Abstract
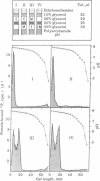
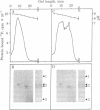
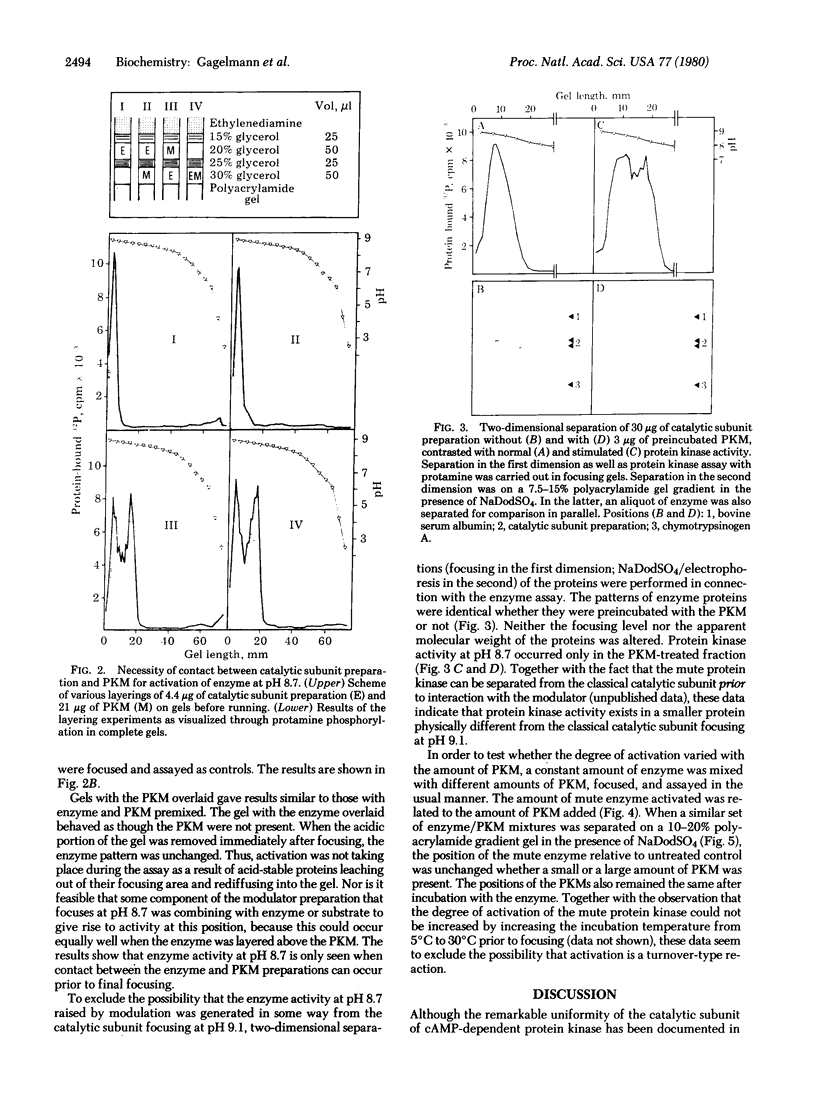
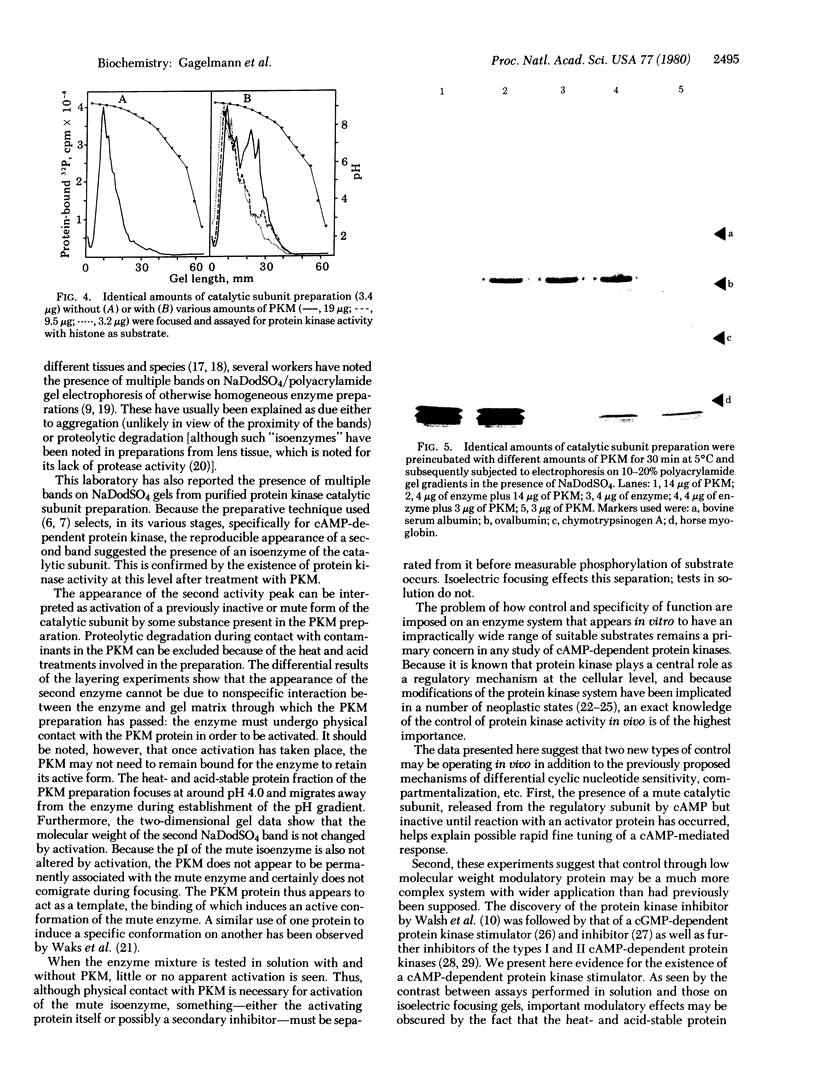
An isoenzyme of the catalytic subunit of type II cyclic AMP-dependent protein kinase from rat muscle is reported which coelutes with the classical catalytic subunit but differs from it in isoelectric point (pI 8.7 vs pI 9.1) and is enzymmatically inactive. After reaction with a heat- and acid-stable component of the protein kinase modulator fraction from the same tissue, the "mute" isoenzyme displays a high activity when assayed on isoelectric focusing gels. This activation process does not occur through proteolytic degradation and is not characteristic of a turnover-type reaction. The data imply direct interaction between the isoenzyme and a modulating protein which may subsequently be separated from the enzyme without reversal of the activation. The modulator protein thus appears to act as a template, inducing a conformational change. The implications of such a mute isoenzyme and its control through small modulator proteins are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaille J. G., Peters K. A., Fischer E. H. Isolation and properties of the rabbit skeletal muscle protein inhibitor of adenosine 3',5'-monophosphate dependent protein kinases. Biochemistry. 1977 Jul 12;16(14):3080–3086. doi: 10.1021/bi00633a006. [DOI] [PubMed] [Google Scholar]
- Finlayson G. R. Isoelectric focusing in polyacrylamide gel and its preparative application. Anal Biochem. 1971 Apr;40(2):292–311. doi: 10.1016/0003-2697(71)90388-5. [DOI] [PubMed] [Google Scholar]
- Gagelmann M., Pyerin W., Kübler D., Kinzel V. Protein kinase assay on isoelectric focusing gels: evaluation of its quantitative potentials. Anal Biochem. 1979 Feb;93(1):52–59. [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. Role of the receptor in the mechanism of action of adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):786–790. doi: 10.1073/pnas.68.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., Kuo J. F. On the mechanism of action of cyclic AMP. Adv Biochem Psychopharmacol. 1970;3:287–306. [PubMed] [Google Scholar]
- Hashimoto E., Kuroda Y., Ku Y., Nishizuka Y. Stimulation by polydeoxyribonucleotide of histone phosphorylation by guanosine 3':5'-monophosphate-dependent protein kinase. Biochem Biophys Res Commun. 1979 Mar 15;87(1):200–206. doi: 10.1016/0006-291x(79)91666-8. [DOI] [PubMed] [Google Scholar]
- Hirsch A., Rosen O. M. An assay for protein activity on polyacrylamide gels. Anal Biochem. 1974 Aug;60(2):389–394. doi: 10.1016/0003-2697(74)90246-2. [DOI] [PubMed] [Google Scholar]
- Kinzel V., Kübler D. Single step purification of the catalytic subunit(s) of cyclic 3', 5'-adenosine monophosphate-dependent protein kinase(s) from rat muscle. Biochem Biophys Res Commun. 1976 Jul 12;71(1):257–264. doi: 10.1016/0006-291x(76)90276-x. [DOI] [PubMed] [Google Scholar]
- Kumon A., Yamamura H., Nishizuka Y. Mode of action of adenosine 3',5'-cyclic phosphate on protein kinase from rat liver. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1290–1297. doi: 10.1016/0006-291x(70)90228-7. [DOI] [PubMed] [Google Scholar]
- Kuo J. F. Guanosine 3':5'-monophosphate-dependent protein kinases in mammalian tissues. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4037–4041. doi: 10.1073/pnas.71.10.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler D., Gagelmann M., Pyerin W., Kinzel V. Isolation of the catalytic subunits of cyclic AMP-dependent protein kinases from different mammalian tissues on the basis of charge differences of their subunits. Hoppe Seylers Z Physiol Chem. 1979 Oct;360(10):1421–1431. doi: 10.1515/bchm2.1979.360.2.1421. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lassam N. J., Bayley S. T., Graham F. L., Branton P. E. Immunoprecipitation of protein kinase activity from adenovirus 5-infected cells using antiserum directed against tumour antigens. Nature. 1979 Jan 18;277(5693):241–243. doi: 10.1038/277241a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- MacGillivray A. J., Rickwood D. The heterogeneity of mouse-chromatin nonhistone proteins as evidenced by two-dimensional polyacrylamide-gel electrophoresis and ion-exchange chromatography. Eur J Biochem. 1974 Jan 3;41(1):181–190. doi: 10.1111/j.1432-1033.1974.tb03258.x. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Rousseau G. G., De Visscher M. Artifactual stimulation of cyclic AMP-dependent protein kinase activity by the heat-stable protein kinase "inhibitor". Biochem Biophys Res Commun. 1976 Oct 18;72(4):1423–1429. doi: 10.1016/s0006-291x(76)80172-6. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Molecular forms and subunit composition of a cyclic adenosine 3',5'-monophosphate-dependent protein kinase purified from bovine heart muscle. J Biol Chem. 1972 Jan 10;247(1):36–44. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Rübsamen H., Friis R. R., Bauer H. Src Gene product from different strains of avian sarcoma virus: Kinetics and possible mechanism of heat inactivation of protein kinase activity from cells infected by transformation-defective, temperature-sensitive mutant and wild-type virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):967–971. doi: 10.1073/pnas.76.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmigielski A., Guidotti A., Costa E. Endogenous protein kinase inhibitors. Purification, characterization, and distribution in different tissues. J Biol Chem. 1977 Jun 10;252(11):3848–3853. [PubMed] [Google Scholar]
- Takáts A., Antoni F., Faragó A. Cyclic nucleotide binding properties and molecular forms of the cyclic-AMP-dependent protein kinase from bovine eye lens. Biochim Biophys Acta. 1979 Jun 6;568(2):475–483. doi: 10.1016/0005-2744(79)90316-4. [DOI] [PubMed] [Google Scholar]
- Waks M., Yip Y. K., Beychok S. Influence of prosthetic groups on protein folding and subunit assembly. Recombination of separated human alpha-and beta-globin chains with heme and alloplex interactions of globin chains with heme-containing subunits. J Biol Chem. 1973 Sep 25;248(18):6462–6470. [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D. Protein kinases: aspects of their regulation and diversity. Recent Prog Horm Res. 1973;29:329–359. doi: 10.1016/b978-0-12-571129-6.50012-9. [DOI] [PubMed] [Google Scholar]