Abstract
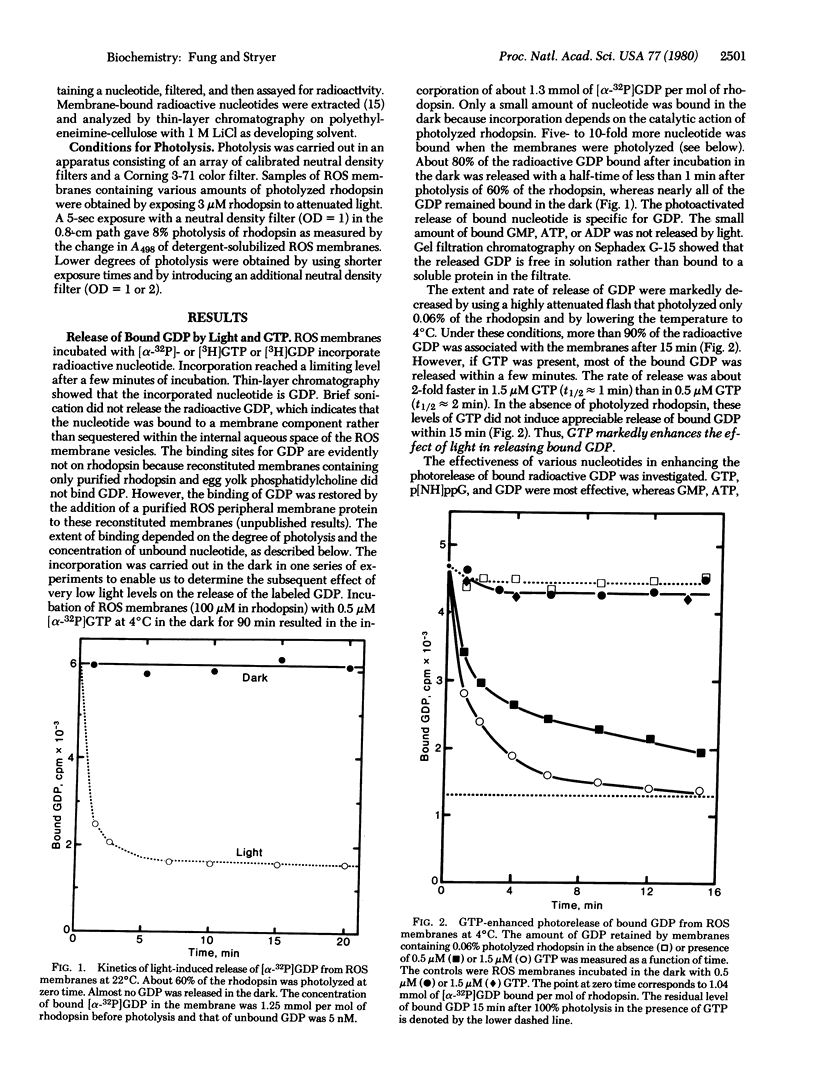
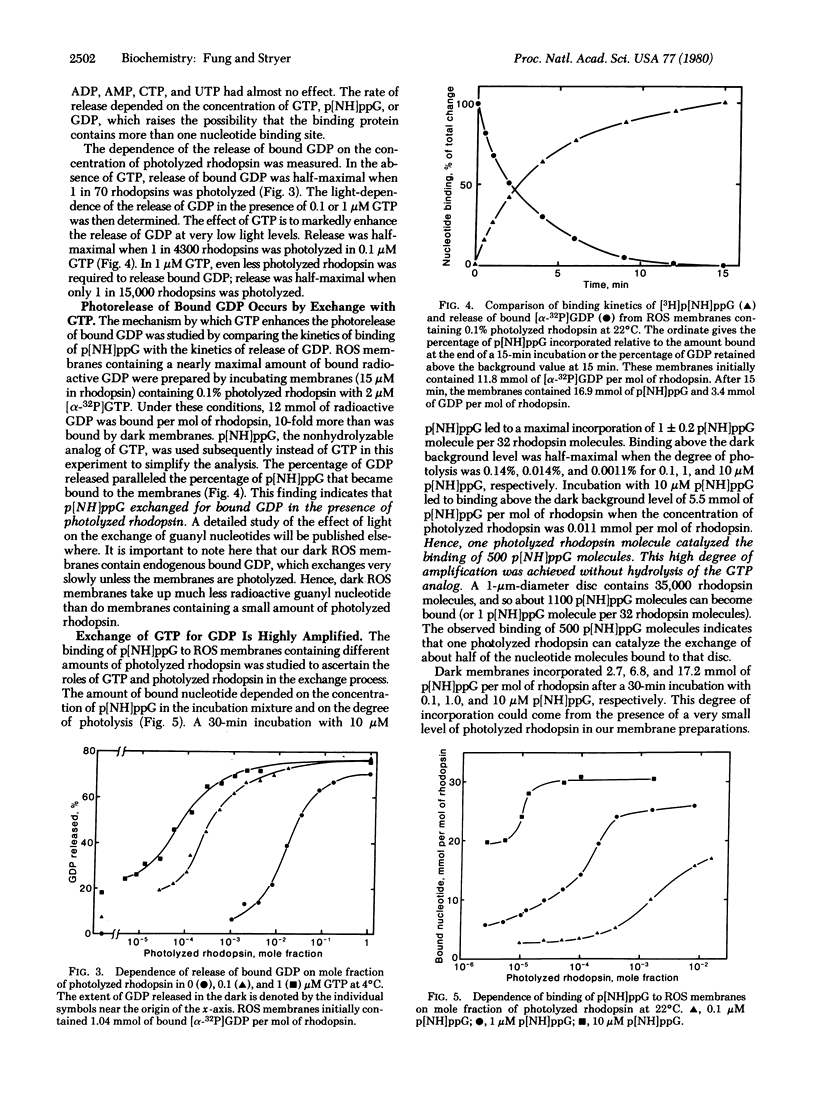
We have studied the binding of guanyl nucleotides to retinal rod outer segment membranes to determine how light activates a cyclic GMP phosphodiesterase and a GTPase. We found that rod outer segment membranes contain tightly bound radioactive GDP after incubation in the dark with [3H]GDP or [alpha-32P]GTP. Reconstituted membranes containing only rhodopsin and phospholipid bind almost no GDP. More than 80% of the radioactive GDP bound to rod outer segment membranes could be released by subsequent illumination. At low light levels, the rate and extent of GDP release were markedly enhanced by the presence of GTP or p[NH]ppG, a nonhydrolyzable analog of GTP. The kinetics of binding of p[NH]ppG paralleled the kinetics of release of bound GDP, indicating that p[NH]ppG was exchanged for bound GDP. The maximal amount of bound p[NH]ppG was 1 per 30 rhodopsins when photolyzed membranes were incubated with 10 micro M nucleotide. Under these conditions, p[NH]ppG binding was half-maximal when only 1 in 90,000 rhodopsins was photolyzed. This corresponds to the catalyzed exchange of 500 p[NH]ppG for bound GDP per photolyzed rhodopsin. We propose a light-activated GTP-GDP amplification cycle involving a guanyl nucleotide binding protein with GTPase activity (E). The essence of this cycle is that photolyzed rhodopsin catalyzes the formation of E . GTP from E . GDP (the major species in the dark) by nucleotide exchange. The formation of several hundred E . GTP per photolyzed rhodopsin may be the first stage of amplification in visual excitation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Bitensky M. W., Wheeler G. L., Aloni B., Vetury S., Matuo Y. Light- and GTP-activated photoreceptor phosphodiesterase: regulation by a light-activated GTPase and identification of rhodopsin as the phosphodiesterase binding site. Adv Cyclic Nucleotide Res. 1978;9:553–572. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chader G. J., Bensinger R., Johnson M., Fletcher R. T. Letter: Phosphodiesterase: an important role in cyclic nucleotide regulation in the retina. Exp Eye Res. 1973 Dec 10;17(5):483–486. doi: 10.1016/0014-4835(73)90229-7. [DOI] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Zimmerman W. F. Membrane-dependent guanine nucleotide binding and GTPase activities of soluble protein from bovine rod cell outer segments. J Biol Chem. 1979 Aug 25;254(16):7874–7884. [PubMed] [Google Scholar]
- Helmreich E. J., Zenner H. P., Pfeuffer T. Signal transfer from hormone receptor to adenylate cyclase. Curr Top Cell Regul. 1976;10:41–87. doi: 10.1016/b978-0-12-152810-2.50009-7. [DOI] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Lipid requirements for Rhodopsin regenerability. Biochemistry. 1973 Oct 23;12(22):4517–4523. doi: 10.1021/bi00746a033. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Helmreich E. J. Hormone-receptor--adenylate cyclase interactions. FEBS Lett. 1979 May 15;101(2):213–219. doi: 10.1016/0014-5793(79)81011-x. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr The control of phosphodiesterase in rod disk membranes: kinetics, possible mechanisms and significance for vision. Vision Res. 1979;19(4):375–380. doi: 10.1016/0042-6989(79)90097-x. [DOI] [PubMed] [Google Scholar]
- Miki N., Baraban J. M., Keirns J. J., Boyce J. J., Bitensky M. W. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975 Aug 25;250(16):6320–6327. [PubMed] [Google Scholar]
- Nicol G. D., Miller W. H. Cyclic GMP injected into retinal rod outer segments increases latency and amplitude of response to illumination. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5217–5220. doi: 10.1073/pnas.75.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannbacker R. G., Fleischman D. E., Reed D. W. Cyclic nucleotide phosphodiesterase: high activity in a mammalian photoreceptor. Science. 1972 Feb 18;175(4023):757–758. doi: 10.1126/science.175.4023.757. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer T. Guanine nucleotide-controlled interactions between components of adenylate cyclase. FEBS Lett. 1979 May 1;101(1):85–89. [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Hagins W. A. GTP hydrolysis in intact rod outer segments and the transmitter cycle in visual excitation. Nature. 1979 Aug 2;280(5721):398–400. doi: 10.1038/280398a0. [DOI] [PubMed] [Google Scholar]
- Shinozawa T., Sen I., Wheeler G., Bitensky M. Predictive value of the analogy between hormone-sensitive adenylate cyclase and light-sensitive photoreceptor cyclic GMP phosphodiesterase: a specific role for a light-sensitive GTPase as a component in the activation sequence. J Supramol Struct. 1979;10(2):185–190. doi: 10.1002/jss.400100208. [DOI] [PubMed] [Google Scholar]
- Wheeler G. L., Bitensky M. W. A light-activated GTPase in vertebrate photoreceptors: regulation of light-activated cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4238–4242. doi: 10.1073/pnas.74.10.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M. L., Bownds M. D. Amplitude, kinetics, and reversibility of a light-induced decrease in guanosine 3',5'-cyclic monophosphate in frog photoreceptor membranes. J Gen Physiol. 1979 May;73(5):629–653. doi: 10.1085/jgp.73.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]



