Abstract
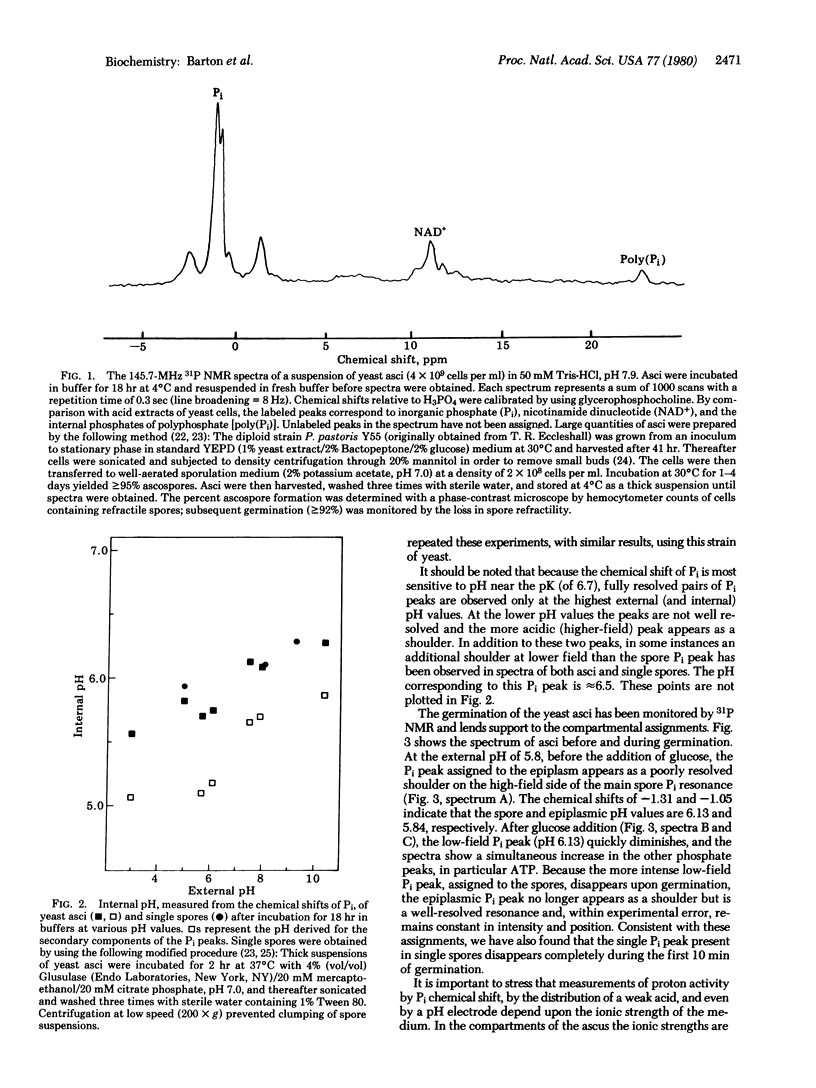
The asci and single spores of the yeast Pichia pastoris were examined by using 31P NMR. The pH within the spores was determined from the pH-dependent chemical shifts of the internal orthophosphate (Pi) peaks. In spectra of asci, two internal Pi peaks corresponding to two compartments were observed. Only small variations in the internal pH values were found upon incubation of the asci in buffers ranging in pH from 3 to 10. For this range in external pH, the internal pH values calculated from the Pi chemical shifts were 5.5-6.3 and 5.1-5.9. The two internal Pi peaks, which have line widths of approximately 60 Hz, have been assigned to spore and epiplasmic compartments, respectively. Spectra of single spores revealed only one Pi peak, and, upon germination of either the single spores or asci, only this Pi peak disappeared. Peaks corresponding to ATP were not found in the dormant spores and asci but did appear upon germination. These NMR studies show the yeast spore to have a mobile and relatively acidic interior.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHURCH B. D., HALVORSON H. Intermediate metabolism of aerobic spores. I. Activation of glucose oxidation in spores of Bacillus cereus var terminalis. J Bacteriol. 1957 Apr;73(4):470–476. doi: 10.1128/jb.73.4.470-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Halvorson H. O. Cell cycle dependency of sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Mar;109(3):1027–1033. doi: 10.1128/jb.109.3.1027-1033.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson T. O., Costello A. J., Omachi A. Phosphate metabolism in intact human erythrocytes: determination by phosphorus-31 nuclear magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2487–2490. doi: 10.1073/pnas.71.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A., Spudich J. A., Nelson D. L., Deutscher M. P. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- Lingappa B. T., Sussman A. S. Endogenous Substrates of Dormant, Activated and Germinating Ascospores of Neurospora Tetrasperma. Plant Physiol. 1959 Jul;34(4):466–472. doi: 10.1104/pp.34.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. High-resolution 31P nuclear magnetic resonance studies of metabolism in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):888–891. doi: 10.1073/pnas.74.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K. B., Baronofsky J. J., Marmur J. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979 Oct 16;18(21):4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. XX. Phosphate metabolism during germination. J Biol Chem. 1970 Mar 10;245(5):1146–1155. [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Rousseau P., Halvorson H. O., Bulla L. A., Jr, St Julian G. Germination and outgrowth of single spores of Saccharomyces cerevisiae viewed by scanning electron and phase-contrast microscopy. J Bacteriol. 1972 Mar;109(3):1232–1238. doi: 10.1128/jb.109.3.1232-1238.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau P., Halvorson H. O. Preparation and storage of single spores of Saccharomyces cerevisiae. J Bacteriol. 1969 Dec;100(3):1426–1427. doi: 10.1128/jb.100.3.1426-1427.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhany J. M., Yamane T., Shulman R. G., Ogawa S. High resolution 31P nuclear magnetic resonance studies of intact yeast cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4966–4970. doi: 10.1073/pnas.72.12.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Measurements of the pH within dormant and germinated bacterial spores. Proc Natl Acad Sci U S A. 1980 May;77(5):2474–2476. doi: 10.1073/pnas.77.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Setlow P. Phosphoglycerate mutase in developing forespores of Bacillus megaterium may be regulated by the intrasporal level of free manganous ion. Biochem Biophys Res Commun. 1978 May 15;82(1):1–5. doi: 10.1016/0006-291x(78)90567-3. [DOI] [PubMed] [Google Scholar]
- Ugurbil K., Rottenberg H., Glynn P., Shulman R. G. 31P nuclear magnetic resonance studies of bioenergetics and glycolysis in anaerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2244–2248. doi: 10.1073/pnas.75.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINBERG E. D. MANGANESE REQUIREMENT FOR SPORULATION AND OTHER SECONDARY BIOSYNTHETIC PROCESSES OF BACILLUS. Appl Microbiol. 1964 Sep;12:436–441. doi: 10.1128/am.12.5.436-441.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]


