Abstract
PCSK9 proprotein convertase subtilisin/kexin type (PCSK9) is a crucial protein in LDL cholesterol (LDL-C) metabolism by virtue of its pivotal role in the degradation of the LDL receptor. In recent years, both in vitro and in vivo studies have greatly supplemented our understanding of the (patho)physiological role of PCSK9 in human biology. In the current review, we summarize studies published or in print before May 2012 concerning the physiological role of PCSK9 in cholesterol metabolism. Moreover, we briefly describe the clinical phenotypes encountered in carriers of mutations in the gene encoding PCSK9. As PCSK9 has emerged as a novel target for LDL-C lowering therapy, methods to inhibit PCSK9 will also be reviewed. Initial data from investigations of PCSK9 inhibition in humans are promising and indicate that PCSK9 inhibition may be a viable new therapeutic option for the treatment of dyslipidemia and associated cardiovascular diseases.
Keywords: antibodies, cholesterol, drug therapy, low density lipoprotein metabolism, lipoprotein receptors, proprotein convertase subtilisin/kexin type 9
Elevated plasma levels of LDL cholesterol (LDL-C) have consistently been shown to be a risk factor for the development of atherosclerosis and associated ischemic cardiovascular disease (CVD), such as myocardial infarction and stroke. Plasma LDL-C levels are highly inheritable, and a number of molecular defects have been shown to underlie extreme levels of LDL-C. One of the pivotal factors in LDL metabolism is the LDL receptor (LDLR) by virtue of its capacity to bind and subsequently clear cholesterol derived from LDL from the circulation. LDL bound to the LDLR is internalized into clathrin-coated pits and subsequently undergoes lysosomal degradation. The LDLR is then recycled back to the plasma membrane where it can bind more LDL. Internalization and reshuttling of the receptor toward the plasma membrane is a continuous process. Proprotein convertase subtilisin kexin type 9 (PCSK9) plays a pivotal role in this process because it promotes the degradation of the LDLR and prevents it from recycling to the membrane. Consequently, PCSK9 has become a novel target for lipid-lowering therapy. The added incentive that inhibition of PCSK9 acts synergistically with existing treatments such as statins has led to a flurry of research to understand the biology of PCSK9.
DISCOVERY OF PCSK9
Familial hypercholesterolemia (FH) is an autosomal dominant form of dyslipidemia characterized by elevated plasma LDL-C levels, which are typically above the 95th percentile for age and sex (1). Apart from pathognomonic clinical signs, such as tendinous xanthomas and a presenile corneal arcus, heterozygous FH patients are at a sharply increased risk for premature CVD, which usually becomes evident in the fourth or fifth decade (2). Molecular defects in the gene encoding the LDLR are identified in the vast majority of FH patients (3), but the exact proportion of LDLR mutations is not known, which might be due to variability in clinical phenotype and referral bias. Approximately 5–10% of individuals with an FH phenotype are found to carry mutations in the ligand-binding domain of apolipoprotein B (apoB), the protein component of the LDL particle that interacts with the LDLR.
In 2003, Abifadel and coworkers identified mutations in the gene encoding PCSK9 (4) in two French families with an autosomal dominant form of FH (5). These mutations were later shown to be “gain-of-function” mutations. Given the finding of this third defect, FH caused by PCSK9 mutations is commonly referred to as “FH3” (OMIM# 603776). The mutation was identified in a kindred previously described by Varret et al. (6) as not carrying a mutation in LDLR or apoB, with linkage analysis having shown a positive logarithm of the odds (LOD) score of 3.13 on a 9 cM interval at 1p34.1-p32. Other PCSK9 mutations were subsequently reported in FH patients from Utah, Norway, and the United Kingdom (7–9), but in general, the prevalence of PCSK9 mutations is very low compared with defects in LDLR and apoB (10). In the latter study, the risk of coronary artery disease (CAD) associated with the rare variant D374Y was shown to be sharply increased and exceeded the risk associated with mutations in the LDLR gene. Currently, information about all reported PCSK9 mutations can be easily accessed online (www.ucl.ac.uk/ldlr).
Subsequent studies showed that PCSK9 was responsible for intracellular LDLR degradation in vivo (11), which is in line with the finding that mutations within the PCSK9 transcription unit that decrease cell surface LDLR expression are associated with increased plasma LDL-C levels.
Large cohort studies have been undertaken to address the role of common PCSK9 sequence variations in lipid metabolism and CAD risk. In 2005, a causative association was established between two relatively common “loss-of-function” mutations in PCSK9 and low plasma LDL-C levels (12). The individuals that carried these mutations (PCSK9-679X or PCSK9-142X) exhibited LDL-C levels of 100 ± 45 mg/dl compared with 138 ± 42 mg/dl for noncarriers, which was accompanied by an astonishing 88% reduction in global coronary heart disease risk (13). No other clinical phenotypes were identified in this patient population. Likewise, persons of European descent carrying the PCSK9-R46L “loss of function” mutation exhibited LDL-C levels of 116 ± 33 mg/dl, compared with 137 ± 37 mg/dl for noncarriers, which was accompanied by a 47% reduction in global coronary heart disease risk (13). As a result of these landmark observations, PCSK9 has become a very attractive drug target and the subject of intensive research.
PCSK9 AND ITS ROLE IN LDLR DEGRADATION
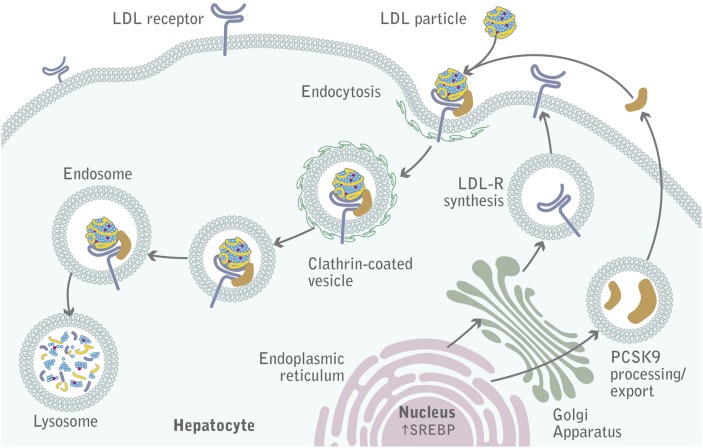
PCSK9 acts by reducing the amount of LDLR at the cell surface of hepatocytes (Fig. 1). This was first demonstrated in mouse models (11, 14–17) and inferred by human genetic studies (18, 19). Numerous overexpression and knockdown/knockout animal studies clearly show that PCSK9 targets the LDLR for degradation (14–17, 20, 21). Probably the most elegant demonstration came from a series of parabiosis experiments showing that PCSK9 is secreted from liver cells, circulates in the plasma, binds to LDLR, and is subsequently internalized together with the LDLR, thereby promoting the cellular degradation of the receptor (22).
Fig. 1.
PCSK9-mediated degradation of LDLR. A complex of LDL-C, LDLR, and PCSK9 is internalized into hepatocytes into clathrin-coated pits and subsequently undergoes lysosomal degradation.
PCSK9 is a serine protease encoded by a gene comprising 12 exons, located on chromosome 1p32.3. It is synthesized primarily by the liver and intestine as a 692-amino acid precursor (∼75 kDa) in which a signal peptide (residues 1–30) and a prodomain (residues 31–152) precede a catalytic domain (residues 153–451) that contains the canonical D-H-S catalytic triad, followed by a C-terminal domain (residues 452–692) (23). Pro-PCSK9 undergoes autocatalytic intramolecular processing between the Q152 and S153 residues in the endoplasmic reticulum to form a mature enzyme (∼62 kDa). The cleavage of the prodomain is required for PCSK9 maturation and secretion. This was demonstrated by experiments in which the prodomain and the catalytically inactive 62 kDa PCSK9 moiety were coexpressed, allowing the exit of a noncovalently bound PCSK9/prodomain complex from the endoplasmic reticulum to the Golgi complex, which ultimately promoted LDLR degradation (24, 25). It is noteworthy that a naturally occurring amino acid substitution within the PCSK9 cleavage site (Q152H) has recently been described. This mutation prevents autocatalytic processing, thereby precluding PCSK9 secretion, and it is associated with a 48% reduction in plasma LDL-C levels (26). After cleavage, the prodomain forms hydrogen bonds with key amino acids of the catalytic domain, thereby preventing access of other potential substrates to the catalytic pocket of PCSK9 (27, 28). The ability of PCSK9 to promote LDLR degradation is, therefore, independent of its catalytic activity, indicating that PCSK9 functions as a chaperone, a mode of action that is unique among serine proteases (24, 25).
PCSK9 BLOCKS THE STRUCTURAL TRANSITION OF THE LDLR IN THE ENDOSOME
The region where secreted PCSK9 interacts with the extracellular domain of the LDLR is located in the first epidermal growth factor-like repeat homology domain (EGFA) of the human LDLR (29, 30). Binding appears to be calcium dependent and occurs with a 1:1 stoichiometry at a Kd of 170–750 nM at the neutral pH of plasma (27, 30–32). At the plasma membrane (i.e., at neutral pH), only the catalytic domain of PCSK9 interacts with the EGFA domain of the receptor (30, 31, 33). The acidic stretch located within the prodomain negatively modulates the binding affinity between PCSK9 and the LDLR (34, 35). In contrast, after endocytosis (i.e., at the acidic pH of endosomes), the affinity between the receptor and PCSK9 is much higher (Kd of 1–8 nM) than that observed at neutral pH (27, 30, 31). Under acidic pH conditions, the prodomain of PCSK9 establishes salt bridges with the β-propeller domain of the receptor (31), and the positively charged C-terminal domain of PCSK9 has been proposed to bind to the negatively charged ligand-binding domain of the LDLR (31, 36, 37). These studies demonstrate that PCSK9 locks the LDLR in an extended (or open) conformation. The failure of the receptor to adopt a closed conformation in the endosome precludes normal recycling to the plasma membrane and targets the LDLR for lysosomal degradation (31, 38) (Fig. 1). In line with this, some amino acid substitutions in PCSK9 (e.g., D374Y and S127R) or, alternatively, in the LDLR (e.g., H306Y) that are causatively associated with FH tighten the molecular interactions within the PCSK9-LDLR complex, thereby enhancing receptor degradation and consequently increasing circulating LDL-C levels among carriers (27, 31, 32, 39–41). In that respect, the D374Y-PCSK9 “gain-of-function” mutation causes an extremely severe FH phenotype that is particularly hard to treat with statins (9). Carriers of this mutation are affected by CVD 10 years earlier than other patients with FH. This mutant was found to bind to the LDLR with a 6- to 30-fold higher affinity compared with wild-type PCSK9, by allowing a hydrogen bond to form between PCSK9 and the EGFA domain of the LDLR (30).
The PCSK9 C-terminal domain plays a pivotal role in targeting LDLR for subsequent degradation (31, 36). This domain, however, is not required for LDLR binding at the cell surface, as demonstrated with a series of deletion mutants (30, 42, 43). The overall positive charge of this domain seems to be a major parameter for PCSK9 function (44). An antibody antigen-binding fragment directed against the C-terminal domain of PCSK9 has recently been shown not to affect LDLR binding but to significantly inhibit the internalization of the PCSK9-LDLR complex (45). Likewise, binding of annexin A2 to the C-terminal domain of PCSK9 prevents PCSK9 from interacting with the LDLR, thereby inhibiting receptor degradation (46). In this process, endocytosis and internalization of the PCSK9-LDLR complex is required, indicating that annexin A2 is secreted to exert its inhibitory function on PCSK9 (46, 47). Besides annexin A2 and the LDLR, the C-terminal domain of PCSK9 also establishes intra- and intermolecular interactions with its own prodomain along the secretory pathway, thereby allowing proper secretion of the protein (43). Because PCSK9 does not (or very weakly) modulate LDLR levels in the adrenals (48, 49) and in some areas of the adult brain (50, 51), it has been proposed that PCSK9 requires tissue-specific partners to efficiently reduce LDLR levels in tissues such as the liver, in which PCSK9 is most efficient (48). A similar discrepancy has been described for the autosomal recessive hypercholesterolemia (ARH) adaptor protein, which was shown to be required for LDLR internalization in hepatocytes but not in some other cell types (22, 52–54). Given its original structure, the C-terminal domain of PCSK9 is very likely to interact with some of these yet unknown but key protein partners (28, 55).
PCSK9, A SECRETED FACTOR UNDER TIGHT CONTROL
The characterization of total and liver-specific PCSK9 knockout mice indicates that hepatocytes are the main source of circulating PCSK9, despite a significant, albeit lower, expression of PCSK9 in the intestine and in the kidney (17, 56). It has been proposed that PCSK9 acts on the LDLR after biosynthesis, before it reaches the basolateral surface of the hepatocyte (40, 47, 57). This was demonstrated by knocking down the clathrin light chains that are critical for vesicular trafficking but not required for endocytosis. In this experiment, only cells that expressed PCSK9 had increased LDLR levels (58), indicating that PCSK9 inhibits the LDLR via an intracellular mechanism. A series of in vivo parabiosis experimental studies, however, have clearly demonstrated that PCSK9 acts on the LDLR primarily as a secreted factor (22, 48).
It is, therefore, clinically relevant to measure circulating PCSK9 plasma levels in humans and to study pharmacological factors affecting its secretion. In that respect, several ELISAs have been developed to measure PCSK9 in human sera (59–66). The mean concentrations varied widely among these studies, likely because of differences in specificities among antibodies to bind plasma PCSK9 and the recombinant PCSK9 standards used in the assays (55, 67). Measuring PCSK9 levels is, however, not an ideal surrogate marker for PCSK9 function, as most antibodies used to detect PCSK9 will also detect inactive truncated PCSK9 forms (21). Despite these limitations, all of these studies have consistently shown positive correlations (r = 0.15–0.58) between circulating PCSK9 and LDL-C levels in the population (59–66, 68). Except for individuals carrying “gain-of-function” mutations in the gene encoding PCSK9, plasma PCSK9 levels should logically correlate with the incidence of CVD events in humans. This has recently been demonstrated in atherosclerosis-prone animal models genetically engineered to express increasing levels of PCSK9 (69). In that respect, we have observed that plasma PCSK9 levels are predictive of recurrent clinical events in a cohort of patients with stable CVD treated with low-dose atorvastatin (70).
Endogenous inactivation of PCSK9
The mature 62 kDa PCSK9 undergoes cleavage after the R218 residue, resulting in the detachment of the prosegment and the formation of a 55 kDa truncated inactive PCSK9 form. This proteolytic cleavage appears to be mediated in hepatocytes by two proprotein convertases, namely, furin and PC5/6A, as shown using a series of hepatic conditional knockout mice (21, 71). In line with this, the naturally occurring PCSK9 R218S missense mutation promotes autosomal dominant hypercholesterolemia among heterozygous carriers (72). Together, these results indicate that a lack of PCSK9 inactivation by furin and/or PC5/6A mechanistically leads to increased/elevated plasma LDL-C levels.
Dietary and hormonal regulation of PCSK9
PCSK9 expression and plasma levels are tightly controlled by hormonal and nutritional status. Hepatic PCSK9 expression and plasma levels are dramatically lowered upon fasting (20, 73–75). Circulating PCSK9 has a marked diurnal rhythm paralleling that of cholesterol biosynthesis in humans, an effect that appears to be mediated by variations in growth hormone secretion. As a result, plasma PCSK9 levels ought to be measured at a defined period of the day (e.g., in the morning after an overnight fast) to perform accurate comparisons between individuals. In addition, PCSK9 plasma levels increase in girls and decrease in boys during their teenage years, paralleling changes in LDL-C levels (76). In adulthood, PCSK9 levels are higher in women than in men, but apparently this does not result from differences in the status of endogenous estrogens (65, 68, 77, 78).
Key regulation of PCSK9 by sterols
Similar to LDLR, the expression of PCSK9 is modulated by intracellular cholesterol levels, and this is mediated predominantly by the transcription factor sterol-responsive element-binding protein 2 (SREBP2) (17, 79–83). The transcription factor hepatocyte nuclear factor 1a (HNF1a) has also been shown to be a potent stimulator of PCSK9 gene expression (83, 84). Because PCSK9 and the LDLR are coordinately regulated at the transcriptional level by cholesterol, they are also coinduced by statin treatment. It is well established in humans and animal models that statin treatment increases plasma PCSK9 levels and, conversely, that attenuation of PCSK9 function enhances the hypolipemic effects of statins (17, 60, 85, 86). Atorvastatin, for example, has been reported to increase circulating PCSK9 levels by 34% in 12 dyslipidemic patients treated with 40 mg daily for 16 weeks (60), and by 47% as early as 4 weeks in 74 normolipemic individuals without CVD treated with 80 mg for 16 weeks (64). In line with this, when the atorvastatin dose was increased from 5 mg to 80 mg daily, plasma PCSK9 levels increased on average by 30% in 53 dyslipidemic patients (62). Recently, daily treatment with 20 mg rosuvastatin has been shown to increase plasma levels of PCSK9 by 28% in men and 35% in women in a large placebo-controlled clinical trial (JUPITER) (66). The concomitant upregulation of PCSK9 and of the LDLR is, therefore, of considerable clinical interest. Inhibiting PCSK9 will probably enhance the LDL-C lowering effects of statins and thereby might be synergistic with statins to further decrease LDL-C levels and CVD risk.
APPROACHES TO PCSK9 INHIBITION
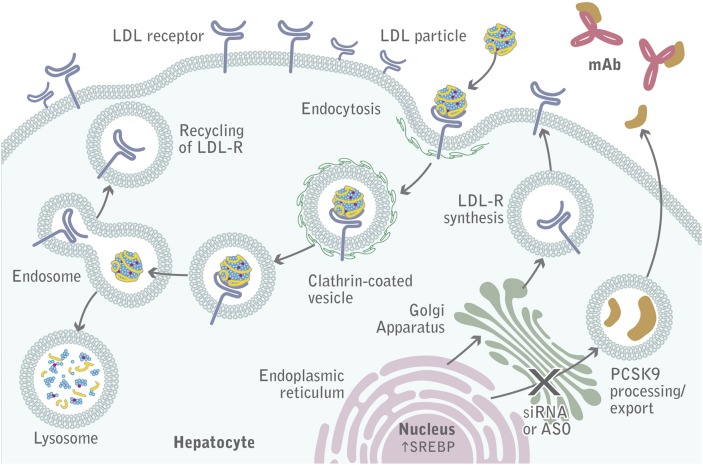
Recently, two cases of homozygous “loss-of-function” mutations in PCSK9 were described. The carriers, who totally lacked PCSK9, were healthy and fertile and presented with very low LDL-C levels (≤15 mg/dl). This finding suggests that pharmacologic interventions that inhibit PCSK9 may be safe (18, 19). Three drug development approaches (Table 1) are currently being tested to pharmacologically inhibit PCSK9 in humans. Gene silencing targets both PCSK9 intra- and extracellular functions, whereas mimetic peptides and monoclonal antibodies exclusively target circulating PCSK9 and therefore its extracellular function (Fig. 2). Other approaches, such as orally active cell permeable small molecules that target PCSK9 processing, have not reached preclinical development yet and have been extensively reviewed elsewhere (87).
TABLE 1.
PCSK9-related therapeutic approaches in development
| Target/Mechanism of Action | Agents in Development | Effects on Lipid Profile | Adverse Events | Notes | References |
| Monoclonal antibodies | SAR236553/REGN727 | Mean LDL-C ↓ 39.6–72.4% | Injection site reactions, leucocytoclastic vasculitis in one patient | REGN727 as add on to atorvastatin in patients with LDL > 100 mg/dl | (101) |
| Non-HDL ↓ 33.6–62.5% | |||||
| Mean LDL-C ↓ 28.9–67.9% | Injection site reactions, generalized pruritis in one patient | Phase II study with REGN727 as add on to statin with or without ezetimibe | (102) | ||
| Non-HDL ↓ 26.8–57.9% | |||||
| ApoB ↓ 20.9–50.2% | |||||
| Mean LDL-C ↓ up to 65.4% in single dose studies, ↓ 39.2–62% in multiple dose study as add on to atorvastatin | Elevated levels of serum creatinine kinase | Three phase I studies in healthy volunteers, FH patients, and non-FH hypercholsterolemic patients | (100) | ||
| AMG145 | Mean LDL-C ↓ 63–75% | Similar to placebo (incl. injection site hematoma, nasopharingitis, viral upper respiratory tract infection) | Phase Ib study AMG145 as add on to statin therapy | http://www.amgen.com (press release) | |
| LDL-C ↓ up to 64% | No specific adverse events reported | First in human single-dose study of AMG145 in healthy volunteers | http://circ.ahajournals.org (abstract) | ||
| RN316 / PF-04950615 (iv) | NA | NA | http://www.pfizer.com; http://clinicaltrials.gov | ||
| LGT209 | NA | NA | http://www.novartis.com | ||
| 1D05-IgG2 | LDL ↓ up to 50% in nonhuman primates | NA | (95) | ||
| Adnexins | BMS-962476 | NA | NA | Currently in preclinical studies. Phase I study is planned to be initiated. | http://clinicaltrials.gov |
| Small molecule inhibitor | SX-PCSK9 | NA | NA | http://www.serometrix.com/ | |
| Gene silencing | LNA-ASO: SPC5001 | Mean LDL-C ↓ 50% in nonhuman primates | NA | Program discontinued | |
| siRNA: ALN-PCS | Mean LDL-C ↓ 41%; up to 50% | Rash, no serious adverse events | Results of phase I single dose study in 32 healthy volunteers | http://www.alnylam.com |
↓, decrease, NA, not available.
Fig. 2.
PCSK9 inhibition. Monoclonal antibodies bound to PCSK9 prevent the association between PCSK9 and the LDLR. The LDLR binds the LDL particle and is internalized, and then the LDL particle is degraded in the lysosome, whereas the LDLR is recycled back to the plasma membrane.
Gene silencing
The subcutaneous administration of the PCSK9 antisense oligonucleotide (ASO) produced by Isis Pharmaceuticals has been shown to result in a 2-fold increase in hepatic expression of the LDLR and a concomitant reduction in circulating total cholesterol levels by 53% in mice (88). Likewise, the intravenous injection of a 13-mer locked nucleic acid (LNA) ASO from Santaris Pharma reduced PCSK9 mRNA levels by ∼60% in mice, thereby promoting a 2.5- to 3-fold increase in hepatic LDLR levels for up to 8 days (89). This compound also decreased circulating PCSK9 up to 50% as well as plasma LDL-C and apoB levels by 35% in nonhuman primates. A 14-mer LNA-ASO specific for a human PCSK9 sequence yielded similar potency in reducing LDL-C levels but displayed longer-lasting effects associated with a significant decrease in liver cholesterol content. This compound did not affect HDL cholesterol (HDL-C) levels (90). PCSK9 gene silencing in mice and monkeys has also been achieved using small interfering RNA (siRNA), a technology developed by Alnylam Pharmaceuticals. The siRNA was incorporated into lipidoid nanoparticles to minimize toxicity and intravenously infused in rats, mice, and monkeys. LDL-C levels were reduced upon administration by more than 50% in monkeys, with reductions lasting for nearly 21 days (91).
Mimetic peptides
Peptides mimicking the EGFA domain of the LDLR that interact with PCSK9 at the plasma membrane have also been developed to inhibit PCSK9 function. A synthetic EGFA peptide has been shown to dose-dependently reduce the cellular degradation of the LDLR induced by exogenously added recombinant PCSK9 (92). Because the naturally occurring H306Y substitution within the LDLR-EGFA domain increases the affinity of the receptor for PCSK9, an EGFA-H306Y peptide was used to efficiently block PCSK9-induced LDLR degradation in hepatoma cells overexpressing either wild-type PCSK9 or the more effective PCSK9-D374Y variant (41). An alternative option is to use PCSK9 peptide sequences that are too short to promote LDLR degradation but long enough to compete with full-length PCSK9. An inhibitory effect of the isolated C-terminal domain of PCSK9 has recently been observed in vitro and in mice on PCSK9-induced LDLR degradation (43), indicating that pharmacological inhibition of PCSK9 extracellularly will lower circulating LDL-C levels in vivo.
Monoclonal antibodies
Duff et al. were able to reverse the PCSK9-mediated effect on cell surface LDLRs by using antibodies that recognize epitopes on PCSK9 in the vicinity of the region within the catalytic domain interacting with the LDLR (93). Likewise, a single 3 mg/kg intravenous infusion of the mAb1 monoclonal antibody specific for the catalytic domain of PCSK9 (developed by Amgen) led to a significant reduction of circulating LDL-C levels as early as 8 h after injection in nonhuman primates. The LDL-C-lowering effects of this antibody reached a nadir of –80% on day 10 postinfusion (94). Incidentally, this was accompanied by a slight decrease in HDL-C levels. Another monoclonal antibody (1D05-IgG2) was developed by Merck to structurally mimic the EGFA domain of the LDLR. This antibody (one single injection at 3 mg/kg) was also found to antagonize PCSK9 function in Rhesus monkeys, reducing plasma LDL-C levels by up to 50% (45, 95). Pfizer-Rinat has recently reported a humanized monoclonal J16 antibody directed against the PCSK9 LDLR-EGFA domain binding site (96). This antibody was injected once intravenously in cynomolgus monkeys and dose-dependently reduced LDL-C levels by 70%, an effect that lasted for 10 days at the 3 mg/kg dose. The magnitude and duration of this effect were similar when the J16 antibody was infused in high fat-fed monkeys. In addition, when these animals were treated with simvastatin (50 mg/day) and subsequently infused with the J16 antibody (3 mg/kg), an additional 65% reduction in LDL-C levels was observed (96). The J16 antibody has been modified to bind PCSK9 in a pH-sensitive manner and thereby escape degradation. The resultant J17 antibody was demonstrated to be as potent as the J10 antibody, the mouse precursor to J16, in mice and monkeys for periods two to three times as long (97).
Several monoclonal antibodies targeting PCSK9 in the circulation are being tested in human clinical trials. Pfizer-Rinat RN316 is currently in a phase II study. Amgen's antibody (AMG145) was evaluated in a phase I ascending single-dose study, which showed that LDL-C was dose-dependently decreased by up to 64% relative to placebo when AMG145 was infused intravenously or subcutaneously in healthy subjects (98). In this study, there were no reports of serious adverse events or discontinuations as a result of an adverse event. The effect of AMG145 administration will be investigated in subjects with hypercholesterolemia in the LAPLACE–TIMI 57 (NCT01380730) trial (99).
In two phase I ascending single-dose studies in healthy subjects, the Sanofi-Aventis/Regeneron SAR236553/REGN727 antibody was associated with a significant (P < 0.001 versus placebo) reduction from baseline in LDL-C, 33–46% when given subcutaneously (50–250 mg) and by 28–65% when given intravenously (0.3–12.0 mg/kg) (100). As an add-on to statin therapy, multiple doses of SAR236553/REGN727 administered subcutaneously (doses 50–150 mg) significantly (P < 0.001 versus placebo) reduced cholesterol levels by 41–58% in patients with FH and by 38–65% in patients with hypercholesterolemia (non-FH) (100). In these phase I trials, no evidence of drug-related adverse events was observed. In a 12-week phase II study of patients with LDL-C levels ≥ 100 mg/dl on a stable dose of atorvastatin (10, 20, or 40 mg/day), add-on of SAR236553/REGN727 doses of 50, 100, or 150 mg administered subcutaneously every 2 weeks resulted in LDL-C reductions of 40–72% (100). Similarly, SAR236553/REGN727 doses of 200 or 300 mg every 4 weeks resulted in reductions of 43% and 48%, respectively (101). SAR236553/REGN727 was generally well tolerated and with similar adverse events for all treatment groups. In an 8-week phase II study of patients with LDL-C levels ≥ 100 mg/dl on a stable dose of atorvastatin 10 mg/day, SAR236553/REGN727 subcutaneously administered in combination with atorvastatin resulted in a 66% reduction in LDL-C (E. M. Roth, personal communication). In the same study, SAR236553/REGN727 as add-on to atorvastatin 80 mg/day resulted in a decrease of 73% compared with a reduction of 17% with atorvastatin 80 mg/day alone (P < 0.0001). In this study, there was a single serious adverse event of dehydration that was not considered related to treatment. These results strongly support the concept that inhibition of circulating PCSK9 in combination with statins will result in sharply decreased plasma levels of LDL-C and be well tolerated.
CONCLUSIONS
PCSK9 inhibition is considered an attractive target for therapy, especially in light of the fact that a large proportion of high-risk patients do not reach the target LDL-C levels despite maximally tolerated forms of currently available lipid-lowering agents. Monoclonal antibodies are currently the most advanced PCSK9 inhibitors in terms of pharmacological development. Recent studies have suggested that pharmacologically induced PCSK9 inhibition is efficacious in the reduction of LCL-C levels. Evaluation of treatment over the long term will determine whether the beneficial effects of PCSK9 inhibition on LDL-C levels will directly translate into CAD risk reduction.
Footnotes
Abbreviations:
- ARH
- autosomal recessive hypercholesterolemia
- ASO
- antisense oligonucleotide
- CAD
- coronary artery disease
- CVD
- cardiovascular disease
- EGFA
- epidermal growth factor-like repeat homology domain
- FH
- familial hypercholesterolemia
- HDL-C
- high-density lipoprotein cholesterol
- HNF1a
- hepatocyte nuclear factor 1a
- LDL-C
- low-density lipoprotein cholesterol
- LDLR
- LDL receptor
- LNA
- locked nucleic acid
- LOD
- logarithm of the odds
- monoclonal antibody
- MAb
- PCSK9
- proprotein convertase subtilisin kexin type 9
- siRNA
- small interfering RNA
- SREBP2
- sterol-responsive element-binding protein 2
This work was supported by National Health and Medical Research Council of Australia Grant 101867 (G.L.), by the Agence Nationale de la Recherche Programme Blanc Physiopathologie (BCNCT) (G.L.), by the Dutch Heart Foundation Lifetime Achievement Award 2010T082 (J.J.P.K.), and by Sanofi and Regeneron Pharmaceuticals, Inc. Technical editorial support was provided by Alexandra Silveira of PPSI. J.J.P.K. is a consultant for Regeneron.
REFERENCES
- 1.Cook S., Auinger P., Huang T. T. 2009. Growth curves for cardio-metabolic risk factors in children and adolescents. J. Pediatr. 155: S6–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobbs H. H., Brown M. S., Goldstein J. L. 1992. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum. Mutat. 1: 445–466 [DOI] [PubMed] [Google Scholar]
- 3.Watts G. F., Lewis B., Sullivan D. R. 2007. Familial hypercholesterolemia: a missed opportunity in preventive medicine. Nat. Clin. Pract. Cardiovasc. Med. 4: 404–405 [DOI] [PubMed] [Google Scholar]
- 4.Seidah N. G., Benjannet S., Wickham L., Marcinkiewicz J., Jasmin S. B., Stifani S., Basak A., Prat A., Chretien M. 2003. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA. 100: 928–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abifadel M., Varret M., Rabes J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., et al. 2003. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34: 154–156 [DOI] [PubMed] [Google Scholar]
- 6.Varret M., Rabes J. P., Saint-Jore B., Cenarro A., Marinoni J. C., Civeira F., Devillers M., Krempf M., Coulon M., Thiart R., et al. 1999. A third major locus for autosomal dominant hypercholesterolemia maps to 1p34.1-p32. Am. J. Hum. Genet. 64: 1378–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timms K. M., Wagner S., Samuels M. E., Forbey K., Goldfine H., Jammulapati S., Skolnick M. H., Hopkins P. N., Hunt S. C., Shattuck D. M. 2004. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Hum. Genet. 114: 349–353 [DOI] [PubMed] [Google Scholar]
- 8.Leren T. P. 2004. Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia. Clin. Genet. 65: 419–422 [DOI] [PubMed] [Google Scholar]
- 9.Naoumova R. P., Tosi I., Patel D., Neuwirth C., Horswell S. D., Marais A. D., van Heyningen C., Soutar A. K. 2005. Severe hypercholesterolemia in four British families with the D374Y mutation in the PCSK9 gene: long-term follow-up and treatment response. Arterioscler. Thromb. Vasc. Biol. 25: 2654–2660 [DOI] [PubMed] [Google Scholar]
- 10.Humphries S. E., Whittall R. A., Hubbart C. S., Maplebeck S., Cooper J. A., Soutar A. K., Naoumova R., Thompson G. R., Seed M., Durrington P. N., et al. 2006. Genetic causes of familial hypercholesterolaemia in patients in the UK: relation to plasma lipid levels and coronary heart disease risk. J. Med. Genet. 43: 943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxwell K. N., Breslow J. L. 2004. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc. Natl. Acad. Sci. USA. 101: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen J., Pertsemlidis A., Kotowski I. K., Graham R., Garcia C. K., Hobbs H. H. 2005. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 37: 161–165 [DOI] [PubMed] [Google Scholar]
- 13.Cohen J. C., Boerwinkle E., Mosley T. H., Jr, Hobbs H. H. 2006. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354: 1264–1272 [DOI] [PubMed] [Google Scholar]
- 14.Park S. W., Moon Y. A., Horton J. D. 2004. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J. Biol. Chem. 279: 50630–50638 [DOI] [PubMed] [Google Scholar]
- 15.Maxwell K. N., Fisher E. A., Breslow J. L. 2005. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc. Natl. Acad. Sci. USA. 102: 2069–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lalanne F., Lambert G., Amar M. J., Chetiveaux M., Zair Y., Jarnoux A. L., Ouguerram K., Friburg J., Seidah N. G., Brewer H. B., Jr, et al. 2005. Wild-type PCSK9 inhibits LDL clearance but does not affect apoB-containing lipoprotein production in mouse and cultured cells. J. Lipid Res. 46: 1312–1319 [DOI] [PubMed] [Google Scholar]
- 17.Rashid S., Curtis D. E., Garuti R., Anderson N. N., Bashmakov Y., Ho Y. K., Hammer R. E., Moon Y. A., Horton J. D. 2005. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. USA. 102: 5374–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Z., Tuakli-Wosornu Y., Lagace T. A., Kinch L., Grishin N. V., Horton J. D., Cohen J. C., Hobbs H. H. 2006. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 79: 514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper A. J., Marais A. D., Tanyanyiwa D. M., Burnett J. R. 2007. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis. 193: 445–448 [DOI] [PubMed] [Google Scholar]
- 20.Lambert G., Jarnoux A. L., Pineau T., Pape O., Chetiveaux M., Laboisse C., Krempf M., Costet P. 2006. Fasting induces hyperlipidemia in mice overexpressing proprotein convertase subtilisin kexin type 9: lack of modulation of very-low-density lipoprotein hepatic output by the low-density lipoprotein receptor. Endocrinology. 147: 4985–4995 [DOI] [PubMed] [Google Scholar]
- 21.Benjannet S., Rhainds D., Hamelin J., Nassoury N., Seidah N. G. 2006. The proprotein convertase (PC) PCSK9 is inactivated by furin and/or PC5/6A: functional consequences of natural mutations and post-translational modifications. J. Biol. Chem. 281: 30561–30572 [DOI] [PubMed] [Google Scholar]
- 22.Lagace T. A., Curtis D. E., Garuti R., McNutt M. C., Park S. W., Prather H. B., Anderson N. N., Ho Y. K., Hammer R. E., Horton J. D. 2006. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J. Clin. Invest. 116: 2995–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjannet S., Rhainds D., Essalmani R., Mayne J., Wickham L., Jin W., Asselin M. C., Hamelin J., Varret M., Allard D., et al. 2004. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 279: 48865–48875 [DOI] [PubMed] [Google Scholar]
- 24.Li J., Tumanut C., Gavigan J. A., Huang W. J., Hampton E. N., Tumanut R., Suen K. F., Trauger J. W., Spraggon G., Lesley S. A., et al. 2007. Secreted PCSK9 promotes LDL receptor degradation independently of proteolytic activity. Biochem. J. 406: 203–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNutt M. C., Lagace T. A., Horton J. D. 2007. Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells. J. Biol. Chem. 282: 20799–20803 [DOI] [PubMed] [Google Scholar]
- 26.Mayne J., Dewpura T., Raymond A., Bernier L., Cousins M., Ooi T. C., Davignon J., Seidah N. G., Mbikay M., Chretien M. 2011. Novel loss-of-function PCSK9 variant is associated with low plasma LDL cholesterol in a French-Canadian family and with impaired processing and secretion in cell culture. Clin. Chem. 57: 1415–1423 [DOI] [PubMed] [Google Scholar]
- 27.Cunningham D., Danley D. E., Geoghegan K. F., Griffor M. C., Hawkins J. L., Subashi T. A., Varghese A. H., Ammirati M. J., Culp J. S., Hoth L. R., et al. 2007. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat. Struct. Mol. Biol. 14: 413–419 [DOI] [PubMed] [Google Scholar]
- 28.Piper D. E., Jackson S., Liu Q., Romanow W. G., Shetterly S., Thibault S. T., Shan B., Walker N. P. 2007. The crystal structure of PCSK9: a regulator of plasma LDL-cholesterol. Structure. 15: 545–552 [DOI] [PubMed] [Google Scholar]
- 29.Zhang D. W., Lagace T. A., Garuti R., Zhao Z., McDonald M., Horton J. D., Cohen J. C., Hobbs H. H. 2007. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J. Biol. Chem. 282: 18602–18612 [DOI] [PubMed] [Google Scholar]
- 30.Bottomley M. J., Cirillo A., Orsatti L., Ruggeri L., Fisher T. S., Santoro J. C., Cummings R. T., Cubbon R. M., Lo S. P., Calzetta A., et al. 2009. Structural and biochemical characterization of the wild type PCSK9-EGF(AB) complex and natural familial hypercholesterolemia mutants. J. Biol. Chem. 284: 1313–1323 [DOI] [PubMed] [Google Scholar]
- 31.Surdo P. L., Bottomley M. J., Calzetta A., Settembre E. C., Cirillo A., Pandit S., Ni Y. G., Hubbard B., Sitlani A., Carfi A. 2011. Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH. EMBO Rep. 12: 1300–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher T. S., Lo S. P., Pandit S., Mattu M., Santoro J. C., Wisniewski D., Cummings R. T., Calzetta A., Cubbon R. M., Fischer P. A., et al. 2007. Effects of pH and low density lipoprotein (LDL) on PCSK9-dependent LDL receptor regulation. J. Biol. Chem. 282: 20502–20512 [DOI] [PubMed] [Google Scholar]
- 33.Kwon H. J., Lagace T. A., McNutt M. C., Horton J. D., Deisenhofer J. 2008. Molecular basis for LDL receptor recognition by PCSK9. Proc. Natl. Acad. Sci. USA. 105: 1820–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjannet S., Saavedra Y. G., Hamelin J., Asselin M. C., Essalmani R., Pasquato A., Lemaire P., Duke G., Miao B., Duclos F., et al. 2010. Effects of the prosegment and pH on the activity of PCSK9: evidence for additional processing events. J. Biol. Chem. 285: 40965–40978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holla Ø. L., Laerdahl J. K., Strom T. B., Tveten K., Cameron J., Berge K. E., Leren T. P. 2011. Removal of acidic residues of the prodomain of PCSK9 increases its activity towards the LDL receptor. Biochem. Biophys. Res. Commun. 406: 234–238 [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto T., Lu C., Ryan R. O. 2011. A two-step binding model of PCSK9 interaction with the low density lipoprotein receptor. J. Biol. Chem. 286: 5464–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tveten K., Holla Ø. L., Cameron J., Strom T. B., Berge K. E., Laerdahl J. K., Leren T. P. 2012. Interaction between the ligand-binding domain of the LDL receptor and the C-terminal domain of PCSK9 is required for PCSK9 to remain bound to the LDL receptor during endosomal acidification. Hum. Mol. Genet. 21: 1402–1409 [DOI] [PubMed] [Google Scholar]
- 38.Blacklow S. C. 2007. Versatility in ligand recognition by LDL receptor family proteins: advances and frontiers. Curr. Opin. Struct. Biol. 17: 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandit S., Wisniewski D., Santoro J. C., Ha S., Ramakrishnan V., Cubbon R. M., Cummings R. T., Wright S. D., Sparrow C. P., Sitlani A., et al. 2008. Functional analysis of sites within PCSK9 responsible for hypercholesterolemia. J. Lipid Res. 49: 1333–1343 [DOI] [PubMed] [Google Scholar]
- 40.Homer V. M., Marais A. D., Charlton F., Laurie A. D., Hurndell N., Scott R., Mangili F., Sullivan D. R., Barter P. J., Rye K. A., et al. 2008. Identification and characterization of two non-secreted PCSK9 mutants associated with familial hypercholesterolemia in cohorts from New Zealand and South Africa. Atherosclerosis. 196: 659–666 [DOI] [PubMed] [Google Scholar]
- 41.McNutt M. C., Kwon H. J., Chen C., Chen J. R., Horton J. D., Lagace T. A. 2009. Antagonism of secreted PCSK9 increases low density lipoprotein receptor expression in HepG2 cells. J. Biol. Chem. 284: 10561–10570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D. W., Garuti R., Tang W. J., Cohen J. C., Hobbs H. H. 2008. Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor. Proc. Natl. Acad. Sci. USA. 105: 13045–13050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du F., Hui Y., Zhang M., Linton M. F., Fazio S., Fan D. 2011. Novel domain interaction regulates secretion of proprotein convertase subtilisin/kexin type 9 (PCSK9) protein. J. Biol. Chem. 286: 43054–43061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holla Ø. L., Cameron J., Tveten K., Strom T. B., Berge K. E., Laerdahl J. K., Leren T. P. 2011. Role of the C-terminal domain of PCSK9 in degradation of the LDL receptors. J. Lipid Res. 52: 1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ni Y. G., Condra J. H., Orsatti L., Shen X., Di M. S., Pandit S., Bottomley M. J., Ruggeri L., Cummings R. T., Cubbon R. M., et al. 2010. A proprotein convertase subtilisin-like/kexin type 9 (PCSK9) C-terminal domain antibody antigen-binding fragment inhibits PCSK9 internalization and restores low density lipoprotein uptake. J. Biol. Chem. 285: 12882–12891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayer G., Poirier S., Seidah N. G. 2008. Annexin A2 is a C-terminal PCSK9-binding protein that regulates endogenous low density lipoprotein receptor levels. J. Biol. Chem. 283: 31791–31801 [DOI] [PubMed] [Google Scholar]
- 47.Nassoury N., Blasiole D. A., Tebon O. A., Benjannet S., Hamelin J., Poupon V., McPherson P. S., Attie A. D., Prat A., Seidah N. G. 2007. The cellular trafficking of the secretory proprotein convertase PCSK9 and its dependence on the LDLR. Traffic. 8: 718–732 [DOI] [PubMed] [Google Scholar]
- 48.Grefhorst A., McNutt M. C., Lagace T. A., Horton J. D. 2008. Plasma PCSK9 preferentially reduces liver LDL receptors in mice. J. Lipid Res. 49: 1303–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo Y., Warren L., Xia D., Jensen H., Sand T., Petras S., Qin W., Miller K. S., Hawkins J. 2009. Function and distribution of circulating human PCSK9 expressed extrahepatically in transgenic mice. J. Lipid Res. 50: 1581–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu M., Wu G., Baysarowich J., Kavana M., Addona G. H., Bierilo K. K., Mudgett J. S., Pavlovic G., Sitlani A., Renger J. J., et al. 2010. PCSK9 is not involved in the degradation of LDL receptors and BACE1 in the adult mouse brain. J. Lipid Res. 51: 2611–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rousselet E., Marcinkiewicz J., Kriz J., Zhou A., Hatten M. E., Prat A., Seidah N. G. 2011. PCSK9 reduces the protein levels of the LDL receptor in mouse brain during development and after ischemic stroke. J. Lipid Res. 52: 1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lambert G. 2007. Unravelling the functional significance of PCSK9. Curr. Opin. Lipidol. 18: 304–309 [DOI] [PubMed] [Google Scholar]
- 53.Fasano T., Sun X. M., Patel D. D., Soutar A. K. 2009. Degradation of LDLR protein mediated by ‘gain of function’ PCSK9 mutants in normal and ARH cells. Atherosclerosis. 203: 166–171 [DOI] [PubMed] [Google Scholar]
- 54.Horton J. D., Cohen J. C., Hobbs H. H. 2009. PCSK9: a convertase that coordinates LDL catabolism. J. Lipid Res. 50(Suppl.): S172–S177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lambert G., Charlton F., Rye K. A., Piper D. E. 2009. Molecular basis of PCSK9 function. Atherosclerosis. 203: 1–7 [DOI] [PubMed] [Google Scholar]
- 56.Zaid A., Roubtsova A., Essalmani R., Marcinkiewicz J., Chamberland A., Hamelin J., Tremblay M., Jacques H., Jin W., Davignon J., et al. 2008. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 48: 646–654 [DOI] [PubMed] [Google Scholar]
- 57.Holla Ø. L., Cameron J., Berge K. E., Ranheim T., Leren T. P. 2007. Degradation of the LDL receptors by PCSK9 is not mediated by a secreted protein acted upon by PCSK9 extracellularly. BMC Cell Biol. 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poirier S., Mayer G., Poupon V., McPherson P. S., Desjardins R., Ly K., Asselin M. C., Day R., Duclos F. J., Witmer M., et al. 2009. Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route. J. Biol. Chem. 284: 28856–28864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alborn W. E., Cao G., Careskey H. E., Qian Y. W., Subramaniam D. R., Davies J., Conner E. M., Konrad R. J. 2007. Serum proprotein convertase subtilisin kexin type 9 is correlated directly with serum LDL cholesterol. Clin. Chem. 53: 1814–1819 [DOI] [PubMed] [Google Scholar]
- 60.Careskey H. E., Davis R. A., Alborn W. E., Troutt J. S., Cao G., Konrad R. J. 2008. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J. Lipid Res. 49: 394–398 [DOI] [PubMed] [Google Scholar]
- 61.Lambert G., Ancellin N., Charlton F., Comas D., Pilot J., Keech A., Patel S., Sullivan D. R., Cohn J. S., Rye K. A., et al. 2008. Plasma PCSK9 concentrations correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clin. Chem. 54: 1038–1045 [DOI] [PubMed] [Google Scholar]
- 62.Dubuc G., Tremblay M., Pare G., Jacques H., Hamelin J., Benjannet S., Boulet L., Genest J., Bernier L., Seidah N. G., et al. 2010. A new method for measurement of total plasma PCSK9: clinical applications. J. Lipid Res. 51: 140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan D. C., Lambert G., Barrett P. H., Rye K. A., Ooi E. M., Watts G. F. 2009. Plasma proprotein convertase subtilisin/kexin type 9: a marker of LDL apolipoprotein B-100 catabolism? Clin. Chem. 55: 2049–2052 [DOI] [PubMed] [Google Scholar]
- 64.Welder G., Zineh I., Pacanowski M. A., Troutt J. S., Cao G., Konrad R. J. 2010. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J. Lipid Res. 51: 2714–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui Q., Ju X., Yang T., Zhang M., Tang W., Chen Q., Hu Y., Haas J. V., Troutt J. S., Pickard R. T., et al. 2010. Serum PCSK9 is associated with multiple metabolic factors in a large Han Chinese population. Atherosclerosis. 213: 632–636 [DOI] [PubMed] [Google Scholar]
- 66.Awan Z., Seidah N. G., MacFadyen J. G., Benjannet S., Chasman D. I., Ridker P. M., Genest J. 2012. Rosuvastatin, proprotein convertase subtilisin/kexin type 9 concentrations, and LDL cholesterol response: the JUPITER trial. Clin. Chem. 58: 183–189 [DOI] [PubMed] [Google Scholar]
- 67.Tibolla G., Norata G. D., Artali R., Meneghetti F., Catapano A. L. 2011. Proprotein convertase subtilisin/kexin type 9 (PCSK9): from structure-function relation to therapeutic inhibition. Nutr. Metab. Cardiovasc. Dis. 21: 835–843 [DOI] [PubMed] [Google Scholar]
- 68.Lakoski S. G., Lagace T. A., Cohen J. C., Horton J. D., Hobbs H. H. 2009. Genetic and metabolic determinants of plasma PCSK9 levels. J. Clin. Endocrinol. Metab. 94: 2537–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Denis M., Marcinkiewicz J., Zaid A., Gauthier D., Poirier S., Lazure C., Seidah N. G., Prat A. 2012. Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation. 125: 894–901 [DOI] [PubMed] [Google Scholar]
- 70.Huijgen R., Boekholdt S. M., Arsenault B. J., Bao W., Davaine J., Tabet R., Petrides F., Rye K., DeMicco D. A., Barter P. J., et al. 2012. Plasma PCSK9 levels and clinical outcomes in the treating to new targets trial: a nested case-control study. J. Am. Coll. Cardiol. 59: 1778–1784 [DOI] [PubMed] [Google Scholar]
- 71.Essalmani R., Susan-Resiga D., Chamberland A., Abifadel M., Creemers J. W., Boileau C., Seidah N. G., Prat A. 2011. In vivo evidence that furin from hepatocytes inactivates PCSK9. J. Biol. Chem. 286: 4257–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allard D., Amsellem S., Abifadel M., Trillard M., Devillers M., Luc G., Krempf M., Reznik Y., Girardet J. P., Fredenrich A., et al. 2005. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum. Mutat. 26: 497. [DOI] [PubMed] [Google Scholar]
- 73.Costet P., Cariou B., Lambert G., Lalanne F., Lardeux B., Jarnoux A. L., Grefhorst A., Staels B., Krempf M. 2006. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J. Biol. Chem. 281: 6211–6218 [DOI] [PubMed] [Google Scholar]
- 74.Browning J. D., Horton J. D. 2010. Fasting reduces plasma proprotein convertase, subtilisin/kexin type 9 and cholesterol biosynthesis in humans. J. Lipid Res. 51: 3359–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Persson L., Cao G., Stahle L., Sjoberg B. G., Troutt J. S., Konrad R. J., Galman C., Wallen H., Eriksson M., Hafstrom I., et al. 2010. Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler. Thromb. Vasc. Biol. 30: 2666–2672 [DOI] [PubMed] [Google Scholar]
- 76.Baass A., Dubuc G., Tremblay M., Delvin E. E., O'Loughlin J., Levy E., Davignon J., Lambert M. 2009. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin. Chem. 55: 1637–1645 [DOI] [PubMed] [Google Scholar]
- 77.Mayne J., Raymond A., Chaplin A., Cousins M., Kaefer N., Gyamera-Acheampong C., Seidah N. G., Mbikay M., Chretien M., Ooi T. C. 2007. Plasma PCSK9 levels correlate with cholesterol in men but not in women. Biochem. Biophys. Res. Commun. 361: 451–456 [DOI] [PubMed] [Google Scholar]
- 78.Persson L., Henriksson P., Westerlund E., Hovatta O., Angelin B., Rudling M. 2012. Endogenous estrogens lower plasma PCSK9 and LDL cholesterol but Not Lp(a) or bile acid synthesis in women. Arterioscler. Thromb. Vasc. Biol. 32: 810–814 [DOI] [PubMed] [Google Scholar]
- 79.Maxwell K. N., Soccio R. E., Duncan E. M., Sehayek E., Breslow J. L. 2003. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J. Lipid Res. 44: 2109–2119 [DOI] [PubMed] [Google Scholar]
- 80.Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., Goldstein J. L. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 100: 12027–12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dubuc G., Chamberland A., Wassef H., Davignon J., Seidah N. G., Bernier L., Prat A. 2004. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 24: 1454–1459 [DOI] [PubMed] [Google Scholar]
- 82.Jeong H. J., Lee H. S., Kim K. S., Kim Y. K., Yoon D., Park S. W. 2008. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J. Lipid Res. 49: 399–409 [DOI] [PubMed] [Google Scholar]
- 83.Dong B., Wu M., Li H., Kraemer F. B., Adeli K., Seidah N. G., Park S. W., Liu J. 2010. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J. Lipid Res. 51: 1486–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li H., Dong B., Park S. W., Lee H. S., Chen W., Liu J. 2009. Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 284: 28885–28895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berge K. E., Ose L., Leren T. P. 2006. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler. Thromb. Vasc. Biol. 26: 1094–1100 [DOI] [PubMed] [Google Scholar]
- 86.Mayne J., Dewpura T., Raymond A., Cousins M., Chaplin A., Lahey K. A., Lahaye S. A., Mbikay M., Ooi T. C., Chretien M. 2008. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis. 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seidah N. G., Prat A. 2012. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 11: 367–383 [DOI] [PubMed] [Google Scholar]
- 88.Graham M. J., Lemonidis K. M., Whipple C. P., Subramaniam A., Monia B. P., Crooke S. T., Crooke R. M. 2007. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J. Lipid Res. 48: 763–767 [DOI] [PubMed] [Google Scholar]
- 89.Gupta N., Fisker N., Asselin M. C., Lindholm M., Rosenbohm C., Orum H., Elmen J., Seidah N. G., Straarup E. M. 2010. A locked nucleic acid antisense oligonucleotide (LNA) silences PCSK9 and enhances LDLR expression in vitro and in vivo. PLoS ONE. 5: e10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lindholm M. W., Elmen J., Fisker N., Hansen H. F., Persson R., Moller M. R., Rosenbohm C., Orum H., Straarup E. M., Koch T. 2012. PCSK9 LNA antisense oligonucleotides induce sustained reduction of LDL cholesterol in nonhuman primates. Mol. Ther. 20: 376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frank-Kamenetsky M., Grefhorst A., Anderson N. N., Racie T. S., Bramlage B., Akinc A., Butler D., Charisse K., Dorkin R., Fan Y., et al. 2008. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl. Acad. Sci. USA. 105: 11915–11920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shan L., Pang L., Zhang R., Murgolo N. J., Lan H., Hedrick J. A. 2008. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem. Biophys. Res. Commun. 375: 69–73 [DOI] [PubMed] [Google Scholar]
- 93.Duff C. J., Scott M. J., Kirby I. T., Hutchinson S. E., Martin S. L., Hooper N. M. 2009. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem. J. 419: 577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chan J. C., Piper D. E., Cao Q., Liu D., King C., Wang W., Tang J., Liu Q., Higbee J., Xia Z., et al. 2009. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc. Natl. Acad. Sci. USA. 106: 9820–9825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ni Y. G., Di M. S., Condra J. H., Peterson L. B., Wang W., Wang F., Pandit S., Hammond H. A., Rosa R., Cummings R. T., et al. 2011. A PCSK9-binding antibody that structurally mimics the EGF(A) domain of LDL-receptor reduces LDL cholesterol in vivo. J. Lipid Res. 52: 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang H., Chaparro-Riggers J., Strop P., Geng T., Sutton J. E., Tsai D., Bai L., Abdiche Y., Dilley J., Yu J., et al. 2012. Proprotein convertase substilisin/kexin type 9 antagonism reduces low-density lipoprotein cholesterol in statin-treated hypercholesterolemic nonhuman primates. J. Pharmacol. Exp. Ther. 340: 228–236 [DOI] [PubMed] [Google Scholar]
- 97.Chaparro-Riggers J., Liang H., Devay R. M., Bai L., Sutton J. E., Chen W., Geng T., Lindquist K., Galindo C. M., Boustany L. M., et al. 2012. Increasing serum half-life and extending cholesterol lowering in vivo by engineering an antibody with pH-sensitive binding to PCSK9. J. Biol. Chem. 287: 11090–11097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dias C., Shaywitz A., Smith B., Emery M., Bing G., Gibbs J., Wishner B., Stolman D., Crispino C., Cook B., et al. 2011. A phase 1, randomized, double-blind, placebo-controlled, ascending single dose study to evaluate the safety, tolerability and pharmacodynamics of AMG145. Circulation. 124 (Abstract 10701) [Google Scholar]
- 99.Kohli P., Desai N. R., Giugliano R. P., Kim J. B., Somaratne R., Huang F., Knusel B., McDonald S., Abrahamsen T., Wasserman S. M., et al. 2012. Design and rationale of the LAPLACE-TIMI 57 trial: a phase II, double-blind, placebo-controlled study of the efficacy and tolerability of a monoclonal antibody inhibitor of PCSK9 in subjects with hypercholesterolemia on background statin therapy. Clin. Cardiol. 35: 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stein E. A., Mellis S., Yancopoulos G. D., Stahl N., Logan D., Smith W., Lisbon E., Gutierrez M., Webb C., Wu R., et al. 2012. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N. Engl. J. Med. 366: 1108–1118 [DOI] [PubMed] [Google Scholar]
- 101.McKenney J. M., Koren M. J., Kereiakes D. J., Hanotin C., Ferrand A. C., Stein E. A. 2012. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J. Am. Coll. Cardiol. 59: 2344–2353 [DOI] [PubMed] [Google Scholar]
- 102.Stein E. A., Gipe D., Bergeron J., Gaudet D., Weiss R., Dufour R., Wu R., Pordy R. 2012. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 380: 29–36 [DOI] [PubMed] [Google Scholar]