Abstract
Steroidogenic acute regulatory-related lipid transfer (START) domain proteins are involved in the nonvesicular intracellular transport of lipids and sterols. The STARD1 (STARD1 and STARD3) and STARD4 subfamilies (STARD4–6) have an internal cavity large enough to accommodate sterols. To provide a deeper understanding on the structural biology of this domain, the binding of sterols to STARD5, a member of the STARD4 subfamily, was monitored. The SAR by NMR [1H-15N heteronuclear single-quantum coherence (HSQC)] approach, complemented by circular dichroism (CD) and isothermal titration calorimetry (ITC), was used. Titration of STARD5 with cholic (CA) and chenodeoxycholic acid (CDCA), ligands of the farnesoid X receptor (FXR), leads to drastic perturbation of the 1H-15N HSQC spectra and the identification of the residues in contact with those ligands. The most perturbed residues in presence of ligands are lining the internal cavity of the protein. Ka values of 1.8·10−4 M−1 and 6.3·104 M−1 were measured for CA and CDCA, respectively. This is the first report of a START domain protein in complex with a sterol ligand. Our original findings indicate that STARD5 may be involved in the transport of bile acids rather than cholesterol.
Keywords: lipid transport, cholesterol metabolism, bile acids, steroidogenic acute regulatory protein, isothermal titration calorimetry, circular dichroism, NMR spectroscopy
Cholesterol is an essential constituent of mammalian cell membranes; it also serves as a precursor to bile acids, steroid hormones, and vitamin D. Cholesterol homeostasis within the body is controlled through different mechanisms involving its uptake, biosynthesis, transport/trafficking, sorting, storage, secretion, and catabolism to bile acids (1–3). In recent years, the focus of many studies has been on mechanisms involved in inclusion and exclusion of cholesterol from various intracellular organelles. In this respect, specialized nonvesicular lipid transporters of the superfamily of proteins with a steroidogenic acute regulatory (StAR)-related lipid transfer (START) domain were shown to be involved in lipids and cholesterol trafficking between intracellular membranes (4–7).
The START superfamily is defined by the presence of a conserved amino acid sequence of typically 210 amino acids that folds into an α/β helix-grip structure forming a hydrophobic pocket for ligand binding (5, 8–16). This module is conserved throughout the evolution and is involved in the transport of ligands, namely lipids, in mammals.
Fifteen mammalian proteins, divided into six subfamilies possess a START domain (4, 5, 11, 17, 18), and two of these subfamilies, STARD1 and STARD4, are reported to bind sterols. The STARD1 subfamily is composed of STARD1 and STARD3. STARD1 (StAR) is the archetype of START domain-containing protein; it binds cholesterol, possesses a mitochondrial leader peptide, and is involved in the transfer of cholesterol into mitochondria in steroidogenic tissues (11, 19–30) and in hepatocytes (31–33).
The second STARD1 subfamily member, STARD3 (metastatic lymph node 64, MLN64), also binds cholesterol (16). STARD3 is a membrane protein that is targeted to the late endosomes by an N-terminal region domain (34). The subcellular localization of STARD1 and STARD3 suggests different roles in cellular cholesterol trafficking between these two proteins. It was also suggested that STARD3 might serve to maintain cholesterol at the membrane of late endosomes prior to its shuttle to cytoplasmic acceptor(s) through the START domain (34).
The members of STARD4 subfamily, STARD4, STARD5, and STARD6, contain 205–233 amino acid residues and share 26–32% identity between each other (18). In contrast to STARD1 and STARD3, STARD4, STARD5, and STARD6 do not have an N-terminal targeting sequence to direct them to specific cellular organelles; they are thus essentially composed of a START domain. In vitro studies indicated that STARD4 and STARD5 are able to bind cholesterol (6, 35). STARD6 expression has been revealed in the testis (germinal cells); it is not present in steroidogenic tissues (18, 36–38). STARD6 is also widely distributed in the nervous system (36, 39, 40), and it was mainly detected in developing Purkinje cells (41).
In contrast to selective cholesterol binding by STARD1 and STARD3, STARD4 also binds 7α-hydroxycholesterol and 7-hydroperoxycholesterol, whereas STARD5 was reported to also bind 25-hydroxycholesterol (6). STARD4 expression has been revealed in several tissues, including liver, kidneys (18), and keratinocytes (42); STARD4 was also found in macrophages, Kupffer cells, and hepatocytes (43). Recent studies indicate an important role for STARD4 in cholesterol transport and homeostasis. In fact, it was reported that STARD4 overexpression in primary mouse hepatocytes led to a marked increase in intracellular cholesteryl ester concentration by delivering cholesterol to ACAT for esterification (35, 43). Furthermore, STARD4 overexpression enhanced sterol transport to the endocytic recycling compartment and to the endoplasmic reticulum (ER). STARD4 was found very efficient in transporting sterol between membranes in vitro (44). Taken together, these results suggest that cholesterol transport mediated by STARD4 is an important component of the cholesterol homeostasis regulatory machinery.
STARD5 is highly expressed in the liver (Kupffer cells) and kidneys (renal proximal tubules) (6, 18, 45, 46). STARD5 is also present in macrophages, monocytes, promyelocytic cells, mast cells, and basophils (46). In the human renal proximal tubule cell line HK-2, ER stressors increased STARD5 mRNA levels and induced a relocalization of STARD5 from a diffuse cytoplasmic pattern to a perinuclear and cell periphery distribution (18, 45). STARD5 overexpression promotes an increase in free cholesterol levels in mouse hepatocytes (35). Also, in HK-2 cells, STARD5 expression is higher in cells with greater cholesterol content (17); STARD5 mRNA levels were significantly increased in cholesterol-loaded mouse macrophages (45, 47). The above data support a positive correlation between cellular free cholesterol content and STARD5 expression. Although these studies demonstrate concomitance between cholesterol and STARD5 levels, the actual role of STARD5 remains to be elucidated.
The crystal structures of STARD1 and STARD5 have been recently reported (15); they are almost identical to the crystal structures of the START domains of STARD3 and STARD4 and of those involved in the transfer of ceramides and phospholipids, namely, STARD2, STARD11, STARD13, and STARD14 (9, 10, 13–16). However, to date, no structure of a START domain in complex with a sterol has been determined. In addition, the mechanism of the binding and release of ligand has yet to be fully elucidated.
To improve our understanding of STARD5 structure, dynamics and functions, we undertook the characterization of STARD5 bound to sterol by circular dichroism (CD) and by multinuclear and multidimensional solution-state NMR spectroscopy. Very unexpectedly, we found no indications of cholesterol binding to STARD5. However, we report for the first time that STARD5 can specifically bind cholic acid (CA) and chenodeoxycholic acid (CDCA). As expected from a specific ligand binding reaction, STARD5 has increased thermodynamic stability in presence of CA and CDCA. Moreover, by monitoring the chemical shift displacement (CSD) of backbone amide correlations on 1H-15N heteronuclear single-quantum coherence (HSQC), we were able to locate residues inside the cavity participating to CA and CDCA binding. Titration analyses obtained by Isothermal Titration Calorimetry revealed that STARD5 binds CA and CDCA acid with Ka values of 1.8·104 M−1 and 6.3·104 M−1, respectively.
MATERIALS AND METHODS
Cloning, expression, and purification
The cDNA for the human STARD5 was generously provided by the Structural Genomics Consortium (Karolinska Institutet, Stockholm, Sweden). The construct was modified to remove the N-terminal TEV protease cleavage site and to add a hexahistidine tag at the C terminus. This new construct was sequenced and cloned into the expression vector pET-3a (Novagen).
For 15N- or 13C, 15N-double labeling, 15N ammonium chloride (1 g/l), and 13C glucose (3 g/l) (Cambridge Isotopes) as the sole nitrogen and carbon sources were used. E. coli BL21(DE3) was transformed with the plasmid, grown at room temperature (23°C) in M9 medium (100% H2O or 20% H2O: 80% D2O), and induced with 1 mM isopropyl-1-thio-b-Dgalactopyranoside (IPTG) when OD600 reached 0.6. After induction, cells were incubated for an additional 18 h at room temperature prior to harvesting by centrifugation. Cells were then resuspended in lysis buffer (3 ml/g of pellets; buffer composition: 50 mM K-Phosphate, 500 mM KCl, 10 mM imidazole, 2 mM TCEP, pH 7.4) with 2 mM TCEP, protease inhibitors (complete Mini EDTA-free inhibitors; Roche), and 1 mM PMSF, and frozen at −80°C.
Bacterial pellets were lysed by thawing at 37°C followed by addition of lysosyme (2 mg/ml) and DNase (50 μg/ml). The cell lysate was centrifuged at 19000 g for 30 min, and the supernatant was loaded onto a Ni-NTA column (Qiagen) during 2 h at room temperature. The resin was washed twice with lysis buffer, and the STARD5 recombinant protein was eluted with elution buffer (50 mM K-Phosphate, 500 mM KCl, 250 mM imidazole, 2 mM TCEP, pH7.4). The buffer was exchanged, and the protein was concentrated using Millipore UltraCel ultracentrifugation filters (10,000 Da MWCO; Amicon, Canada) device into NMR buffer (50 mM K-Phosphate, 50 mM KCl, 2 mM TCEP, pH 7.4) complemented with 10% D2O and 0.01 mM NaN3. The final concentrations of the NMR samples were between 0.8 and 1.2 mM. The identity and integrity of the final protein sample was confirmed by SDS-PAGE.
CD spectropolarimetry
CD measurements were performed on a Jasco J-810 spectropolarimeter equipped with a Peltier-type thermostat. Routine calibration of the instrument was done with an aqueous solution of d-10-(+)-camphor-sulfonic acid at 290.5 nm. Experiments were performed using quartz cells with a path length of 1.0 mm. For CD spectra and temperature denaturation measurements of STARD5, the protein was dissolved in 10 mM phosphate at pH 7.4, to a final concentration of 10 μM. The protein concentration was determined spectrophotometrically at 280 nm using an extinction coefficient of 30,940 M−1 cm−1. The CD spectra presented are the results of the accumulation of 10 scans at 0.1 nm intervals. Scan speeds and time constants were chosen to allow sufficient response time and achieve favorable signal-to-noise ratios. Temperature-induced denaturation curves were performed in the temperature range from 5°C to 95°C with a rate heating of 1°C/min. The raw mdeg values were transformed in mean residue molar ellipticity (deg·cm2·dmol−1) using the following equation: [Θ]222 = CD signal (deg)·MRW/concentration (g/l)·l·10, where MRW is the mean residue weight and l is the path length of the CD cell in cm. The determination of the apparent melting temperature (T°), temperature-dependent enthalpy of unfolding (ΔH°u), and temperature-dependent Gibbs free energy of unfolding (ΔG°u) was performed by the simulation of the temperature denaturation curves with a model describing the equilibrium of a two-state unfolding mechanism and assuming a ΔCp,u of 1 kcal·mol−1·K−1 (a typical value for a protein the size of STARD5) as described in Roostae et al. (48). Measurements with the ligand in appropriate buffer were performed at a protein to ligand molar ratio of 1:100, and spectra were taken at a molar ratio of 1:100. In cholesterol studies, a 5 mM stock solution in ethanol was used; the final concentration of ethanol was 0.2%. After 90 min equilibrium time, samples were analyzed by CD. Each spectrum was baseline corrected for buffer and ligand.
NMR spectroscopy
NMR experiments were performed at 298 K on a Varian 600 MHz spectrometer equipped with a Z-axis pulsed-field gradient triple resonance probes. The backbone sequence-specific assignments of 1HN, 13Cα, 13C′, 15N, and 1Hα, and side chain 13Cβ for the STARD5 were obtained using 1H-15N HSQC, HNHA, and standard triple resonance NMR experiments [HNCACB, HNCA, HNCO, HN(CO)CA, CBCA(CO)NH, CC(CO)NH, HC(CO)NH, HCCH-TOCSY]. {1H}-15N nuclear Overhauser effect (NOE) measurements were done by comparing HSQC spectra with 10 s proton saturation and without proton saturation. The experiments were repeated twice, and the average of the two sets is reported.
NMR data were processed using NMRPipe (49) and analyzed with CCPNmr Analysis (50). The assignments are deposited in the BioMagResBank (http://www.bmrb.wisc.edu/) with accession number 17909. The spectra were referenced as described by Wishart et al. (51). The chemical shift values of 15N, 13Cα, and 13Cβ have been corrected for the deuterium isotopic effect using the values described in Gardner et al. (52).
NMR titration experiments were performed at a protein concentration of 0.2 mM in NMR buffer at pH 7.4. NMR titrations were carried out by acquiring 1H-15N HSQC spectra on samples of 15N-labeled STARD5 with ligand concentration ranging from 0 to 0.4 mM, for a final protein:ligand molar ratio of 1:2. The peak intensities of the disappearing and/or appearing peaks as a function of titration progression were measured. The observed difference (ΔOBS) between the intensities of each point and the intensity of the initial point over the maximal difference in intensities (ΔMAX: difference between the intensities of final and the initial points) have been determined and plotted as function of the ratio of the total concentration of ligand (L) and protein (P). Assuming a 1:1 stoichiometry, for the ascending curves, the ratio (ΔOBS/ΔMAX) increases from 0 to 1 and gives the fraction of bound protein (FB). FB is given by Equation 1 (53). By fitting ΔOBS/ΔMAX as a function of L/P with the right-end term of Equation 1, one can determine Ka:
| (Eq. 1) |
However, because ΔOBS/ΔMAX monitored from the decrease in peak intensities of the free form gives a descending curve or (1 − FB), we fitted those curves with Equation 2 to obtain Ka:
| (Eq. 2) |
Isothermal titration calorimetry
Isothermal titration calorimetry (ITC) measurements were performed using a VP-ITC (GE Healthcare-MicroCal, Northampton, MA) at 25°C. Protein concentration were chosen to have a c value, defined as the product of the binding constant (Ka), the protein concentration ([P]), and the stoichiometric parameter (n) between 10 and 100 and a peak height of more than 0.5 µcal/sec. The ligand concentrations used were below their respective CMC values. Each experiment consisted of 49 injections into the experimental chamber of 6 μl of the ligand solution at 3 min intervals at a stirring speed of 260 rpm. Heats of dilution were subtracted from the raw titration data before analysis. Experiments were performed in triplicate, and data were fitted by least-squares procedures assuming a one-site binding model using Microcal Origin version 7.0.
Molecular modeling and docking
Coordinates for the missing residues in the α3 helix of the crystal structure of STARD5 (PDB code: 2R55) were generated by introducing them in the model in an α-helical conformation. Then the potential energy in that region was minimized while restraining of the rest of the molecule. A second round of minimization was performed on the entire model. This procedure lead to a model that was superimposable on the initial coordinates with a backbone RMSD of less than 0.2 Å. This was done using the InsightII suite (Acceleris, CA). We used the program FlexAID to perform the molecular docking (http://bcb.med.usherbrooke.ca). FlexAID uses a genetic algorithm and allows for side-chain and ligand flexibility and optimizes a surface area complementarity-based scoring function. One thousand poses of CA and CDCA in the binding site of STARD5 were generated. The poses were rescored with the MM-GBSA force field (54). The top (lowest potential energy) 100 poses were analyzed to uncover potential recurrent H-bond patterns between the ligands and the residues of the binding site.
RESULTS
The 3D structure and the flexible regions of the crystal structure of STARD5 are conserved in solution
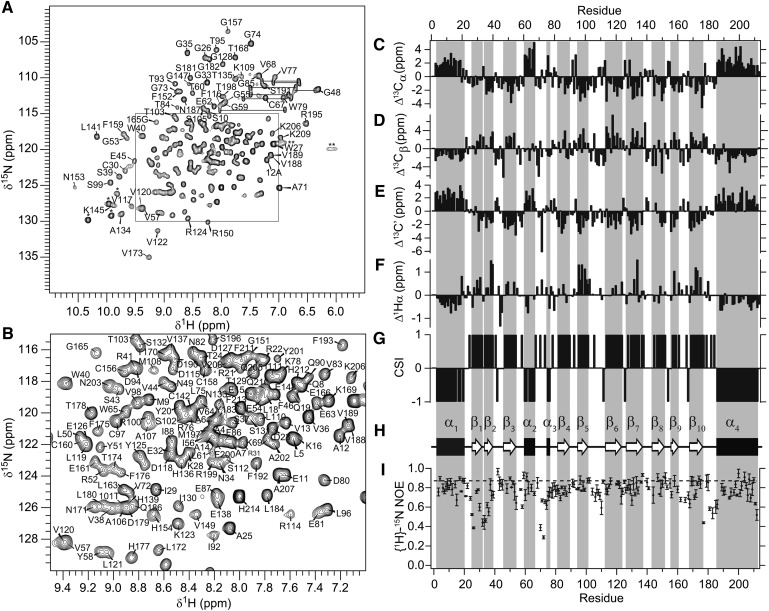
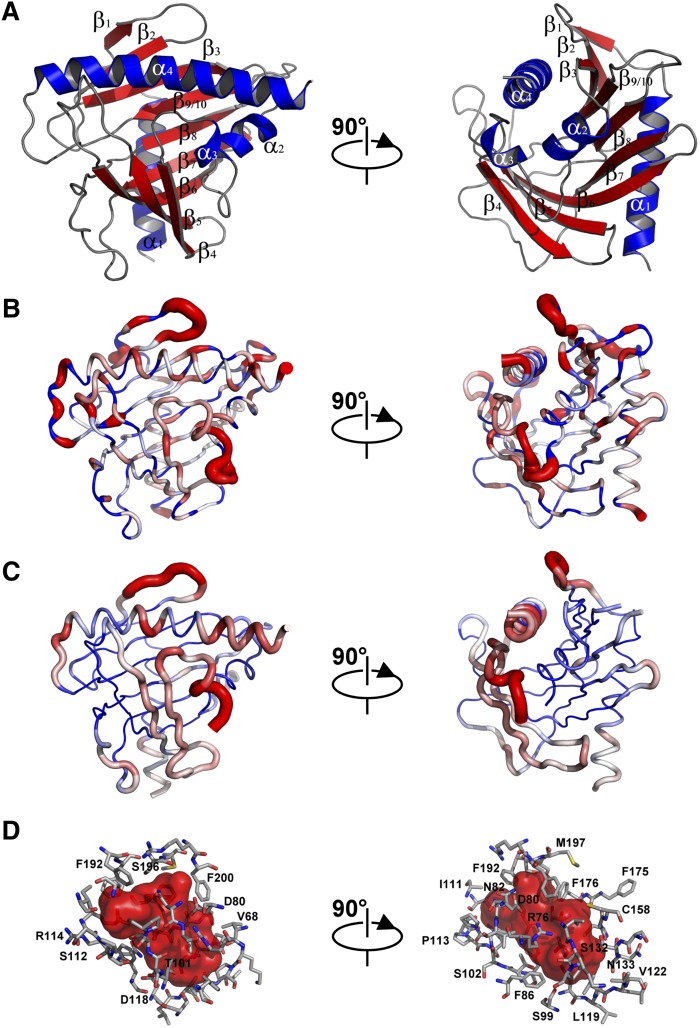
The assigned 1H-15N HSQC of STARD5 recorded at 25°C and pH 7.4 are presented in Fig. 1A, B. As expected for a stable tertiary structure, the chemical shift dispersion is excellent with little overlap. The assignments of the backbone 1H, 15N, and 13C-chemical shifts have been reported by us recently (55). The secondary chemical shifts for the Cα, Cβ, C′, and Hα are depicted in Fig. 1C–F, respectively. For Cα and C′, positive and negative secondary chemical shifts (difference between the assigned chemical shift of a given residue and its random coil value) indicate α-helical and β-strand conformation, respectively. This relationship is reversed for Cβ and Hα secondary chemical shifts. Using the chemical shift index (CSI) (56, 57), the sequence specific secondary structure elements of STARD5 have been determined (Fig. 1G, H). The location of the secondary structure elements in STARD5 in solution is identical to that observed in the crystal structure (Fig. 2A). Hence, this confirms that the structure is practically identical in the different milieu. Moreover, we determined the extent of motion of the backbone amides on the ps-ns timescale by measuring the {1H}-15N heteronuclear NOE (Fig. 1G). The {1H}-15N heteronuclear NOE is sensitive to internal motions (in addition to the overall molecular tumbling) of the backbone 15N-1H vector on the ps-ns timescale. In absence of internal motion, the {1H}-15N heteronuclear NOE is maximal (0.87 at 600 MHz). However, the presence of internal motion lowers the {1H}-15N heteronuclear NOE. Hence, the {1H}-15N heteronuclear NOE values for 15N-1H vectors in stable secondary structure is expected to be close to the maximum, whereas those in flexible loops and unfolded regions are expected to tend toward zero and negative values, respectively. Fig. 2B illustrates the flexible regions of STARD5 in absence of ligand. In this representation, for a large value of the heteronuclear NOE, the diameter of the ribbon is minimal, indicating low mobility of the 15N-1H vector. Inversely, 15N-1H vectors with low heteronuclear NOE values are depicted by larger ribbon diameters. As can be observed, the regions of STARD5 with regular secondary structures have low mobility on the ps-ns timescale, whereas loops and unstructured regions have higher mobility. Note the presence of significant movement in the loops connecting α1 and β1, β1 and β2, α2 and α3 (and part of α3), β5 and β6 (Ω-loop 1), and β9/10 and α4. Similarly, the atoms in the above regions also have the largest B-factors in the crystal structure of STARD5 (PDB code: 2R55), with no electron density observed for part of the loop between α2 and α3 (Fig. 2C). This strongly suggests that these regions are true flexible regions in STARD5 on the ps-ns timescale. However, α1, β1, and β2 do not participate to the internal cavity assigned to the binding site of STARD5 (Fig. 2B, D).
Fig. 1.
Solution structure and dynamics of apo-STARD5. (A) 1H-15N HSQC spectrum of STARD5 (pH 7.4) at 600 MHz and 25°C. Assignments of the cross-peaks are given with the residue number and one-letter code for amino acids. Horizontal lines connect the side-chain resonances of asparagines and glutamines. Tryptophan side-chain indole NH cross-peaks are marked with an asterisk (*). The double asterisks (**) denote protected guanidino (Hη) protons from arginine side-chains. (B) Detailed view of the congested region of the spectrum. (C–G) Backbone chemical shift assignments of apo-STARD5. Secondary chemical shifts of 13Cα, 13Cβ, 13C′, and Hα and CSI for STARD5. α-helices and β-strands are identified by consecutive CSI values of −1 and 1, respectively. (H) Secondary structure profile from CSI values. (I) {1H}-15N heteronuclear NOE. Dashed line is the maximum NOE value at 600 MHz in absence of internal motion. Values smaller than the maximum indicate internal motions of the corresponding backbone amide on the ps-ns time scale. Concentrations of STARD5 samples were 1.2 mM.
Fig. 2.
Structure, backbone mobility, and binding cavity of STARD5. (A) X-ray structure of STARD5 (PDB: 2R55). Note that no coordinates are present for residues Ala71, Val72, Gly165, or E166 in the pdb file. These residues were modeled as described in Materials and Methods. (B) {1H}-15N heteronuclear NOE (Fig. 1I) mapped onto the STARD5 structure. Worm representation with worm radius proportional to 1− the NOE values and coded in a blue-to-red gradient; regions of lower mobility (NOE > 0.7, blue) have a thinner backbone worm, regions of higher mobility (NOE < 0.5, red) have a thicker backbone worm, and intermediate regions (0.5 < NOE > 0.7) are white. (C) Crystallographic B-factors from the Cα backbone of the X-ray structure mapped on the backbone worm as in (B). (D) Internal cavity of STARD5 and residue identification lining the cavity. Cavity inner surface colored red and lining residues shown as sticks. The figure was created with Pymol (http://www.pymol.org/).
STARD5 does not bind cholesterol
We previously demonstrated the binding of cholesterol to STARD1 using CD (48) and NMR (58); essentially, addition of cholesterol led to the thermodynamic stabilization of the START domain of STARD1 and to drastic changes in the chemical shifts of the 1H-15N correlations in its HSQC spectrum. However, under the same experimental conditions, addition of cholesterol to STARD5 did not induce noticeable changes in the far-UV CD spectrum, in the thermodynamic stability (shift in the temperature denaturation curve), or in the 1H-15N HSQC (see supplementary data). We did not observe binding of 25-hydroxycholesterol either (data not shown). As described in earlier, STARD5 is expressed in Kupffer cells, circulating macrophages, and renal proximal tubules. These cells are exposed to bile acids and are involved in their transport, reabsorption, and metabolism. In this context, we verified whether STARD5 could bind bile acids; we chose to test cholic acid (CA) and chenodeoxycholic acid (CDCA). The rationale for this choice resides in the fact that CA and CDCA are ligands of the farnesoid X receptor (FXR) involved in the regulation of bile acid levels (59–61) and that STARD5 and FXR are both expressed in macrophages and the kidneys (18, 46, 62, 63).
STARD5 binds CA and CDCA
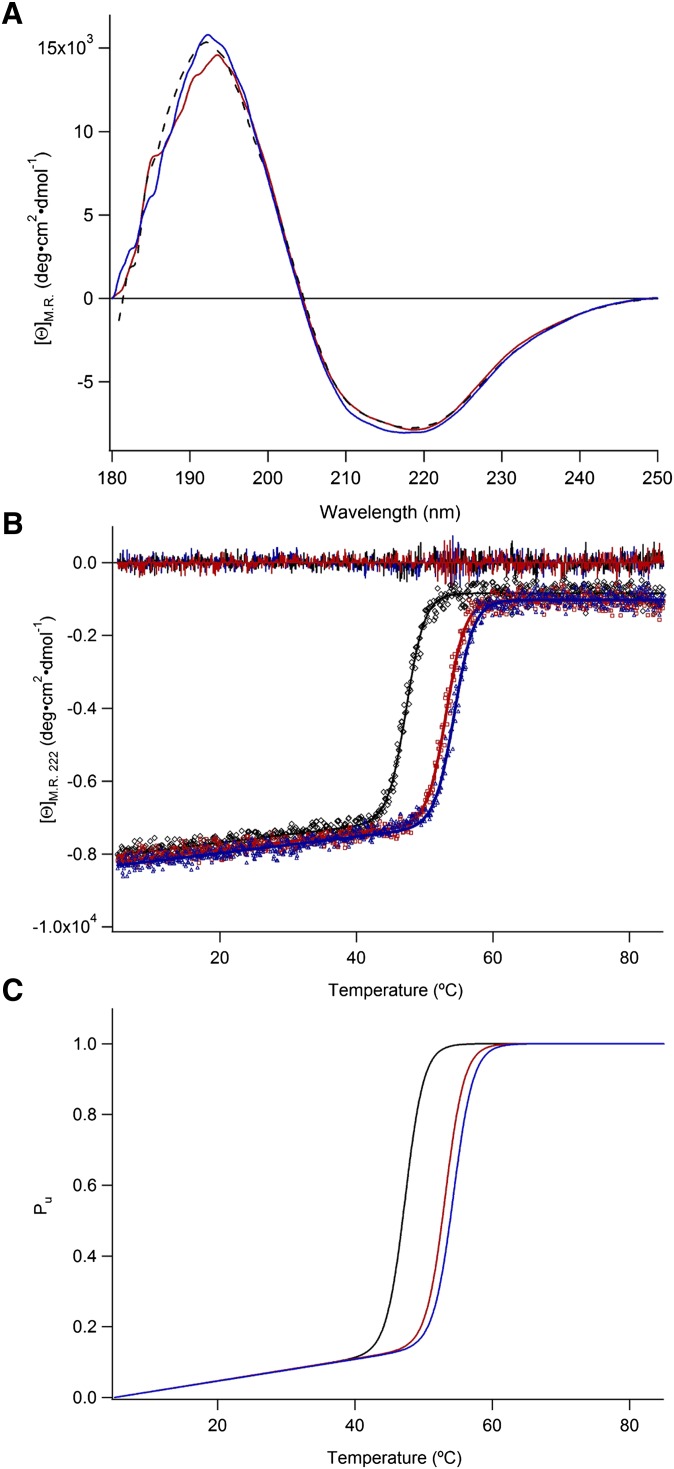
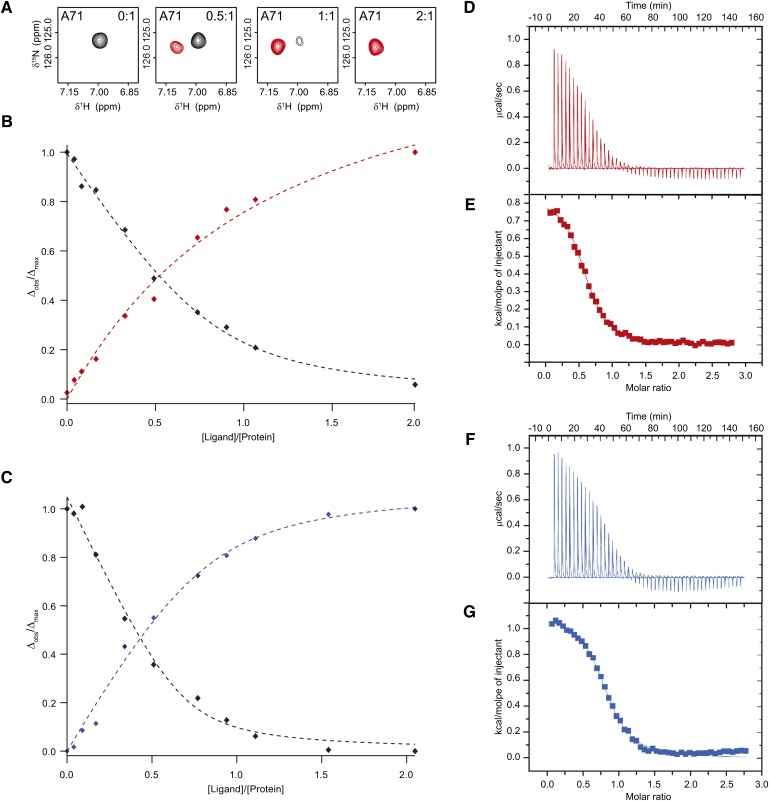
As shown in Fig. 3A, CA (red) and CDCA (blue) induce only minute changes in the far-UV CD spectrum and more pronouncedly on the thermodynamic stability of STARD5 (Fig. 3B, C). Moreover, the 1H-15N HSQC of STARD5 is drastically perturbed by the presence of CA and CDCA (Fig. 4A, B). These results clearly indicate binding and prompted us to further characterize, structurally and thermodynamically, the binding reaction.
Fig. 3.
Far-UV CD spectra of STARD5. (A) Mean residue ellipticity of STARD5 with CA or CDCA, black curve; apo-STARD5, red curve; STARD5 with CA and blue curve; STARD5 with CDCA. (B) Thermal melting curve recorded at 222 nm: apo-STARD5 (black), STARD5 with CA (red), and STARD5 with CDCA (blue). Residuals for the curve fitting are shown with the same color coding. (C) The concentrations were 10 μM for STARD5 and 1 mM for CA and CDCA. Considering Ka values in the 104 M−1 range, it can be calculated that the population of complex at 25°C is 100% with such concentration of STARD5 and ligands.
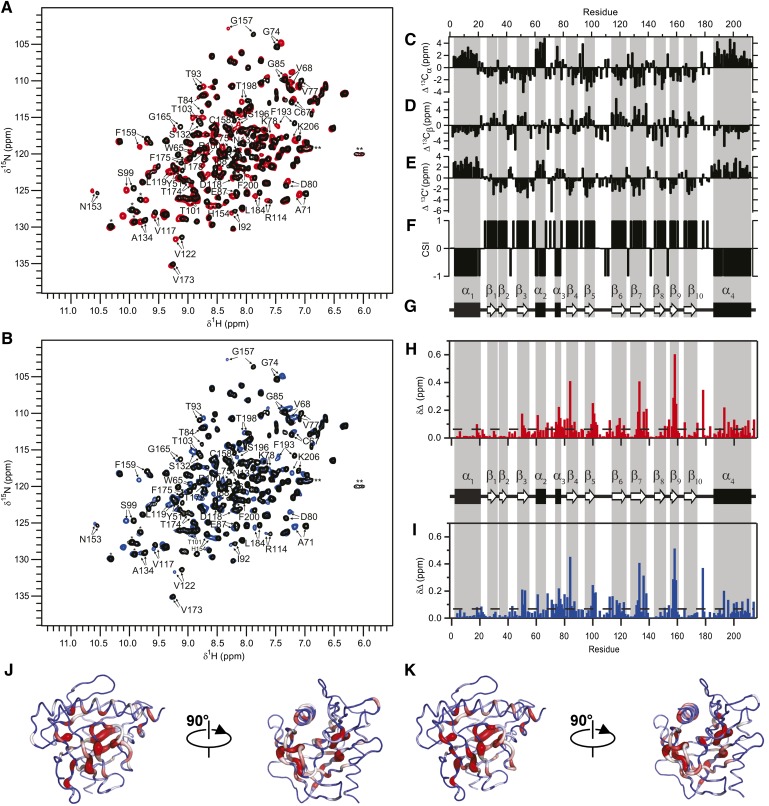
Fig. 4.
Binding of primary bile salt to STARD5. Overlay of 1H-15N HSQC spectra of STARD5 (0.8 mM in NMR buffer at pH 7.4) alone (black) and in the presence of 2 equivalents of (A) CA (red) or (B) CDCA (blue). Residues perturbed are labeled. (C–F) Backbone chemical shift assignments of STARD5 bound to CA. Secondary chemical shifts of 13Cα, 13Cβ, and 13C′ and CSI for STARD5. α-helices and β-strands are identified by consecutive CSI values of −1 and 1, respectively. (G) Secondary structure profile from CSI values. (H, I) Weighted CSD map upon binding of CA (H) and CDCA (I) to STARD5. Dashed line represented the mean value of CSD for each ligand. (J, K) Mapping of CSD upon binding of CA (J) and CDCA (K). Worm representation with worm radius proportional to the CSD values and coded in a blue-to-red gradient; regions unaffected (blue) have a thinner backbone worm, and regions perturbed (red) have a thicker backbone worm, whereas intermediate regions are white.
Thermodynamic stability of STARD5 and characterization of the binding of CA and CDCA by CD
To verify the thermodynamic stability of the tertiary structure of STARD5 with and without ligands, we measured its far-UV CD (Fig. 3A, black dashed line) spectrum and its temperature denaturation (Fig. 3B) monitored by CD (see below). The apparent minimum, starting from 202 nm in the far-UV CD spectrum, indicates a significant mixture of α-helical and β-sheet structure. Spectral analysis, using the program SELCON3 (64), allowed us to simulate (data not shown) the experimental spectrum with contents of α-helix, β-sheet, turns, and disordered structures of 0.18, 0.33, 0.24, and 0.25, respectively. These values are in excellent agreement with those estimated directly from the 3D structure (Fig. 2A).
The simulation of the temperature denaturation of STARD5 with a two-state model (see Materials and Methods), in which the folded state of STARD5 is in equilibrium with its unfolded state, is shown in Fig. 3B (black). Fig. 3C (black) illustrates the corresponding population of the unfolded state (Pu) versus temperature. From this simulation, we determined apparent values for T° and ΔH°u(T°) of 47.3 ± 0.3°C and 150.1 ± 3.2 kcal·mol−1, respectively (Table 1). From these parameters and the Gibbs-Helmholtz equation, ΔG°u(25°C) and ΔG°u(37°C) values of 9.7 ± 0.4 and 4.6 ± 0.3 kcal·mol−1 were calculated. The corresponding populations of the unfolded state (Pu) are 8·10−8 and 5·10−4 at 25 and 37°C, respectively. This indicates that STARD5 is stably folded at both temperatures.
TABLE 1.
Thermodynamic parameters of stability of STARD5
| T°(°C) | ΔH°u(T°)a | ΔG°u(25°C) | ΔG°u(37°C) | |
| apo | 47.3 (± 0.3)b | 150.1 (± 3.2) | 9.7 (± 0.4) | 4.6 (± 0.3) |
| CA | 53.2 (± 0.3) | 152.1 (± 3.3) | 11.9 (± 0.5) | 7.1 (± 0.3) |
| CDCA | 54.4 (± 0.3) | 151.1 (± 3.3) | 12.2 (± 0.5) | 7.3 (± 0.3) |
Thermodynamic parameters of stability of STARD5 were obtained from the simulation of the temperature denaturation apo-STARD5 and STARD5 in the presence of CA and CDCA.
Values are in kcal·mol−1.
Standard deviation of the fits.
Although CA (red) and CDCA (blue) induce slight changes in the far-UV CD spectrum of STARD5 (Fig. 3A), no apparent change in secondary structure content as determined by NMR has been observed (vide infra). However, Fig. 3B, C demonstrate that the apparent T° of STARD5 is significantly increased in presence of CA (ΔT° = 5.9°C) and CDCA (ΔT° = 7.1°C); this indicates that the stabilization free energy provided by the binding CDCA [ΔΔG°u(25°C) = 2.5 kcal·mol−1] is slightly larger than that provided by the binding of CA [ΔΔG°u(25°C) = 2.2 kcal·mol−1] (Table 1). Since there is a direct relationship between ΔT° induced by the binding of ligands and their actual Ka (65), the affinity (or Ka) of CDCA for STARD5 is apparently larger than that of CA; this is shown to be the case below.
Localization of the binding site of CA and CDCA in STARD5 by NMR
To locate the binding sites of CA and CDCA in STARD5, we monitored the titration of the START domain with the two bile acids by NMR. More precisely, we determined the backbone amides of STARD5 that are perturbed by the addition of CA and CDCA using the assigned 1H-15N HSQC. Because the 3D structure of STARD5 is known, the identification of the perturbed amides allows for the identification of the residues in contact with the bile acids or that undergo a conformational change upon complex formation. This approach is commonly called SAR by NMR (66). The titration of STARD5 with both molecules causes the displacement of numerous cross-peaks (labeled in Fig. 4A, B) on the 1H-15N HSQC of STARD5; indicating that the chemical environment of many backbone amides is perturbed by the presence of the bile acids in the complexes. The addition of CA and CDCA causes the displacement of the same cross-peaks on STARD5 HSQCs, demonstrating that a single subset of residues is perturbed by the presence of both ligands and, hence, suggesting the existence of a common binding site.
Interestingly, free and bound states of STARD5 are in the so-called slow exchange regime: the gradual addition of ligand causes a proportional increase in the intensity of the bound cross-peaks and a concomitant decrease in the intensity of the free form (vide infra). Usually, as a rule of thumb, high- to moderate-affinity complexes (Kd < 10 μM) are in “slow exchange” and low-affinity complexes (Kd > 100 μM) are in “fast exchange” on the NMR chemical shift timescale (67). In the latter case, the actual chemical shifts of the cross-peaks is a population weighted average of those of the free and bound states. To unambiguously identify the backbone amides of all the residues in the HSQCs of the bound form of STARD5, we reassigned the backbone 1H, 13C, and 15N chemical shifts of the bound state STARD5 (BMRB access, 18721) as described previously (55). Fig. 4C–F shows the secondary chemical shifts for the Cα, Cβ, and C′ and CSI, respectively. It is important to note, as indicated by the CSI (Fig. 4F), that the presence of the ligand does not induce changes in the location and extent of the secondary structure elements in STARD5. This suggests, as pointed out by CD (Fig. 3), that the 3D structure of STARD5 in solution is minimally affected by the presence of CA and CDCA.
The assignment of the backbone chemical shifts allows the identification of the backbone amide cross-peaks significantly shifted by the presence of CA and CDCA, respectively. From those assignments, CSD profiles can be established (Fig. 4H, I). The CSD is calculated with the following equation: CSD = ((Δ1H)2 + (Δ15N/(SW1H/SW15N))2/2)1/2, where SW is the spectral width of both dimensions. Significant displacements are attributed to cross-peaks that have CSD values one SD above the mean (dotted lines in Fig. 4H, I). The patterns of CSD are virtually identical for both ligands. The residues (and regions) most perturbed are located around Arg76(α3), Thr84(β4), Arg100(β5), Val122(β6), Ser132(β7), Cys158(β9), Thr178, and Ser196. In Fig. 4J, K, worm representations of the CSDs are displayed on the backbone of STARD5 for CA and CDCA, respectively. The residues with significant CSDs are located on contiguous secondary structure elements and residues that define the internal cavity of STARD5 (Fig. 2D). To the best of our knowledge, this is the first experimental validation of a ligand binding site in a START domain of the STARD1 and STARD4 subfamilies.
Determination of the apparent binding constant (Ka) of CA and CDCA to STARD5 by NMR
As stated above, the titration experiments monitored by NMR indicate that the free and bound forms of STARD5 interchange in the slow-exchange regime on the NMR timescale. In this regime, the cross-peaks of the residues affected by the presence of the ligand in both states can be observed simultaneously on 1H-15N HSQC. The intensities of the cross-peaks corresponding to the free and bound forms are directly proportional to the population of both states (Fig. 5A). Hence, the fraction of bound state (FB = ΔOBS/ΔMAX) can be directly determined as a function of ratio of the concentration of ligand over protein (L/P) when monitoring the appearance of the cross-peaks corresponding to the bound state. From such binding isotherms, an apparent Ka can be determined (see Materials and Methods). Inversely, by monitoring the decrease in the intensity of the cross-peaks of the free form, 1-FB can be determined and used to obtain Ka. We selected 10 residues in STARD5 that have both their free and bound cross-peaks well resolved to determine the apparent Ka of CA (Fig. 5B) and CDCA (Fig. 5C). The averages values obtained by NMR (Ka, NMR) for CA and CDCA are 3.7 (± 3.1)·104 M−1 and 7.7 (± 4.8)·104 M−1, respectively (Table 2). Although not significantly different, as suggested by the temperature denaturation of the complexes (Fig. 3B, C), STARD5 appears to have a larger affinity for CDCA than for CA.
Fig. 5.
Determination of the Ka of CA and CDCA for STARD5. (A–C) Determination of the Ka of CA and CDCA for STARD5 using 1H-15N HSQC spectra performed at a protein concentration of 0.2 mM in NMR buffer (pH 7.4) with a ligand concentration ranging from 0 to 0.4 mM, for a final protein:ligand molar ratio of 1:2. (A) The free state (black cross-peaks) and bound state of STARD5 are interchanging slowly on the NMR timescale. The cross-peaks of the backbone amide correlation of A71 is shown to have different chemical shifts in the free (red cross-peaks) and the bound (black cross-peaks) states, respectively. In the slow exchange regime, the intensity of the cross-peak is proportional to the population of each states. Plotting ΔOBS/ΔMAX (observed/maximal differences in intensity) as a function of the ratio of the total concentration of ligand/protein gives the evolution of the fraction bound (FB) or 1-FB when the intensities of the bound and free states are monitored along the titration, respectively. (B, C) Fitting of the isotherms obtained by monitoring the ΔOBS/ΔMAX of A71 upon titration of STARD5 with CA (B) and CDCA (C). (D–G) ITC measurements of STARD5 (protein concentration of 0.4 mM and 0.5 mM for CA and CDCA, respectively). Raw data with (D) CA and (F) CDCA at 25°C. Integrated heat changes, corrected for the heat of dilution, fitted to a single site model for (E) CA and (G) CDCA.
TABLE 2.
Thermodynamic parameters of the binding of CA and CDCA to STARD5
| Ka,NMRa | Ka,ITCa | ΔHab | T·ΔS°ab | ΔG°ab | n | |
| CA | 3.7 (± 3.1) | 1.8 (± 0.2) | 0.68 (± 0.02) | 6.49 (± 0.2) | −5.81 (± 0.90) | 0.90 (± 0.02) |
| CDCA | 7.7 (± 4.9) | 6.2 (± 0.2) | 0.97 (± 0.02) | 7.51 (± 0.2) | −6.54 (± 0.90) | 0.80 (± 0.02) |
Thermodynamic parameters of the binding of CA and CDCA to STARD5 determined by NMR and ITC at 25°C.
104 M−1.
Values are in kcal·mol−1.
Determination of the thermodynamics of binding of CA and CDCA to STARD5 by ITC
To further define the binding parameters, we titrated STARD5 with CA and CDCA using ITC, which allows direct determination of the stoichiometry, ΔHa(T), ΔSa(T) and ΔGa(T), and Ka(T). Fig. 5D, F show the binding isotherms corrected for the heat of dissolution and the nonlinear fits (Fig. 5E, G) to a single binding site model for CA and CDCA, respectively. These results indicate that the binding reaction of CA and CDCA is entropy driven with favorable ΔSa and unfavorable ΔHa, respectively (Table 2). The corresponding Ka,ITC values of 1.8 (± 0.2)·104 M−1 and 6.3 (± 0.2)·104 M−1, for CA and CDCA, respectively, are in agreement with the Ka,NMR values above and confirm the fact that STARD5 has a slightly larger affinity for CDCA than for CA.
Docking of CA and CDCA inside the STARD5 cavity
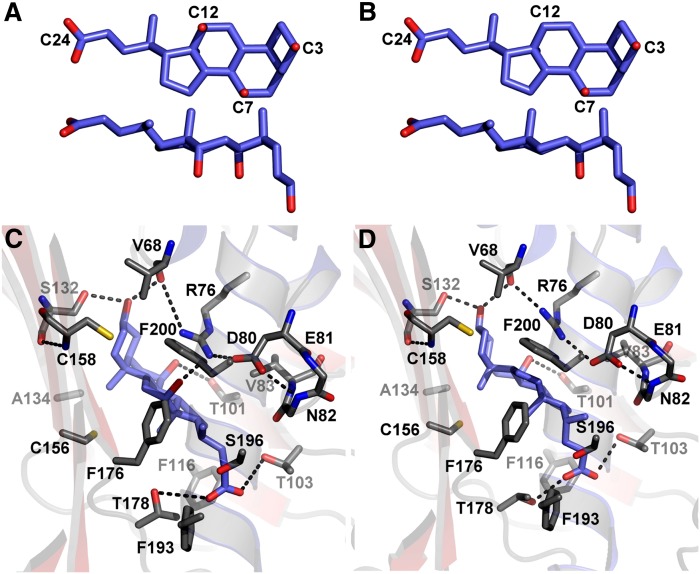
As established earlier for STARD3 (16), STARD4 (14), and more recently, STARD1 and STARD5 (15), the internal cavity of START domains is large enough to accommodate a sterol molecule (Fig. 2D). Nevertheless, no structure of STARD1 and STARD4 subfamilies in complex with a sterol ligand has been solved thus far. Hence, to generate possible configuration(s) of the complexes between STARD5 and CA and CDCA, we exploited our CSD data (Fig. 4H–K) and combined them with molecular docking studies using the program FlexAID (see Materials and Methods).
In the selection process, we searched for docking configurations in which CA and CDCA formed H-bonds with residues bearing significant CSDs. Note that the CSDs observed are not necessarily caused by a direct H-bond between the backbone amide and the ligands. In fact, the backbone amides of most of the residues affected by the presence of the ligands are involved in secondary structure H-bonds. Moreover, potential H-bond formation by CA (Fig. 6A) and CDCA (Fig. 6B) is limited to the carboxylate in C24 and carbons bearing an hydroxyl group. The representative poses that optimally fulfill the H-bond potential for CA and CDCA in the binding site of STARD5 are shown in Fig. 6C, D. Interestingly, similar features are observed in both complexes; noteworthy are the H-bonds between the γ-hydroxyl of the side-chain of Ser132 and the C3-hydroxyl, and between the γ1-hydroxyl of Thr101 and the C7-hydroxyl. Also, in both complexes, the C24 carboxylate receives H-bonds from the γ1-hydroxyls of Thr103 and Thr178, leading to the internal solvation of this ionizable moiety. Also, note the H-bond between the NηH2 of the guanidino group of Arg76 and the C12-hydroxyl of CA (absent in CDCA). Interestingly, this H-bond appears to be facilitated by the formation of an intramolecular salt bridge between Arg76 and Asp80.
Fig. 6.
Ligand structure and binding site of STARD5. Structure of CA (A) and CDCA (B). Representative and corresponding poses of CA (C) and CDCA (D) found to fulfill, in accordance with the CSD data (Fig. 4H–K), optimal H-bond potential in the binding site of STARD5. The H-bonds suggested to lead to specific binding are highlighted with dashed lines.
DISCUSSION
To gain insight into the function of STARD5, we characterized its solution structure, dynamics (on the ps-ns timescale), and ligand binding using CD, NMR, and ITC. We found that the α/β helix fold of STARD5 in solution is identical to that of the crystal structure (15). Similar to the crystallographic B-factors, motions of significant amplitudes on the ps-ns timescale were observed in the α2–α3 region (Fig. 2B). Whether this suggests an entry or exit route for the ligands remains to be elucidated by more in-depth relaxation studies, such as relaxation dispersion experiments and molecular dynamic simulations with and without ligands.
Using the same techniques (CD and NMR) previously reported for the demonstration of the binding of cholesterol to STARD1 (48, 58), we were unable to detect cholesterol binding to STARD5 (supplementary Fig. I, A–C), contrasting with a previous report (6). However, we report for the first time that STARD5 specifically binds the primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA). We determined that STARD5 has a larger affinity for CDCA than for CA [Ka of 6.2·104 M−1 (Kd = 1.6·10−5 M) and 1.8·104 M−1 (Kd = 5.5·10−5 M), respectively]. For comparison, the affinity of STARD5 for CDCA is in the same order of magnitude reported for the bile acids nuclear receptor (FXR), whereas the affinity of STARD5 for CA is 10 times higher than FXR for CA (68).
The chemical structure of bile acids (Fig. 6A, B) differs from that of other steroids by their nonplanar shape due to their cis A/B ring junction. Bile acids are amphipathic molecules with a concave hydrophilic α-face and a convex hydrophobic β-face. The hydroxyl groups oriented toward the α-face and the carboxylic side-chain confer them their hydrophilic character, while the methyl groups oriented toward the β-face afford them their hydrophobic properties (69). CA possesses three hydroxyl groups in position C3, C7, and C12, two methyl groups (C18 and C19), and an acidic group in C24; the 12-hydroxyl group is absent in CDCA (Fig. 6A, B). Particularly, the binding of both bile acids to STARD5 is associated with positive enthalpy (Fig. 5D–G), indicating that the reaction is entropy driven. The positive ΔH°a observed for CA and CDCA could be caused by lack of complete compensation of the important dehydration enthalpy associated with the burial of the charged carboxylate and the hydroxyls in CA and CDCA; this dehydration enthalpy can amount to up to 70 kcal·mol−1 for a charged group (70). From an entropic point of view, the burial of the hydrophobic side for both bile acids is expected to be favorable with respect to entropy increase of water.
Using an NMR titration approach and CSD data, we identified the residues of STARD5 perturbed by the presence of the ligands and located the binding site of CA and CDCA. These residues are localized in the internal cavity present in all START domains with known structures (Figs. 2 and 6). This is the first experimental validation of the binding of a sterol molecule inside the internal cavity of the START domain of the STARD1 and STARD4 subfamilies. By combining this information with molecular docking, we generated model complexes of STARD5 with CA and CDCA and selected those that best fulfilled the CSD data. In these complexes, all the hydroxyls of CA and CDCA are involved in H-bonds, and the carboxylate is surrounded by side-chains that can help in its internal solvation.
The present study clearly establishes that STARD5 binds CA and CDCA in vitro; the physiological function of this finding remains to be explored. However, STARD5 is highly expressed in Kupffer cells, peripheral macrophages, and kidney proximal tubule cells. These three cell types are bile acid targets and contain specific receptors or transporters for bile acid uptake and secretion. Hence, we can speculate that STARD5 modulates the activity of putative bile acid-regulated molecules by competing for or delivering CDCA.
In conclusion, this is the first detailed report on the elucidation of a START domain-sterol complex. We also define for the first time that STARD5 specifically binds primary bile acids. These findings will contribute to the understanding of the binding mechanism of a START domain to a sterol and will help to gain more insight into the function of STARD5 in sterol homeostasis.
Supplementary Material
Acknowledgments
The authors thank Dr Lari Lehtiö (Åbo Akademi University, Turku, Finland) for kindly providing us with the STARD5 cDNA.
Footnotes
Abbreviations:
- CA
- cholic acid
- CD
- circular dichroism
- CDCA
- chenodeoxycholic acid
- CSD
- chemical shift displacement
- CSI
- chemical shift index
- ER
- endoplasmic reticulum
- FXR
- farnesoid X receptor
- HSQC
- heteronuclear single-quantum coherence
- ITC
- isothermal titration calorimetry
- NOE
- nuclear Overhauser effect
- StAR
- steroidogenic acute regulatory
- START
- steroidogenic acute regulatory-related lipid transfer
This work supported by Canadian Institutes of Health Research Grant MT-10983 (to J.G.L. and P.L.) and by the Regroupement Stratégique sur la Structure, la Fonction et l'Ingénierie des Protéines (PROTEO).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two fi gures.
REFERENCES
- 1.Hylemon P. B., Stravitz R. T., Vlahcevic Z. R. 1994. Molecular genetics and regulation of bile acid biosynthesis. Prog. Liver Dis. 12: 99–120 [PubMed] [Google Scholar]
- 2.Russell D. W. 2003. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72: 137–174 [DOI] [PubMed] [Google Scholar]
- 3.Soccio R. E., Breslow J. L. 2004. Intracellular cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 24: 1150–1160 [DOI] [PubMed] [Google Scholar]
- 4.Alpy F., Tomasetto C. 2005. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J. Cell Sci. 118: 2791–2801 [DOI] [PubMed] [Google Scholar]
- 5.Ponting C. P., Aravind L. 1999. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem. Sci. 24: 130–132 [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Agudo D., Ren S., Hylemon P. B., Redford K., Natarajan R., Del Castillo A., Gil G., Pandak W. M. 2005. Human StarD5, a cytosolic StAR-related lipid binding protein. J. Lipid Res. 46: 1615–1623 [DOI] [PubMed] [Google Scholar]
- 7.Strauss J. F., 3rd, Kishida T., Christenson L. K., Fujimoto T., Hiroi H. 2003. START domain proteins and the intracellular trafficking of cholesterol in steroidogenic cells. Mol. Cell. Endocrinol. 202: 59–65 [DOI] [PubMed] [Google Scholar]
- 8.Iyer L. M., Koonin E. V., Aravind L. 2001. Adaptations of the helix-grip fold for ligand binding and catalysis in the START domain superfamily. Proteins. 43: 134–144 [DOI] [PubMed] [Google Scholar]
- 9.Kudo N., Kumagai K., Matsubara R., Kobayashi S., Hanada K., Wakatsuki S., Kato R. 2010. Crystal structures of the CERT START domain with inhibitors provide insights into the mechanism of ceramide transfer. J. Mol. Biol. 396: 245–251 [DOI] [PubMed] [Google Scholar]
- 10.Kudo N., Kumagai K., Tomishige N., Yamaji T., Wakatsuki S., Nishijima M., Hanada K., Kato R. 2008. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc. Natl. Acad. Sci. USA. 105: 488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavigne P., Najmanivich R., Lehoux J. G. 2010. Mammalian StAR-related lipid transfer (START) domains with specificity for cholesterol: structural conservation and mechanism of reversible binding. Subcell. Biochem. 51: 425–437 [DOI] [PubMed] [Google Scholar]
- 12.Mathieu A. P., Lavigne P., LeHoux J. G. 2002. Molecular modeling and structure-based thermodynamic analysis of the StAR protein. Endocr. Res. 28: 419–423 [DOI] [PubMed] [Google Scholar]
- 13.Roderick S. L., Chan W. W., Agate D. S., Olsen L. R., Vetting M. W., Rajashankar K. R., Cohen D. E. 2002. Structure of human phosphatidylcholine transfer protein in complex with its ligand. Nat. Struct. Biol. 9: 507–511 [DOI] [PubMed] [Google Scholar]
- 14.Romanowski M. J., Soccio R. E., Breslow J. L., Burley S. K. 2002. Crystal structure of the Mus musculus cholesterol-regulated START protein 4 (StarD4) containing a StAR-related lipid transfer domain. Proc. Natl. Acad. Sci. USA. 99: 6949–6954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorsell A. G., Lee W. H., Persson C., Siponen M. I., Nilsson M., Busam R. D., Kotenyova T., Schuler H., Lehtio L. 2011. Comparative structural analysis of lipid binding START domains. PLoS ONE. 6: e19521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsujishita Y., Hurley J. H. 2000. Structure and lipid transport mechanism of a StAR-related domain. Nat. Struct. Biol. 7: 408–414 [DOI] [PubMed] [Google Scholar]
- 17.Clark B. J. 2012. The mammalian START domain protein family in lipid transport in health and disease. J. Endocrinol. 212: 257–275 [DOI] [PubMed] [Google Scholar]
- 18.Soccio R. E., Adams R. M., Romanowski M. J., Sehayek E., Burley S. K., Breslow J. L. 2002. The cholesterol-regulated StarD4 gene encodes a StAR-related lipid transfer protein with two closely related homologues, StarD5 and StarD6. Proc. Natl. Acad. Sci. USA. 99: 6943–6948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bens S., Mohn A., Yuksel B., Kulle A. E., Michalek M., Chiarelli F., Nuri Ozbek M., Leuschner I., Grotzinger J., Holterhus P. M., et al. 2010. Congenital lipoid adrenal hyperplasia: functional characterization of three novel mutations in the STAR gene. J. Clin. Endocrinol. Metab. 95: 1301–1308 [DOI] [PubMed] [Google Scholar]
- 20.Bhangoo A., Gu W. X., Pavlakis S., Anhalt H., Heier L., Ten S., Jameson J. L. 2005. Phenotypic features associated with mutations in steroidogenic acute regulatory protein. J. Clin. Endocrinol. Metab. 90: 6303–6309 [DOI] [PubMed] [Google Scholar]
- 21.Bose H. S., Sugawara T., Strauss J. F., 3rd, Miller W. L. 1996. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N. Engl. J. Med. 335: 1870–1878 [DOI] [PubMed] [Google Scholar]
- 22.Chen X., Baker B. Y., Abduljabbar M. A., Miller W. L. 2005. A genetic isolate of congenital lipoid adrenal hyperplasia with atypical clinical findings. J. Clin. Endocrinol. Metab. 90: 835–840 [DOI] [PubMed] [Google Scholar]
- 23.Clark B. J., Wells J., King S. R., Stocco D. M. 1994. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J. Biol. Chem. 269: 28314–28322 [PubMed] [Google Scholar]
- 24.Flück C. E., Pandey A. V., Dick B., Camats N., Fernandez-Cancio M., Clemente M., Gussinye M., Carrascosa A., Mullis P. E., Audi L. 2011. Characterization of novel StAR (steroidogenic acute regulatory protein) mutations causing non-classic lipoid adrenal hyperplasia. PLoS ONE. 6: e20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gassner H. L., Toppari J., Quinteiro Gonzalez S., Miller W. L. 2004. Near-miss apparent SIDS from adrenal crisis. J. Pediatr. 145: 178–183 [DOI] [PubMed] [Google Scholar]
- 26.Katsumata N., Kawada Y., Yamamoto Y., Noda M., Nimura A., Horikawa R., Tanaka T. 1999. A novel compound heterozygous mutation in the steroidogenic acute regulatory protein gene in a patient with congenital lipoid adrenal hyperplasia. J. Clin. Endocrinol. Metab. 84: 3983–3987 [DOI] [PubMed] [Google Scholar]
- 27.Khoury J., Amundsen A. L., Tonstad S., Henriksen T., Ose L., Retterstol K., Iversen P. O. 2009. Evidence for impaired physiological decrease in the uteroplacental vascular resistance in pregnant women with familial hypercholesterolemia. Acta Obstet. Gynecol. Scand. 88: 222–226 [DOI] [PubMed] [Google Scholar]
- 28.Lehoux J. G., Mathieu A., Lavigne P., Fleury A. 2003. Adrenocorticotropin regulation of steroidogenic acute regulatory protein. Microsc. Res. Tech. 61: 288–299 [DOI] [PubMed] [Google Scholar]
- 29.Lin D., Sugawara T., Strauss J. F., 3rd, Clark B. J., Stocco D. M., Saenger P., Rogol A., Miller W. L. 1995. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 267: 1828–1831 [DOI] [PubMed] [Google Scholar]
- 30.Nakae J., Tajima T., Sugawara T., Arakane F., Hanaki K., Hotsubo T., Igarashi N., Igarashi Y., Ishii T., Koda N., et al. 1997. Analysis of the steroidogenic acute regulatory protein (StAR) gene in Japanese patients with congenital lipoid adrenal hyperplasia. Hum. Mol. Genet. 6: 571–576 [DOI] [PubMed] [Google Scholar]
- 31.Hall E. A., Ren S., Hylemon P. B., Rodriguez-Agudo D., Redford K., Marques D., Kang D., Gil G., Pandak W. M. 2005. Detection of the steroidogenic acute regulatory protein, StAR, in human liver cells. Biochim. Biophys. Acta. 1733: 111–119 [DOI] [PubMed] [Google Scholar]
- 32.Pandak W. M., Ren S., Marques D., Hall E., Redford K., Mallonee D., Bohdan P., Heuman D., Gil G., Hylemon P. 2002. Transport of cholesterol into mitochondria is rate-limiting for bile acid synthesis via the alternative pathway in primary rat hepatocytes. J. Biol. Chem. 277: 48158–48164 [DOI] [PubMed] [Google Scholar]
- 33.Sugawara T., Lin D., Holt J. A., Martin K. O., Javitt N. B., Miller W. L., Strauss J. F., 3rd 1995. Structure of the human steroidogenic acute regulatory protein (StAR) gene: StAR stimulates mitochondrial cholesterol 27-hydroxylase activity. Biochemistry. 34: 12506–12512 [DOI] [PubMed] [Google Scholar]
- 34.Alpy F., Tomasetto C. 2006. MLN64 and MENTHO, two mediators of endosomal cholesterol transport. Biochem. Soc. Trans. 34: 343–345 [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Agudo D., Ren S., Wong E., Marques D., Redford K., Gil G., Hylemon P., Pandak W. M. 2008. Intracellular cholesterol transporter StarD4 binds free cholesterol and increases cholesteryl ester formation. J. Lipid Res. 49: 1409–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang I. Y., Kim J. K., Lee S. M., Kim J. N., Soh J., Kim J. W., Yoon S. P. 2009. The changed immunoreactivity of StarD6 after pilocarpine-induced epilepsy. Neuroreport. 20: 963–967 [DOI] [PubMed] [Google Scholar]
- 37.Chang I. Y., Shin S. Y., Kim J. W., Yu J. M., Kim J. S., Song P. I., Yoon S. P. 2007. The changed immunolocalization of START-domain-containing 6 (StarD6) during the development of testes in rat perinatal hypothyroidism. Acta Histochem. 109: 315–321 [DOI] [PubMed] [Google Scholar]
- 38.Gomes C., Oh S. D., Kim J. W., Chun S. Y., Lee K., Kwon H. B., Soh J. 2005. Expression of the putative sterol binding protein Stard6 gene is male germ cell specific. Biol. Reprod. 72: 651–658 [DOI] [PubMed] [Google Scholar]
- 39.Chang I. Y., Jeon Y. J., Jung S. M., Jang Y. H., Ahn J. B., Park K. S., Yoon S. P. 2010. Does the StarD6 mark the same as the StAR in the nervous system? J. Chem. Neuroanat. 40: 239–242 [DOI] [PubMed] [Google Scholar]
- 40.Chang I. Y., Kim J. H., Hwang G., Song P. I., Song R. J., Kim J. W., Yoon S. P. 2007. Immunohistochemical detection of StarD6 in the rat nervous system. Neuroreport. 18: 1615–1619 [DOI] [PubMed] [Google Scholar]
- 41.Chang I. Y., Ohn T., Ko G. S., Yoon Y., Kim J. W., Yoon S. P. 2012. Immunolocalization of steroidogenic acute regulatory protein-related lipid transfer (START) domain-containing proteins in the developing cerebellum of normal and hypothyroid rats. J. Chem. Neuroanat. 43: 28–33 [DOI] [PubMed] [Google Scholar]
- 42.Elbadawy H. M., Borthwick F., Wright C., Martin P. E., Graham A. 2011. Cytosolic StAR-related lipid transfer domain 4 (STARD4) protein influences keratinocyte lipid phenotype and differentiation status. Br. J. Dermatol. 164: 628–632 [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Agudo D., Calderon-Dominguez M., Ren S., Marques D., Redford K., Medina-Torres M. A., Hylemon P., Gil G., Pandak W. M. 2011. Subcellular localization and regulation of StarD4 protein in macrophages and fibroblasts. Biochim. Biophys. Acta. 1811: 597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mesmin B., Pipalia N. H., Lund F. W., Ramlall T. F., Sokolov A., Eliezer D., Maxfield F. R. 2011. STARD4 abundance regulates sterol transport and sensing. Mol. Biol. Cell. 22: 4004–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y. C., Meier R. K., Zheng S., Khundmiri S. J., Tseng M. T., Lederer E. D., Epstein P. N., Clark B. J. 2009. Steroidogenic acute regulatory-related lipid transfer domain protein 5 localization and regulation in renal tubules. Am. J. Physiol. Renal Physiol. 297: F380–F388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Agudo D., Ren S., Hylemon P. B., Montanez R., Redford K., Natarajan R., Medina M. A., Gil G., Pandak W. M. 2006. Localization of StarD5 cholesterol binding protein. J. Lipid Res. 47: 1168–1175 [DOI] [PubMed] [Google Scholar]
- 47.Soccio R. E., Adams R. M., Maxwell K. N., Breslow J. L. 2005. Differential gene regulation of StarD4 and StarD5 cholesterol transfer proteins. Activation of StarD4 by sterol regulatory element-binding protein-2 and StarD5 by endoplasmic reticulum stress. J. Biol. Chem. 280: 19410–19418 [DOI] [PubMed] [Google Scholar]
- 48.Roostaee A., Barbar E., Lehoux J. G., Lavigne P. 2008. Cholesterol binding is a prerequisite for the activity of the steroidogenic acute regulatory protein (StAR). Biochem. J. 412: 553–562 [DOI] [PubMed] [Google Scholar]
- 49.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. 1995. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 6: 277–293 [DOI] [PubMed] [Google Scholar]
- 50.Vranken W. F., Boucher W., Stevens T. J., Fogh R. H., Pajon A., Llinas M., Ulrich E. L., Markley J. L., Ionides J., Laue E. D. 2005. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins. 59: 687–696 [DOI] [PubMed] [Google Scholar]
- 51.Wishart D. S., Bigam C. G., Yao J., Abildgaard F., Dyson H. J., Oldfield E., Markley J. L., Sykes B. D. 1995. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J. Biomol. NMR. 6: 135–140 [DOI] [PubMed] [Google Scholar]
- 52.Gardner K. H., Rosen M. K., Kay L. E. 1997. Global folds of highly deuterated, methyl-protonated proteins by multidimensional NMR. Biochemistry. 36: 1389–1401 [DOI] [PubMed] [Google Scholar]
- 53.Fielding L. 2007. NMR methods for the determination of protein-ligand dissociation constants. Prog. Nucl. Magn. Reson. Spectrosc. 51: 219–242 [Google Scholar]
- 54.Hou T., Wang J., Li Y., Wang W. 2011. Assessing the performance of the molecular mechanics/Poisson Boltzmann surface area and molecular mechanics/generalized Born surface area methods. II. The accuracy of ranking poses generated from docking. J. Comput. Chem. 32: 866–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorin A., Letourneau D., Lefebvre A., Lehoux J. G., Lavigne P. 2012. (1)H, (13)C, and (15)N backbone chemical shift assignments of StAR-related lipid transfer domain protein 5 (STARD5). Biomol. NMR Assign Epub ahead of print. March 6, 2012; doi:10.1007/s12104-012-9368-z. [DOI] [PubMed] [Google Scholar]
- 56.Wishart D. S., Sykes B. D. 1994. Chemical shifts as a tool for structure determination. Methods Enzymol. 239: 363–392 [DOI] [PubMed] [Google Scholar]
- 57.Wishart D. S., Sykes B. D. 1994. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J. Biomol. NMR. 4: 171–180 [DOI] [PubMed] [Google Scholar]
- 58.Barbar E., Lehoux J. G., Lavigne P. 2009. Toward the NMR structure of StAR. Mol. Cell. Endocrinol. 300: 89–93 [DOI] [PubMed] [Google Scholar]
- 59.Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. 1999. Identification of a nuclear receptor for bile acids. Science. 284: 1362–1365 [DOI] [PubMed] [Google Scholar]
- 60.Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. 1999. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 3: 543–553 [DOI] [PubMed] [Google Scholar]
- 61.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., et al. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science. 284: 1365–1368 [DOI] [PubMed] [Google Scholar]
- 62.Mencarelli A., Renga B., Distrutti E., Fiorucci S. 2009. Antiatherosclerotic effect of farnesoid X receptor. Am. J. Physiol. Heart Circ. Physiol. 296: H272–H281 [DOI] [PubMed] [Google Scholar]
- 63.Jiang T., Wang X. X., Scherzer P., Wilson P., Tallman J., Takahashi H., Li J., Iwahashi M., Sutherland E., Arend L., et al. 2007. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 56: 2485–2493 [DOI] [PubMed] [Google Scholar]
- 64.Sreerama N., Woody R. W. 2004. Computation and analysis of protein circular dichroism spectra. Methods Enzymol. 383: 318–351 [DOI] [PubMed] [Google Scholar]
- 65.Layton C. J., Hellinga H. W. 2010. Thermodynamic analysis of ligand-induced changes in protein thermal unfolding applied to high-throughput determination of ligand affinities with extrinsic fluorescent dyes. Biochemistry. 49: 10831–10841 [DOI] [PubMed] [Google Scholar]
- 66.Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. 1996. Discovering high-affinity ligands for proteins: SAR by NMR. Science. 274: 1531–1534 [DOI] [PubMed] [Google Scholar]
- 67.Zuiderweg E. R. 2002. Mapping protein-protein interactions in solution by NMR spectroscopy. Biochemistry. 41: 1–7 [DOI] [PubMed] [Google Scholar]
- 68.Lew J. L., Zhao A., Yu J., Huang L., De Pedro N., Pelaez F., Wright S. D., Cui J. 2004. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J. Biol. Chem. 279: 8856–8861 [DOI] [PubMed] [Google Scholar]
- 69.Monte M. J., Marin J. J., Antelo A., Vazquez-Tato J. 2009. Bile acids: chemistry, physiology, and pathophysiology. World J. Gastroenterol. 15: 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lazaridis T., Archontis G., Karplus M. 1995. Enthalpic contribution to protein stability: insights from atom-based calculations and statistical mechanics. Adv. Protein Chem. 47: 231–306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.