Abstract
Organophosphorous (OP) pesticides are used frequently in agriculture, particularly in Asian countries over the past decades. Poisoning by these agents, either as acute or chronic in these nations, is a serious health problem. OP pesticides residue in fruits and vegetables that may not induce early clinical features, could also affect the human health. Therefore, medical and health professionals should be aware and learn more on the toxicology, prevention and proper management of OP poisoning. The well-known mechanism of OP toxicity is the inhibition of acetyl cholinesterase, resulting in an accumulation of acetylcholine and continued stimulation of acetylcholine receptors. Therefore, they are also called anticholinesterase agents. Determination of blood acetyl cholinesterase and butyryl cholinesterase activities remains a mainstay for the rapid initial screening of OP pesticides. Quantitative analysis of OP and their degradation products in plasma and urine by mass spectrometric methods is a more specific method, but is expensive and limited to specialized laboratories. Therefore, history of OP pesticides exposure and clinical manifestations of a cholinergic syndrome is sufficient for management of the exposed patients. However, electrophysiological tests may be required for the diagnosis of delayed neuropathy of OP poisoning. The standard management of OP poisoning includes decontamination, atropine sulphate with an oxime. Recent advances focus on blood alkalinisation and magnesium sulphate as promising adjunctive therapies. Preventive measures in OP exposure are of great importance in human health in developing countries. Therefore, regulations and controls on safe use of OP particularly in Asian countries are recommended.
Keywords: Health, Organophosphorous, Toxicity, Poisoning, Pesticides residues, Asia
Introduction
Background
Over the past decades, organophosphorous (OP) pesticides were commonly used in agriculture, especially in Asian countries. Organophosphorous pesticides poisoning, either in its acute or chronic forms is a serious health threat. Although OP pesticides residues in fruits and vegetables may not induce early clinical features, nonetheless they could still affect the human health (1, 2). Hence, raising awareness among medical and health professionals is imperative. This awareness should not only cover the toxicology behind poisoning, but it should also include methods of prevention and appropriate management of OP poisoning.
How this review article was prepared
This review was prepared based on the experience of the fist author and the available literature between 1980 and 2011. Pub Med was searched with different names of organophosphorous, organophosphate, pesticides, insecticides and search terms including human exposure, organophosphorous pesticides poisoning and its complications. The first author’s collections of literature on OP including the books, monographs and historically relevant articles were also used for this review. It was aimed to describe the brief basic chemistry and toxicology of OP as well as its clinical effects and long term complications. Special attention was given to the studies and reports from Asian countries. Therefore, relevant references were selected and the headings and subheadings were chosen based on the above objectives. This is neither a Meta analysis nor a critical review on OP. Herein we present a descriptive text on health aspects of OP for health professionals with special reference to the Asian countries.
History and size of the problem
Aside from the large scale use of OP pesticides in agriculture, OP compounds have also been used as chemical warfare agents (tabun and sarin) by the Iraqi army against the Iranian troops (Majnoon Island) and civilians in Halabjah (1). A few OP compounds (glyphosate, merphos) are used as herbicides, but they are structurally different from the OP pesticides (2).
Von Hofmann synthesized an OP compound called methyl phosphor chloride in 1837. Michaels introduced a compound with P-CN bond, which led to the synthesis of a number of insecticides and the nerve agent tabun before the World War II (3, 4).
Over the past decades, numerous structurally different OP compounds have been synthesized and used as pesticides, which poisoned even the children of agricultural workers in India (2, 5). Currently, over 100 different OP compounds are used worldwide as insecticides (6). Low level exposures either as occupational or via the consumption of OP contaminated fruits and vegetables have been reported (7–10). OP compounds are not ideal pesticides because of the lack of target vector selectivity, and severe toxicity in human beings and domestic animals. On the other hand, OP pesticides are readily available and are widely used which explains the high number of reported intoxications (11, 12).
In some parts of the developing world particularly in Asian countries, pesticide poisoning causes more deaths than infectious diseases (11). Use of pesticides is poorly regulated and controlled in the Asian countries and thus, their easy availability may induce self-poisoning. In 1985, Food and Agriculture Organization (FAO) of the United Nation produced a voluntary code of conduct for the pesticide industry to limit the harmful effects of pesticides. Unfortunately, inadequate governmental attention in some developing countries has made this code ineffective (1, 11). The World Health Organization (WHO) has recommended restricting highly toxic pesticides, but this was not applied in a number of developing countries. As a result, OP pesticides exposure has remained common in these countries and continues to induce health problems as well as environmental hazards (8–16).
It was estimated that in 2002–2005, around 3,000,000 human beings were intoxicated by OP pesticides worldwide (6, 14, 15).
It was later indicated that OP poisoned 3 million and kills at least 250,000 people every year, mostly in Asian countries (17, 18). In 25 villages of three townships of Jiangsu and Shandong provinces in China, more than 5000 cases of OP exposure as sprayers were reported (19). From July 1985 through December 2006, 4799 OP exposures were recorded in Taiwan (20). An outbreak of food poisoning in Singapore of an imported green leafy vegetable (Brossica alboglabra) contaminated by two OP (metamidophos and profenfos) poisoned 105 people (21).
The above information clearly shows that OP pesticides are the cause of high number of human morbidities and mortalities particularly in the Asian region. Therefore, health authorities should act on the regulation, control and prevention of this common serious poisoning.
Chemical structures and classification
OP compounds including organophosphates are derived from phosphoric, phosphonic, phosphinic and thiophosphoric acids (2, 22). Organophosphates are usually esters, amides or thiol derivatives of phosphoric, phosphonic or phosphinic acids. The general structure of an OPcompound is shown in Fig. 1 (22, 23).
Fig. 1:
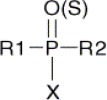
General chemical structure of an organophosphorous compound. R1 and R2 are alkyl-, alkoxy-, alkylthio- or amido groups. X is the acyl residue (cyano-, substituted or branched aliphatic, aromatic or heterocyclic groups)
OP compounds are divided into two main groups; pesticides and chemical warfare nerve agents. OP pesticides have varied chemical structures. Variations in the chemical structure of main groups of OP pesticides are summarized in Table 1.
Table 1:
Chemical structures of main groups of OP pesticides and examples in each group
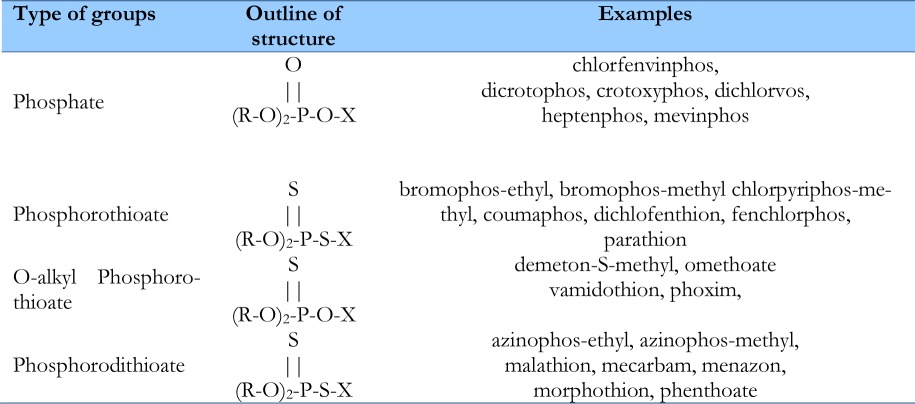
|
OP compounds exhibit markedly different actions depending on the specific substituents at R1, R2, and X. For example, parathion is 1000 times more toxic than malathion (22).
Mechanism of action
The well known mechanism of action of OP is the inhibition of cholinesterases. Two types of cholinesterase are involved:
Acetyl cholinesterase (AChE), which is a specific enzyme for the diagnosis of OP poisoning and is referred to as true cholinesterase. It is usually estimated in red blood cells (RBC) and as such it is also called RBC AChE or erythrocyte acetyl cholinesterase.
Butyryl cholinesterase (BChE); which is less specific but more sensitive than AChE is also called pseudo cholinesterase. BChE is usually estimated in Plasma and is thus known as plasma cholinesterase.
Some proteolytic enzymes (e.g. trypsin, chymotrypsin) may also be inhibited by OP. OP pesticides like parathion are detoxified in part through a two-step pathway involving bioactivation of the parent compound by the cytochrome P450 system, followed by hydrolysis of the resulting oxygenating metabolite (Oxon) by serum and liver paraoxygeanse 1 (PON1). Serum PON1 has been shown to be polymorphic in human populations (24).
Although all OP compounds have a common mechanism of action, their effectiveness as inhibitors of AChE, can be significantly different. OP compounds are classified as direct or indirect inhibitors of AChE. Direct inhibitors are effective without undergoing any further metabolic modifications in the body, whereas, indirect inhibitors need to be transformed in the body to become active (22).
People with BChE genetic variations may be at risk for taking succinylcholine. The clinically most important variant is atypical (D70G) BChE. The people with this variation may have apnea after receiving a dose of succinylcholine which is intended to paralyze muscles for 3–5 minutes in anaesthesia (23, 25).
Furthermore, OP covalently binds to other serine esterase, such as carboxyl esterase (CaE), neuropathy target esterase (NTE), trypsin and chymotrypsin (26). In OP binding to a tyrosine, residue of human serum albumin has also been reported (27).
The persistence of unbound OP in the body depends on the physico-chemical properties and the activity of endogenous OP hydrolyzing enzymes, such as paraoxonases (25). Lipophilic OP, like parathion and its active form paraoxon may penetrate into deep compartments resulting in long-term toxicity (27).
Toxicokinetics
Absorption
Absorption varies by route of exposure.
Dermal exposure
Absorption by the skin tends to be slow, however as the OP pesticides are hard to remove from the skin. The presence of aqueous dispersing agents or organic solvents in a spray or other formulations may enhance absorption.
On the basis of radio autographic studies, it appears that skin absorption of parathion is trans-epidermal. The rate of dermal absorption of parathion in rabbit is reported 0.059 mg/min/cm2 (16).
Following administration of 14C-malathion to the ventral forearm of 12 volunteers, 8.2% of the total dose was recovered from their urine during the course of 5 days. After IV injection of 14C-malathion, 90.2% of the radioactivity was recovered in the urine (16, 22).
Inhalation exposure
In a study, OP exposure by respiratory and dermal routes was compared in workers spraying parathion. They either breathed a pure air supply but did not wear protective clothing, or wore protective clothing but did not have any respiratory protection. Total urinary output of 4-nitrophenyl as derived from the respiratory source, compared with that derived from the dermal source, was 1.2% and 12%, respectively (16).
Oral exposure
After 32P-dimethoate was given orally to volunteers, 76 to 100% of the radioactivity appeared in the urine over 24 hours (16).
Distribution
The absorbed OP compounds are going under bio-transformations and reactions with tissue constituents. In view of the inherent instability of the OP pesticides, retention in human tissue is not prolonged.
Metabolism
OP compounds are mainly metabolized via oxidation and hydrolysis by esterases and by reaction with glutathione. Demethylation and glucuronidation may also occur. Oxidation of OP pesticides may result in potentially toxic products. In general, phosphorothioates are not directly toxic and require oxidative metabolism to produce toxic metabolite. In reverse, glutathione transferase reactions induce products that are usually less toxic (28).
The half-life of most OP pesticides and their inhibitory metabolites in vivo is relatively short. For instance, the serum half-life of malathion was 2.89 hours in a 24-year-old white male who, in a suicidal attempt, injected approximately 3 ml of 50% malathion intravenously into his right forearm (22).
Excretion
The elimination of the OP and metabolites is mostly by the urine and lesser amounts in the feces and expired air. In summary, the rates of OP excretion usually reach a peak within two days and decline quite rapidly (2, 16).
Clinical Effects
The lipophilic diethyl phosphoryl pesticides such as azinophos-ethyl, bromophos-ethyl, chlorpyriphos, coumaphos, diazinon, parathion, phosalone and sulfotep may remain in the body for many days in severe cases. This may promote a recurrence of clinical effects after an initial period of apparent recovery (2, 28, 29).
Clinical effects of OP exposure are categorized into acute and chronic poisonings.
Acute Poisoning
Initial clinical manifestations following acute exposure vary according to the route of exposure.
Inhalation
Inhalation of OP pesticides depends on volatility of the compound, formulation and the technique of application. However, clinical effects appear immediately or shortly after OP inhalation (22).
Exposure to low vapor concentrations may affect only the eyes, nose and airways. Miosis, copious secretion, visual disturbances, rhinorrhoea and or some degree of dyspnea develop within seconds to several minutes. The extent of severity of dyspnea is dose dependent. Usually, these effects do not progress significantly once the patient is removed from the contamination area. After inhalation of high vapor concentrations, victims lose consciousness within one or two minutes and then have seizures, flaccid paralysis and apnea. Involuntary micturation/defecation may also occur in severe cases (2, 22).
Skin
Symptoms and signs usually appear about 2–3 hours following dermal absorption. A small concentration of OP may retain in fat tissues which can delay the clinical manifestation of symptoms for up to 24 h (2).
Dermal absorption of OP varies depending on the body site exposed and the ambient temperature. Droplets containing near-lethal or lethal dose, may cause loss of consciousness, seizures, flaccid paralysis and apnea. The onset of these effects is sudden, after an asymptomatic interval of 10 to 30 minutes (23, 30).
Eyes
Following splash exposure or eye contact with OP vapor and later if at all after systemic poisoning, miosis may occur. Unilateral miosis can occur, if only one eye has been exposed. Miosis may be accompanied by deep, aching eye pain, conjunctival irritation, and visual disturbances following direct OP exposure (4).
Gastrointestinal
Accidental ingestion may occur in children and even in adults due to improper storage of OP pesticides at home. Following oral ingestion, nausea and vomiting may occur. Abdominal pain and diarrhoea together with a cholinergic syndrome, CNS and cardiovascular effects may be observed in moderate to severe OP poisoning (16). Clinical features of cholinergic syndromes, CNS and cardiovascular effects are described in the following relevant sections.
Parenteral
Intradermal injection of paraoxon or surface application of maloxon or dichlorvos to human skin produced a long-lasting local sweating over a few minutes. Intramuscular administration of an OP pesticide (diisopropylfluorophosphonate or DFP) to people with psychosis, and to normal controls at a rate of 2 mg/man per day (about 0.028 mg/kg per day) for seven days caused anorexia, vomiting, and diarrhea more severe in normal than in psychotic patients (31).
Cholinergic syndromes
These syndromes are due to the acetylcholine accumulation at the nerve endings, stimulating both muscarinic and nicotinic receptors. Muscarinic syndrome include increased bronchial secretion, excessive sweating, salivation, and lachrymation, pinpoint pupils, bronchoconstriction, vomiting, diarrhoea, abdominal cramps urinary frequency, bradycardia and hypotension and in severe intoxicated patients; pulmonary edema may occur. Nicotinic syndrome include tachycardia, hypertension, mydriasis, twitching and fasciculation of muscles, and in more severe cases; paralysis of diaphragm and respiratory muscles (2, 16, 22).
Central nervous system effects
Central nervous system effects include headache, dizziness, restlessness, anxiety, mental confusion, convulsions and coma. Depression of the respiratory and vasomotor centers in brain may occur and further complicate the clinical picture of OP poisoning (3, 16, 32).
Cardiovascular effects
Cholinesterase inhibition increases the vagal nerve and thereby slows the heart rate. In accidentally poisoned patients, however, other factors, such as fear, hypoxia, and ganglionic stimulation, may accelerate the heart rate (16).
ECG abnormalities other than bradycardia have been described in human OP pesticides poisoning. These include atrial fibrillation, idioventricular dysrhythmias, multiform ventricular extrasystoles, torsade de pointes, ventricular fibrillation, and complete heart block (33). Sudden death occurring after a patient has appeared to recover from the respiratory and neurologic effects of acute OP insecticide exposure has been reported (34).
Intermediate Syndrome
The intermediate syndrome consists of marked weakness of the proximal skeletal musculature (including the muscles of respiration-intercostal muscles) and cranial nerve palsies, which may occur within 1–4 days of certain acute OP poisoning. This syndrome is probably a consequence of cholinergic over activity at the neuromuscular junction. It appears that there is a correlation between the acute phase and OP-induced myopathy (32, 35, 36).
Severity Grading of intoxication
Severity grading of OP poisoning can be categorized based on clinical manifestations, cholinesterase activity and initial atropine dose required for atropinisation.
Clinical
The severity of OP poisoning can be divided into four groups of mild, moderate, severe and fatal according to the symptoms and signs of poisoning (Table 2) (2, 22):
Table 2:
Severity grading of OP poisoned patients based on symptoms and signs of poisoning
| Grade | Symptoms | Signs |
|---|---|---|
| Mild | Dizziness, anxiety, headache, nausea, weakness, tightness of breath. | failure of eye accommodation, rhinorrhoea, sweating, salivation, coughing, lachrymation |
| Moderate | (Worsening of the above features plus the followings): Restlessness, confusion, dyspnea, disorientation, abdominal pain, diarrheal | Pallor, miosis, lack of concentration, tachycardia, hypertension, muscle twitching, fasciculation, respiratory depression, bronchorrhea, loss of consciousness, bronchospasm. |
| Severe | (Worsening of the above features plus the followings) Loss of conscious, seizure. | Convulsions, respiratory failure, pulmonary edema, flaccid paralysis, involuntary micturation/defecation, Cyanosis, deep coma. |
| Fatal | (Worsening of the above features plus the followings) Respiratory arrest, cardiac arrest. | Coma, convulsions, miosis hypersecretions and apnea, within a few minute after exposure. |
Inhibition of cholinesterases
The severity of OP poisoning may be divided into three groups according to their cholinesterase activities as shown in Table 3 (2, 16, 22).
Table 3:
Severity grading of poisoned patients based on their cholinesterase activities
| Grade | BChE activity (%) | AChE activity (%) |
|---|---|---|
| 1. Mild | 40–50 | 50–90 |
| 2. Moderate | 10–40 | 10–50 |
| 3. Severe | <10 | <10 |
As it can be observed in Table 3, BChE concentration has less quantitative value than AChE activity.
Atropine dose
Acute OP poisoning can also be divided into three groups according to the initial dose required for atropinisation:
| 1. Mild | <2mg |
| 2. Moderate | <2–10mg |
| 3. Severe | >10mg |
The patients with OP pesticides poisoning require much more atropine doses and thus the above atropine dosing for the severity grading of OP pesticides should be five to ten times higher (13).
Mixed Poisoning
Concomitant poisoning of OP with other compounds such as organochlorine, fungicide, copper sulphate and kerosene may occur. The organic solvents used for dissolution of OP may also potentiate the intoxication. A report of three patients with mixed poisoning who were successfully treated with greater focus on OP poisoning was reported (37).
Chronic Poisoning
Chronic and/or sub-acute OP poisonings are usually occupational and may occur in workers who have daily exposure. Chronic poisoning is most common among agricultural workers involved in spraying of OP pesticide or in sheep divers. Since chronic OP poisoning is much less common than acute poisoning, unless the physician pays attention to the occupation of the patient, the diagnosis will be missed out (2, 22).
The great majority of OP compounds are rapidly metabolized and excreted and thus sub-acute or chronic poisoning may not occur. However, since several OP compounds cause slowly reversible inhibition of cholinesterase, accumulation of this effect can occur (22).
The farmers who exposed to OP revealed lower AChE activity and higher urinary dialkylphosphate and 8-hydrox-29-deoxyguanosine than the controls, indicating that they had chronic OP poisoning. They also had higher DNA damage than the controls (38).
Delayed neuropathy
OP induced delayed neuropathy (OPIND) is a symmetrical sensorimotor axonopathy, tending to be more severe in long axons and occurring 7–14 days after exposure. In severe cases, it is an extremely disabling condition, but is very rare. Inhibition of neuropathy target esterase (NTE) claimed to be necessary for OPIND to develop. However, other mechanisms may be involved e.g. trophic factor (ornithine decarboxylase), a growth related enzyme, which decreased in spinal cord following diisopropylfluorophosphate (DFP) poisoning (2, 22).
Electrodiagnostic procedures including electromyography of the extremities and nerve conduction velocity of the upper and lower nerves of the limbs revealed that sensory nerve dysfunction of the lower extremities was more common than motor nerves, which was predominantly a distal sensory deficit (39).
Diagnosis
Initial diagnosis of OP poisoning can be made based on the history of exposure and clinical manifestations. In low-level exposure, the route of absorption may affect the clinical features, but in high-level exposure, the occurrence time is faster through inhalation than by skin absorption (16).
Estimation of AChE in erythrocytes is required to confirm diagnosis and to estimate severity of OP intoxication. BChE is more sensitive and declined earlier, but it is not specific and may be low naturally in some people due to genetic variations (40).
Reasonable relationships exist between red blood cells or plasma cholinesterase inhibition and the clinical signs of acute intoxication. Monitoring of neuromuscular transmission during the course of severe OP poisoning revealed that disturbances occur when AChE activity reached below 30 % (41).
Toxicological analyses
Cholinesterases determination
Determinations of AChE and BChE activities are well known screening methods to establish exposure to OP pesticides. Modifications of the original colorimetric Elman assay (40) allowed determination of AChE activity in whole blood and of BChE activity in the plasma more accurately (42).
Standard determination of AChE and BChE activity may indicate inhibition of the enzyme which may be due to OP or other cholinesterase inhibitors like carbamates. Thus, reduced activity of AChE and or BChE activity indicate an exposure to a cholinesterase inhibitor without specifying the agent. However, determination of cholinesterase activities is a valuable tool for confirming the clinical diagnosis of OP poisoning. Clinical symptoms are expected at inhibition of more than 50% of brain AChE and 70% of diaphragm AChE, respectively (27). Inhibition levels greater than 90% are associated with severe toxicity.
Erythrocyte AChE showed to be a more specific parameter than BChE for the diagnosis of OP poisoning and the assessment of antidotal efficacy (16, 40, 42, 43).
Detection of OP compounds and their metabolites
Identification and quantitative analysis of trace concentrations of OP compounds in plasma after human exposure requires sophisticated instruments such as gas chromatography-mass spectrometry (GC–MS) or liquid chromatography-mass spectrometry (LC–MS) and well trained operators (44). It is not really required for the clinical management of OP poisoning.
Management
First aid advice for medical and para-medical personnel
The first rule is that the emergency responders must protect themselves to avoid contamination resulting from contact with casualties and the polluted environment. This can be done by appropriate personal protective equipments. At minimum, rescuers should wear a protective mask (mask containing a charcoal filter, not a surgical or similar mask) and heavy rubber gloves and avoid skin contact with victims until decontamination has been carried out (2, 22).
The victims should be removed from the contaminated area as soon as possible, and decontamination must be initiated. Antidotes (e.g. auto injector containing atropine, obidoxime and diazepam) should be used at the onset of clinical symptom/sign. For unconscious or severely intoxicated patients, priorities must follow the ABCs of resuscitation. Oxygen administration and assisted ventilation should be undertaken as soon as possible in those with respiratory insufficiency (2).
It is important to improve tissue oxygenation before atropine administration to minimize the risk of ventricular fibrillation. Advanced life support, including IV line placement, should be provided to all victims with signs of severe OP toxicity (12).
Decontamination
If the eyes have been exposed, they should be irrigated as with running water or normal saline. Skin decontamination should be done by pouring on large amounts of a light chlorine-liberated solution such as 5.0% hypochlorite solution (household bleach) followed by several water rinsing. If bleach is not available, the skin should be washed up by an alkaline soap and water followed by a water rinse. Water alone can be used if nothing else is available as it will dilute and physically wash away the agent, but it will not hydrolyze it. Contaminated clothing and jewellery should be removed before decontamination. Care should be taken to clean under the nails, intertriginous areas, auxilla, groin, and hair (22).
Recombinant DNA-derived AChEs revealed a great improvement as bioscavengers (45). Immobilized Escherichia coli with surface-expressed organophosphorous hydrolase were made to detoxify OP poisoning (46). By protein engineering techniques one butyryl cholinesterase mutant G117H was made to hydrolyze OP but reacts much too slowly (47).
Antidotes
The well known antidotes (atropine, oxime) do not effect in all severe OP pesticides poisoning and may not prevent respiratory insufficiency. However, appropriate administration of these antidotes and intensive care management are the main treatment for acute OP poisoning. Cinical investigations revealed that blood alkalinisation by sodium bicarbonate is effective and should be added to the treatment regime of OP poisoning (48).
Although oximes have been designed to reactivate the inhibited acetylcholinesterase, clinical experience has revealed that they are not always effective and none of them can be regarded as a broad-spectrum antidote (49). Liver function tests should be checked regularly during obidoxime therapy to avoid hepatotoxicity (13,49–52).
Blood alkalinisation by sodium bicarbonate
Effects of sodium bicarbonate in OP pesticide poisoning were studied in patients with moderate to severe intoxication. It was aimed to make a mild alkalinisation to obtain the arterial blood pH of 7.45 to 7.55. The preliminary results were promising (53) and the final results were satisfactory (48).
Roberts and co-workers (54) were concern about the safety of alkalinisation regime but the investigators replied with the evidence of safety data (55).
Intravenous lipid emulsions combined with extracorporeal purification particularly charcoal hemoperfusion has been recommended for adjunct treatment of severe OP poisoning (56). However, this should be investigated by a carefully designed study in severe human different OP poisonings.
Advances in the treatment of acute organophosphorous poisonings were reported in 2006 and 2012 (57, 58).
Pesticides Residues
OP pesticide abuse in growing fruit and vegetable was found in 65% in Tianjin area. Around 32% of the fruits and vegetable samples collected in summer from this city were positive for high toxic OP pesticides. Significant decrease of pesticide residue (up to 80%) in fruits and vegetables was achieved by scaling, immersion in 0.15% and 0.30% detergent solution, as well as peeling and cutting root. The authors concluded that it is necessary to strengthen pesticide abuse control and market surveillance and inspection to reduce the harmful effects of pesticide residue in fruit and vegetables to human health (59).
Over 27,000 tons of pesticides were used in the Islamic Republic of Iran during 2000 and 2001, of which 16,000 tons are formulated in the country. Of the 241 pesticides used in this country, the group order was insecticides 33%, herbicides 30%, fungicides 20%, acaricides 6.2%, rodenticides 3.8%, nematicides 1.5 % and others for 5.5%. The country spent US$125 million on pesticide imports in 2002. Although this huge volume of pesticides is distributed all over the country, 60 % of all pesticides were applied in the three northern provinces, close to the Caspian Sea, while rice production alone accounts for a quarter of the national pesticide usage (60). Unregistered and illegal imports of pesticides, which have not been formally reported, should be added to the size of this health problem.
Contamination of crop samples by organic chemicals, mainly OP pesticides has become a pressing problem in many Arab countries. Safety status was assessed by comparing the current contamination level with that of WHO maximum residue limits (MRL). Low contamination levels were detected in cucumber and tomato in Palestine, Jordan and Egypt. Elevated levels of contamination were detected in vegetables from Pakistan, Egypt and in grapes from Jordan. Several poisonous cases and plant food contamination were reported in Morocco, Egypt, Iraq, Saudi Arabia, Sudan, Syria, Jordan, UAE, Pakistan and Yemen in the past years. Further detailed studies of these problems showed accumulation of these organic contaminants in food consumer bodies and farm workers who deal directly with agricultural products. This problem occurred probably due to misuse and/or overuse of pesticides in the environment. The author concluded that these countries should implement control measures to reduce pesticide use and awareness programs to enhance food safety (61).
Ethion and diazinon concentrations in Iranian pistachio nuts have been analyzed by use of matrix solid-phase dispersion (MSPD) followed by gas chromatography with nitrogen–phosphorus detection (GC–NPD). The limits of detection were 0.02 mg kg−1 for ethion and 0.035 mg kg−1 for diazinon; these were lower than the maximum residue limits (MRL) established by WHO/FAO (62). However, regular pesticides monitoring on this product is recommended.
Preventive measures
The persons are going to use OP pesticides should be provided with safety information and equipments before application. Protective devices for low toxic OP pesticides may include a long-sleeved shirt, long trousers or overall suit with appropriate hat, but for the highly toxic OP pesticides, waterproof suit, thick gloves and rubber boots are needed. The protective devices should be washed out every day after use. The contaminated items must be washed up separately. To protect possible splashing on the eyes, goggles or a face shield should be worn.
Contact lenses are advised to be removed before using an OP pesticide, because it may seep in behind the lenses. For spraying the highly toxic OP pesticides, a respirator may be required. The label of OP pesticides may specify when this is necessary.
More precautions are needed when mixing the highly toxic OP pesticides and thus physical protection must be applied. Mixing the OP pesticides should be carefully done by a stick or paddle. The risk of pesticide spraying, increases on windy days due to possible inhaling spray drift or contaminating the skin and thus appropriate protections are required. After spraying OP pesticides, taking shower and changing the clothes are necessary. Hands should be washed out carefully before eating, drinking or smoking.
All chemicals including OP pesticides should be stored preferably in a locked shed, out of the reach of children and animals. OP pesticides should also be kept away from work areas for other purposes. They also must be stored separated from other materials such as animal food Stuff. OP pesticides as any other chemicals should be kept in their original containers and must have never been transferred to the containers which are normally used for food or drink. The new container must be labelled properly.
Decontamination and clean-up procedures of OP pesticides should be performed by a mild alkaline solution such as 10% sodium carbonate (washing soda solution) to the contaminated area. After bushing well, it should be left for at least 8 hours.
For workers who are employing to use OP pesticides on a regular basis, determination of their baseline cholinesterase levels is advisable. These tests should also be repeated on a regular basis to determine whether exposure is occurring with sub-clinical exposure. If the red blood cell or plasma cholinesterase falls below 25% of baseline levels, workers must be taken off the job and should not return to work until their cholinesterase levels return to normal range (63).
Bacterial enzymes including OP hydrolases have demonstrated prophylactic and antidotal efficacy against a few different OP classes in animal models (18).
Conclusions and recommendations
OP pesticides have induced a lot of human morbidities and mortalities, particularly in the Asian countries. OP pesticides are still used in most parts of the world and unfortunately are easily available in some developing countries and thus occupational and accidental exposure and even intentional ingestion are common and induced health problems.
WHO has recommended that access to highly toxic pesticides be restricted and where this has been done, suicide rates have fallen. Proper legislations and pesticides control particularly on OP compounds which are the most commonly used pesticides are recommended. Biological pest control that has recently been applied in some countries should be extended and advanced to replace the use of OP pesticides.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
Partial financial support of the Medical Toxicology Research Center of Mashhad University of Medical Sciences is acknowledged. The authors declare that there is no conflict of interests.
References
- 1.Balali-Mood M, Balali-Mood K. Neurotoxic disorders of organophosphorous compounds and their managements. Arch Iranian Med. 2008;11(1):65–89. [PubMed] [Google Scholar]
- 2.Jeyaratnam J, Maroni M. Organophosphorous compounds. Toxicology. 1994;91(1):15–27. doi: 10.1016/0300-483x(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 3.Sungurtekin H, Gürses E, Balci C. Evaluation of several clinical scoring tools in organophosphate poisoned patients. Clin Toxicol. 2006;44(2):121–26. doi: 10.1080/15563650500514350. [DOI] [PubMed] [Google Scholar]
- 4.Holmsted B. The third symposium on the prophylaxis and treatment of chemical poisons. Fund Appl Toxicol. 1985;5:S1–S9. [PubMed] [Google Scholar]
- 5.Rastogi SK, Tripathi S, Ravishanker D. A study of neurologic symptoms on exposure to organophosphate pesticides in the children of agricultural workers. Indian J Occup Environ Med. 2010;14(2):54–57. doi: 10.4103/0019-5278.72242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwong T. Organophosphate pesticides: biochemistry and clinical toxicology. Therc Drug Monit. 2002;24(1):144–49. doi: 10.1097/00007691-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Lotti M. Low level exposures to organophosphorus esters and peripheral nerve function. Muscle & Nerve. 2002;25(4):492–504. doi: 10.1002/mus.10086. [DOI] [PubMed] [Google Scholar]
- 8.Amr M. Pesticide monitoring and its health problems in Egypt, a Third World country. Toxicol Lett. 1999;107(1–3):1–13. doi: 10.1016/s0378-4274(99)00026-0. [DOI] [PubMed] [Google Scholar]
- 9.Cocker J, Mason H, Garfitt S, Jones K. Biological monitoring of exposure to organophosphate pesticides. Toxicol Lett. 2002;134(1–3):97–103. doi: 10.1016/s0378-4274(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 10.Brown M, Brix K. Review of health consequences from high-, intermediate-and low-level exposure to organophosphorus nerve agents. J Appl Toxicol. 1998;18(6):393–408. doi: 10.1002/(sici)1099-1263(199811/12)18:6<393::aid-jat528>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Eddleston M, Karalliedde L, Buckley N, Fernando R, Hutchinson G, Isbister G, Konradsen F, Murray D, Piola J, Senanayake N. Pesticide poisoning in the developing world; a minimum pesticides list. The Lancet. 2002;360(9340):1163–67. doi: 10.1016/s0140-6736(02)11204-9. [DOI] [PubMed] [Google Scholar]
- 12.Thiermann H, Szinicz L, Eyer F, Worek F, Eyer P, Felgenhauer N, Zilker T. Modern strategies in therapy of organophosphate poisoning. Toxicol Lett. 1999;107(1–3):233–39. doi: 10.1016/s0378-4274(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 13.Balali-Mood M, Shariat M. Treatment of organophosphate poisoning. Experience of nerve agents and acute pesticide poisoning on the effects of oximes. J of Physiol-Paris. 1998;92(5–6):375–78. doi: 10.1016/S0928-4257(99)80008-4. [DOI] [PubMed] [Google Scholar]
- 14.Forget G. Pesticides and the third world. J ToxicolEnviron Health, Part A. 1991;32(1):11–31. doi: 10.1080/15287399109531462. [DOI] [PubMed] [Google Scholar]
- 15.Eddleston M, Gunnell D, Karunaratne A, De Silva D, Sheriff M, Buckley N. Epidemiology of intentional self-poisoning in rural Sri Lanka. Brit J Psychiat. 2005;187(6):583–584. doi: 10.1192/bjp.187.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worek F, Koller M, Thiermann H, Szinicz L. Diagnostic aspects of organophosphate poisoning. Toxicology. 2005;214(3):182–89. doi: 10.1016/j.tox.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed F, Gawarammana I, Robertson T, Roberts M, Palangasinghe C, Zawahir S, Jayamanne S, Kandasamy J, Eddleston M, Buckley N. Acute human self-poisoning with imidacloprid compound: A neonicotinoid insecticide. PLoS One. 2009;4(4) doi: 10.1371/journal.pone.0005127. e5127(1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bird S, Dawson A, Ollis D. Enzymes and bioscavengers for prophylaxis and treatment of organophosphate poisoning. Front Biosci (Scholar edition) 2010;2(1):209–220. doi: 10.2741/s58. [DOI] [PubMed] [Google Scholar]
- 19.Gan W, Chen S, Tao B. An epidemiological study on pesticide poisoning with mixed preparation. Chin J Prev Med. 2001;35(1):13–15. [PubMed] [Google Scholar]
- 20.Lin T, Walter F, Hung D, Tsai J, Hu S, Chang J, Deng J, Chase P, Denninghoff K, Chan H. Epidemiology of organophosphate pesticide poisoning in Taiwan. Clin Toxicol. 2008;46(9):794–801. doi: 10.1080/15563650801986695. [DOI] [PubMed] [Google Scholar]
- 21.Goh K, Yew F, Ong K, Tan I. Acute organophosphorus food poisoning caused by contaminated green leafy vegetables. Arch Environ Health. 1990;45(3):180–184. doi: 10.1080/00039896.1990.9936713. [DOI] [PubMed] [Google Scholar]
- 22.Bradbery SM, Vale JA. Organophosphorous insecticides. In: Brent, Wallace, Bukhart, Philips, Donovan, editors. Critical Care Toxicology. 1st ed. Elsevier Mosby; Philadelphia, USA: pp. 937–942. [Google Scholar]
- 23.Ooms A, Van Dijk C. The reaction of organophosphorus compounds with hydrolytic enzymes-III: The inhibition of chymotrypsin and trypsin. Biochem Pharmacol. 1966;15(9):1361–1377. doi: 10.1016/0006-2952(66)90106-7. [DOI] [PubMed] [Google Scholar]
- 24.Josse D, Lockridge O, Xie W, Bartels C, Schopfer L, Masson P. The active site of human paraoxonase (PON1) J Appl Toxicoly. 2001;21(S1):S7–S11. doi: 10.1002/jat.789. [DOI] [PubMed] [Google Scholar]
- 25.Boter H, Ooms A. Stereospecificity of hydrolytic enzymes in their reaction with optically active organophosphorus compounds--II:: The inhibition of aliesterase, acetylesterase, chymotrypsin and trypsin by S-alkyl p-nitrophenyl met-hylphosphonothiolates. Biochem Pharmacol. 1967;16(8):1563–69. doi: 10.1016/0006-2952(67)90134-7. [DOI] [PubMed] [Google Scholar]
- 26.Sogorb M, Vilanova E. Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol Lett. 2002;128(1–3):215–28. doi: 10.1016/s0378-4274(01)00543-4. [DOI] [PubMed] [Google Scholar]
- 27.Eyer P, Worek F, Kiderlen D, Sinko G, Stuglin A, Simeon-Rudolf V, Reiner E. Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal Biochem. 2003;312(2):224–27. doi: 10.1016/s0003-2697(02)00506-7. [DOI] [PubMed] [Google Scholar]
- 28.Young G, Koplovitz I. Acute toxicity of cyclohexylmethylphosphonofluoridate (CMPF) in rhesus monkeys: serum biochemical and hematologic changes. Arch Toxicol. 1995;69(6):379–83. doi: 10.1007/s002040050187. [DOI] [PubMed] [Google Scholar]
- 29.Ecobichon D, Comeau A. Pseudocholinesterases of mammalian plasma: Physicochemical properties and organophosphate inhibition in eleven species. Toxicol Appl Pharmacol. 1973;24(1):92–100. doi: 10.1016/0041-008x(73)90184-1. [DOI] [PubMed] [Google Scholar]
- 30.Gordon R, Feaster S, Russell A, LeJeune K, Maxwell D, Lenz D, Ross M, Doctor B. Organophosphate skin decontamination using immobilized enzymes. Chem-Biol Interact. 1999;119–120:463–70. doi: 10.1016/s0009-2797(99)00059-9. [DOI] [PubMed] [Google Scholar]
- 31.Rowntree DW, Nevin S, Wilson A. The effects of diisopropylfluorophosphonate in schizophrenia and manic depressive psychosis. J Neurol Neurosurg Psychiat. 1950;13(1):47–62. doi: 10.1136/jnnp.13.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senanayake N, Karalliede L. Neurotoxic effects of organophosphorous insecticides. An intermediate syndrome. N Engl J Med. 1987;316:761–63. doi: 10.1056/NEJM198703263161301. [DOI] [PubMed] [Google Scholar]
- 33.Vijayakumar S, Fareedullah M, Ashok Kumar E, Mohan Rao K. A prospective study on electrocardiographic findings of patients with organophosphorus poisoning. Cardiovasc Toxicol. 2011;11(2):113–17. doi: 10.1007/s12012-011-9104-4. [DOI] [PubMed] [Google Scholar]
- 34.De Candole C, Douglas W, Evans C, Holmes R, Spencer K, Torrance R, Wilson K. The failure of respiration in death by anticholinesterase poisoning. Br J Pharmacol Chemother. 1953;8(4):466–475. doi: 10.1111/j.1476-5381.1953.tb01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furlong C, Li W, Richter R, Shih D, Lusis A, Alleva E, Costa L. Genetic and temporal determinants of pesticide sensitivity: role of paraoxonase (PON1) Neurotoxicology. 21(1–2):91. [PubMed] [Google Scholar]
- 36.De Bleecker J, Van Den Neucker K, Willems J. The intermediate syndrome in organophosphate poisoning: Presentation of a case and review of the literature. Clin Toxicol. 1992;30(3):321–29. doi: 10.3109/15563659209021546. [DOI] [PubMed] [Google Scholar]
- 37.Thunga G, Sam K, Khera K, Xavier V, Verma M. Profile of acute mixed organophosphorus poisoning. Am J Emerg Med. 2009;27(5) doi: 10.1016/j.ajem.2008.08.030. 628 (e1–3) [DOI] [PubMed] [Google Scholar]
- 38.Atherton K, Williams F, Egea González F, Glass R, Rushton S, Blain P, Mutch E. DNA damage in horticultural farmers: a pilot study showing an association with organophosphate pesticide exposure. Biomarkers. 2009;14(7):443–51. doi: 10.3109/13547500903137265. [DOI] [PubMed] [Google Scholar]
- 39.Jalali N, Balali Mood M, Jalali I, Shakeri M. Electrophysiological Changes in Patients with Acute Organophosphorous Pesticide Poisoning. Basic Clin Pharmacol Toxicol. 2010;108(4):251–255. doi: 10.1111/j.1742-7843.2010.00652.x. [DOI] [PubMed] [Google Scholar]
- 40.Ellman G, Courtney K. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88–90. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 41.Thiermann H, Zilker T, Eyer F, Felgenhauer N, Eyer P, Worek F. Monitoring of neuromuscular transmission in organophosphate pesticide-poisoned patients. Toxicol Lett. 2009;191(2–3):297–304. doi: 10.1016/j.toxlet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Worek F, Mast U, Kiderlen D, Diepold C, Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta. 1999;288(1–2):73–90. doi: 10.1016/s0009-8981(99)00144-8. [DOI] [PubMed] [Google Scholar]
- 43.Mortensen S, Brimijoin S, Hooper M, Padilla S. Comparison of thein VitroSensitivity of Rat Acetylcholinesterase to Chlorpyrifosoxon: What Do Tissue IC50 Values Represent? Toxicol Appl Pharmacol. 1998;148(1):46–49. doi: 10.1006/taap.1997.8287. [DOI] [PubMed] [Google Scholar]
- 44.Katagi M, Nishikawa M, Tatsuno M, Tsuchihashi H. Determination of the main hydrolysis product of O-ethylS-2-diisopropylaminoethyl methylphosphonothiolate, ethyl methylphosphonic acid, in human serum. J Chromatograph B: Biomed Sci Appl. 1997;689(2):327–33. doi: 10.1016/s0378-4347(96)00356-8. [DOI] [PubMed] [Google Scholar]
- 45.Wolfe A, Rush R, Doctor B, Koplovitz I, Jones D. Acetylcholinesterase prophylaxis against organophosphate toxicity. Fundam Appl Toxicol. 1987;9(2):266–70. doi: 10.1016/0272-0590(87)90048-0. [DOI] [PubMed] [Google Scholar]
- 46.Caranto G, Waibel K, Asher J, Larrison R, Brecht K, Schutz M, Raveh L, Ashani Y, Wolfe A, Maxwell D. Amplification of the effectiveness of acetylcholinesterase for detoxification of organophosphorus compounds by bis-quaternary oximes. Biochem Pharmacol. 1994;47(2):347–57. doi: 10.1016/0006-2952(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 47.Caranto GR, Waibel KH, Asher JM, Larrison RW, Brecht KM, Schutz MB, Raveh L, Ashani Y, Wolfe AD, Maxwell DM, Bhupedra P. Amplification of the effectiveness of acetylcholinesterase for detoxification of organophosphorus compounds by bis-quaternary oximes. Biochem Pharmacol. 1994;47(2):347–57. doi: 10.1016/0006-2952(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 48.Balali-Mood M, Ayati M, Ali-Akbarian H. Effect of high doses of sodium bicarbonate in acute organophosphorous pesticide poisoning. Clin Toxicol. 2005;43(6):571–74. doi: 10.1081/clt-200068845. [DOI] [PubMed] [Google Scholar]
- 49.Van Helden H, Busker R, Melchers B, Bruijnzeel P. Pharmacological effects of oximes: how relevant are they? Archiv Toxicol. 1996;70(12):779–86. doi: 10.1007/s002040050340. [DOI] [PubMed] [Google Scholar]
- 50.Corvino T, Nahata M, Angelos M, Tschampel M, Morosco R, Zerkle J, Nelson R. Availability, Stability, and Sterility of Pralidoxime for Mass Casualty Use. Annals Emerg Med. 2006;47(3):272–77. doi: 10.1016/j.annemergmed.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 51.Worek F, Widmann R, Knopff O, Szinicz L. Reactivating potency of obidoxime, pralidoxime, HI 6 and HLö 7 in human erythrocyte acetylcholinesterase inhibited by highly toxic organophosphorus compounds. Arch Toxicol. 1998;72(4):237–43. doi: 10.1007/s002040050495. [DOI] [PubMed] [Google Scholar]
- 52.Dawson R. Review of oximes available for treatment of nerve agent poisoning. J Appl Toxicol. 1994;14(5):317–31. doi: 10.1002/jat.2550140502. [DOI] [PubMed] [Google Scholar]
- 53.Balali-Mood M, Salimifar H, Shariate M. Effects of sodium bicarbonate in human organophosphate poisoning. “Proceedings of the third international Chemical and Biological Medical treatment symposium; Spiez, Switzerland. May 7–12; 2000. pp. 18–27. [Google Scholar]
- 54.Roberts DM, Dawson AH, Hittarage A, Jeganathan K, Sheriff MH, Buckley NA. Plasma alkalinization for acute organophosphorus poisoning--is it a reality in the developing world? Clin Toxicol (Phila) 2007;45(1):90–91. doi: 10.1080/15563650601033342. [DOI] [PubMed] [Google Scholar]
- 55.Balali-Mood M, Afshari R, Kahrom M, Ayati M, Ali-Akbarian H, Zare G. Letter to the Editor: Use of high doses of sodium bicarbonate in acute organophosphorous pesticide poisoning is advancing”. Clin Toxicol. 2007;45(1):92–93. doi: 10.1081/clt-200068845. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Y, Zhan C, Li Y, Zhong Q, Pan H, Yang G. Intravenous lipid emulsions combine extracorporeal blood purification: a novel therapeutic strategy for severe organophosphate poisoning. Med Hypotheses. 2010;74(2):309. doi: 10.1016/j.mehy.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Balali-Mood M, Balali-Mood K, Hosseini Shirazi F. Recent advances in treatment of acute organophosphorous nerve agents poisoning. Iran J Pharm Res. 2006;2:82–88. [Google Scholar]
- 58.Balali-Mood M, Saber MR. Recent advances in the treatment of acute organophosphorous poisonings. Iran J Med Sci. 2012;37(2):74–91. [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang G, Huo F, Li J, Wang Y, Cao H. Studies on use and residue levels of pesticides in fruit and vegetable in Tianjin Area and its control measures. Chin J Prev Med. 2003;37(5):351–54. [PubMed] [Google Scholar]
- 60.Heidari H. Farmer field schools (FFS) slash pesticide use and exposure in Islamic republic of Iran. Agro-Chemicals Report. 2003;3(1):23–26. [Google Scholar]
- 61.El Nahhal Y. Contamination and safety status of plant and food in Arab countries. J Appl Sci. 2004;4(3):411–7. [Google Scholar]
- 62.Husain S, Kiarostami V, Morrovati M, Tagebakhsh M. Multiresidue determination of diazinon and ethion in pistachio nuts by use of matrix solid phase dispersion with a lanthanum silicate co-column and gas chromatography. Acta Chromatographica. 2003;13:208–14. [Google Scholar]
- 63.Temple WA, Smith NA. 1989. last updated by Szinicz L, Karalliedde L, Ostapenko Y, Jonitz W, Balali-Mood M, Wong A, Socrates J (1999). Organophosphorous pesticides PIM, IPCS/WHO. http://www.inchem.org/documents/pims/chemical/pimg001.htm.


