Abstract
Primary adipocyte isolation by collagenase digestion is a widely used technique to study metabolic regulation and insulin action in adipocytes. However, induction of a proinflammatory response characterized by enhanced secretion of interleukin (IL)-6 has been tightly linked to the isolation process itself. To test the hypothesis that the shaking mechanical force exerted on adipocytes stimulates inflammation during isolation, rat primary adipocytes were prepared by collagenase digestion in orbital shaking incubators maintained at varying speeds. Contrary to expectation, the isolation-induced release of IL-6 was attenuated by increasing the rotational speed of digestion and the concentration of collagenase, both of which resulted in rapid dissociation of adipocytes from the vasculature. In addition, the attenuation of IL-6 secretion was associated with decreased phosphorylation of the stress-related p38 mitogen-activated protein kinase (p38 MAPK) and preserved insulin action. The data suggest that optimization of parameters including, but not limited to, mincing technique, time of digestion, and collagenase concentration will make it possible to isolate primary adipocytes without activation of a proinflammatory response leading to elevated secretion of IL-6.
Keywords: obesity, inflammation, digestion, collagenase and insulin sensitivity
Dysregulation of adipose tissue has been shown to play a critical role in the etiologies of numerous pathologies, including obesity, insulin resistance, and atherosclerosis (1, 2). Adipose tissue is composed of not only adipocytes but also a variety of immune cells and an elaborate stromal-vascular matrix (3, 4). Considering the cellular heterogeneity of this tissue, dissecting out the contribution of different cell types to disease phenotypes is not a trivial endeavor. Isolating primary adipocytes from other adipose-resident cell types allows for the measurement of adipocyte-specific gene expression, metabolism, and responses to various hormones. The primary adipocyte isolation technique was pioneered in the 1960s by Martin Rodbell, who showed that a homogeneous preparation of adipocytes could be made by dissecting out the fat pads of rodents, cutting the adipose tissue into pieces, digesting it with a combination of collagenase and mechanical rotation, and subsequently removing the stromal-vascular cells via centrifugation (5).
Primary adipocyte isolation techniques based on Rodbell's original protocol have been widely applied, with a particular focus on insulin sensitivity as measured by levels of GLUT4 translocation, glucose and lipid metabolism, and more recently, adipokine secretion and action (6–13). It is, therefore, important to understand how the isolation process itself and its constituent variables affect insulin sensitivity in liberated adipocytes.
Previous studies have demonstrated that the isolation process itself has negative effects on metrics of primary adipocyte inflammation and insulin sensitivity. Ruan et al. showed that isolating adipocytes by collagenase digestion induced an acute and chronic release of the proinflammatory factors tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), respectively (14). Importantly, treatment with these cytokines has been shown to promote insulin resistance in 3T3-L1 adipocytes (15–19). Consistent with these findings, Ruan et al. also showed that collagenase digestion of mouse epididymal adipose tissue triggered a downregulation of the expression of several genes involved in insulin signaling and sensitivity in adipocytes, including, insulin receptor substrate 2 (IRS2), phosphoinositide 3-kinase (PI3K), glucose transporter 4 (GLUT4), adiponectin, and peroxisome proliferator-activated receptor γ (PPAR-γ) (14). Interestingly, expression of genes involved in insulin responsiveness were downregulated even in adipocytes isolated from TNF-α(−/−) knockout mice, suggesting that increased IL-6, not TNF-α, secretion is the more likely cause of isolation-induced inflammation and subsequent insulin desensitization (14). Data from humans also suggest a prominent role for IL-6 in obesity-induced adipocyte insulin resistance given that adipose tissue IL-6 content, but not TNF-α or leptin content, is strongly inversely correlated with insulin-stimulated glucose transport in isolated adipocytes (20).
The observation that the adipocyte isolation process leads to the downregulation of genes involved in insulin responsiveness while concomitantly inducing the release of the potent proinflammatory and insulin-desensitizing cytokine IL-6 underscores the need to reevaluate this procedure in order to mitigate this response to prepare the most physiologically relevant adipocyte populations ex vivo. Accordingly, the goal of this study was to determine conditions for the primary adipocyte isolation technique that mitigate the IL-6 release inherent to this procedure.
MATERIALS AND METHODS
Animals
Retired male Sprague Dawley breeders (∼300 g) were obtained from Charles River Laboratories and maintained on a normal chow diet and a 12 h light-dark cycle. The Institutional Animal Care and Use Committee of the University of Arizona in accordance with the Public Health Service Animal Welfare Policy approved all experimental protocols.
Primary adipocyte isolation and quantification of primary adipocyte yield
Following anesthesia by CO2 inhalation and euthanasia by decapitation, approximately 2.0–3.0 g of epididymal fat were dissected out and placed in Krebs-Ringer-Henseleit buffer (KRHB, 30 mM HEPES, pH 7.4, 1 mM CaCl2, 120 mM NaCl, 4 mM KH2PO4, 1 mM MgSO4, 10 mM NaCO3, 200nM adenosine) containing 2% Fraction-V BSA (Sigma) prior to being weighed. Adipose tissue was then placed in digestion solution [KRHB containing 2% BSA and 3.3 mg/ml collagenase (≥125 U/mg, Type II, Sigma) unless otherwise indicated] at a 1:1 ratio of milliliters of digestion solution to grams of adipose tissue. The tissue was then minced with surgical scissors for ∼2 min (at ∼3 openings and closings of blades per second), resulting in small (∼1–2 mm3) pieces of adipose tissue. The digestion solution-tissue mixture was then transferred to a 250 ml siliconized-glass Erlenmeyer flask and incubated in orbital shaking incubators at 37°C, rotating at 30, 75, or 120 rpm. Digestions were deemed complete when no large clumps of tissue were visible and ≥ 90% of the digestion solution-tissue mixture formed a uniform film of cells when the mixture was tilted and allowed to run down the bottom of the flask. Upon digestion completion, the cells were poured into a siliconized-glass separating funnel for delicate washing and removal of stromal-vascular components without the need for centrifugation. Cells were washed four times in the separating funnel with 10–15 ml of KRHB or RPMI 1640 media (Invitrogen). The cells were then poured through sterile gauze to remove any remaining clumps of nondissociated adipocytes or stromal-vascular components into graduated 15 ml polypropylene conical centrifuge tubes. Any remaining liquid below the layer of adipocytes was aspirated off using a pipetting needle (Popper and Sons). The volume of isolated adipocytes per gram of tissue digested was estimated using the printed graduations on the conical tubes. At this point, the isolated adipocytes were incubated further or lysed as described below.
Counting of cells released from adipose tissue during collagenase digestion
Fifty microliter aliquots of adipose tissue digestion mixtures (containing 3.3 mg collagenase/ml and rotating at 120 rpm at 37°C) were removed using wide bore tips at the specified time points following the addition of the digestion solution. The digestion mixture was then spotted onto a 40 µm mesh cell strainer. Using a clean pipette tip, the cell suspension was drawn into the tip from the opposite side of the mesh to remove clumps of tissue. KRHB with 2% BSA was added to the cell suspensions to obtain cell densities suitable for the Countess automated cell counter (Invitrogen). Cell suspensions were then diluted 1:1 with a 0.4% Trypan Blue solution (Invitrogen). Ten microliters of these mixtures were added to Countess cell counting slides with 100 µm chamber depth prior to initiation of cell counting. Only live cells were counted.
Primary adipocyte incubation
Isolated adipocytes were incubated for 22 h at 37°C in incubation media (RPMI 1640 media (Invitrogen) containing 2% Fraction-V BSA, 100 U/ml penicillin, and 100 µg/ml streptomycin, pH 7.4) at a 1:1 or 1:1.5 ratio of milliliters of adipocytes to milliliters of incubation media.
Primary adipocyte size determination
One microliter of Nile red (AdipoRed, Lonza) was added to a 50 µL aliquot of isolated adipocytes in KRHB. Images of at least 45 cells from each group were taken from a minimum of six independent fields. Cells were visualized using a Leica DM IL inverted microscope equipped with a mercury arc lamp and an Omega XF100 filter set. Images were taken with a Qimaging CCD camera and QCapture imaging software. Cell diameters were measured using NIH ImageJ.
Insulin stimulation of primary adipocytes
Following the 22 h postdigestion incubation, isolated adipocytes were treated with or without 100 nM insulin (Humulin R, Eli Lilly) for 10 min at 37°C. Cells were then diluted 7-fold in KRHB and centrifuged for 3 min at 150 rcf. The buffer below the top adipocyte layer was aspirated off, and the pellet of cells was frozen at −80°C until lysate preparation.
Cell lysate preparation and Western blotting
Adipocytes were isolated for lysate preparation either at the time of digestion completion or at the end of the 22 h postdigestion incubation following treatment with or without insulin. Homogenization buffer (5× 25 mM HEPES pH 7.4, 125 mM NaCl, 10 mM sodium orthovanadate, 500 mM NaF, 10 µM microcystine LR, 1:10 Sigma protease inhibitor cocktails 1 and 2 supplemented with one Roche mini-Complete protease inhibitor cocktail without EDTA per 2 ml lysis buffer) was added to aliquots of isolated adipocytes at one-fifth the volume of cells. The cells were then disrupted using a tissue homogenizer (Eberbach Con-Torque) with a Teflon pestle rotating at medium-high speed for fifteen 2 s strokes. Homogenates were then sonicated for 8 s at 30% amplitude (0.5 s ON and 0.5 s OFF) at 4°C, and then centrifuged at 14,000 rcf for 30 min at 4°C to separate the lipids from the supernatant, which was collected using a needle and syringe. Total protein concentrations were determined using the Bradford assay (Bio-Rad). Proteins were fractionated via SDS-PAGE and transferred to nitrocellulose paper. Immunoblotting was performed using purified polyclonal rabbit antibodies against p38 mitogen-activated protein kinase (p38 MAPK), pan-Akt, and β-actin (all from Cell Signaling) and purified monoclonal mouse antibodies against phospho-p38 MAPK (Thr180/Tyr182) (Cell Signaling) and phospho-Akt (Thr308) (BD Biosciences). Secondary antibodies were IRDye 800 conjugated goat anti-mouse and IRDye 680 conjugated goat anti-rabbit antibodies (LI-COR). Imaging was performed using the Odyssey® Infrared Imaging System (LI-COR). To ensure equal protein loading, some blots were stained with 0.5% Ponceau S and then destained with ddH2O prior to blocking and immunoblotting.
ELISAs
Aliquots of the digestion solution were collected and flash frozen in liquid nitrogen at the time of digestion completion. Aliquots of the incubation media were collected and flash frozen in liquid nitrogen 22 h after the start of the incubation. The concentrations of IL-6 and IL-1β in the digestion solution and incubation media samples (adjusted for the cell:media volume) were determined using Quantikine rat IL-6 (R and D Systems) and rat IL-1β (Signosis) ELISA kits, respectively.
Statistical analysis
Data are presented as mean ± SE. Statistical comparisons between three groups were performed using one-way ANOVA with Tukey post hoc test (Figs. 1B, 2A–E, 4C, 5A). Statistical comparisons between two groups were performed using Student's paired two-tailed t-test (Figs. 3A–C, 4B) or one sample Student's two-tailed t-test (Fig. 5C). Groups not sharing a common letter are statistically different.
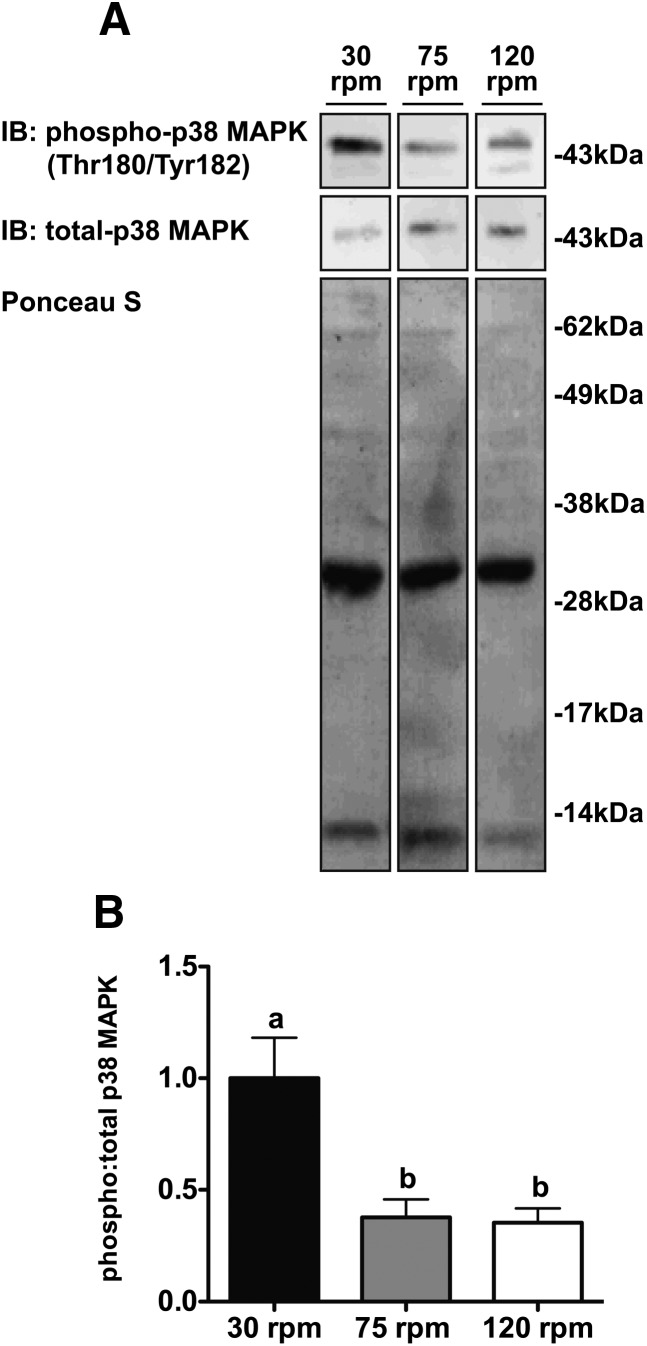
Fig. 1.
High rotational speeds of digestion attenuate p38 MAPK activation in isolated adipocytes. (A) Representative Western blot and (B) densitometry quantifying the ratio of phospho-p38 MAPK (Thr180/Ty182) to total p38 MAPK intensity at the time of digestion completion in adipocytes isolated from tissue digested at a rotational speed of 30, 75, or 120 rpm. The relative amount of total protein loaded in the different lanes was assessed by staining with Ponceau S prior to blocking and immunoblotting (n = 4 per group). Data were normalized to phospho:total p38 MAPK ratio at 30 rpm. LC/MS/MS was performed on the predominant band running at ∼35 kDa in the Ponceau S stain. Proteins identified by Scaffold 3 software as being present with greater than 99.9% probability included carbonic anhydrase 3, 14-3-3 protein γ, electron transfer flavoprotein subunit β, phosphoglycerate mutase 1, 14-3-3 protein β/alpha, and adiponectin. One-way ANOVA with Tukey's post hoc test was used for all between-group analyses. Groups not sharing a common letter are significantly different (P < 0.007).
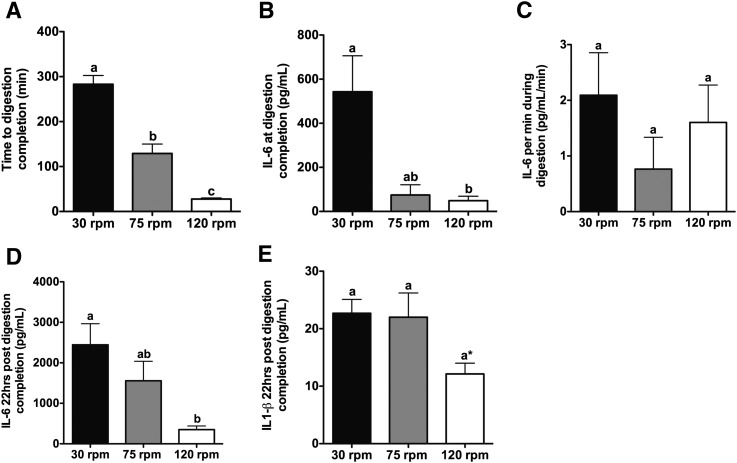
Fig. 2.
High rotational speeds of digestion reduce the time to digestion completion and attenuate IL-6 secretion at digestion completion and 22 h post digestion completion. (A) Time to digestion completion, (B) IL-6 secreted at digestion completion, (C) IL-6 secreted per min during digestion, (D) IL-6 secreted 22 h post digestion completion, and (E) IL-1β secreted 22 h post digestion completion for tissues digested at rotational speeds of 30, 75, or 120 rpm (n = 3–6 per group). One-way ANOVA with Tukey's post hoc test was used for all between-group analyses. Groups not sharing a common letter are significantly different. (A) P < 0.0001 for 30 versus 75 rpm and 30 versus 120 rpm, whereas P < 0.001 for 75 versus 120 rpm. (B) P < 0.02. (D) P < 0.04. The asterisk (*) in (E) denotes P = 0.0507 for 30 versus 120 rpm.
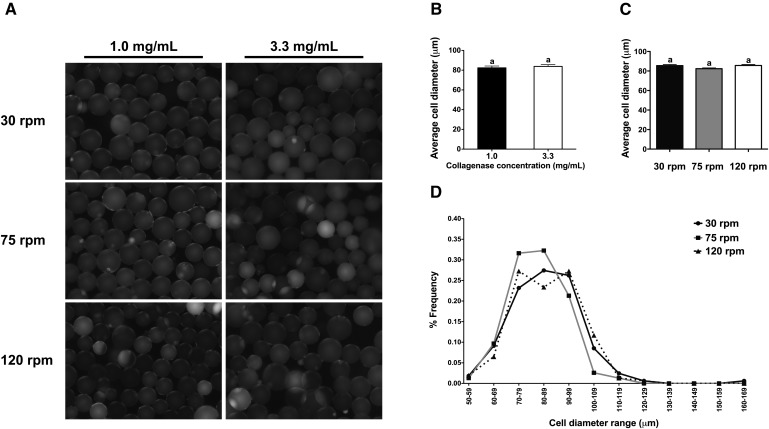
Fig. 4.
The concentration of collagenase and rotational speed of digestion have no effect on cell diameter. (A) Representative fluorescent images of adipocytes stained with Nile red and (B) average cell diameter of adipocytes isolated from tissues digested at rotational speeds of 30, 75, or 120 rpm with 1.0 or 3.3 mg collagenase/ml (n = 46–118 per rotational speed per collagenase concentration). Student paired two-tailed t-test was used for between-group analyses. (C) Average cell diameter of adipocytes isolated from tissues digested at 30, 75, or 120 rpm with 3.3 mg collagenase/ml (n = 154–164 per group). One-way ANOVA with Tukey's post hoc test was used for all between-group analyses. (D) Distribution of cell diameters of adipocytes isolated from tissues digested at 30, 75, or 120 rpm with 3.3 mg collagenase/ml (n = 154–164 per group). Groups not sharing a common letter are significantly different (P < 0.05).
Fig. 5.
Effect of rotational speed of digestion on adipocyte yield and insulin-stimulated Akt phosphorylation. (A) Average adipocyte yield from tissues digested at 30, 75, or 120 rpm with 3.3 mg collagenase/ml (n = 8 per group). One-way ANOVA with Tukey's post hoc test was used for all between-group analyses. (B) Representative Western blot and (C) densitometry quantifying the ratio of phospho-Akt (Thr308) to total-Akt 22 h post digestion completion in adipocytes isolated from tissues digested at rotational speeds of 30, 75, or 120 rpm with 3.3 mg collagenase/ml in the absence or presence of 100 nM insulin for 10 min at 37°C (n = 4 per rotational speed per treatment). The relative amount of total protein loaded in the different lanes was assessed by immunoblotting for β-actin. Data were normalized to “no insulin” at each rotational speed for each experiment. For each rotational speed, a one sample Student two-tailed t-test with hypothetical mean set to 1 for groups not treated with insulin was used to compare phospho:total Akt ratio in the absence or presence of insulin. Groups not sharing a common letter are significantly different (P < 0.04).
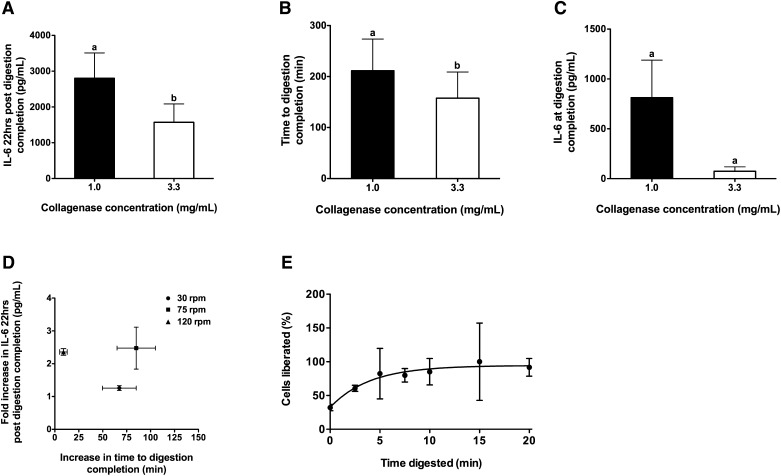
Fig. 3.
High collagenase concentration reduces the time to digestion completion and attenuates IL-6 secretion 22 h post digestion completion. (A) IL-6 secreted 22 h post digestion completion, (B) time to digestion completion, and (C) IL-6 secreted at digestion completion for tissues digested with 1.0 or 3.3 mg collagenase/ml. Equal numbers of paired observations at rotational speeds of 30, 75, or 120 rpm were combined to assess the overall effect of collagenase independent of rotational speed. A, B: n = 6 per collagenase concentration with two observations per rotational speed and C, n = 5 in total with two observations each for 30 and 120 rpm and 1 observation for 75 rpm. Student paired two-tailed t-tests were used for all between-group analyses. (D) Scattergram of the increase in the time to reach digestion completion (x axis) versus the fold increase in IL-6 secreted 22 h post digestion completion (y axis) for tissues digested with 1.0 versus 3.3 mg collagenase/ml when rotated at 30, 75, or 120 rpm (n = 2 per group). (E) Fraction of Trypan Blue-negative cells liberated as a function of digestion time using 3.3 mg collagenase/ml and rotation at 120 rpm (the curve was derived from nonlinear regression analysis (exponential one-phase association) with n = 2 per time point and r2 = 0.35). Groups not sharing a common letter are significantly different. (A) P < 0.02. (B) P < 0.03. (C) P = 0.1044.
RESULTS
p38 MAPK activation and IL-6 secretion are attenuated in rapidly dissociated adipocytes
The traditional primary adipocyte isolation procedure employs both enzymatic and mechanical digestion of adipose tissue. Previous studies have shown that shearing mechanical stress induces p38 MAPK phosphorylation, which subsequently leads to increased IL-6 production (21). Therefore, we hypothesized that delicate digestion at a slow rotational speed would reduce shearing mechanical stress as measured by p38 MAPK phosphorylation and thereby reduce IL-6 secretion.
Contrary to our expectations, adipocytes isolated by rotating ∼2.0–3.0 g of adipose tissue at 30 rpm had ∼2.7-fold (P < 0.007) greater p38 MAPK phosphorylation than adipocytes isolated by rotating at 75 or 120 rpm (Fig. 1A, B). As expected, increasing the rotational speed and, therefore, mechanical digestion, reduced the time to digestion completion. On average, it took tissue rotated at 30 rpm 154 min (P < 0.0001) and 255 min (P < 0.0001) longer to reach digestion completion than tissue rotated at 75 and 120 rpm, respectively. On average, it took tissue rotated at 75 rpm 101 min (P < 0.001) longer to reach digestion completion than tissue rotated at 120 rpm (Fig. 2A).
Consistent with the observation that slow dissociation at 30 rpm is associated with greater p38 MAPK activation, at the time of digestion completion, adipose tissue digested by rotating at 30 rpm secreted 7.3- and 11.1-fold (P < 0.02) more IL-6 than adipose tissue digested by rotating at 75 or 120 rpm, respectively (Fig. 2B). Taking into consideration both the concentration of IL-6 at the time of digestion completion and the time to digestion completion, the rate of IL-6 secretion was not significantly different when tissues were rotated at 30, 75, or 120 rpm (Fig. 2C).
To determine how rotational speed influences IL-6 production in adipocytes after cessation of the digestion procedure, isolated adipocytes were cultured for 22 h following washing and removal of the stromal-vascular components. Adipocytes isolated by rotating at 30 rpm secreted 1.6- and 7.0-fold (P < 0.04) more IL-6 than adipocytes isolated by rotating at 75 or 120 rpm, respectively (Fig. 2D). Importantly, the concentrations of IL-6 secreted from adipocytes isolated by rotating at 30 and 75 rpm (∼2,500 pg/ml and ∼1,500 pg/ml, respectively) approach the concentrations reported to reduce insulin sensitivity in adipocytes (5,000–20,000 pg/ml) (16, 19).
Interleukin-1 β (IL-1β), like IL-6, has been shown to reduce insulin sensitivity in adipocytes (22, 23). As insulin sensitivity is a common metric assessed in isolated primary adipocytes, we sought to measure the secretion of IL-1β in response to various rotational speeds of digestion. At the time of digestion completion, the absolute concentrations of IL-1β at all three rotational speeds were below the assay's limit of detection (data not shown). Twenty-two hours post digestion completion, IL-1β secretion showed a strong tendency to be lower in adipocytes isolated by rotating at 120 rpm compared with adipocytes isolated by rotating at 30 or 75 rpm (P = 0.0507, Fig. 2E). The highest concentration of IL-1β secreted by isolated adipocytes in these experiments (≤23 pg/ml), however, was much lower than the concentration of IL-1β reported to reduce insulin sensitivity in adipocytes (20,000 pg/ml) (22).
High collagenase concentration during digestion is associated with reduced IL-6 secretion in dissociated adipocytes
A search of the literature for studies employing a primary adipocyte isolation procedure revealed substantial variation regarding technical details, including the mincing technique, collagenase concentration, time to digestion completion, rotational shaking speed, digestion container, digestion buffer, the ratio of tissue to volume of digestion buffer, and the amount of tissue digested (Table 1). Many of these studies used a lower collagenase concentration (0.5–1.0 mg/ml) than that used in the studies presented here or in Rodbell's initial studies (3.3 mg/ml). Given that a lower collagenase concentration is expected to decrease the rate of enzymatic digestion and our observation that a more rapid dissociation of adipocytes leads to attenuated IL-6 secretion, we directly compared the time to digestion completion and IL-6 secretion between tissues digested with 1.0 versus 3.3 mg collagenase/ml in a subset of our experiments.
TABLE 1.
Adipose tissue digestion conditions in a sampling of studies employing a primary adipocyte isolation procedure
| Reference | Mincing Technique | Collagenase Concentration | DigestionTime (h) | Rotational Speed | Digestion Container | Digestion Buffer (g tissue/ml buffer) | Tissue Amount |
| (5) | “Distal portions from each pad were cut into three pieces” | 3.3 mg/ml | 1.0 | NDA | Siliconized 25 ml flask | BB 4% BSA, 3 mM glucose (1/3) | ≤1.0 g |
| (6) | “Minced” | 0.5 mg/ml | 1.0 | Stirred with a 6 × 15 mm smooth Teflon bar | Polystyrene 30 ml cylinder | Buffer containing 3.5% BSA (NDA) | NDA |
| (28) | NDA | NDA | NDA | 30 rpm | NDA | KHB 2% BSA, 2 mM glucose (1/10–1/20) | 0.5–1.0 g |
| (8) | “Pads were chopped with a Mickle tissue mincer” | 1.0 mg/ml | 0.5 | NDA | NDA | KRH 1% BSA, 2 mM glucose (1/3) | 1.0 g |
| (29) | “Minced with sharp dissecting scissors [until] pieces are ∼2 mm in size” | 3.3 mg/ml | 1.0 | 100 spm | Soft-sided 1 oz polypropylene vial | KRBH 1% BSA (1/1) | 6.0 g |
| (10) | NDA | NDA | NDA | NDA | NDA | KRBH 1% BSA (NDA) | NDA |
| (30) | “Finely cut” | 5.0 mg/ml | 0.5 | 160 cpm | NDA | KRBH 1% BSA, 2.7 mM glucose (1/1) | 3.0 g |
| (14) | “Minced” | NDA | 2.0 | NDA | NDA | NDA (NDA) | ∼10 cells/ml |
| (31) | NDA | 1.0 mg/ml | NDA | NDA | NDA | NDA (NDA) | NDA |
| (26) | “Tissue was cut with scissors into small pieces (10–20 mg)” | 0.65 mg/ml | 2.0 | 100 rpm | NDA | Buffer + BSA (1/2) | 1.0 g |
| (32) | “Minced” | 100 U/ml | 1.0 | NDA | NDA | KRP 4% BSA (NDA) | NDA |
| (33) | “Mince the tissue into small pieces, approximately 5–10 mg per piece (1–2 mm ), using sterile sharp scissors” | 1.0 mg/ml | 0.5–1.0 | 100 rpm | Polypropylene 50 cc tube | KRB or M199 4% BSA (1/2–1/3) | NDA |
| Thompson et al. 2012 | “Minced with surgical scissors for ∼2 min (at ∼3 openings and closings of blades per second) resulting in small (∼1–2 mm ) pieces of adipose tissue” | 3.3 mg/ml | 0.5a or 2.0b | 120 or 75 rpm | Siliconized 250 ml glass flask | KRHB 2% BSA (1/1) | ∼2.0–3.0 g |
BB, bicarbonate buffer; CPM, cycles per minute; KHB, Krebs-Henseleit buffer; KRB, Krebs-Ringer bicarbonate buffer; KRBH, Krebs-Ringer bicarbonate-HEPES buffer; KRH, Krebs-Ringer media buffered with HEPES; KRHB, Krebs-Ringer-Henseleit buffer; KRP, Krebs-Ringer phosphate buffer; NDA, no data available; SPM, strokes per minute.
Corresponding digestion time for rotational speed of 120 rpm.
Corresponding digestion time for rotational speed of 75 rpm.
To determine the effects of collagenase concentration on the time to digestion completion and IL-6 secretion independent of rotational speed, paired observations from an equal number of experiments with rotation at 30, 75, and 120 rpm were pooled. These experiments showed that adipocytes isolated with 1.0 mg collagenase/ml secreted 1.8-fold (P < 0.02) more IL-6 at 22 h post digestion completion than adipocytes isolated with 3.3 mg collagenase/ml (Fig. 3A). This was accompanied by a 54 min (P < 0.03) increase in the amount of time for digestion to reach completion (Fig. 3B) and a tendency for increased IL-6 concentrations at the time of digestion completion (10.8-fold increase, P = 0.1044, Fig. 3C) when adipose tissue was digested with 1.0 versus 3.3 mg collagenase/ml. Interestingly, while increases in the time to digestion completion and IL-6 secreted after overnight culture were observed in groups treated with 1.0 versus 3.3 mg collagenase/ml, regardless of rotational speed, adipocytes prepared by rotating at 120 rpm secreted 2.4-fold more IL-6 after overnight culture, even though they only spent on average 9 additional minutes (23–32 min) in the collagenase digestion mixture (Fig. 3D). These data suggest that the prolongation of the time to reach digestion completion due to lowering the collagenase concentration could be responsible for the increased IL-6 secretion at 22 h post digestion completion, but it is likely not the only factor.
Importantly, upon quantifying the number of cells liberated at specific times during digestion at 120 rpm using 3.3 mg collagenase/ml we found that greater than 50% of the cells were liberated after just 2.5 min and 100% of the cells were liberated after 10–15 min (Fig. 3E).
Effects of collagenase concentration and rotational speed on adipocyte integrity
Both rotation speed and duration of digestion could have a negative impact on the physical and functional integrity of isolated adipocytes. Therefore, the following metrics of integrity were assessed in adipocytes isolated under the various conditions: i) cell diameter, ii) adipocyte yield, and iii) insulin-stimulated Akt phosphorylation. There was no difference in the average diameter of adipocytes isolated with 1.0 versus 3.3 mg collagenase/ml (Fig. 4A, B). In addition, there was no difference in the average diameter or the distribution of diameters of adipocytes isolated using 3.3 mg collagenase/ml with rotation at 30, 75, or 120 rpm (Fig. 4C, D). There was no difference in adipocyte yield when adipocytes were isolated using 3.3 mg collagenase/ml with rotation at 30, 75, or 120 rpm (Fig. 5A). Lastly, insulin-stimulated Akt phosphorylation at 22 h post digestion completion was highest in adipocytes isolated using 3.3 mg collagenase/ml with rotation at 120 rpm (P < 0.04) (Fig. 5B, C). Surprisingly, there was essentially no insulin-stimulated Akt phosphorylation observed in adipocytes isolated using 3.3 mg collagenase/ml with rotation at 30 and 75 rpm.
DISCUSSION
Metabolic studies of isolated primary adipocytes have become widespread as the need to determine the mechanisms of metabolic diseases, including obesity and diabetes, has risen. The primary adipocyte isolation procedure used in these various studies varies little with regard to the primary steps of i) tissue dissection and mincing, ii) collagenase digestion, and iii) removal of stromal-vascular components. However, technical details of the procedure, including the mincing technique, collagenase concentration, time to digestion completion, rotational shaking speed, digestion container, digestion buffer, the ratio of tissue to volume of digestion buffer, and the amount of tissue digested appear to vary considerably among studies (Table 1). Many of these details are often not recorded, presumably because they are not considered important.
Previous work by Ruan et al. has shown that the primary adipocyte isolation process itself induces an inflammatory response as measured by secretion of TNF-α and IL-6, which have been shown to have insulin-desensitizing effects (14–19, 24). This prior study additionally demonstrated that the isolation process leads to the downregulation of several genes involved in insulin signaling. Considering that insulin sensitivity is a commonly assessed metric in primary adipocyte studies, these discoveries highlighted the need to minimize the dysregulation of liberated adipocytes to ensure that experimental outcomes are physiologically relevant.
Here we show that isolation-induced inflammation in primary adipocytes, as measured by IL-6 secretion, appears to arise primarily from the length of digestion time and is associated with p38 MAPK activation. For tissues weighing ∼2.0–3.0 g, digesting at a high rotational speed (75 or 120 rpm) resulted in lower p38 MAPK phosphorylation compared with rotation at 30 rpm (Fig. 1). Consistent with the observation that p38 MAPK activation induces IL-6 production (21), digestion at these high rotational speeds also resulted in lower IL-6 secretion at the time of digestion completion and 22 h post digestion completion compared with rotating at 30 rpm. In addition, the reduction in IL-6 secretion at both of these time points was correlated with a faster time to digestion completion (Fig. 2).
The rate of IL-6 secretion during the digestion process was comparable for tissues rotated at 30, 75, and 120 rpm (Fig. 2C). Therefore, because it took tissues rotated at 30 rpm longer to reach digestion completion than tissues rotated at 75 rpm, which took longer than tissues rotated at 120 rpm (Fig. 2A), the hierarchy of the absolute yield of IL-6 at the time of digestion completion was 30 > 75 > 120 rpm (Fig. 2B). Given that adipocytes isolated by rotating at 30, 75, and 120rpm were incubated for the same amount of time during the post-digestion experiments (22 h), the differences in IL-6 concentrations 22 h post digestion completion (Fig. 2D) must have been due to differences in the rate of IL-6 secretion between adipocytes isolated at the various rotational speeds. The rate of IL-6 secretion during the 22 h incubation may have been influenced by the rotational speed of digestion, the time to reach digestion completion, or the absolute amount of IL-6 produced during the digestion. Interestingly, chronic IL-6 treatment of differentiating 3T3-F442A adipocytes has been shown to increase endogenous IL-6 secretion in the resultant mature adipocytes (25).
Consistent with the observed positive correlation between time to digestion completion and IL-6 secretion in our initial studies, when a lower concentration of collagenase (1.0 mg/ml) was used, digestion at all three rotational speeds took longer to reach digestion completion and resulted in higher IL-6 secretion 22 h post digestion completion compared with digestions using a higher concentration of collagenase (3.3 mg/ml, Fig. 3AndashC). However, the length of time to reach digestion completion is likely not the only factor influencing IL-6 secretion. For adipocytes digested at 120 rpm in 1.0 mg collagenase/ml, just a small (9 min) increase in the absolute amount of time for digestion to reach completion resulted in a disproportionate increase in IL-6 secretion after overnight culture compared with digestion in 3.3 mg collagenase/ml (Fig. 3D). This likely reflects the inherent lack of precision in assessing the completeness of digestion by visual inspection. At the high rotational speed of 120 rpm, mechanical agitation may have caused most of the adipocytes to become separated from each other, leading to the appearance of digestion completion by visual inspection. However, the relative lack of extracellular matrix breakdown in low collagenase concentration could allow some macrophages to remain attached to single adipocytes, leading to increased secretion of IL-6 in overnight cultures. Future experiments should examine the relationship between collagenase concentration and the presence of macrophage-specific markers in adipocyte preparations.
Adipocyte integrity, as measured by adipocyte size and yield, were comparable between tissues digested with 3.3 mg collagenase/ml at 30, 75, and 120 rpm (Fig. 4C, D and Fig. 5A). Importantly, however, adipocytes isolated with rotation at 120 rpm exhibited the highest insulin sensitivity 22 h post digestion completion (Fig. 5B, C). The absence of insulin-stimulated Akt phosphorylation in adipocytes isolated with rotation at 30 or 75 rpm was unexpected. One possible explanation is that the commercial BSA stock was contaminated with some insulin. Therefore, the groups not treated with insulin may not reflect true basal, noninsulin-stimulated conditions. Despite this possibility, the insulin stimulation assay conditions were identical for all adipocytes isolated at the various rotational speeds and the adipocytes isolated with rotation at 120 rpm did indeed exhibit a ∼2-fold increase in Akt phosphorylation upon insulin stimulation.
The very short time (27 min) that it took adipose tissue digestions rotated at 120 rpm to reach digestion completion was likely due to three primary factors: i) the mincing technique, ii) the high concentration of collagenase (3.3 mg/ml), and iii) the high rotational speed. The mincing technique employed in the primary adipocyte isolation procedure described herein involved the mincing of adipose tissue bathed in the digestion solution for ∼2 min with surgical scissors, resulting in small (∼1–2 mm3) pieces of adipose tissue. This mincing technique greatly increases the surface area of the adipose tissue, thereby increasing the rate of both enzymatic and mechanical digestion. Given the strong impact of the mincing technique on the rate of digestion, this step should be standardized in future primary adipocyte isolation protocols.
As previously alluded to, the benefit of decreasing the time to adipose tissue digestion completion could be due to influences from cells other than adipocytes within the adipose tissue. It has been previously shown that >90% of the proinflammatory cytokines produced by the adipose tissue originates from nonfat cell types (26). It is possible that the cells within the adipose tissue stromal-vascular matrix and resident immune cells produce the majority of proinflammatory cytokines in response to the stress of the digestion process. Readily removing these cells from the proximity of adipocytes using conditions for rapid dissociation may diminish their ability to induce an inflammatory response in adipocytes in a paracrine manner. In addition, rapid digestion of adipose tissue may reduce isolation-induced inflammation by shortening the time in which the inner region of the tissue pieces are exposed to a hypoxic environment, which has been shown to induce IL-6 expression (27).
The observation that rotational speed and duration of digestion are likely not the only factors influencing IL-6 secretion underscores the need to evaluate multiple factors in the adipocyte isolation process. For example, the degree of mechanical force exerted on the adipose tissue likely contributes to the rate of digestion. Consideration of the centrifugal force equation (Fc = mv2/r, where Fc = centrifugal force, m = mass, v = velocity, r = radius) suggests that parameters, such as the radius of the digestion vessel, orbit size of the orbital shakers, and mass of the digestion mixture, could affect the rate of adipocyte dissociation. Indeed, we observed that shaking larger adipose tissue samples of ∼5.0–6.5 g in 3.3 mg collagenase/ml at 75 rpm led to a faster time to digestion completion and lower adipocyte IL-6 secretion after 22 h in culture compared with shaking tissues weighing ∼2.0–3.0 g (data not shown). However, when both rotational speed and digestion mixture mass are sufficiently high, the centrifugal force may become too large and cross a mechanical stress threshold beyond which adipocytes may not tolerate well. Consistent with this hypothesis, digesting ∼5.0–6.5 g of adipose tissue at 120 rpm led to increased adipocyte IL-6 secretion 22 h post digestion completion compared with digesting ∼2.0–3.0 g of tissue (data not shown). These observations suggest that there may be a fine balance between rapidity of digestion and induction of stress via mechanical force.
Taken together, the evidence presented here suggests that the proinflammatory IL-6 secretion associated with the primary adipocyte isolation procedure can be mitigated if adipocytes are rapidly dissociated. Without sacrificing yield or adipocyte insulin sensitivity, this rapid dissociation can be achieved by thoroughly mincing the adipose tissue, using a high concentration of collagenase (3.3 mg/ml), and optimizing the speed of rotation to deliver an appropriately high degree of mechanical force (120 rpm for ∼2.0–3.0 g of adipose tissue). Under these conditions, the duration of the digestion can be modified depending on the yield required (∼100% of cells are liberated after 15 min). These suggested guidelines will hopefully enhance the reproducibility and physiological relevance of future primary adipocyte studies.
Acknowledgments
The authors thank Dr. Erik Henriksen for valuable discussions and Michael Pham, David Briggs, and Rebecca Giron for technical assistance.
Footnotes
Abbreviations:
- GLUT4
- glucose transporter 4
- IL
- interleukin
- IRS2
- insulin receptor substrate 2
- PI3K
- phosphoinositide 3-kinase
- p38 MAPK
- p38 mitogen-activated protein kinase
- PPAR
- peroxisome proliferator-activated receptor
- TNF
- tumor necrosis factor
This work was supported by American Diabetes Association Junior Faculty Award 1-08-JF-54 (to T.-S.T.), by an Arizona Biomedical Research Commission grant (to T.-S.T.), by Graduate Training Grants in Biochemistry and Molecular and Cellular Biology GM-08659 (to A.C.S.T. and T.H.) and GM-08659 (to K.S.), and by Minority Access to Research Careers Grant GM-008718. Mass spectrometry and proteomics data were acquired by the Arizona Proteomics Consortium supported by National Institutes of Health Grants ES-06694 (to Southwest Environmental Health Sciences Center) and CA-023074 (to Arizona Cancer Center) and by the BIO5 Institute of the University of Arizona. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Kahn S. E., Hull R. L., Utzschneider K. M. 2006. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 444: 840–846 [DOI] [PubMed] [Google Scholar]
- 2.Allende-Vigo M. Z. 2010. Pathophysiologic mechanisms linking adipose tissue and cardiometabolic risk. Endocr. Pract. 16: 692–698 [DOI] [PubMed] [Google Scholar]
- 3.Sun K., Kusminski C. M., Scherer P. E. 2011. Adipose tissue remodeling and obesity. J. Clin. Invest. 121: 2094–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson E. K., Gutierrez D. A., Hasty A. H. 2010. Adipose tissue recruitment of leukocytes. Curr. Opin. Lipidol. 21: 172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodbell M. 1964. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 239: 375–380 [PubMed] [Google Scholar]
- 6.Foley J. E., Foley R., Gliemann J. 1980. Rate-limiting steps of 2-deoxyglucose uptake in rat adipocytes. Biochim. Biophys. Acta. 599: 689–698 [DOI] [PubMed] [Google Scholar]
- 7.Gliemann J., Rees W. D., Foley J. A. 1984. The fate of labelled glucose molecules in the rat adipocyte. Dependence on glucose concentration. Biochim. Biophys. Acta. 804: 68–76 [DOI] [PubMed] [Google Scholar]
- 8.Honnor R. C., Dhillon G. S., Londos C. 1985. cAMP-dependent protein kinase and lipolysis in rat adipocytes. II. Definition of steady-state relationship with lipolytic and antilipolytic modulators. J. Biol. Chem. 260: 15130–15138 [PubMed] [Google Scholar]
- 9.Joost H. G., Weber T. M., Cushman S. W. 1988. Qualitative and quantitative comparison of glucose transport activity and glucose transporter concentration in plasma membranes from basal and insulin-stimulated rat adipose cells. Biochem. J. 249: 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malide D., Ramm G., Cushman S. W., Slot J. W. 2000. Immunoelectron microscopic evidence that GLUT4 translocation explains the stimulation of glucose transport in isolated rat white adipose cells. J. Cell Sci. 113: 4203–4210 [DOI] [PubMed] [Google Scholar]
- 11.Phillips S. A., Kung J., Ciaraldi T. P., Choe C., Christiansen L., Mudaliar S., Henry R. R. 2009. Selective regulation of cellular and secreted multimeric adiponectin by antidiabetic therapies in humans. Am. J. Physiol. Endocrinol. Metab. 297: E767–E773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao L., Kinney B., Schaack J., Shao J. 2011. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes. 60: 1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fantuzzi G. 2005. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 115: 911–919 [DOI] [PubMed] [Google Scholar]
- 14.Ruan H., Zarnowski M. J., Cushman S. W., Lodish H. F. 2003. Standard isolation of primary adipose cells from mouse epididymal fat pads induces inflammatory mediators and down-regulates adipocyte genes. J. Biol. Chem. 278: 47585–47593 [DOI] [PubMed] [Google Scholar]
- 15.Ruan H., Hacohen N., Golub T. R., Van Parijs L., Lodish H. F. 2002. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 51: 1319–1336 [DOI] [PubMed] [Google Scholar]
- 16.Rotter V., Nagaev I., Smith U. 2003. Interleukin-6 (IL-6) induces insulin resistance in 3T3–L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 278: 45777–45784 [DOI] [PubMed] [Google Scholar]
- 17.Uno T., He J., Usui I., Kanatani Y., Bukhari A., Fujisaka S., Yamazaki Y., Suzuki H., Iwata M., Ishiki M., et al. 2008. Long-term interleukin-1alpha treatment inhibits insulin signaling via IL-6 production and SOCS3 expression in 3T3-L1 adipocytes. Horm. Metab. Res. 40: 8–12 [DOI] [PubMed] [Google Scholar]
- 18.Serrano-Marco L., Rodríguez-Calvo R., El Kochairi I., Palomer X., Michalik L., Wahli W., Vázquez-Carrera M. 2011. Activation of peroxisome proliferator-activated receptor-β/-δ (PPAR-β/-δ) ameliorates insulin signaling and reduces SOCS3 levels by inhibiting STAT3 in interleukin-6-stimulated adipocytes. Diabetes. 60: 1990–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie L., Ortega M. T., Mora S., Chapes S. K. 2010. Interactive changes between macrophages and adipocytes. Clin. Vaccine Immunol. 17: 651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastard J-P., Maachi M., Van Nhieu J. T., Jardel C., Bruckert E., Grimaldi A., Robert J-J., Capeau J., Hainque B. 2002. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J. Clin. Endocrinol. Metab. 87: 2084–2089 [DOI] [PubMed] [Google Scholar]
- 21.Zampetaki A., Zhang Z., Hu Y., Xu Q. 2005. Biomechanical stress induces IL-6 expression in smooth muscle cells via Ras/Rac1-p38 MAPK-NF-kappaB signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 288: H2946–H2954 [DOI] [PubMed] [Google Scholar]
- 22.Jager J., Grémeaux T., Cormont M., Le Marchand-Brustel Y., Tanti J-F. 2007. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 148: 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagathu C., Yvan-Charvet L., Bastard J-P., Maachi M., Quignard-Boulangé A., Capeau J., Caron M. 2006. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia. 49: 2162–2173 [DOI] [PubMed] [Google Scholar]
- 24.Ruan H., Miles P. D. G., Ladd C. M., Ross K., Golub T. R., Olefsky J. M., Lodish H. F. 2002. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes. 51: 3176–3188 [DOI] [PubMed] [Google Scholar]
- 25.Lagathu C., Bastard J-P., Auclair M., Maachi M., Capeau J., Caron M. 2003. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem. Biophys. Res. Commun. 311: 372–379 [DOI] [PubMed] [Google Scholar]
- 26.Fain J. N., Madan A. K., Hiler M. L., Cheema P., Bahouth S. W. 2004. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 145: 2273–2282 [DOI] [PubMed] [Google Scholar]
- 27.Wang B., Wood I. S., Trayhurn P. 2007. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 455: 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kono T., Suzuki K., Dansey L. E., Robinson F. W., Blevins T. L. 1981. Energy-dependent and protein synthesis-independent recycling of the insulin-sensitive glucose transport mechanism in fat cells. J. Biol. Chem. 256: 6400–6407 [PubMed] [Google Scholar]
- 29.Weber T. M., Joost H. G., Simpson I. A., Cushman S. W.1988. Methods for assessment of glucose transport activity and the number of gluscose transporters in isolated rat adipose cells and membrane fractions. In Insulin Receptors: Clinical assessment, Biological Responses, and Comparison to the IGF-I Receptor. C. R. Kahn and L. C. Harrison, editors. A. R. Liss, NY. 171–187.
- 30.Wang Y., Han J., Zang Y., Guo W. 2002. Effects of vanadate on leptin production from isolated rat adipocytes. Biol. Trace Elem. Res. 85: 171–182 [DOI] [PubMed] [Google Scholar]
- 31.Blüher M., Patti M-E., Gesta S., Kahn B. B., Kahn C. R. 2004. Intrinsic heterogeneity in adipose tissue of fat-specific insulin receptor knock-out mice is associated with differences in patterns of gene expression. J. Biol. Chem. 279: 31891–31901 [DOI] [PubMed] [Google Scholar]
- 32.Skurk T., Alberti-Huber C., Herder C., Hauner H. 2007. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 92: 1023–1033 [DOI] [PubMed] [Google Scholar]
- 33.Carswell K. A., Lee M-J., Fried S. K. 2012. Culture of isolated human adipocytes and isolated adipose tissue. Methods Mol. Biol. 806: 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]