhang Y., Shmelkov S. V., Hackett N. R., Rabbany S., Boyer J. L., Rafii S. (2008) Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc. Natl. Acad. Sci. U.S.A. 105, 19288–19293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harada T., Batsford S., Morioka T., Yao J., Arakawa M., Gejyo F., Oite T. (2005) Establishment of immortalized human glomerular endothelial cell lines and their application. Nephron Exp. Nephrol. 99, e38–45 [DOI] [PubMed] [Google Scholar]
- 8. Satchell S. C., Tasman C. H., Singh A., Ni L., Geelen J., von Ruhland C. J., O'Hare M. J., Saleem M. A., van den Heuvel L. P., Mathieson P. W. (2006) Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int. 69, 1633–1640 [DOI] [PubMed] [Google Scholar]
- 9. Singh A., Satchell S. C., Neal C. R., McKenzie E. A., Tooke J. E., Mathieson P. W. (2007) Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J. Am. Soc. Nephrol. 18, 2885–2893 [DOI] [PubMed] [Google Scholar]
- 10. Ramalingam R., Rafii S., Worgall S., Brough D. E., Crystal R. G. (1999) E1(-)E4(+) adenoviral gene transfer vectors function as a “pro-life” signal to promote survival of primary human endothelial cells. Blood 93, 2936–2944 [PubMed] [Google Scholar]
- 11. Zhang F., Cheng J., Hackett N. R., Lam G., Shido K., Pergolizzi R., Jin D. K., Crystal R. G., Rafii S. (2004) Adenovirus E4 gene promotes selective endothelial cell survival and angiogenesis via activation of the vascular endothelial-cadherin/Akt signaling pathway. J. Biol. Chem. 279, 11760–11766 [DOI] [PubMed] [Google Scholar]
- 12. Shay J. W., Pereira-Smith O. M., Wright W. E. (1991) A role for both RB and p53 in the regulation of human cellular senescence. Exp. Cell Res. 196, 33–39 [DOI] [PubMed] [Google Scholar]
- 13. Yates J. R., 3rd, Eng J. K., McCormack A. L., Schieltz D. (1995) Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal. Chem. 67, 1426–1436 [DOI] [PubMed] [Google Scholar]
- 14. Volpert O. V., Zaichuk T., Zhou W., Reiher F., Ferguson T. A., Stuart P. M., Amin M., Bouck N. P. (2002) Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat. Med. 8, 349–357 [DOI] [PubMed] [Google Scholar]
- 15. Hutchings H., Maitre-Boube M., Tombran-Tink J., Plouët J. (2002) Pigment epithelium-derived factor exerts opposite effects on endothelial cells of different phenotypes. Biochem. Biophys. Res. Commun. 294, 764–769 [DOI] [PubMed] [Google Scholar]
- 16. Rafii S., Shapiro F., Pettengell R., Ferris B., Nachman R. L., Moore M. A., Asch A. S. (1995) Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood 86, 3353–3363 [PubMed] [Google Scholar]
- 17. Yang J., Chang E., Cherry A. M., Bangs C. D., Oei Y., Bodnar A., Bronstein A., Chiu C. P., Herron G. S. (1999) Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 274, 26141–26148 [DOI] [PubMed] [Google Scholar]
- 18. O'Hare M. J., Bond J., Clarke C., Takeuchi Y., Atherton A. J., Berry C., Moody J., Silver A. R., Davies D. C., Alsop A. E., Neville A. M., Jat P. S. (2001) Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 98, 646–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garlanda C., Parravicini C., Sironi M., De Rossi M., Wainstok de Calmanovici R., Carozzi F., Bussolino F., Colotta F., Mantovani A., Vecchi A. (1994) Progressive growth in immunodeficient mice and host cell recruitment by mouse endothelial cells transformed by polyoma middle-sized T antigen: implications for the pathogenesis of opportunistic vascular tumors. Proc. Natl. Acad. Sci. U.S.A. 91, 7291–7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramalingam R., Rafii S., Worgall S., Hackett N. R., Crystal R. G. (1999) Induction of endogenous genes following infection of human endothelial cells with an E1(-) E4(+) adenovirus gene transfer vector. J. Virol. 73, 10183–10190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malard V., Chardan L., Roussi S., Darolles C., Sage N., Gaillard J. C., Armengaud J. (2012) Analytical constraints for the analysis of human cell line secretomes by shotgun proteomics. J. Proteomics 75, 1043–1054 [DOI] [PubMed] [Google Scholar]
- 22. Zhang A., Sun H., Wang P., Han Y., Wang X. (2012) Recent and potential developments of biofluid analyses in metabolomics. J. Proteomics 75, 1079–1088 [DOI] [PubMed] [Google Scholar]
- 23. Yamagishi S. I., Matsui T., Kawaguchi T., Sata M. (2010) Pathophysiological role of pigment epithelium-derived factor (PEDF) in hepatic disorders. Curr. Med. Chem. 17, 1995–2000 [DOI] [PubMed] [Google Scholar]
- 24. Cai J., Jiang W. G., Grant M. B., Boulton M. (2006) Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J. Biol. Chem. 281, 3604–3613 [DOI] [PubMed] [Google Scholar]
- 25. Kanda S., Mochizuki Y., Nakamura T., Miyata Y., Matsuyama T., Kanetake H. (2005) Pigment epithelium-derived factor inhibits fibroblast-growth-factor-2-induced capillary morphogenesis of endothelial cells through Fyn. J. Cell Sci. 118, 961–970 [DOI] [PubMed] [Google Scholar]
- 26. Sheikpranbabu S., Ravinarayanan H., Elayappan B., Jongsun P., Gurunathan S. (2010) Pigment epithelium-derived factor inhibits vascular endothelial growth factor-and interleukin-1beta-induced vascular permeability and angiogenesis in retinal endothelial cells. Vascul. Pharmacol. 52, 84–94 [DOI] [PubMed] [Google Scholar]
- 27. Fujimura T., Yamagishi S., Ueda S., Fukami K., Shibata R., Matsumoto Y., Kaida Y., Hayashida A., Koike K., Matsui T., Nakamura K., Okuda S. (2009) Administration of pigment epithelium-derived factor (PEDF) reduces proteinuria by suppressing decreased nephrin and increased VEGF expression in the glomeruli of adriamycin-injected rats. Nephrol. Dial. Transplant. 24, 1397–1406 [DOI] [PubMed] [Google Scholar]
- 28. Brenchley P. E. (2003) Vascular permeability factors in steroid-sensitive nephrotic syndrome and focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 18, vi21–25 [DOI] [PubMed] [Google Scholar]
- 29. Sipkens J. A., Hahn N., van den Brand C. S., Meischl C., Cillessen S. A., Smith D. E., Juffermans L. J., Musters R. J., Roos D., Jakobs C., Blom H. J., Smulders Y. M., Krijnen P. A., Stehouwer C. D., Rauwerda J. A., van Hinsbergh V. W., Niessen H. W. (2012) Homocysteine-Induced Apoptosis in Endothelial Cells Coincides With Nuclear NOX2 and Peri-nuclear NOX4 Activity. Cell. Biochem. Biophys, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lener B., Koziel R., Pircher H., Hutter E., Greussing R., Herndler-Brandstetter D., Hermann M., Unterluggauer H., Jansen-Dürr P. (2009) The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem. J. 423, 363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murakami Y., Ikeda Y., Yonemitsu Y., Onimaru M., Nakagawa K., Kohno R., Miyazaki M., Hisatomi T., Nakamura M., Yabe T., Hasegawa M., Ishibashi T., Sueishi K. (2008) Inhibition of nuclear translocation of apoptosis-inducing factor is an essential mechanism of the neuroprotective activity of pigment epithelium-derived factor in a rat model of retinal degeneration. Am. J. Pathol. 173, 1326–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. M'Bika-Binzangi J. P., Achour A., Khazen W., Gillibert M., Chany C. (2006) Effects of sarcolectin (SCL) on human peripheral blood mononuclear cells. Biochimie 88, 683–692 [DOI] [PubMed] [Google Scholar]
- 33. Kaighn M. E., Reddel R. R., Lechner J. F., Peehl D. M., Camalier R. F., Brash D. E., Saffiotti U., Harris C. C. (1989) Transformation of human neonatal prostate epithelial cells by strontium phosphate transfection with a plasmid containing SV40 early region genes. Cancer Res. 49, 3050–3056 [PubMed] [Google Scholar]
- 34. Schägger H., Bentlage H., Ruitenbeek W., Pfeiffer K., Rotter S., Rother C., Böttcher-Purkl A., Lodemann E. (1996) Electrophoretic separation of multiprotein complexes from blood platelets and cell lines: technique for the analysis of diseases with defects in oxidative phosphorylation. Electrophoresis. 17, 709–714 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.