Abstract
Human platelet-type 12-lipoxygenase (12-LOX) has recently been shown to play an important role in regulation of human platelet function by reacting with arachidonic acid (AA). However, a number of other fatty acids are present on the platelet surface that, when cleaved from the phospholipid, can be oxidized by 12-LOX. We sought to characterize the substrate specificity of 12-LOX against six essential fatty acids: AA, dihomo-γ-linolenic acid (DGLA), eicosapentaenoic acid (EPA), α-linolenic acid (ALA), eicosadienoic acid (EDA), and linoleic acid (LA). Three fatty acids were comparable substrates (AA, DGLA, and EPA), one was 5-fold slower (ALA), and two showed no reactivity with 12-LOX (EDA and LA). The bioactive lipid products resulting from 12-LOX oxidation of DGLA, 12-(S)-hydroperoxy-8Z,10E,14Z-eicosatrienoic acid [12(S)-HPETrE], and its reduced product, 12(S)-HETrE, resulted in significant attenuation of agonist-mediated platelet aggregation, granule secretion, αIIbβ3 activation, Rap1 activation, and clot retraction. Treatment with DGLA similarly inhibited PAR1-mediated platelet activation as well as platelet clot retraction. These observations are in surprising contrast to our recent work showing 12(S)-HETE is a prothrombotic bioactive lipid and support our hypothesis that the overall effect of 12-LOX oxidation of fatty acids in the platelet is dependent on the fatty acid substrates available at the platelet membrane.
Keywords: thrombin, fatty acid oxidation, eicosanoids, thrombosis
In the human platelet, hydroperoxidation of polyunsaturated fatty acids using molecular oxygen is accomplished by the human lipoxygenase (LOX) isozyme family (1). 5-LOX, 12-LOX, and 15-LOX are the three main LOX and are named according to their positional specificity on arachidonic acid (AA), producing their respective hydroperoxyeicosatetraenoic acid (HPETE) products. The HPETE products are subsequently reduced by cellular peroxidases to the bioactive lipid, hydroxyeicosatetraenoic acid (HETE). The LOX products are responsible for human inflammatory responses (2), and they are also implicated in a variety of human diseases. 5-LOX is involved in asthma (3) and cancer (4, 5). 12-LOX is involved in psoriasis (6), hypertension (7, 8), hemostasis (9–12), diabetes (13, 14), and cancer (5, 15, 16), and 15-LOX is involved in atherosclerosis (17) and cancer (5, 18).
Two 12-LOX isozymes are expressed in humans, platelet-type 12-LOX (12-LOX) (19) and epithelial 12-LOX (20). The former isozyme (12-LOX) makes the S-product and is primarily expressed in megakaryocytes and platelets, whereas the latter makes the R-product and is primarily expressed in epithelial tissue. Recently, 12-LOX has been implicated in platelet activation, which is known to play a central role in the pathophysiology of cardiovascular disease (12, 21–24). Following initial activation of human platelets by primary signals, such as thrombin and collagen, secondary signals mediated by bioactive lipids [thromboxane A2 and 12(S)-HETE] and secreted molecules (ADP) are essential for recruitment and activation of platelets.
12-LOX acts upon fatty acid substrates that are released from the phospholipid membrane in the platelet by cytosolic phospholipase A2 (cPLA2) (25). The composition of the phospholipids in the platelet membrane is dynamic in nature, and because many of the fatty acids that make up the phospholipid bilayer are not produced in the body, their content is primarily regulated by dietary intake (26–29). Although AA makes up a large proportion of the phospholipid content, other fatty acids are also available from the platelet membrane, and their content, relative to AA, has been shown to fluctuate depending on diet (27, 29, 30). Whereas the catalysis of 12-LOX with AA has been studied previously (19, 21, 31, 32), little is known about the relative 12-LOX kinetic rates of the other fatty acid substrates and their physiological effects on platelet activation. LOX are known to have promiscuous substrate selectivity and can react with a variety of fatty acids, ranging from 18 to 22 carbons long and having 2 to 6 sites of unsaturation. Based on this knowledge, the current study investigated the substrate specificity and reactivity of 12-LOX with six fatty acids. In addition, the substrates and their 12-LOX products were screened for the potential to regulate platelet reactivity and thrombosis. Interestingly, we were able to determine that some, but not all, of the fatty acids and their bioactive metabolites directly regulate the level of agonist-mediated platelet activation, albeit in a different manner than the AA product 12(S)-HETE.
MATERIALS AND METHODS
Materials
All commercial fatty acids were purchased from Nu-Check prep with purities of 99.0% or greater. HPLC grade solvents were used for both semipreparative HPLC purification and analytical HPLC analysis of 12-LOX products. Large-scale product purification was achieved by using a C18 HAIsil 250 × 10 mm semipreparative column, whereas the C18 HAIsil 250 × 4.6 mm analytical column was used for product separation in tandem with MS/MS analysis. Both columns were purchased from Higgins Analytical (Mountain View, CA). All other chemicals were reagent grade or better and were used without further purification. PAR1-AP (SFLLRN) was purchased from GL Biochem (Shanghai, China). Aggregometers, chronolume reagent, collagen, and other aggregation supplies were purchased from Chrono-Log Corp. (Havertown, PA). Anti-Rap1 primary antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Thrombin was purchased from Enyzme Research Labs (South Bend, IN). Anti-Rabbit 800 nM secondary antibody was purchased from Rockland Antibodies and Assays (Gilbertsville, PA). ADP and acetylsalicylic acid (aspirin) was purchased from Sigma-Aldrich (St. Louis, MO). PAC1 and CD62P were purchased from BD Biosciences (San Jose, CA). Convulxin was purchased from Centerchem (Stamford, CT).
Large-scale generation of 12-LOX products
LOX products were generated by reacting an individual substrate with 12-LOX in 1,000 ml of 25 mM HEPES (pH 8.0) with 25–50 μM substrate and run to completion. Reactions were quenched with 5 ml acetic acid, extracted three times with 30% volume of dichloromethane, evaporated to dryness, and reconstituted in methane for HPLC purification. The reduced products were generated by selectively reducing the hydroperoxide product to the alcohol with trimethylphosphite. Products were purified by HPLC using a C18 HAIsil 250 × 10 mm semiprep column. Solution A was 99.9% methane and 0.1% acetic acid; solution B was 99.9% H2O and 0.1% acetic acid. An isocratic gradient of 75% A and 25% B was used to purify products. Products were stored at −80°C for a maximum of 6 months. The purity and identity of the products were confirmed by using a Thermo-Electron LTQ LC-MS/MS.
Overexpression and purification of 12-LOX
Human platelet-type 12-LOX was expressed as an N-terminally, His6-tagged protein and purified to greater than 90% purity, as evaluated by SDS-PAGE analysis (33, 34). Concentration of 12-LOX was determined by using the method of Bradford with a BSA standard, in which 1 mg/ml solutions were equivalent. Iron content of 12-LOX was determined with a Finnigan inductively coupled plasma mass spectrometer (ICP-MS), using cobalt-EDTA as an internal standard. Iron concentrations were compared with standardized iron solutions and used to normalize enzyme concentrations. Protein expression yields and iron content of 12-LOX were similar to previously published results (31, 32).
12-LOX product identification and distribution
Product identification was achieved by using a Thermo-Electron LTQ LC-MS/MS. Product separation was achieved by using a C18 HAIsil 250 × 4.6 mm analytical column from Higgins Analytical. Solution A was 99.9% acetonitrile and 0.1% acetic acid; solution B was 99.9% H2O and 0.1% acetic acid. An isocratic gradient of 55% A and 45% B was used to purify products. The identification of 12-LOX products was achieved by comparing MS/MS fragments with known standards at www.lipidmaps.org. In cases where MS/MS fragmentation standards were not available, products were identified by comparing fragment masses with predicted fragment masses mediated by the hydroxy group near an unsaturated carbon (35). The mechanism is described as charge remote allylic fragmentation (supplementary Fig. I) and gives rise to a negatively charged carboxylic fragment that can identify the regio-specificity of the product. Product distribution of the products was analyzed by comparing the total ion count of single oxygenated product versus double and triple oxygenated products by parent mass and fragment mass.
Steady-state kinetic measurements
LOX rates were determined by following the formation of the conjugated diene product at 234 nm with either a Perkin-Elmer Lambda 40 or a Shimadzu (UV-2401PC) UV-Vis spectrophotometer. Molar extinction coefficients for 12-LOX products of each substrate at 234 nm were measured in the enzymatic buffer and are as follows: 13(S)-HPOTrE (ALA product), ϵ = 22,500 M−1 cm−1; 12(S)-HPETE (AA product), ϵ = 22,500 M−1 cm−1; 12(S)-HPETrE (DGLA product), ϵ = 14,300 M−1 cm−1; and 12(S)-HPEPE (EPA product), ϵ = 18,400 M−1 cm−1. Molar extinction coefficients were calculated by first weighing the substrate on an analytical balance, then dissolving the substrate with a measured mass of HPLC grade methanol to achieve a stock substrate/methanol solution. Small aliquots of this solution were then diluted into a quartz cuvette containing a Teflon stir bar and 2 ml of 25 mM HEPES buffer (pH 7.5) at room temperature (22°C). Diluted samples were mixed thoroughly for 20 min, and baseline stability was monitored at 234 nm before proceeding. The substrate buffer solution was reacted with small amounts of purified 12-LOX, and the final absorbance at 234 nm was determined. A small aliquot of soybean LOX-1 was added at the end of the reaction to ensure all substrate was converted to product. At minimum, three independently weighed substrate measurements and UV-Vis assays were performed and averaged to achieve the extinction coefficients listed above. The extinction coefficient of 12(S)-HPETE determined by this method was within error of the published values by Graff et al., after correcting for differences in solvent conditions (36). Kinetic assays were carried out in 25 mM HEPES buffer (pH 7.5) with substrate concentrations ranging from 1 μM to 20 μM, and they were initiated by the addition of enzyme (approximately 20 nM 12-LOX). The 12-LOX displays variable behavior at low substrate concentrations (<1 µM), resulting in large errors in the KM values. To circumvent this inherent problem, we determined that adding the 12-LOX first, and then quickly initiating the reaction with the addition of the appropriate amount of substrate yielded significantly more reproducible results. Substrate concentrations were quantitatively determined by allowing the enzymatic reaction to go to completion with soybean LOX-1. Kinetic data were obtained by recording initial enzymatic rates at each substrate concentration and then fitting them to the Michaelis-Menten equation using the KaleidaGraph (Synergy) program to determine kcat and kcat/KM values. Note that the steady-state kinetics with AA were slightly higher than our previously published data (32), which we ascribe to differences in the extinction coefficients used and a more active 12-LOX preparation.
Determination of LOX product stereochemistry
Determining the stereochemistry of the secondary alcohol LOX products was achieved by mosher ester analysis (37). HPLC-purified LOX product (evaporated in a glass vial) was reacted with 39 equivalents of anhydrous pyridine, 100 μl of anhydrous deuterated chloroform, and 16 equivalents of either (S)-(+)-α-Methoxy-α-trifluoromethylphenylacetyl chloride or (R)-(–)-α-Methoxy-α-trifluoromethylphenylacetyl chloride. The reaction was completed after 1 h and esterfication was checked by thin layer chromatography. Samples were diluted with deuterated chloroform to a final volume of 700 μl, transferred to a 5 mm NMR tube, and then both 1D proton and 2D COSY spectra were taken (Varian 600 MHz NMR). The nonmodified LOX product was also analyzed in a similar manner for comparison, and the proton assignments determined. The differences in proton chemical shifts between R and S mosher esterfied products were tabulated (supplementary Figs. II–XXII and Tables II–XXII). Subtracting the chemical shifts between S and R spectra yields a positive or negative value, which indicates the priority of each side of the alcohol in regards to the Chan Ingold Prelog convention and thus allows the determination of the absolute configuration of the secondary alcohol. A LOX product with known stereochemistry, 13-(S)-HODE from soybean LOX-1 catalysis, was first analyzed by mosher ester analysis before proceeding with the analysis of 12(S)-HETrE and 13(S)-HOTrE. The stereochemistry of the vinyl groups was also assigned based on the proton coupling constants, with the Z-vinyl protons having J-values of approximately 10 Hz and those of the E-vinyl protons having approximately 16 Hz. These coupling values, along with the proton signal positions, were consistent with the values found for our standard LOX product, 13(S)-HODE.
Human platelets
Human platelets were obtained from healthy volunteers from within the Thomas Jefferson University community and the Philadelphia area. These studies were approved by the Thomas Jefferson University Institutional Review Board, and informed consent was obtained from all donors before blood draw. Washed platelets were prepared as previously described (21, 38). The final platelet concentration of washed platelets in Tyrodes buffer was adjusted to a physiological concentration of 3 × 108 platelets/ml. Reported results are the data obtained using platelets from at least three different subjects.
Platelet aggregation
Washed platelets (250 μl) were pretreated with or without varying concentrations (0–40 μM) of fatty acid metabolites for 7 min, followed by stimulation with 20 μM protease-activated receptor-activating peptide (PAR1-AP), 20 μM ADP, or 100 ng/ml convulxin. The aggregation response was measured in real time using a lumi-aggregometer with stirring at 1,100 rpm at 37°C.
Human platelet dense granule secretion
ATP release was used as a measure of dense granule secretion in washed platelets. For ATP studies, 240 µl aliquots of washed platelets were pretreated with or without varying concentrations of fatty acid metabolites for 7 min. After addition of 10 µl of chronolume reagent, ATP release in response to 20 μM PAR1-AP was measured in real time using a lumi-aggregometer at 37°C with stirring at 1,100 rpm.
Measurement of Rap1 activity
Rap1 activity was measured using GST-RalGDS-Rap1-binding domain that specifically interacts with activated Rap1 as described elsewhere (39, 40). Activated Rap1 was detected by immunoblotting with the anti-Rap1 antibody. In parallel experiments using whole-platelet lysate, Rap1 expression was analyzed to confirm equal protein loading.
Flow cytometric measurements of alphaIIbbeta3 and P-selectin
αIIbβ3 activity and surface expression of P-selectin were measured by flow cytometry with the FITC-PAC1, an antibody that only recognizes the active conformation of αIIbβ3, and PE-conjugated CD62P, which recognizes P-selectin on the surface of the cell. Aliquots (40 μl) of washed platelets adjusted to a final concentration of 2.5 × 107 platelets/ml were pretreated with metabolites for 15 min followed by addition of 2 μl PAC1 and 2 μl CD62P. Platelets were stimulated with agonist for 10 min and diluted to a final volume of 500 μl using tyrodes buffer. The fluorescence intensity of platelets was immediately measured using an Accuri flow cytometer.
Clot retraction assay
Clot retraction experiments were performed as previously described (41). Briefly, platelet-rich plasma (PRP) was adjusted to a platelet count of 3 × 108 platelets/ml in glass tubing. Following treatment with fatty acids or metabolites, clot formation and retraction was initiated with 10 nM thrombin. Pictures were taken of the clots at 10 min intervals, and the size of the clot was quantified using Image-J software. Statistical significance was determined by Student t-test.
Statistical analysis
Comparison between experimental groups was made using paired t-test or two-way ANOVA with posttest analysis with Prism software. Differences in mean values ± SEM were considered significant at P < 0.05. All experiments were repeated at least three times using different subjects.
RESULTS
12-LOX product identification and distribution
Of the six fatty acids tested as substrates for 12-LOX catalysis, two fatty acids, linoleic acid (LA) and eicosapentaenoic acid (EDA), were found not to be suitable as substrates for 12-LOX. This was possibly due to improper positioning of the activated methylene, whose position near the active site iron is critical for hydrogen atom abstraction. As shown in Fig. 1, the substrates are positioned with the methyl-end of the substrate entering the cavity first, as previously determined (42). In this configuration, the activated methylene moieties for EDA and LA are not positioned properly due to length for the iron-hydroxide moiety to abstract a hydrogen atom. However, note that other factors, such as conjugation, could also play an important part in substrate activity. The four other fatty acids that were screened [AA, dihomo-γ-linolenic acid (DGLA), eicosapentaenoic acid (EPA), and α-linolenic acid (ALA)] all produced over 90% of a single oxygenated product, whose retention time, parent peak mass, and main fragmentation peaks are listed in Tables 1 and 2. Interestingly, ALA produces predominately one product, 13(S)-HPOTrE, but it is unclear how it is positioned for hydrogen atom abstraction (vide infra). The methyl-end first binding of ALA positions it in a similar manner to that of LA and EDA; however, those fatty acids are not substrates.
Fig. 1.
12-LOX product regio-specificity and substrate comparison. (A) The substrates are positioned methyl-end first, relative to the active site iron. The carbon atoms that are labeled with numbers indicate location of oxygenation. (B) Stereochemical structures of 12(S)-HETrE and 13(S)-HOTrE.
TABLE 1.
Primary product distribution
| Substrate | Product Name |
| AA | 12(S)-HPETE (99.0%) |
| DGLA | 12(S)-HPETrE (99.0%) |
| EPA | 12(S)-HPEPE (99.0%) |
| ALA | 13(S)-HPOTrE (99.0%) |
| EDA | No reaction |
| LA | No reaction |
The primary products for 12-LOX are singly oxygenated species. The percentage distribution was determined by tabulating total ion count of the singly oxygenated species in comparison with double- or triple-oxygenated species. Note noted that the S-configuration of 12(S)-HPEPE was not directly determined but assumed, since the configuration of 12(S)-HPETrE and 13(S)-HPOTrE was determined directly.
TABLE 2.
Primary product identification by mass spectrometry
| 12-LOX Reduced Product (Substrate) | Retention Time (Min) | Mass (Da) | MS-MS Fragment Mass (Da) |
| 12(S)-HETE (AA) | 44.97 | 319 | 179 |
| 12(S)-HETrE (DGLA) | 54.53 | 321 | 181 |
| 12(S)-HEPE (EPA) | 28.09 | 317 | 179 |
| 13(S)-HOTrE (ALA) | 21.73 | 293 | 195 |
The retention times are for the method described in the text, with subsequent MS/MS analysis. The mass of the primary identifying fragment is listed.
Kinetics of 12-LOX
The kinetics of 12-LOX with the four active substrates were measured (Table 3). The kcat and kcat/KM values indicate that the substrates can be categorized into two groups. The longer substrates, with 20 carbon atoms (AA, DGLA, and EPA) have over 10-fold greater kcat and kcat/KM values than the shorter substrate, with 18 carbon atoms (ALA), suggesting that length is a key factor in the rate of substrate capture and product release. These data could be due to the fact that ALA appears not to have an activated methylene positioned for hydrogen atom abstraction (vida supra, Fig. 1), and thus ALA may have a less than optimal binding conformation.
TABLE 3.
Steady-state kinetics of substrates
| Substrate | kcat (sec−1) | kcat/KM (sec−1μM−1) |
| AA | 12 (0.23) | 18 (1.9) |
| DGLA | 19 (0.91) | 9.3 (1.8) |
| EPA | 8.4 (0.30) | 19 (4.8) |
| ALA | 1.3 (0.08) | 0.36 (0.08) |
| EDA | No reaction | |
| LA | No reaction |
Kinetic measurements were measured in 25 mM HEPES buffer (pH 7.5) at room temperature. Error values are shown in parentheses.
Determination of LOX product stereochemistry
LOX typically generate one stereospecific product due to the antrafacial oxygen insertion (43). It has been previously reported that 12-LOX generates an S-configured product [12(S)-HPETE] when AA is used as a substrate (34). DGLA is similar to AA with respect to length, and one would predict that the oxygenated DGLA product [12(S)-HPETrE] would have the same stereochemistry as the oxygenated AA product [12(S)-HPETE], due to the stereospecific nature of the active site. Mosher ester analysis of the reduced 12(S)-HETrE product indicates that this is indeed the case and it does have an S-configuration, secondary alcohol. For 13(S)-HPOTrE, the 12-LOX product of ALA, the stereochemistry was determined in the same manner and also found to be an S-configuration. The stereochemistry of the vinyl groups were assigned based on the proton signal positions and their coupling constants, compared with our standard, 13(S)-HODE. The 12(S)-HETrE configuration was determined to be 12-(S)-hydroxy-8Z,10E,14Z-eicosatrienoic acid and the 13(S)-HOTrE configuration was 13-(S)-hydroxy-9Z,11E,15Z-octadecatrienoic acid (Fig. 1B).
Human platelet aggregation dependence of fatty acid metabolites
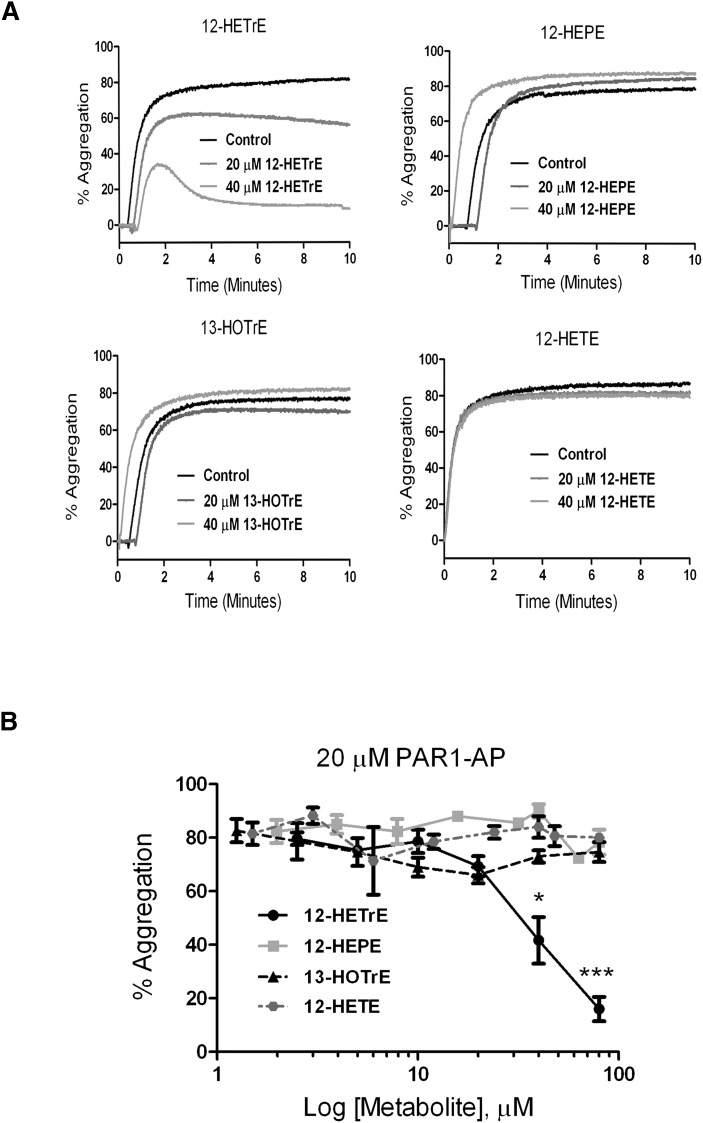
Fatty acid metabolites play an important role in regulation of platelet activation and normal hemostasis. Although AA is widely studied in platelets, 12-LOX may oxidize a number of other fatty acids on the platelet membrane following stimulation (22, 44). Because thrombin is the most potent activator of platelet function, we investigated the ability of these metabolites to modulate platelet activation following stimulation of the thrombin receptor protease-activated receptor-1 (PAR1). The hydroperoxide metabolites of 12-LOX catalysis are reduced to hydroxymetabolites in the presence of peroxidases; therefore, the hydroxymetabolites were evaluated for their potential regulation of human platelet function. Platelets were treated with increasing concentrations of each fatty acid metabolite followed by stimulation with 20 μM PAR1-activating peptide (PAR1-AP), and subsequent platelet aggregation was measured. PAR1-AP alone resulted in more than 80% aggregation in less than 1 min, which was stable for the duration of the experiment (Fig. 2). Platelet aggregation was sensitive to treatment with 12(S)-HETrE, showing inhibition in the presence of 40 μM 12(S)-HETrE (Fig. 2A), a clinically relevant concentration following fatty acid supplementation (45, 46). Not all metabolites were shown to attenuate platelet aggregation, as 12(S)-HEPE and 13(S)-HOTrE had no observable effect on PAR1-AP-mediated aggregation. Even at higher concentrations, only 12(S)-HETrE was shown to attenuate PAR1-AP-induced platelet aggregation (Fig. 2B). In comparison, exposure to increasing concentrations of 12(S)-HETE, the predominant product of AA and 12-LOX in human platelets, did not result in attenuation of PAR1-AP-mediated platelet aggregation at any concentration tested. This is consistent with the observation that 12(S)-HETE is prothrombotic in human platelets (23).
Fig. 2.
Fatty acid metabolites regulate platelet aggregation. Washed human platelets were treated with or without increasing concentrations of the exogenously added fatty acid metabolites 12(S)-HETrE, 12(S)-HEPE, 13(S)-HOTrE, or 12(S)-HETE. (A) Representative curves for platelets treated with 20 μM PAR1-AP in the absence (control) or presence of each metabolite. The level of platelet aggregation was measured for 10 min poststimulation (N = 3 for each condition). (B) Composite for all replicates in the presence of increasing concentrations of each metabolite, ranging from 0 to 80 μM (N = 3). Replicates were graphed for the maximal level of platelet aggregation 10 min poststimulation for each condition. *P < 0.05; ***P = 0.002.
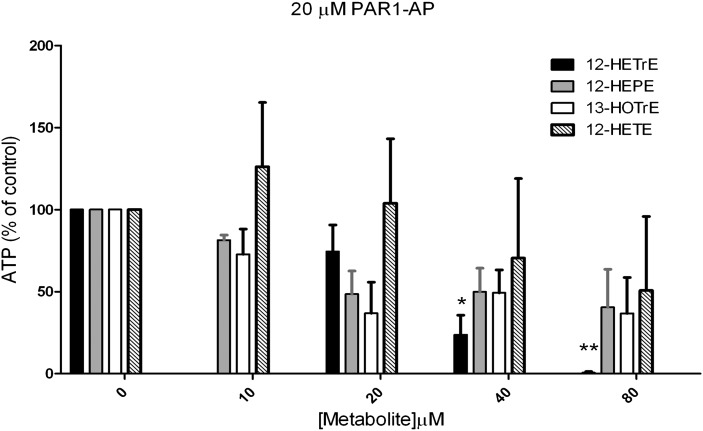
Human platelet dense granule secretion is sensitive to fatty acid metabolites
Thrombin activation of human platelets involves a number of biochemical steps. One of the biochemical steps important for the stability of clot formation is secretion of the dense granule, which contains small molecules that are essential for autocrine and paracrine reinforcement of the platelet clot (including ATP, ADP, and 5-HT). PAR1-AP-mediated ATP secretion was measured as a surrogate for dense granule secretion (Fig. 3). Only the presence of increasing concentrations of 12(S)-HETrE resulted in complete inhibition of dense granule secretion clearly showing 12(S)-HETrE signals in a specific, dose-dependent manner, unlike the other metabolites tested.
Fig. 3.
Fatty acid regulation of platelet dense granule secretion. Washed human platelets were treated with or without increasing concentrations of the exogenously added fatty acid metabolites 12(S)-HETrE, 12(S)-HEPE, 13(S)-HOTrE, or 12(S)-HETE. The maximal level of ATP secretion was measured poststimulation (N = 3 independent donors for each condition). *P < 0.05; **P < 0.01.
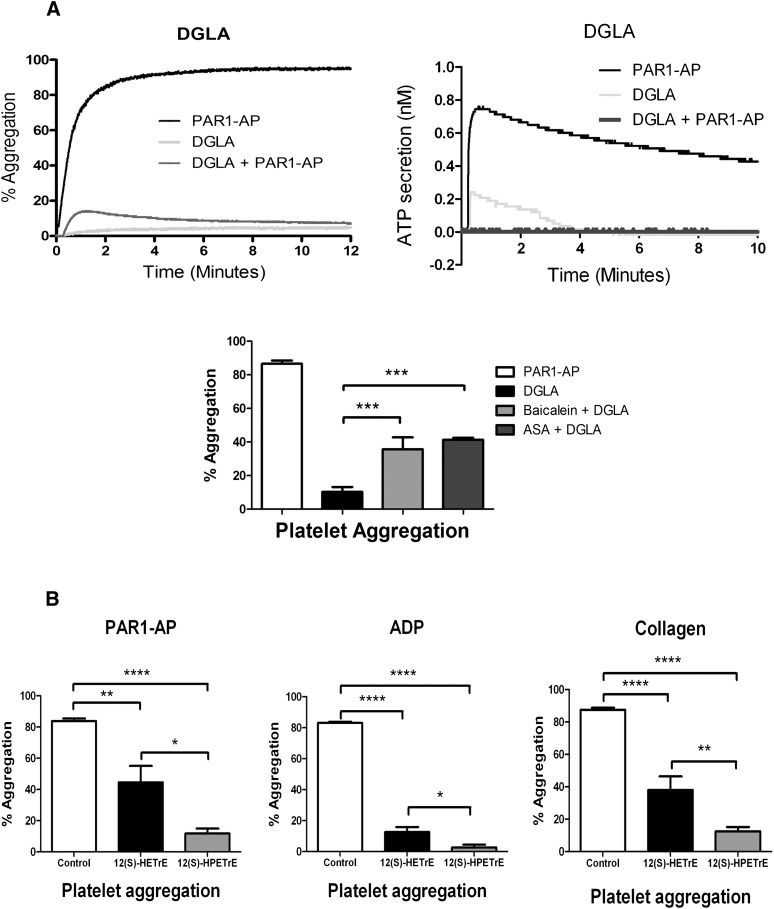
DGLA inhibits PAR-mediated platelet activation
It is possible that although the bioactive metabolite 12(S)-HETrE inhibits platelet aggregation, the DGLA substrate responsible for its formation is not oxidized by 12-LOX in the platelet and therefore has no effect on platelet function. To confirm that the observed inhibition of platelet activation in the presence of 12(S)-HETrE is due to 12-LOX oxidation of DGLA, 10 μM DGLA was added to washed platelets, followed by addition of 20 μM PAR1-AP (Fig. 4A). When the platelets were incubated with DGLA for 10 min followed by addition of PAR1-AP, maximal platelet aggregation was attenuated and quickly reversed within 1 min following addition of PAR1-AP. This observation is similar to an earlier study, which showed that addition of DGLA to the human platelet resulted in a partial inhibition of collagen-mediated platelet aggregation (47). Likewise, DGLA completely inhibited PAR1-AP-mediated dense granule secretion, similar to what was observed when treating platelets with 12(S)-HETrE, the reduced 12-LOX oxidation product of DGLA (Figs. 2 and 3). Finally, to confirm that DGLA regulates platelet aggregation in part through 12-LOX, washed platelets were stimulated with PAR1-AP following treatment with DGLA or DGLA in combination with baicalein (12-LOX inhibitor) or aspirin. The absence of either 12-LOX or COX-1 activity resulted in a partial rescue of platelet aggregation (Fig. 4A).
Fig. 4.
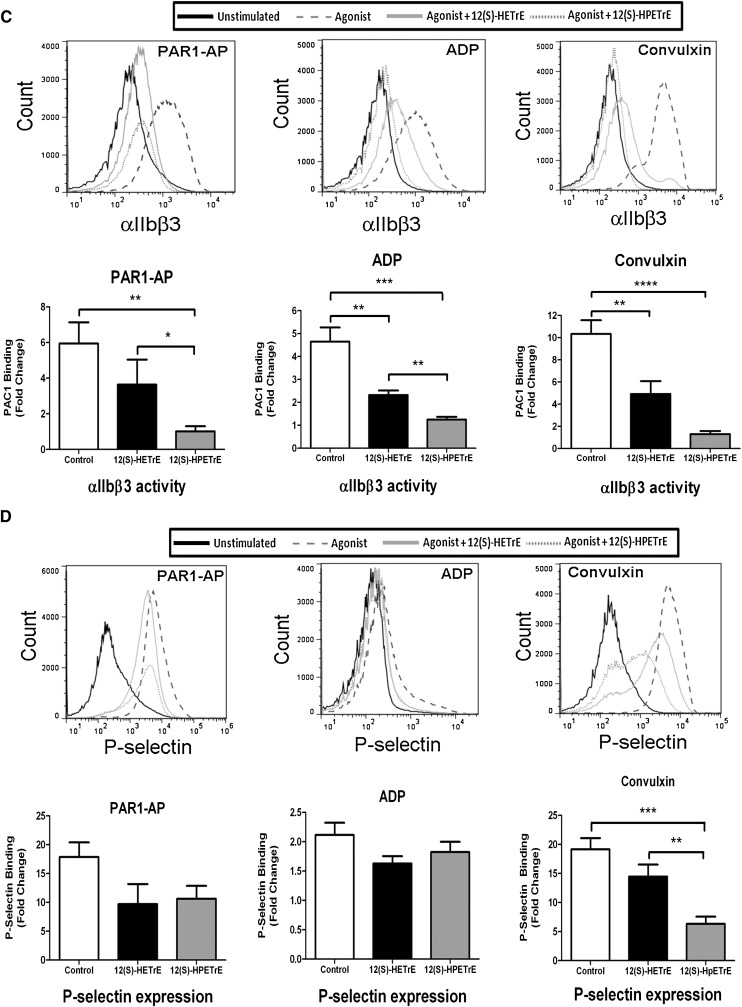
Agonist-independent regulation of platelet activity by 12-LOX metabolites. (A) Washed human platelets were treated with or without 5 μM DGLA for 10 min followed by stimulation with 20 μM PAR1-AP (PAR1-AP) for 10 min. PAR1-AP induced an immediate and stable platelet aggregation when added to platelets. Maximal and final platelet aggregation following treatment with DGLA was significantly attenuated. Additionally, treatment of DGLA resulted in inhibition of dense granule secretion in the presence of PAR1-AP (N = 3). Treatment with either a COX-1 inhibitor [100 µM aspirin (ASA)] or a 12-LOX inhibitor (100 µM baicalein) partially rescued DGLA-induced inhibition of PAR1-AP-mediated platelet aggregation (N = 3). ***P < 0.001. (B) Platelet aggregation was measured following 10 min stimulation with PAR1-AP, 20 μM ADP, or 5 μg/ml collagen in the presence of 40 μM 12(S)-HPETrE or 40 μM 12(S)-HETrE (N = 4–9). (C) αIIbβ3 activation was measured by flow cytometry following 10 min stimulation with PAR1-AP, ADP, or 100 ng/ml convulxin in the presence of 12(S)-HPETrE or 12(S)-HETrE (N = 4–10). (D) α-Granule secretion was assessed by measuring P-selectin surface expression on the platelet by flow cytometry following 10 min stimulation with PAR1-AP, ADP, or 100 ng/ml convulxin in the presence of 12(S)-HPETrE or 12(S)-HETrE (N = 4–10). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
12-LOX metabolite inhibition of ADP- and GPVI-induced platelet activation
To identify whether 12-LOX eicosanoids produced from DGLA regulate platelet activation through multiple agonist pathways, platelets were treated with either 12(S)-HETrE or its peroxidated precursor 12(S)-HPETrE, followed by stimulation with PAR1-AP, collagen, or ADP (Fig. 4B–D). Similar to the observed sensitivity to 12(S)-HETrE in Fig. 2, platelet aggregation induced by ADP and collagen were attenuated following treatment with 12(S)-HETrE. Additionally, treatment with 12(S)-HPETrE significantly attenuated platelet aggregation following stimulation with ADP, collagen, or PAR1-AP (Fig. 4B). To determine whether these eicosanoids affected activation of the integrin αIIbβ3, an essential integrin in the activation of platelets, platelets treated with either eicosanoid were stimulated with ADP, PAR1-AP, or convulxin (direct activator of the collagen receptor GPVI). αIIbβ3 activation was attenuated in the presence of either 12(S)-HETrE or 12(S)-HPETrE (Fig. 4C). Finally, to identify whether α granule secretion is affected by the presence of 12(S)-HETrE or 12(S)-HPETrE, P-selectin (a marker of α granule secretion) surface expression was measured following treatment with either eicosanoid (Fig. 4D). Treatment with either eicosanoid attenuated α granule secretion following stimulation with ADP, PAR1-AP, or convulxin. Although both metabolites attenuate platelet function, 12(S)-HPETrE was found to have a lower IC50 compared with 12(S)-HETrE for inhibition of platelet aggregation (5 μM versus 40 μM, respectively). Considering the only difference between 12(S)-HPETrE and 12(S)-HETrE is their oxidation state, it is possible either that the structural difference between the hydroperoxide and the alcohol affect receptor binding or that the increased reactivity of the hydroperoxide could generate a different species (i.e., oxidation to the ketone). These two explanations are currently being investigated.
Bioactive metabolite regulation of Rap1 activity
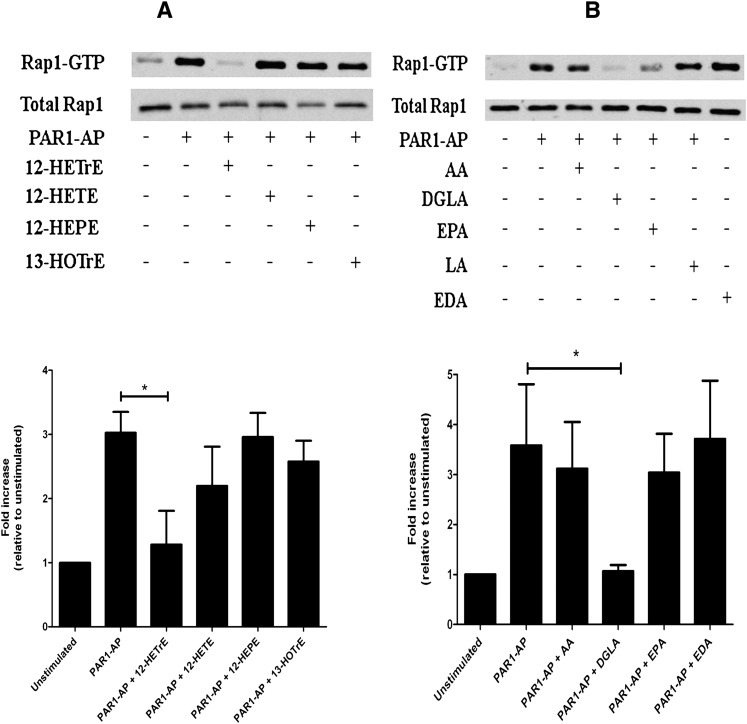
Platelet activation is regulated in large part by the small GTPase Rap1 (39). To identify whether 12-LOX metabolites from the various fatty acids regulate the level of Rap1 activation, washed platelets were treated with or without 40 μM of each metabolite [12(S)-HETE, 12(S)-HETrE, 12(S)-HEPE, or 13(S)-HOTrE] or 10 μM fatty acid (AA, DGLA, EPA, LA, or EDA) followed by stimulation with PAR1-AP for 5 min (Fig. 5). The level of Rap1 activation was measured as a fold change relative to the unstimulated condition. Whereas PAR1-AP induced a large increase in the level of active Rap1, treatment with 12(S)-HETrE prior to PAR1-AP stimulation fully inhibited Rap1 activation (Fig. 5A). No significant difference in the level of Rap1 activity was observed with the other metabolites tested (N = 3). Similarly, when platelets were treated with fatty acids prior to stimulation with PAR1-AP, DGLA completely inhibited Rap1 activation. Although treatment with EPA reduced Rap1 activation levels, the reduction was not statistical significant compared with PAR1-AP alone. Treatment with the other fatty acids did not appear to significantly alter the level of PAR1-AP-induced Rap1 activation (Fig. 5B).
Fig. 5.
12-LOX metabolite regulation of Rap1. Washed platelets were treated with 12-LOX metabolites or various fatty acids for 10 min prior to stimulation with 20 μM PAR1-AP to determine their regulatory effects on the level of PAR1-AP-mediated Rap1 activation. (A) Platelets were treated with or without 40 μM 12(S)-HETrE, 12(S)-HETE, 12(S)-HEPE, or 13(S)-HOTrE for 10 min. Following 12-LOX metabolite treatment, Rap1 activation was measured after stimulation with PAR1-AP for 5 min (N = 3 independent experiments). PAR1-AP alone induced a significant increase in Rap1-GTP levels. Treatment with 12(S)-HETrE significantly attenuated PAR1-AP-induced Rap1 activation. Treatment with 12(S)-HETE, 12(S)-HEPE, or 13(S)-HOTrE had no significant effect on PAR1-AP-induced Rap1 activation. (B) Platelets were treated with or without 10 μM AA, DGLA, EPA, LA, or EDA for 10 min. Following fatty acid treatment, Rap1 activation was measured after stimulation with PAR1-AP for 5 min (N = 6). Treatment with DGLA significantly attenuated PAR1-AP-induced Rap1 activation. Treatment with AA, EPA, LA, or EDA had no significant effect on the level of Rap1 activity. *P < 0.05.
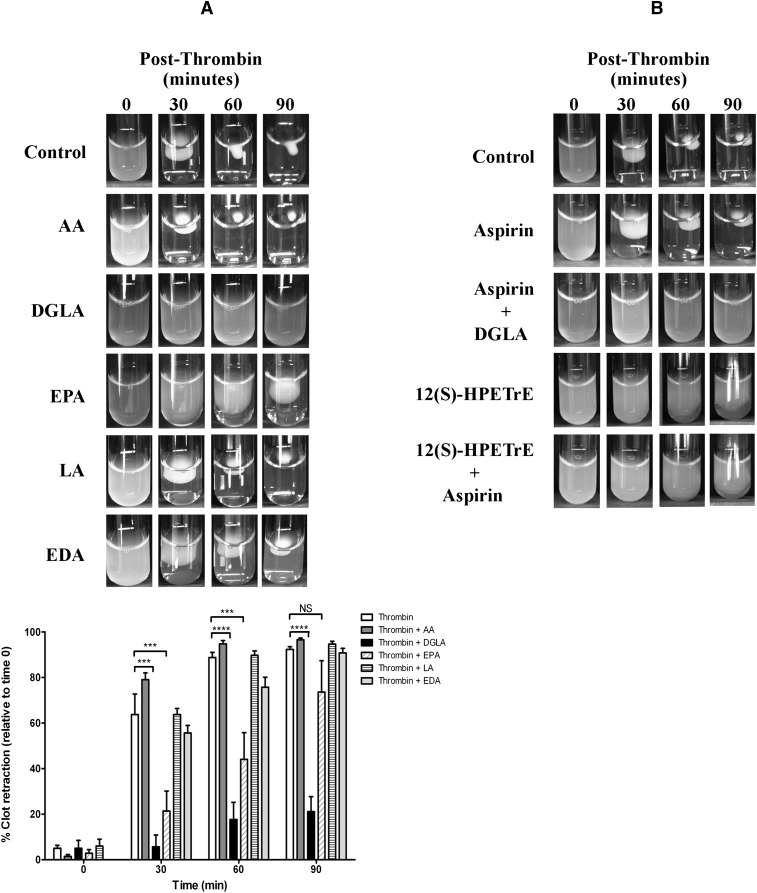
DGLA and EPA prevent clot retraction
As Rap1 is an upstream regulator of integrin activation in platelets, we sought to determine whether the fatty acids regulate integrin-dependent platelet-mediated clot retraction. To determine whether any of the fatty acids play a role in clot retraction, which involves the consolidation of integrin-dependent retraction of platelets within the clot (48), PRP was treated with each fatty acid (Fig. 6). Thrombin (10 nM) was added to the PRP for each condition in the presence or absence of 25 μM fatty acid (Fig. 6A), and a picture of the clot was taken at several time points following stimulation. The size of the clot was quantified using Image J. The PRP clotted with thrombin in the absence of fatty acid within 60 min. Following treatment with DGLA or EPA, clot retraction was significantly delayed and attenuated. However, pretreatment with AA, LA, or EDA had no effect. To identify whether DGLA inhibition of clot retraction was due to COX-1 or 12-LOX activity, platelets were treated with aspirin, 12(S)-HPETrE, aspirin + 12(S)-HPETrE, or aspirin + DGLA, followed by stimulation with thrombin (Fig. 6B). Inhibition of COX-1 with aspirin did not significantly inhibit thrombin-induced clot retraction. Additionally, aspirin did not rescue clot retraction in the presence of DGLA. However, treatment with a high concentration of 12(S)-HPETrE (200 μM) in the presence or absence of aspirin significantly inhibited thrombin-induced clot retraction, suggesting that 12-LOX oxidation of DGLA plays at least a partial role in DGLA inhibition of platelet clot retraction.
Fig. 6.
Fatty acid regulation of platelet-mediated clot retraction. PRP was treated with fatty acids, inhibitors, or eicosanoids, followed by stimulation with thrombin. The rate at which thrombin caused a platelet-dependent clot retraction of the PRP was determined. Pictures were taken at several time points following stimulation (0, 30, 60, and 90 min). The size of the clot was quantified using Image J. PRP formed a complete clot retraction within 60 min without fatty acid treatment. (A) PRP treated with 25 μM fatty acid (AA, DGLA, EPA, LA, or EDA), followed by stimulation with 10 nM thrombin. The rate of clot retraction was not affected by treatment with AA, LA, or EDA, but it was significantly attenuated by DGLA and EPA (N = 4–6). (B) PRP treated with 100 μM aspirin for 40 min, 25 μM DGLA for 10 min, or 200 μM 12(S)-HPETrE for 10 min was stimulated with thrombin (N = 3–5). NS, not significant; ***P < 0.001; ****P < 0.0001.
DISCUSSION
Although fatty acid regulation of hemostatic function, including platelets, has been studied by various research groups (28), a consensus is lacking on the importance of fatty acid intake to the role of platelets in regulating hemostasis. This is in part due to the small number of studies, small number of subjects in each study, and the differences in study design among research groups. However, human subject studies support an important role in the type of fatty acid supplemented in the diet to overall regulation of platelet function and hemostasis (27, 49). GLA supplementation has been shown to increase DGLA content in the serum by 3-fold, as GLA is quickly converted to DGLA enzymatically (27). Although a significant increase in the amount of DGLA was shown to be incorporated into the neutrophil membrane, no observable change in AA was measured, suggesting DGLA incorporation does not displace AA on the membrane (27).
DGLA was initially identified as a fatty acid that produced potentially inhibitory metabolites, such as PGE1 through COX-1 and 12(S)-HETrE through 12-LOX (50, 51). Although PGE1 is known to be a potent anti-platelet eicosanoid, its role in platelet function deriving from DGLA as a substrate is controversial with research groups having determined that although PGE1 and thromboxane B1 are produced by DGLA through COX-1, their levels are much smaller than that of the inactive metabolite 12-hydroxyheptadecadienoic acid and may not play a significant role in DGLA-induced inhibition of platelet activity (47, 52). 12(S)-HETrE formation, through 12-LOX oxidation of DGLA, was shown here to play an important role in DGLA-mediated inhibition of platelet function by significantly attenuating a number of biochemical endpoints. Hence, inhibitory activity of DGLA is most likely mediated through a number of eicosanoids produced from both the 12-LOX and COX-1 oxidation of DGLA in the platelet. Formation of these metabolites results in attenuation of agonist-induced platelet activation.
Polyunsaturated fatty acid content in the platelet membranes varies, depending on diet. The most abundant phospholipid in the platelet is phosphatidylcholine, containing a number of polyunsaturated fatty acids (PUFA), including AA (13.5%), LA (7.9%), DGLA (2.1%), EDA (0.6%), docosahexaenoic acid (DHA) (0.6%), EPA (0.2%), ALA (0.1%), and γ-linolenic acid (GLA) (0.07%) (26). Our laboratories investigated the kinetic and biological effects of these fatty acids as potential 12-LOX substrates. Other PUFAs found in the platelet, such as DHA and GLA, displayed multiple enzymatic products and due to this complexity will be the subject of subsequent investigations. From this work, it was determined that 12-LOX has selective substrate preferences, with two fatty acids not being substrates (LA and EDA) and four producing only one oxygenated fatty acid (AA, DGLA, ALA, and EPA). The fatty acids with 20 carbon atoms and more than two sites of unsaturation had the fastest kcat/KM values. These relative kinetic values can be directly related to fatty acid metabolism in the platelet, where substrate concentration is low. ALA is 10-fold slower than the other four fatty acid substrates, which could be indicative of a distinct binding mode. In an analysis of the placement of these substrates in the active site with the substrate binding methyl-end first as previously determined (42), there was no activated methylene close to the iron-hydroxide moiety for LA, ALA, or EDA (Fig. 1). This is in contrast to what is seen for AA, DGLA, and EPA, where an activated methylene is located in close proximity to the iron. Therefore, to position the C10 of ALA close to the iron to generate the 13-product, the substrate would have to be inserted only partially into the active site. In contrast, 12-LOX does not react with LA, so it appears that the additional ω-3 unsaturation for ALA affects its positioning in the active site, possibly by allowing the carboxylate or pi-bond active site interaction to supersede the methyl-end active site interaction (53), leading to partial insertion of ALA into the active site. Another possible explanation could be that ALA binds in the opposite direction with its carboxylate moiety entering the active site first, producing the R-isomer of the product, which has been previously seen with soybean LOX-1 (54). This hypothesis, however, is unlikely because only the S-isomer of 13(S)-HPOTrE is observed, which supports the partial substrate, active site insertion hypothesis.
In addition to being catalyzed at different rates, the effects of these 12-LOX products on the platelets were markedly different. Following treatment with PAR1-AP, 12(S)-HETrE significantly attenuated platelet aggregation, the release of ATP, and Rap1 activation at 40 μM concentration. This result is in contrast with comparable concentrations of 12(S)-HETE, in which agonist-mediated aggregation and ATP release were potentiated, suggesting a substrate-specific regulation of 12-LOX in platelet reactivity. Under ex vivo conditions, platelets spiked with high levels of DGLA or its 12-LOX metabolites inhibited platelet activation following stimulation with a number of agonists. Similarly, an increase in DGLA concentration resulted in an increase in 12(S)-HETrE production in the platelet, suggesting that 12-LOX can produce 12(S)-HETrE in the platelet (data not shown). Although it may be possible to reach circulating fatty acid concentrations of 40 μM following fatty acid supplementation (45), it is unlikely that all of the DGLA would be incorporated into the platelet membrane and converted by 12-LOX to 12(S)-HETrE, as several 12-LOX and COX-1 products have been previously identified through catalysis of DGLA (51). Further, the readout of these assays may be overestimated due to the fact that endogenous AA is present in the platelet, leading to production of 12(S)-HETE. The endogenous 12(S)-HETE could then compete with the exogenous 12(S)-HETrE, thus counterbalancing both of their effects and dampening the inhibitory effect of 12(S)-HETrE on the platelet. Nonetheless, 12(S)-HPETrE is over 8-fold more potent than 12(S)-HETrE, making its concentration biologically relevant. However, due to the high level of peroxidases present in the platelet, the biological effect of 12(S)-HPETrE on the platelet clot may be limited to localized regions of metabolite production. Taken together, these observations further support a potential role for unique pools of AA and DGLA in the platelet that are independently regulated to mediate either a prothrombotic or antithrombotic environment depending on a number of factors, including 12-LOX localization, fatty acid compartmentalization, and specific agonist used (21).
The mechanism by which these fatty acids regulate platelet function through catalysis by 12-LOX is not well understood. Although the receptor for 12(S)-HETE has been recently identified (55), the underlying mechanism by which other metabolites, such as 12(S)-HETrE, regulate the platelet are unknown. Here we have shown that 12(S)-HETrE, as well as its substrate DGLA, inhibit Rap1 activation. Rap1 is an essential GTPase involved in regulation of platelet function, and inhibiting its activation in the presence of a strong activating agonist, such as PAR1-AP, suggests an important regulatory role for 12-LOX metabolites, such as 12(S)-HETrE, in mediating this process. Our previous work showing that direct inhibition of 12-LOX activity attenuates calcium mobilization (12), a key step in activation of Rap1, further supports a role for 12-LOX products in regulation of platelet function through Rap1. Additionally, as Rap1 is known to be important for integrin activation, it is not surprising that addition of DGLA results in attenuation of thrombin-induced clot retraction (41). Finally, 12-LOX metabolite formation from catalysis of DLGA attenuates platelet aggregation, integrin αIIbβ3 activation, and α granule secretion. Inhibition of these biochemical endpoints in the presence of 12(S)-HPETrE and 12(S)-HETrE supports a potentially protective role for 12-LOX metabolites in preventing unwanted platelet activation resulting in occlusive thrombotic events. As both the peroxidated and nonperoxidated products of DGLA attenuate platelet function and DGLA inhibition of platelet activity is stronger than either of the 12-LOX products, it is likely that multiple 12-LOX-dependent and independent products of DGLA are involved in protection against platelet activation and thrombosis (51, 56, 57).
It has long been postulated that fatty acid content in the diet can affect human health (58). Dietary fatty acid formulation has been shown to inhibit leukotriene biosynthesis (59), and DGLA specifically can be converted to the anti-inflammatory prostaglandin PGE1 by COX (60), which is implicated in the inhibition of platelet activation (57) and in diminishing atherosclerosis (61, 62) and ocular discomfort symptoms (63). In this study, we have shown a direct antiplatelet phenotype of the 12-LOX product of DGLA, which supports the role of this and potentially other fatty acids as protective agents against unwanted platelet activation and thrombosis. Considering the recent deorphanization of GPR31 as the 12(S)-HETE receptor (55), the data presented here support the possibility that a unique receptor may exist on the surface of the platelet that, like PGE1, may act as a protective signal inhibiting excessive platelet activation and thrombosis. Given the similar 12-LOX kinetics against AA and DGLA and the fact that the concentration of DGLA can become elevated in the blood with specific diets (27, 64), it is possible that the level of coagulation and thrombosis in circulating blood could be regulated in part by modifications in the diet (65, 66). Currently, we are testing this hypothesis in animal models in the hope of confirming both the beneficial and detrimental roles of 12-LOX in blood coagulation as well as the level of metabolite produced in vivo.
Supplementary Material
Acknowledgments
The authors thank Qingli Zhang for her assistance with mass spectrometry measurements of 12-LOX metabolites.
Footnotes
Abbreviations:
- 12-LOX
- platelet-type 12-lipoxygenase
- AA
- arachidonic acid
- ALA
- α-linolenic acid
- DGLA
- dihomo-γ-linolenic acid
- EDA
- eicosadienoic acid
- EPA
- eicosapentaenoic acid
- HETE
- hydroxyeicosatetraenoic acid
- HPETE
- hydroperoxyeicosatetraenoic acid
- LA
- linoleic acid
- PAR1
- protease-activated receptor-1
- PAR1-AP
- PAR1-activating peptide
- PRP
- platelet-rich plasma
This work was supported by National Institutes of Health Grants S10-RR-20939, S10-RR-19918, HL-089457 (M.H.), and GM-56062 (T.R.H.); National Science Foundation Grant CHE-0342912; Sigma-Xi (J.Y.); and Parenteral Drug Association Foundation (M.H.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of 22 figures and 22 tables.
REFERENCES
- 1.Solomon E. I., Zhou J., Neese F., Pavel E. G. 1997. New insights from spectroscopy into the structure/function relationships of lipoxygenases. Chem. Biol. 4: 795–808 [DOI] [PubMed] [Google Scholar]
- 2.Serhan C. N. 1994. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim. Biophys. Acta. 1212: 1–25 [DOI] [PubMed] [Google Scholar]
- 3.Nakano H., Inoue T., Kawasaki N., Miyataka H., Matsumoto H., Taguchi T., Inagaki N., Nagai H., Satoh T. 2000. Synthesis and biological activities of novel antiallergic agents with 5-lipoxygenase inhibiting action. Bioorg. Med. Chem. 8: 373–380 [DOI] [PubMed] [Google Scholar]
- 4.Ghosh J., Myers C. E. 1998. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc. Natl. Acad. Sci. USA. 95: 13182–13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steele V. E., Holmes C. A., Hawk E. T., Kopelovich L., Lubet R. A., Crowell J. A., Sigman C. C., Kelloff G. J. 1999. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol. Biomarkers Prev. 8: 467–483 [PubMed] [Google Scholar]
- 6.Hussain H., Shornick L. P., Shannon V. R., Wilson J. D., Funk C. D., Pentland A. P., Holtzman M. J. 1994. Epidermis contains platelet-type 12-lipoxygenase that is overexpressed in germinal layer keratinocytes in psoriasis. Am. J. Physiol. 266: C243–C253 [DOI] [PubMed] [Google Scholar]
- 7.Quintana L. F., Guzman B., Collado S., Claria J., Poch E. 2006. A coding polymorphism in the 12-lipoxygenase gene is associated to essential hypertension and urinary 12(S)-HETE. Kidney Int. 69: 526–530 [DOI] [PubMed] [Google Scholar]
- 8.Zink M. H., Oltman C. L., Lu T., Katakam P. V., Kaduce T. L., Lee H., Dellsperger K. C., Spector A. A., Myers P. R., Weintraub N. L. 2001. 12-lipoxygenase in porcine coronary microcirculation: implications for coronary vasoregulation. Am. J. Physiol. Heart Circ. Physiol. 280: H693–H704 [DOI] [PubMed] [Google Scholar]
- 9.Fonlupt P., Croset M., Lagarde M. 1991. 12-HETE inhibits the binding of PGH2/TXA2 receptor ligands in human platelets. Thromb. Res. 63: 239–248 [DOI] [PubMed] [Google Scholar]
- 10.Nyby M. D., Sasaki M., Ideguchi Y., Wynne H. E., Hori M. T., Berger M. E., Golub M. S., Brickman A. S., Tuck M. L. 1996. Platelet lipoxygenase inhibitors attenuate thrombin- and thromboxane mimetic-induced intracellular calcium mobilization and platelet aggregation. J. Pharmacol. Exp. Ther. 278: 503–509 [PubMed] [Google Scholar]
- 11.Olas B., Wachowicz B., Stochmal A., Oleszek W. 2005. Inhibition of blood platelet adhesion and secretion by different phenolics from Yucca schidigera Roezl. bark. Nutrition. 21: 199–206 [DOI] [PubMed] [Google Scholar]
- 12.Yeung J., Apopa P. L., Vesci J., Kenyon V., Rai G., Jadhav A., Simeonov A., Holman T. R., Maloney D. J., Boutaud O., et al. 2012. Protein kinase C regulation of 12-lipoxygenase-mediated human platelet activation. Mol. Pharmacol. 81: 420–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma K., Nunemaker C. S., Wu R., Chakrabarti S. K., Taylor-Fishwick D. A., Nadler J. L. 2010. 12-Lipoxygenase products reduce insulin secretion and {beta}-cell viability in human islets. J. Clin. Endocrinol. Metab. 95: 887–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tohjima T., Honda N., Mochizuki K., Kinoshita J., Watanabe K., Arisaka T., Kawamori R., Nakamura M., Kurahashi Y., Yoshimoto T., et al. 1998. Decreased activity of arachidonate 12-lipoxygenase in platelets of Japanese patients with non-insulin-dependent diabetes mellitus. Metabolism. 47: 257–263 [DOI] [PubMed] [Google Scholar]
- 15.Connolly J. M., Rose D. P. 1998. Enhanced angiogenesis and growth of 12-lipoxygenase gene-transfected MCF-7 human breast cancer cells in athymic nude mice. Cancer Lett. 132: 107–112 [DOI] [PubMed] [Google Scholar]
- 16.Natarajan R., Nadler J. 1998. Role of lipoxygenases in breast cancer. Front. Biosci. 3: E81–E88 [DOI] [PubMed] [Google Scholar]
- 17.Harats D., Shaish A., George J., Mulkins M., Kurihara H., Levkovitz H., Sigal E. 2000. Overexpression of 15-lipoxygenase in vascular endothelium accelerates early atherosclerosis in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 20: 2100–2105 [DOI] [PubMed] [Google Scholar]
- 18.Kamitani H., Geller M., Eling T. 1998. Expression of 15-lipoxygenase by human colorectal carcinoma Caco-2 cells during apoptosis and cell differentiation. J. Biol. Chem. 273: 21569–21577 [DOI] [PubMed] [Google Scholar]
- 19.Chen X-S., Brash A. R., Funk C. D. 1993. Purification and characterization of recombinant histidine-tagged human platelet 12-lipoxygenase expressed in a baculovirus/insect cell system. Eur. J. Biochem. 214: 845–852 [DOI] [PubMed] [Google Scholar]
- 20.Boeglin W. E., Kim R. B., Brash A. R. 1998. A 12R-lipoxygenase in human skin: mechanistic evidence, molecular cloning, and expression. Proc. Natl. Acad. Sci. USA. 95: 6744–6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holinstat M., Boutaud O., Apopa P. L., Vesci J., Bala M., Oates J. A., Hamm H. E. 2011. Protease-activated receptor signaling in platelets activates cytosolic phospholipase A2alpha differently for cyclooxygenase-1 and 12-lipoxygenase catalysis. Arterioscler. Thromb. Vasc. Biol. 31: 435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan L. T., Thomas C. P., Kuhn H., O'Donnell V. B. 2010. Thrombin-activated human platelets acutely generate oxidized docosahexaenoic-acid-containing phospholipids via 12-lipoxygenase. Biochem. J. 431: 141–148 [DOI] [PubMed] [Google Scholar]
- 23.Thomas C. P., Morgan L. T., Maskrey B. H., Murphy R. C., Kuhn H., Hazen S. L., Goodall A. H., Hamali H. A., Collins P. W., O'Donnell V. B. 2010. Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. J. Biol. Chem. 285: 6891–6903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung J., Holinstat M. 2011. 12-lipoxygenase: a potential target for novel anti-platelet therapeutics. Cardiovasc. Hematol. Agents Med. Chem. 9: 154–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burke J. E., Dennis E. A. 2009. Phospholipase A2 biochemistry. Cardiovasc. Drugs Ther. 23: 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barre D. E., Holub B. J. 1992. The effect of borage oil consumption on the composition of individual phospholipids in human platelets. Lipids. 27: 315–320 [DOI] [PubMed] [Google Scholar]
- 27.Johnson M. M., Swan D. D., Surette M. E., Stegner J., Chilton T., Fonteh A. N., Chilton F. H. 1997. Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J. Nutr. 127: 1435–1444 [DOI] [PubMed] [Google Scholar]
- 28.Kris-Etherton P., Daniels S. R., Eckel R. H., Engler M., Howard B. V., Krauss R. M., Lichtenstein A. H., Sacks F., St Jeor S., Stampfer M., et al. 2001. Summary of the scientific conference on dietary fatty acids and cardiovascular health: conference summary from the nutrition committee of the American Heart Association. Circulation. 103: 1034–1039 [DOI] [PubMed] [Google Scholar]
- 29.Simopoulos A. P. 2006. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed. Pharmacother. 60: 502–507 [DOI] [PubMed] [Google Scholar]
- 30.Kapoor R., Huang Y. S. 2006. Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol. 7: 531–534 [DOI] [PubMed] [Google Scholar]
- 31.Segraves E. N., Holman T. R. 2003. Kinetic investigations of the rate-limiting step in human 12- and 15-lipoxygenase. Biochemistry. 42: 5236–5243 [DOI] [PubMed] [Google Scholar]
- 32.Wecksler A. T., Jacquot C., van der Donk W. A., Holman T. R. 2009. Mechanistic investigations of human reticulocyte 15- and platelet 12-lipoxygenases with arachidonic acid. Biochemistry. 48: 6259–6267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amagata T., Whitman S., Johnson T. A., Stessman C. C., Loo C. P., Lobkovsky E., Clardy J., Crews P., Holman T. R. 2003. Exploring sponge-derived terpenoids for their potency and selectivity against 12-human, 15-human, and 15-soybean lipoxygenases. J. Nat. Prod. 66: 230–235 [DOI] [PubMed] [Google Scholar]
- 34.Chen X. S., Funk C. D. 1993. Structure-function properties of human platelet 12-lipoxygenase: chimeric enzyme and in vitro mutagenesis studies. FASEB J. 7: 694–701 [DOI] [PubMed] [Google Scholar]
- 35.Murphy R. C., Barkley R. M., Zemski Berry K., Hankin J., Harrison K., Johnson C., Krank J., McAnoy A., Uhlson C., Zarini S. 2005. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal. Biochem. 346: 1–42 [DOI] [PubMed] [Google Scholar]
- 36.Graff G., Anderson L. A., Jaques L. W. 1990. Preparation and purification of soybean lipoxygenase-derived unsaturated hydroperoxy and hydroxy fatty acids and determination of molar absorptivities of hydroxy fatty acids. Anal. Biochem. 188: 38–47 [DOI] [PubMed] [Google Scholar]
- 37.Hoye T. R., Jeffrey C. S., Shao F. 2007. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat. Protoc. 2: 2451–2458 [DOI] [PubMed] [Google Scholar]
- 38.Holinstat M., Voss B., Bilodeau M. L., McLaughlin J. N., Cleator J., Hamm H. E. 2006. PAR4, but not PAR1, signals human platelet aggregation via Ca2+ mobilization and synergistic P2Y12 receptor activation. J. Biol. Chem. 281: 26665–26674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holinstat M., Preininger A. M., Milne S. B., Hudson W. J., Brown H. A., Hamm H. E. 2009. Irreversible platelet activation requires protease-activated receptor 1-mediated signaling to phosphatidylinositol phosphates. Mol. Pharmacol. 76: 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Triest M., de Rooij J., Bos J. L. 2001. Measurement of GTP-bound Ras-like GTPases by activation-specific probes. Methods Enzymol. 333: 343–348 [DOI] [PubMed] [Google Scholar]
- 41.Gong H., Shen B., Flevaris P., Chow C., Lam S. C., Voyno-Yasenetskaya T. A., Kozasa T., Du X. 2010. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science. 327: 340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivanov I., Heydeck D., Hofheinz K., Roffeis J., O'Donnell V. B., Kuhn H., Walther M. 2010. Molecular enzymology of lipoxygenases. Arch. Biochem. Biophys. 503: 161–174 [DOI] [PubMed] [Google Scholar]
- 43.Knapp M. J., Seebeck F. P., Klinman J. P. 2001. Steric control of oxygenation regiochemistry in soybean lipoxygenase-1. J. Am. Chem. Soc. 123: 2931–2932 [DOI] [PubMed] [Google Scholar]
- 44.Gomolka B., Siegert E., Blossey K., Schunck W. H., Rothe M., Weylandt K. H. 2011. Analysis of omega-3 and omega-6 fatty acid-derived lipid metabolite formation in human and mouse blood samples. Prostaglandins Other Lipid Mediat. 94: 81–87 [DOI] [PubMed] [Google Scholar]
- 45.Harper C. R., Edwards M. J., DeFilippis A. P., Jacobson T. A. 2006. Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J. Nutr. 136: 83–87 [DOI] [PubMed] [Google Scholar]
- 46.Holy E. W., Forestier M., Richter E. K., Akhmedov A., Leiber F., Camici G. G., Mocharla P., Luscher T. F., Beer J. H., Tanner F. C. 2011. Dietary alpha-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler. Thromb. Vasc. Biol. 31: 1772–1780 [DOI] [PubMed] [Google Scholar]
- 47.Siess W., Siegel F. L., Lapetina E. G. 1984. Dihomogammalinolenic acid, but not eicosapentaenoic acid, activates washed human platelets. Biochim. Biophys. Acta. 801: 265–276 [DOI] [PubMed] [Google Scholar]
- 48.Flevaris P., Stojanovic A., Gong H., Chishti A., Welch E., Du X. 2007. A molecular switch that controls cell spreading and retraction. J. Cell Biol. 179: 553–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Schacky C., Weber P. C. 1985. Metabolism and effects on platelet function of the purified eicosapentaenoic and docosahexaenoic acids in humans. J. Clin. Invest. 76: 2446–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergstroem S., Danielsson H., Klenberg D., Samuelsson B. 1964. The enzymatic conversion of essential fatty acids into prostaglandins. J. Biol. Chem. 239: PC4006–PC4008 [PubMed] [Google Scholar]
- 51.Falardeau P., Hamberg M., Samuelsson B. 1976. Metabolism of 8,11,14-eicosatrienoic acid in human platelets. Biochim. Biophys. Acta. 441: 193–200 [DOI] [PubMed] [Google Scholar]
- 52.Needleman P., Whitaker M. O., Wyche A., Watters K., Sprecher H., Raz A. 1980. Manipulation of platelet aggregation by prostaglandins and their fatty acid precursors: pharmacological basis for a therapeutic approach. Prostaglandins. 19: 165–181 [DOI] [PubMed] [Google Scholar]
- 53.Gan Q. F., Browner M. F., Sloane D. L., Sigal E. 1996. Defining the arachidonic acid binding site of human 15-lipoxygenase. Molecular modeling and mutagenesis. J. Biol. Chem. 271: 25412–25418 [DOI] [PubMed] [Google Scholar]
- 54.Rickert K. W., Klinman J. P. 1999. Nature of hydrogen transfer in soybean lipoxygenase-1: separation of primary and secondary isotope effects. Biochemistry. 38: 12218–12228 [DOI] [PubMed] [Google Scholar]
- 55.Guo Y., Zhang W., Giroux C., Cai Y., Ekambaram P., Dilly A-K., Hsu A., Zhou S., Maddipati K. R., Liu J., et al. 2011. Identification of the orphan G protein coupled receptor GPR31 as a receptor for 12(S)hydroxyeicosatetraenoic acid. J. Biol. Chem. 286: 33832–33840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zafiriou M. P., Deva R., Ciccoli R., Siafaka-Kapadai A., Nigam S. 2007. Biological role of hepoxilins: upregulation of phospholipid hydroperoxide glutathione peroxidase as a cellular response to oxidative stress? Prostaglandins Leukot. Essent. Fatty Acids. 77: 209–215 [DOI] [PubMed] [Google Scholar]
- 57.Kernoff P. B., Willis A. L., Stone K. J., Davies J. A., McNicol G. P. 1977. Antithrombotic potential of dihomo-gamma-linolenic acid in man. BMJ. 2: 1441–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan Y. Y., Chapkin R. S. 1998. Importance of dietary gamma-linolenic acid in human health and nutrition. J. Nutr. 128: 1411–1414 [DOI] [PubMed] [Google Scholar]
- 59.Surette M. E., Koumenis I. L., Edens M. B., Tramposch K. M., Clayton B., Bowton D., Chilton F. H. 2003. Inhibition of leukotriene biosynthesis by a novel dietary fatty acid formulation in patients with atopic asthma: a randomized, placebo-controlled, parallel-group, prospective trial. Clin. Ther. 25: 972–979 [DOI] [PubMed] [Google Scholar]
- 60.Levin G., Duffin K. L., Obukowicz M. G., Hummert S. L., Fujiwara H., Needleman P., Raz A. 2002. Differential metabolism of dihomo-gamma-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem. J. 365: 489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takai S., Jin D., Kawashima H., Kimura M., Shiraishi-Tateishi A., Tanaka T., Kakutani S., Tanaka K., Kiso Y., Miyazaki M. 2009. Anti-atherosclerotic effects of dihomo-gamma-linolenic acid in ApoE-deficient mice. J. Atheroscler. Thromb. 16: 480–489 [DOI] [PubMed] [Google Scholar]
- 62.Willis A. L., Smith D. L. 1989. Therapeutic impact of eicosanoids in atherosclerotic disease. Eicosanoids. 2: 69–99 [PubMed] [Google Scholar]
- 63.Aragona P., Bucolo C., Spinella R., Giuffrida S., Ferreri G. 2005. Systemic omega-6 essential fatty acid treatment and pge1 tear content in Sjogren's syndrome patients. Invest. Ophthalmol. Vis. Sci. 46: 4474–4479 [DOI] [PubMed] [Google Scholar]
- 64.Laidlaw M., Holub B. J. 2003. Effects of supplementation with fish oil-derived n-3 fatty acids and gamma-linolenic acid on circulating plasma lipids and fatty acid profiles in women. Am. J. Clin. Nutr. 77: 37–42 [DOI] [PubMed] [Google Scholar]
- 65.Hurtado de Catalfo G. E., de Gomez Dumm I. N. 1996. Spontaneously hypertensive rats: eicosa-8,11,14-trienoic acid metabolism and arachidonic acid biosynthesis. Biochem. Mol. Biol. Int. 40: 759–768 [DOI] [PubMed] [Google Scholar]
- 66.Manku M. S., Oka M., Horrobin D. F. 1979. Differential regulation of the formation of prostaglandins and related substances from arachidonic acid and from dihomogammalinolenic acid. II. Effects of vitamin C. Prostaglandins Med. 3: 129–137 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.