Abstract
Many drugs, including some commonly used medications, can cause abnormal heart rhythms and sudden death, as manifest by a prolonged QT interval in the electrocardiogram. Cardiac arrhythmias caused by drug-induced long QT syndrome are thought to result mainly from reductions in the delayed rectifier potassium ion (K+) current IKr. Here, we report a mechanism for drug-induced QT prolongation that involves changes in multiple ion currents caused by a decrease in phosphoinositide 3-kinase (PI3K) signaling. Treatment of canine cardiac myocytes with inhibitors of tyrosine kinases or PI3Ks caused an increase in action potential duration that was reversed by intracellular infusion of phosphatidylinositol 3,4,5-trisphosphate. The inhibitors decreased the delayed rectifier K+ currents IKr and IKs, the L-type calcium ion (Ca2+) current ICa,L, and the peak sodium ion (Na+) current INa and increased the persistent Na+ current INaP. Computer modeling of the canine ventricular action potential showed that the drug-induced change in any one current accounted for less than 50% of the increase in action potential duration. Mouse hearts lacking the PI3K p110α catalytic subunit exhibited a prolonged action potential and QT interval that were at least partly a result of an increase in INaP. These results indicate that down-regulation of PI3K signaling directly or indirectly via tyrosine kinase inhibition prolongs the QT interval by affecting multiple ion channels. This mechanism may explain why some tyrosine kinase inhibitors in clinical use are associated with increased risk of life-threatening arrhythmias.
INTRODUCTION
Long QT syndrome is a disorder of the electrical activity of the heart that can lead to torsades de pointes arrhythmia and sudden death (1). As seen on an electrocardiogram (ECG), activation (depolarization) of the ventricle begins with the Q wave and the final repolarization of each beat occurs at the end of the T wave. Changes in ion fluxes that delay repolarization are detected clinically as an increase in the QT interval and can also be seen in vitro as an increase in the action potential duration (APD) in individual cardiac myocytes. Although understanding of the pathogenic mechanism is incomplete, it is thought that excessive lengthening of the APD allows the L-type Ca2+ channel to recover from inactivation and initiate an early after-depolarization (EAD) whose probability of occurrence is enhanced by high sympathetic tone. Once produced, the EAD can be conducted slowly through the ventricle, leading to its reentry into regions already activated by the normal sinus beat, generating a macroscopic arrhythmia and possible sudden death.
Long QT syndrome can arise from congenital mutations that affect the function of individual ion channels that form the action potential or, in the acquired form, from drug inhibition of these channels. Most cases of congenital long QT syndrome are due to loss-of-function mutations in genes encoding the repolarizing K+ channels that conduct the outward delayed rectifier currents IKr or IKs (1). Gain-of-function mutations in the gene encoding the depolarizing Na+ channel that conducts the persistent Na+ current (INaP) are found in a smaller number of patients (1). In addition, a mutation in ankyrin-B affecting multiple ion channels also leads to a long QT syndrome (2). Acquired long QT syndrome can be caused by many commonly used medications and limits the use of marketed drugs and the development of new drugs (3). Drugs that induce long QT syndrome are believed to almost invariably target IKr, and regulatory agencies recommend that all new drug candidates undergo in vitro testing for effects on IKr early in development (4). Drug binding to Kv11.1, the pore-forming subunit of the ion channel encoded by KCNH2 (also called hERG), is the major mechanism for IKr inhibition (5, 6), although some drugs disrupt channel trafficking (7–9).
Tyrosine kinase inhibitors have recently entered clinical use as anti-cancer drugs (10, 11). Prescribing information for two of these drugs, dasatinib and sunitinib, warns that they can cause QT prolongation, and prescribing information for nilotinib contains a “black box” warning about the risk of QT prolongation and sudden death. Class IA phosphoinositide 3-kinases (PI3Ks), consisting of a catalytic subunit (p110α, p110β, or p110δ) bound to a p85 regulatory subunit, are activated by tyrosine kinases in many cell types by binding of Src homology 2 domains in p85 to tyrosine-phosphorylated proteins (12, 13). Here, we test the effects of dasatinib, sunitinib, and nilotinib on PI3K and APD in cardiac myocytes and the QT interval in isolated hearts to examine the mechanism by which these agents affect the QT interval.
RESULTS
APD prolongation induced by tyrosine kinase inhibitors is reversed by phosphatidylinositol 3,4,5-trisphosphate
The canine heart is the best-accepted animal model for the study of human cardiac electrophysiology (14). Canine ventricular myocytes are used by pharmaceutical companies and accepted by regulatory agencies as a screen for compounds for human use for the potential side effect of drug-induced long QT syndrome (15). The tyrosine kinase inhibitors nilotinib, dasatinib, and sunitinib cause long QT syndrome in humans. As expected, treatment of canine ventricular myocytes for 2 hours with these drugs induced a significant increase in APD90 (90% repolarization) (Fig. 1, A and B). Acute application of nilotinib for up to 5 min did not result in APD prolongation (fig. S1, A and C), indicating that the effect was most likely not a result of direct blockade of ion channels that determine the action potential. Two-hour treatment with the tyrosine kinase inhibitor imatinib, which does not cause long QT in humans, did not increase APD90 in canine myocytes (fig. S2A). The same concentration of drug completely blocked BCR-Abl autophosphorylation in human leukemia cells (fig. S2B), showing that Abl kinase was inhibited at this dose. To further demonstrate the usefulness of the canine model, treatment with terfenadine, the iconic long QT syndrome–inducing drug in humans, also pro-longed the APD90 in canine myocytes (fig. S3).
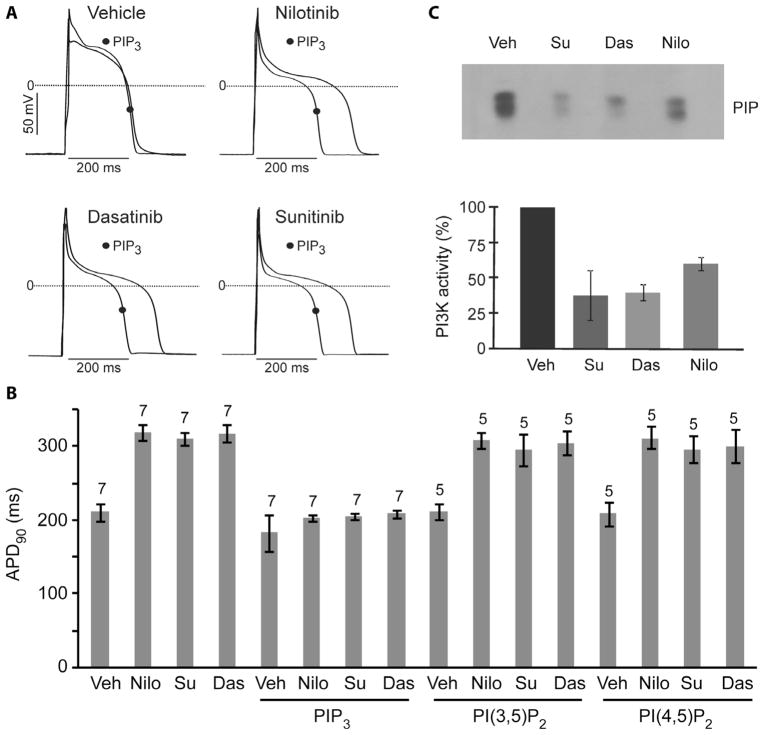
Fig. 1.
Prolonged APD caused by tyrosine kinase inhibitors in canine myocytes and reversal by PIP3 infusion. (A) Sample traces of action potentials in myocytes treated with vehicle or drugs (1 μM for 2 hours) with or without intracellular infusion of 1 μM PIP3. (B) Summary data of APD90 in myocytes treated with tyrosine kinase inhibitors with or without infusion of 1 μM phospholipids. Data are means ± SE. The number of cells studied is above each bar. (C) Myocytes were treated with vehicle (Veh) or dasatinib (Das), nilotinib (Nilo), or sunitinib (Su) at 1 μM for 1 hour and then stimulated with 7.5% fetal bovine serum for 5 min. PI3K activity was assayed in anti-phosphotyrosine immunoprecipitates of cell lysates. The upper panel is an autoradiograph from a representative assay showing [32P]phosphatidylinositol 3-phosphate (PIP), and the lower graph summarizes data from three independent experiments. Data are means ± SE.
Because class IA PI3Ks can be activated by tyrosine kinases, we wondered whether suppression of PI3K activity by nilotinib, dasatinib, and sunitinib might contribute to the ability of these drugs to prolong the QT interval. First, we tested whether these tyrosine kinase inhibitors blocked serum activation of PI3K in isolated canine ventricular myocytes. Indeed, PI3K activity associated with tyrosine-phosphorylated proteins was substantially decreased in drug-treated myocytes compared to vehicle-treated cells (Fig. 1C). By contrast, imatinib did not cause a decrease in PI3K activity (fig. S2C). When phosphatidylinositol 3,4,5-trisphosphate (PIP3), the second messenger produced by PI3K, was added to the patch pipette to dialyze the interior of cells treated with nilotinib, dasatinib, or sunitinib, the APD90 was shortened to control levels (Fig. 1, A and B). Intra-cellular infusion of control phospholipids phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2] or phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] did not have this effect (Fig. 1B). These results indicate that inhibition of PI3K signaling is responsible for prolongation of the APD by these tyrosine kinase inhibitors that induce long QT syndrome in humans.
PI3K inhibitors induce APD prolongation and EADs
We next tested whether inhibitors that directly target PI3K also prolong the APD. Potent inhibitors of PI3K, such as BEZ235 (16), have already entered clinical trials for cancer therapy. We incubated canine myocytes for 2 hours with BEZ235 or with PI-103 (17), a chemically distinct PI3K inhibitor that is widely used in vitro, and both compounds significantly prolonged the APD90 (Fig. 2, A and B). The effect on APD was dose-dependent for both inhibitors, and BEZ235 had a smaller effect than PI-103 at each concentration (Fig. 2B). APD90 prolongation caused by PI3K inhibitors was larger than that caused by tyrosine kinase inhibitors. Infusion with PIP3, but not PI(4,5)P2 or PI(3,5)P2, completely reversed the drug effects, confirming that the increase in APD was due to inhibition of PI3K (Fig. 2, A and B). As with nilotinib, acute application of PI-103 did not cause APD prolongation (fig. S1, B and C).
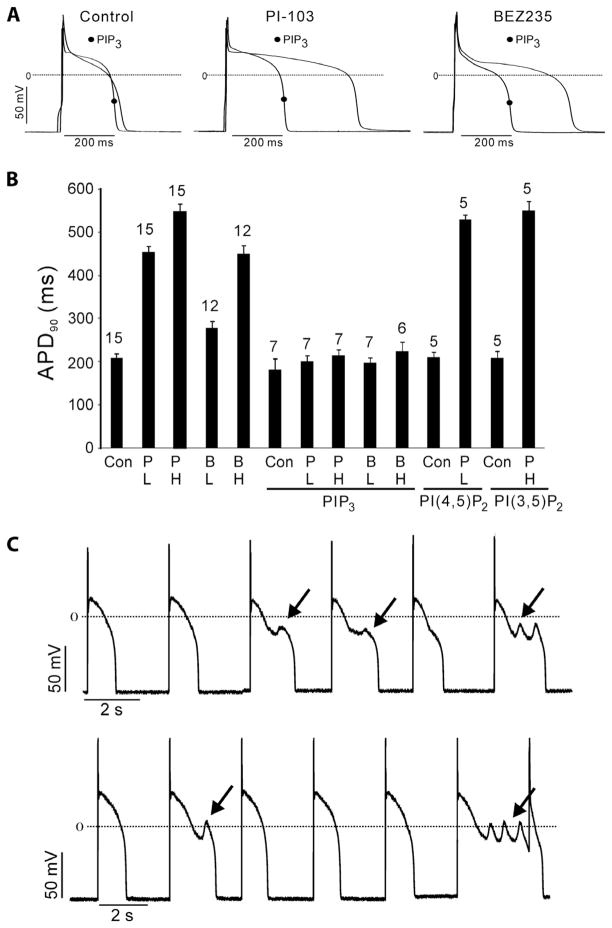
Fig. 2.
Prolonged APD and EADs caused by PI3K inhibitors in canine myocytes. (A) Sample traces of action potentials in myocytes treated with inhibitor (500 nM for 2 hours) or left untreated as control with or without intracellular infusion of 1 μM PIP3. (B) Summary data of APD90 in myocytes treated with 50 nM (L) or 500 nM (H) PI-103 (P) or BEZ235 (B) with or without infusion of 1 μM phospholipids. Data are means ± SE. The number of cells studied is above each bar. (C) ISO (5 μM)–induced EADs (arrows) in myocytes treated with 50 nM (upper panel) or 500 nM (lower panel) PI-103. Action potentials were initiated in current clamp mode by applying a 180-pA depolarizing stimulus for 15 ms at a cycle length of 3 s and were recorded at 34°C.
APD prolongation is associated with the development of EADs that may trigger arrhythmias. Because the probability of occurrence of EADs is enhanced by high sympathetic tone, we tested whether EADs are produced in myocytes exposed to PI3K inhibitors in the presence of isoproterenol (ISO). In canine myocytes exposed to ISO alone, there was a decrease in the plateau height and some APD shortening compared to untreated cells (fig. S4), but no EADs were induced in any of the control cells (0 of 10). In contrast, ISO induced EADs in the presence of 50 nM (8 of 10 cells) or 500 nM PI-103 (10 of 10 cells) (Fig. 2C). These data indicate that direct inhibition of PI3K might predispose to ventricular arrhythmias in the presence of increased sympathetic tone.
Multiple ion currents are affected by nilotinib and PI-103
Although nilotinib has been reported to reduce IKr (18), there is no a priori reason to assume that drug inhibition of PI3K signaling would affect only this current. We therefore looked for drug effects on other currents that regulate APD in canine myocytes treated with nilotinib or PI-103. Representative tracings (Fig. 3A) and current density–voltage (I-V) relationships (Fig. 3B) for the total time-dependent out-ward delayed rectifier current IK show that the current density was smaller in cells incubated with nilotinib or PI-103 than in controls at test potentials greater than +10 mV. To discriminate between effects on the IKr or IKs component of IK, we applied selective blockers of IKs (10 μM chromanol 293B) or IKr (1 μM dofetilide) to determine each current. The data show that the time-dependent chromanol-sensitive IKs density in nilotinib- or PI-103–treated cells was smaller than in controls at potentials greater than +10 mV, as was the time-dependent dofetilide-sensitive IKr density at all test potentials (Fig. 3, A and B).
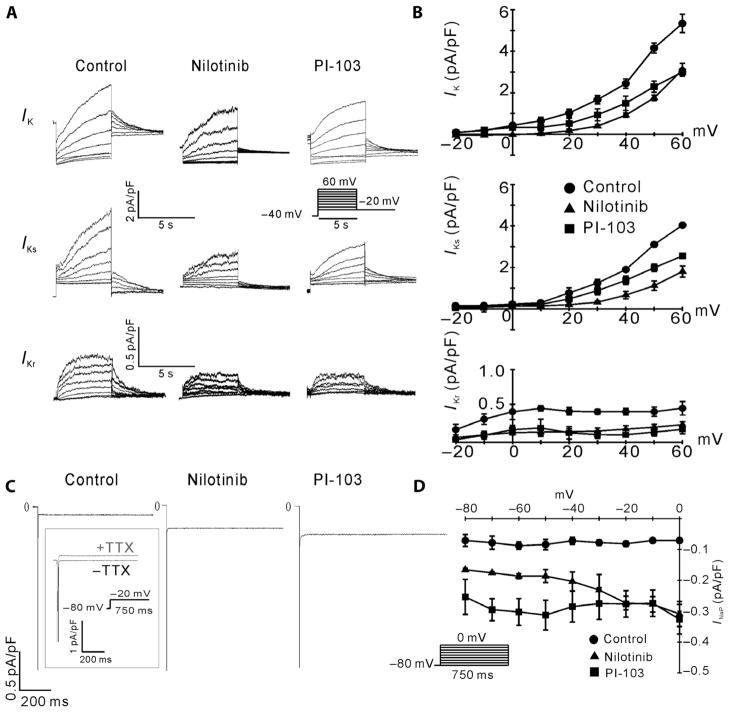
Fig. 3.
Effects of nilotinib and PI-103 on canine cardiac ion currents. (A) Sample traces of total outward rectifier current (IK), chromanol 293B–sensitive component (IKs), and dofetilide-sensitive component (IKr) for canine myocytes treated with vehicle (control), 1 μM nilotinib, or 500 nM PI-103. (B) Leak-subtracted time-dependent I-V relationships of IK, IKs, and IKr. n = 5 cells for each group. (C) Sample traces of tetrodotoxin (TTX)–sensitive INaP for myocytes treated with vehicle, 1 μM nilotinib, or 500 nM PI-103 in 50 mM external Na+. TTX-sensitive currents were obtained by subtracting traces obtained in the presence of 10 μM TTX from the traces obtained in its absence (boxed traces). (D) I-V relationships of INaP. n = 7 cells for each group. The insets show the pulse protocols.
Prolongation of the APD can also be caused by an increase in net inward currents during the action potential plateau. We therefore examined the inward Na+ and Ca2+ currents in canine myocytes treated with nilotinib or PI-103. Representative tracings (Fig. 3C) and I-V relationships (Fig. 3D) show that both drugs increased the tetrodotoxin (TTX)–sensitive persistent Na+ current INaP in 50 mM external Na+ at all potentials tested. This concentration of external Na+ was used because the magnitude of INaP is larger and thus the measurements more robust even though there can be escape from the membrane voltage clamp under these conditions. We also measured INaP with 10 mM external Na+ when membrane voltage was well controlled and observed similar drug-induced increases in INaP (fig. S5, A and B). The peak Na+ current INa was reduced by both nilotinib and PI-103 (fig. S5C). When normalized, the I-V relationships superimposed (fig. S5D), suggesting that the drugs cause a reduction in peak Na+ conductance and indicating that INa was well clamped at 10 mM external Na+. We previously reported that PI-103 causes a decrease in ICa,L in canine myocytes (19). Nilotinib treatment also decreased ICa,L at most of the potentials examined (fig. S6, A and B). These results show that direct inhibition of PI3K with PI-103 or indirect inhibition with nilotinib affects multiple ion channels that control the APD.
PIP3 infusion or drug washout reverses the effect of nilotinib on IKr and INaP
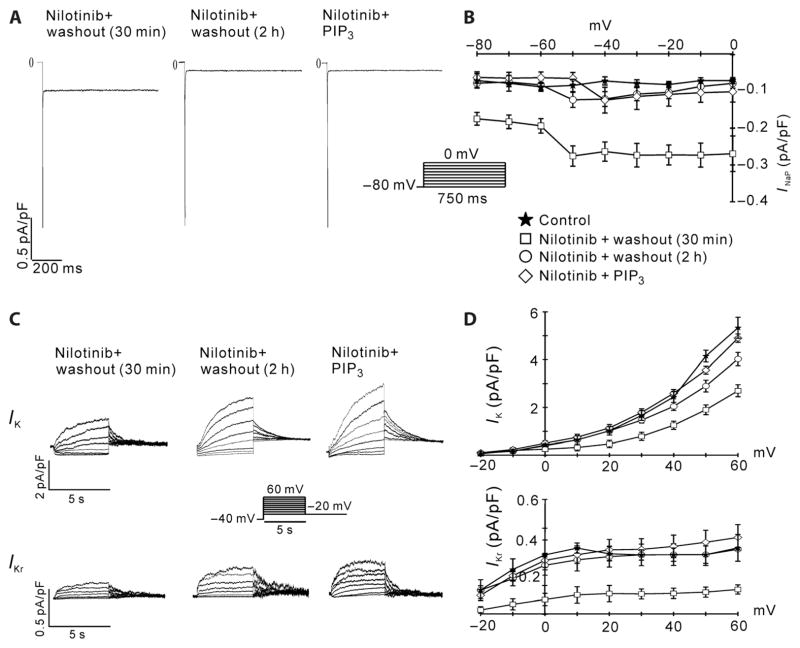
We next investigated whether the effects of nilotinib on IKr and INaP are reversed after intracellular PIP3 infusion or drug washout. In cells incubated with nilotinib, PIP3 reversed the positive effect of the drug on INaP (Fig. 4, A and B) and the inhibitory effect of the drug on IKr (Fig. 4, C and D). Similarly, after the drug was washed away for 2 hours, both INaP (Fig. 4, A and B) and IKr (Fig. 4, C and D) returned to nearly control levels. However, both currents were still almost maximally affected after the drug was washed away for only 30 min (Fig. 4, A to D). Together with the PIP3 infusion data and the lack of an acute effect of nilotinib on APD, the parsimonious explanation for the washout results is that these currents are regulated by PIP3, which is slowly depleted after incubating myocytes with nilotinib and then gradually replenished after washing away the drug.
Fig. 4.
Reversal of nilotinib effects on INaP and IKr. Canine myocytes were treated with 1 μM nilotinib for 2 hours, and then the drug was washed out for either 30 min or 2 hours before patch clamping. In a separate experiment, cells were treated with nilotinib for 2 hours and then infused with 1 μM PIP3 through the patch pipette. (A) Representative traces of INaP measured with 50 mM external Na+. (B) I-V relationships of INaP. n = 7 cells for each condition. (C) Representative traces of IK and IKr. (D) I-V relationships of IK and IKr. n = 6 cells for each condition.
PI3K deletion increases INaP in mouse cardiac myocytes
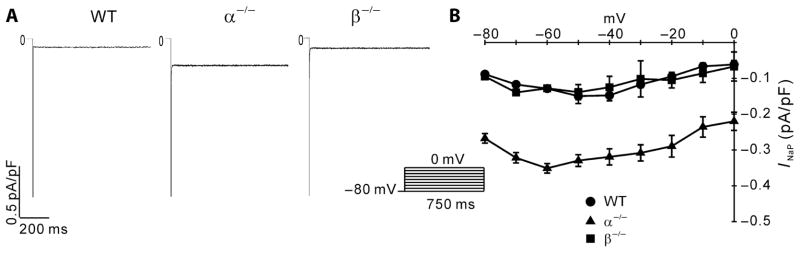
Next, we used mouse strains lacking p110α or p110β in cardiac myocytes (19) to test the effect of decreased PI3K signaling on ion currents and the action potential without using pharmacological inhibitors. We reported previously that ICa,L in mouse cardiac myocytes is inhibited by deletion of p110α but not p110β (19). Delayed rectifier currents in mouse myocytes are very small and are thought to contribute little to the mouse APD, so they are not considered here. We therefore tested whether the sodium currents affected by nilotinib and PI-103 in dog myocytes are similarly affected by p110α ablation in the mouse. As in canine cells, INaP was markedly enhanced in p110α-null mouse myocytes when measured with either 50 mM (Fig. 5, A and B) or 10 mM (fig. S7, A and B) external Na+. INa was also reduced in p110α−/− myocytes compared to wild-type myocytes (fig. S7C). When normalized, the INa-V relationships superimposed (fig. S7D), indicating that INa was well clamped at 10 mM external Na+. In contrast, ablation of p110β did not affect INaP or INa (Fig. 5 and fig. S7).
Fig. 5.
Effects of PI3K ablation on mouse INaP measured with 50 mM external Na+. (A) Sample traces of TTX-sensitive INaP for wild-type (WT), α−/−, and β−/−mouse myocytes. (B) I-V relationships for INaP in the three groups of mouse myocytes. The inset shows the pulse protocol for the activation of the Na+ currents. n = 7 cells per group.
Decreased PI3K signaling causes increased APD and QT prolongation in the mouse
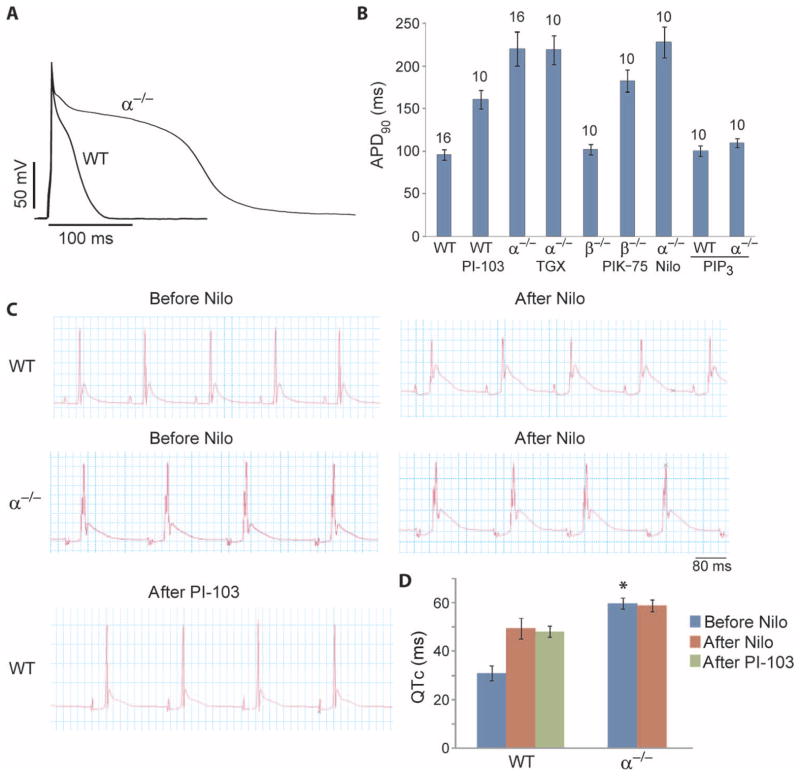
We also tested whether decreased PI3K signaling leads to prolongation of the APD in the mouse. Mouse APD was measured in the presence of 4-aminopyridine (4-AP) to reduce the large transient outward K+ current that allows the rapid heart rate in this species. Under these conditions, APD90 in p110α−/− myocytes was markedly longer than in wild-type cells, and APD90 in wild-type cells treated with PI-103 was almost as long as in p110α−/− myocytes (Fig. 6, A and B). Treatment of p110α−/− myocytes with a p110β-specific inhibitor (TGX-221) or nilotinib did not further prolong the APD90, but, as expected, intracellular dialysis of PIP3 shortened the APD (Fig. 6B). In contrast, ablation of p110β had minimal effects on the APD90, and treatment of p110β −/− myocytes with a p110α-specific inhibitor (PIK-75) lengthened the APD90 to nearly the level observed in p110α−/− myocytes (Fig. 6B). Together, these results indicate that p110α rather than p110β is the dominant PI3K that regulates the APD in mouse myocytes and suggest that APD prolongation induced by nilotinib, PI-103, or p110α ablation is mediated by the common mechanism of reduced PI3K signaling.
Fig. 6.
Effect of PI3K ablation on APD and the QT interval. APD90 was measured in the presence of 2 mM 4-AP. ECG recordings were obtained from spontaneously beating mouse hearts mounted on a Langendorff apparatus. (A) Representative action potentials recorded in cardiac myocytes isolated from α−/− and WT mice. (B) Summary data of APD90 shown as means ± SE. The number of cells studied is above each bar. Where indicated, myocytes were incubated with 500 nM PI-103, 500 nM TGX-221, 100 nM PIK-75, or 1 μM nilotinib for 2 hours before measurements or dialyzed with 1 μM PIP3 through the patch pipette. (C) Representative ECG tracings from α−/− and WT hearts recorded before and after addition of 1 μM nilotinib or 1 μM PI-103 to the circulating bath. (D) Summary data of QT interval corrected for heart rate (QTc). Data are means ± SE. n = 3 hearts per group. *P < 0.05, t test, significantly different from the WT before nilotinib group.
To determine whether p110α ablation results in prolongation of the QT interval, we recorded ECGs from isolated hearts. The QT interval corrected for heart rate (QTc) was almost twice as long in p110α−/− hearts (60 ms) than in wild-type hearts (31 ms) (Fig. 6, C and D). Nilotinib increased the QTc of wild-type hearts but did not have an additional effect on p110α−/− hearts (Fig. 6, C and D). Last, we confirmed that PI-103 also increased QTc in wild-type hearts (Fig. 6, C and D).
Alterations in multiple ion currents account for APD prolongation caused by nilotinib and PI-103
Nilotinib and PI-103 affected multiple ion channels that could exert opposing effects on the APD. The decrease in IKr and IKs and increase in INaP could lengthen the APD, whereas inhibition of ICa,L and INa could shorten the APD. To determine the theoretical impact of the sum total of these current changes on the action potential, we used a modified Hund-Rudy model of the canine ventricular action potential (20). Figure 7A shows the fractional change in each current that we measured in cells treated with nilotinib or PI-103, and Fig. 7B shows the action potentials generated by the computer simulation incorporating these changes. The control action potential generated an APD90 of 216 ms, whereas the APD90 with nilotinib or PI-103 was 343 or 323 ms, respectively. These results agree with the experimental data showing that these compounds produce a lengthening of the APD. Although nilotinib and PI-103 affected multiple channels, it was still possible that most of the effect on APD prolongation was due to the 60% reduction in IKr and that the long QT syndrome induced by inhibition of PI3K would still be predominantly an IKr disease. Also shown in Fig. 7 is the result of a simulation in which the only parameter change was a reduction in IKr to 40% of control (ΔIKr), which generated an APD90 of 256 ms. Thus, less than half of the change in APD90 induced by either drug is due to the reduction in IKr. Similarly, the APD90s generated from single-parameter changes in the other currents (ΔIKs, ΔINaP, etc.) were all less than 256 ms (Fig. 7A). On the other hand, mathematical modeling showed that alterations in just IKr and INaP account for about 80% of APD90 prolongation due to PI3K inhibition in canine myocytes (Fig. 7B). These simulations indicate that inhibition of PI3K lengthens the APD by affecting multiple ion currents, especially IKr plus INaP, and not an individual current. These results are consistent with a report in which Noble’s group used computer modeling to illustrate how alterations in multiple ion currents by drugs could be a better predictor of long QT–induced arrhythmias than inhibition only of IKr (21).
Fig. 7.
Computer simulation of the effects of nilotinib and PI-103 on the canine ventricular action potential. (A) Ratio of ion current densities to control values based on experimental measurements (see Materials and Methods). Data for INa and INaP are from measurements in 10 mM external Na+. Data for ICa,L with PI-103 are from (19). (B) Simulations of the canine ventricular action potential using the modified Hund-Rudy model (see Materials and Methods). Control (black), nilotinib (blue), PI-103 (red), PI-103 effects on IKr and INaP only (dashed red), and ΔIKr (green).
Suppression of INaP ameliorates the QT prolongation by PI3K inhibition
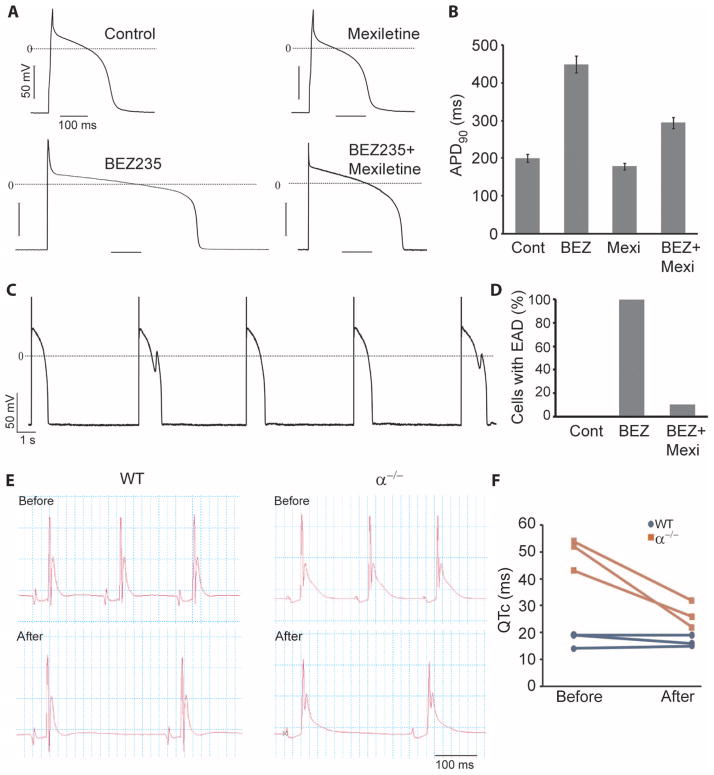
We next sought to confirm experimentally that the increase in INaP caused by PI3K inhibition contributes to APD prolongation and EAD generation in canine myocytes. Cells were treated with BEZ235 in the presence or absence of mexiletine, a relatively selective INaP inhibitor. Mexiletine caused a small decrease in APD90 in control cells, but it reduced the APD90 in BEZ235-treated myocytes from 450 ms to about 300 ms (Fig. 8, A and B). These data support the conclusion of the computer simulations that an increase in INaP plays animportantrole indrug-induced APD prolongation. Mexiletine also prevented EADs in canine myocytes treated with BEZ235. ISO stimulation of BEZ235-treated cells induced EADs in 10 of 10 myocytes (Fig. 8, C and D). When the cells were treated with mexiletine in conjunction with BEZ235, ISO stimulation induced EADs in only 1 of 10 of the myocytes (Fig. 8D). These results suggest that selective blockers of INaP could be used to counter-act drug-induced long QT syndrome involving the PI3K signaling pathway.
Fig. 8.
Reversal of APD and QT prolongation and EADs by mexiletine. (A) Representative action potentials recorded in canine myocytes treated with or without 500 nM BEZ235 in the presence or absence of mexiletine (4 μg/ml) for 2 hours. (B) Summary data of APD90 are shown in the graph. n = 10 cells for each group. (C) EADs induced by 5 μM ISO in BEZ235-treated myocytes. (D) Summary data of percentage of cells with EADs. n = 10 cells for each condition. (E) Representative ECG tracings from WT and p110α−/− (α−/−) hearts before and after addition of mexiletine (4 μg/ml) to the circulating bath. (F) QT interval corrected for heart rate (QTc) from three hearts in each group.
We also tested whether the increase in INaP contributes to QTc prolongation in p110α−/− hearts. We found that mexiletine markedly reduced the QTc interval in p110α−/− hearts but had no effect on QTc in wild-type hearts (Fig. 8, E and F). These results indicate that an increase in INaP also plays a role in the long QT phenotype caused by down-regulation of PI3K signaling in the mouse heart.
DISCUSSION
Reports in the 1980s and 1990s that Seldane (terfenadine), the first antihistamine free of soporific side effects, induced life-threatening arrhythmias associated with sudden death markedly changed how the pharmaceutical industry tests candidate drugs to meet Food and Drug Administration safety requirements (22). The prevailing view regarding drug-induced long QT syndrome has been that it is mainly an IKr disease resulting from direct blockade of the Kv11.1 ion channel by pharmaceutical agents (3). Our study introduces an alternative view for the basis of drug-induced long QT syndrome. We show that inhibition of PI3K signaling can be arrhythmogenic and is the major cause of nilotinib-induced action potential prolongation. Decreased PI3K signaling affects multiple currents in cardiac myocytes, and this complex alteration of both inward and outward ionic fluxes leads to prolongation of the action potential and the QT interval.
Acute treatment of rodent cardiac my-ocytes with the PI3K inhibitor LY294002 caused APD prolongation and EADs (23, 24). These effects were attributed to direct inhibition of outward K+ currents by LY294002 rather than inhibition of PI3K. In contrast, we found that APD prolongation in canine myocytes was elicited only after prolonged exposure to inhibitors of tyrosine kinases or PI3K. The slow reversal of the effects of nilotinib on IKr and INaP after drug washout, together with the rapid PIP3-induced reversal of the effects of inhibitors, supports our conclusion that PI3K inhibition underlies the effects of these drugs.
Some studies have examined modulation of individual ion channels relevant to this work by PI3K and its downstream effector, the protein kinase Akt. Kv11.1 expressed in human embryonic kidney (HEK) 293 cells was highly phosphorylated (25). Zhang et al. (26) showed that PI3K/Akt signaling in HEK293 cells maintained the Kv11.1-induced current, and expression of constitutively active forms of PI3K p110α or Akt caused an increase in current density. These investigators speculated that Akt might regulate the current by modifying consensus Akt phosphorylation sites identified in Kv11.1 (26). We showed that PI3K/Akt inhibition decreases ICa,L by reducing the number of channels on the myocyte surface (19), and Viard et al. (27) demonstrated that Ca2+ channel trafficking to the cell surface is enhanced by Akt-dependent phosphorylation. IKs is also modulated by trafficking (28). The increase in INaP after PI3K inhibition is probably not due to trafficking of Nav1.5 sodium channels to the plasma membrane because peak INa was concomitantly decreased. Instead, it is more likely due to an increase in open probability of the persistent gating state. One potential mechanism to induce such a gating change is phosphorylation of Akt consensus sites in Nav1.5.
Because of electrophysiological differences between species, mouse models of congenital K+ channel long QT syndromes in general have not been highly informative with regard to the human diseases. On the other hand, mouse models of sodium channel mutations that cause an increase in INaP exhibit most of the phenotypes seen in patients with type 3 congenital long QT syndrome (LQT3) who have gain-of-function mutations in Nav1.5 (encoded by SCN5A) (29, 30). Expression of two different SCN5A mutants found in human LQT3 led to an increase in INaP, significant prolongation of the QT interval, and development of cardiac arrhythmias in mice (31, 32). Mexiletine treatment reversed the APD prolongation in myocytes expressing a Nav1.5 mutant but did not affect APD in myocytes from wild-type mice (32). Our finding that mexiletine shortened QTc in p110α-null hearts but not in wild-type hearts is consistent with a prominent role of PI3K in regulating INaP.
Mexiletine shortens QTc in LQT3 patients (33). Our results suggest that mexiletine may serve as a useful adjuvant to ameliorate some of the APD lengthening and EADs induced by inhibition of PI3K. The use of β-adrenergic receptor blockers to reduce the probability of EAD initiation could have serious side effects on contractility because PI3K inhibition already induces a significant reduction in ICa,L. However, reduction of ICa,L probably has an anti–long QT effect, because it tends to shorten the APD.
The incidence of QT prolongation in patients taking nilotinib was reported to be 1 to 10% (34). Cancer patients often have multiple risk factors, such as electrolyte disturbances, heart disease, and use of other medications that prolong the QT interval that might make them especially vulnerable to long QT syndrome induced by tyrosine kinase or PI3K inhibitors. Our results suggest that patients treated with tyrosine kinase inhibitors, PI3K inhibitors, or other drugs that target PI3K signaling in the heart should be closely monitored for QT prolongation and cardiac arrhythmias. Some tyrosine kinase inhibitors such as imatinib might be innocuous because the enzymes they target do not regulate cardiac PI3K.
Our results suggest that known long QT syndrome–inducing drugs should be reinvestigated to determine whether they affect PI3K signaling. Indeed, we found that infusion with PIP3 reversed the terfenadine-induced APD prolongation by ~80% (fig. S3). Furthermore, terfenadine increased INaP, and this effect on the sodium current was completely reversed by PIP3 infusion (fig. S8). These results suggest that this iconic long QT syndrome–inducing drug not only directly blocks IKr but also affects the PI3K signaling pathway to prolong the QT interval.
Patients receiving 400 mg of nilotinib twice daily exhibited mean peak and trough serum concentrations of 3.6 and 1.7 μM, respectively (35). Patients taking 1600 mg of BEZ235 per day had a maximal median steady-state serum concentration of 3.8 μM (36). Although some of the compounds we tested might directly block ion channels at higher pharmacological concentrations, our results indicate that inhibition of PI3K is the dominant factor that causes APD prolongation. However, when considering the role PI3K plays in drug-induced long QT syndrome, it is important to realize the limitations in applying our results to testing of new compounds during drug development. First, like all studies of this kind, results were obtained from animal models, which might not translate to the human condition when dealing with new compounds. Second, to make a definitive statement concerning the safety of a drug candidate, it is necessary to know the therapeutic concentration of drug candidate compared to the dose-response curves for PI3K inhibition and direct channel blockade. Nevertheless, our results may necessitate changes in the safety testing requirements for new drugs and indicate that drugs in clinical use that inhibit PI3K signaling could pose significant cardiac risks.
MATERIALS AND METHODS
Animals
Mixed-breed dogs of either sex more than 12 months old were purchased from R&R Research. The MerCreMer;p110αFlox/Flox and MerCreMer; p110βFlox/Flox mouse models were described earlier (19). At 8 to 9 weeks of age, mice were injected intraperitoneally with 1 mg of tamoxifen (Sigma) five times a week for 4 weeks to knock out the Pik3ca or Pik3cb gene, and the animals were analyzed at 5 to 6 months of age. All animal-related experimental protocols were approved by the Stony Brook University Institutional Animal Care and Use Committee.
Ventricular myocyte isolation
Canine ventricular cells were isolated from the mid-myocardium as described (19). Mouse ventricular myocytes were isolated as described (37).
Electrophysiology
Isolated myocytes were stored in KB (Kraftbrühe) solution and then placed in a temperature-controlled chamber. Recordings were made at room temperature unless otherwise indicated. Only relaxed quiescent cells displaying clear cross striations were used. Standard whole-cell patch-clamp techniques were performed with an Axopatch-1D amplifier with a CV-4 1/100 headstage (Axon Instruments). A PC equipped with 12-bit AD/DA converters (model 1360, Cambridge Electronic Design) was used for data acquisition, generation of pulse protocols, and data analysis. Currents were filtered with a four-pole Bessel filter at 2 kHz and digitized at 1 kHz. Current amplitude was normalized to cell capacitance to obtain current density. Pipette and external solutions are described in the Supplementary Materials. In some experiments, 1 μM phospholipids (all di-C8, Echelon Biosciences) were added to the pipette solution. Chromanol 293B, dofetilide, 4-AP, and ISO were obtained from Sigma and were freshly prepared before experiments. Dasatinib monohydrate (ChemieTek), nilotinib (ChemieTek), sunitinib malate (LC Laboratories), imatinib mesylate (Selleckchem), terfenadine (Sigma), mexiletine hydrochloride (Sigma), PI-103 (Cayman Chemical), or BEZ235 (Cayman Chemical) was added to cells for 2 hours at room temperature before patch clamping, except where otherwise noted.
Recording of action potentials in canine myocytes was initiated in current clamp mode by applying a 180-pA depolarizing stimulus for 15 ms with cycle lengths from 1 to 3 s. The pulses were 120 pA in amplitude and 10 ms in duration for mouse myocytes with a cycle length of 1 s. The APD was determined at 90% repolarization. At least 10 consecutive stable action potentials were recorded within 5 min after going into whole-cell configuration. A sufficient number of stimuli were applied at each frequency before measurements were taken so that the APD was at steady state. Because of the shorter APD in mouse than in canine ventricular myocytes, mainly caused by a larger transient outward current Ito, minor changes in APD caused by drugs may be missed or underestimated. Thus, 2 mM 4-AP was added to the external solution for mouse myocytes to block most of the transient outward current and prolong the APD for easier comparison to the canine APD (38).
IK was generated by 5-s depolarizing test voltage pulses ranging from −20 to 60 mV in 10-mV increments and then returned to −20 mV for 5 s. The pulse frequency was 0.05 Hz and the holding potential was −40 mV to inactivate Na+ current. Typically, total IK was measured first. Then, the cell was exposed to chromanol 293B (10 μM, Sigma), and IKs was determined by subtracting IK plus chromanol 293B from total IK. Further addition of dofetilide (1 μM, Sigma) to the same cell allowed IKr to be determined by subtracting the current traces in the presence of chromanol 293B plus dofetilide from those in the presence of chromanol 293B. For the reversal experiments where only IKr was measured, dofetilide was added alone. The amplitudes of IK, IKr, and IKs were measured as the difference between the instantaneous current immediately after the application of the depolarizing voltage step and the current level at the end of the test pulse. Ca2+ current and Ito were inhibited by including CdCl2 and 4-AP, respectively, in the external solution.
The TTX-sensitive Na+ current was elicited by 750-ms depolarizing voltage steps ranging from −80 to +50 mV at 10-mV increments from a holding potential of −80 mV. INa was measured as the peak negative current, and INaP was measured as the main inward current between 700 and 750 ms at the end of depolarization. TTX-sensitive currents were measured by subtracting a trace obtained in the presence of 10 μM TTX from a trace obtained in the absence of TTX. INaP records were filtered at 20 Hz. For the single trace of TTX-sensitive current shown in the figures, the current was activated at a test voltage of −20 mV from a holding potential of −80 mV.
Recording of ICa,L was performed as described (19).
ECG recordings from mouse hearts ex vivo
Isolated mouse hearts were mounted on the Harvard Apparatus isolated heart perfusion system (IH-SR) and perfused with Krebs-Henseleit solution (118 mM NaCl, 4.7 mM KCl, 2.52 mM CaCl2, 1.64 mM MgSO4, 24.88 mM NaHCO3, 1.18 mM KH2PO4, 5.55 mM glucose, and 2 mM sodium pyruvate aerated with 5% CO2 and 95% O2) at 37°C for 30 min to reach a stable baseline before data collection. For ECG recording, we placed one electrode at the base of the heart next to the left atrium and a second electrode at the heart apex. Recordings were collected under control conditions, and then drugs were added to the perfusate reservoir and circulated through the system for 30 min before collecting another set of ECG recordings. QT intervals were measured automatically by the LabChart 7.1.2 (ADInstruments) software system from >30 consecutive heartbeats, and QTc was calculated with the correction described by Mitchell et al. (39).
PI3K activity
Lysates prepared from canine myocytes were immunoprecipitated with an anti-phosphotyrosine antibody (Millipore) and then subjected to PI3K activity assays as described (40).
Computer simulation of canine action potential
A modified version (41) of the Hund-Rudy mathematical model (20) that describes action potentials in isolated ventricular myocytes was used in computer simulations. Computing was performed in the MATLAB computing environment. The model was integrated with library routine ode15s, an adaptive algorithm that adjusts integration time increments to maintain a relative tolerance of better than 10−3, or an absolute tolerance of better than 10−6. In all cases, the model was paced at 1 Hz to a steady state. IK, IKs, and IKr were measured at a test voltage of +60 mV from a holding potential of −40 mV. INaP and INa were measured at test voltages of −40 and 0 mV, respectively. The holding potential was −80 mV. ICa,L was measured at a test voltage of +10 mV from a holding potential of −50 mV. All currents were normalized to cell capacitance. In all cases, we assumed a change in conductance that is not voltage-dependent for this initial computation. This assumption is roughly valid for all but IKs, which is affected only at positive potentials but has only a small effect on the computed APD by itself.
Supplementary Material
Acknowledgments
We thank J. Zuckerman for preparing the cardiac myocytes and M. Rosen for his helpful discussion.
Funding: This work was funded by NIH grants DK62722 (R.Z.L.), HL67101 (I.S.C.), and HL94410 (I.S.C.); a Veterans Affairs Merit Award (R.Z.L.); and grants from the American Diabetes Association (R.Z.L.) and the Juvenile Diabetes Research Foundation (R.Z.L.).
Footnotes
Competing interests: The authors declare that they have no competing interests.
Author contributions: Z.L., C.-Y.C.W., and Y.-P.J. performed the experiments and analyzed the data. C.C. and I.S.C. performed the computer simulation. L.M.B. contributed to the discussion, analyzed the data, and wrote the manuscript. R.Z.L. and I.S.C. supervised the study, analyzed the data, provided the funding, and wrote the manuscript.
REFERENCES AND NOTES
- 1.Hedley PL, Jørgensen P, Schlamowitz S, Wangari R, Moolman-Smook J, Brink PA, Kanters JK, Corfield VA, Christiansen M. The genetic basis of long QT and short QT syndromes: A mutation update. Hum Mutat. 2009;30:1486–1511. doi: 10.1002/humu.21106. [DOI] [PubMed] [Google Scholar]
- 2.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogné K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 3.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 4.The Non-Clinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; Geneva. 2005. [PubMed] [Google Scholar]
- 5.Mitcheson JS. hERG potassium channels and the structural basis of drug-induced arrhythmias. Chem Res Toxicol. 2008;21:1005–1010. doi: 10.1021/tx800035b. [DOI] [PubMed] [Google Scholar]
- 6.Mitcheson JS, Chen J, Sanguinetti MC. Trapping of a methanesulfonanilide by closure of the HERG potassium channel activation gate. J Gen Physiol. 2000;115:229–240. doi: 10.1085/jgp.115.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ficker E, Kuryshev YA, Dennis AT, Obejero-Paz C, Wang L, Hawryluk P, Wible BA, Brown AM. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol. 2004;66:33–44. doi: 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Kuryshev YA, Ficker E, Wang L, Hawryluk P, Dennis AT, Wible BA, Brown AM, Kang J, Chen XL, Sawamura K, Reynolds W, Rampe D. Pentamidine-induced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther. 2005;312:316–323. doi: 10.1124/jpet.104.073692. [DOI] [PubMed] [Google Scholar]
- 9.Dennis A, Wang L, Wan X, Ficker E. hERG channel trafficking: Novel targets in drug-induced long QT syndrome. Biochem Soc Trans. 2007;35:1060–1063. doi: 10.1042/BST0351060. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal M, Garg RJ, Cortes J, Quintás-Cardama A. Tyrosine kinase inhibitors: The first decade. Curr Hematol Malig Rep. 2010;5:70–80. doi: 10.1007/s11899-010-0045-y. [DOI] [PubMed] [Google Scholar]
- 11.Adams VR, Leggas M. Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors. Clin Ther. 2007;29:1338–1353. doi: 10.1016/j.clinthera.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Backer JM, Myers MG, Jr, Shoelson SE, Chin DJ, Sun XJ, Miralpeix M, Hu P, Margolis B, Skolnik EY, Schlessinger J, White MF. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter CL, Auger KR, Chanudhuri M, Yoakim M, Schaffhausen B, Shoelson S, Cantley LC. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J Biol Chem. 1993;268:9478–9483. [PubMed] [Google Scholar]
- 14.Skadsberg ND, Hill AJ, Iaizzo PA. Isolated heart models. In: Sigg DC, Iaizzo PA, Xiao Y-F, He B, editors. Cardiac Electrophysiology Methods and Models. Springer; New York: 2010. pp. 249–260. [Google Scholar]
- 15.Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: Evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 16.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, Garcia-Echeverria C. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 17.Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S, Henley A, Di-Stefano F, Ahmad Z, Guillard S, Bjerke LM, Kelland L, Valenti M, Patterson L, Gowan S, de Haven Brandon A, Hayakawa M, Kaizawa H, Koizumi T, Ohishi T, Patel S, Saghir N, Parker P, Waterfield M, Workman P. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67:5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 18.Freebern WJ, Fang HS, Slade MD, Wells S, Canale J, Megill J, Grubor B, Shi H, Fletcher A, Lombardo L, Levesque P, Lee FY, Sasseville VG. In vitro cardiotoxicity potential comparative assessments of chronic myelogenous leukemia tyrosine kinase inhibitor therapies: Dasatinib, imatinib and nilotinib. Blood. 2007;110:abstract 4582. [Google Scholar]
- 19.Lu Z, Jiang YP, Wang W, Xu XH, Mathias RT, Entcheva E, Ballou LM, Cohen IS, Lin RZ. Loss of cardiac phosphoinositide 3-kinase p110α results in contractile dysfunction. Circulation. 2009;120:318–325. doi: 10.1161/CIRCULATIONAHA.109.873380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 2004;110:3168–3174. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirams GR, Cui Y, Sher A, Fink M, Cooper J, Heath BM, McMahon NC, Gavaghan DJ, Noble D. Simulation of multiple ion channel block provides improved early prediction of compounds’ clinical torsadogenic risk. Cardiovasc Res. 2011;91:53–61. doi: 10.1093/cvr/cvr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monahan BP, Ferguson CL, Killeavy ES, Lloyd BK, Troy J, Cantilena LR., Jr Torsades de pointes occurring in association with terfenadine use. JAMA. 1990;264:2788–2790. [PubMed] [Google Scholar]
- 23.Sun H, Oudit GY, Ramirez RJ, Costantini D, Backx PH. The phosphoinositide 3-kinase inhibitor LY294002 enhances cardiac myocyte contractility via a direct inhibition of Ik, slow currents. Cardiovasc Res. 2004;62:509–520. doi: 10.1016/j.cardiores.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Cockerill SL, Tobin AB, Torrecilla I, Willars GB, Standen NB, Mitcheson JS. Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits. J Physiol. 2007;581:479–493. doi: 10.1113/jphysiol.2006.123414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Wang H, Wang J, Han H, Nattel S, Wang Z. Normal function of HERG K+ channels expressed in HEK293 cells requires basal protein kinase B activity. FEBS Lett. 2003;534:125–132. doi: 10.1016/s0014-5793(02)03804-8. [DOI] [PubMed] [Google Scholar]
- 27.Viard P, Butcher AJ, Halet G, Davies A, Nurnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- 28.Strutz-Seebohm N, Henrion U, Steinke K, Tapken D, Lang F, Seebohm G. Serum- and glucocorticoid-inducible kinases (SGK) regulate KCNQ1/KCNE potassium channels. Channels. 2009;3:88–90. doi: 10.4161/chan.3.2.8086. [DOI] [PubMed] [Google Scholar]
- 29.Salama G, London B. Mouse models of long QT syndrome. J Physiol. 2007;578:43–53. doi: 10.1113/jphysiol.2006.118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charpentier F, Bourgé A, Mérot J. Mouse models of SCN5A-related cardiac arrhythmias. Prog Biophys Mol Biol. 2008;98:230–237. doi: 10.1016/j.pbiomolbio.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, Smits JF, Flameng W, Clancy CE, Moons L, Vos MA, Dewerchin M, Benndorf K, Collen D, Carmeliet E, Carmeliet P. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med. 2001;7:1021–1027. doi: 10.1038/nm0901-1021. [DOI] [PubMed] [Google Scholar]
- 32.Tian XL, Yong SL, Wan X, Wu L, Chung MK, Tchou PJ, Rosenbaum DS, Van Wagoner DR, Kirsch GE, Wang Q. Mechanisms by which SCN5A mutation N1325S causes cardiac arrhythmias and sudden death in vivo. Cardiovasc Res. 2004;61:256–267. doi: 10.1016/j.cardiores.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantù F, Towbin JA, Keating MT, Hammoude H, Brown AM, Chen LS. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 34.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 35.Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A, Griffin JD, Hoelzer D, Albitar M, Dugan M, Cortes J, Alland L, Ottmann OG. Nilotinib in imatinib-resistant CML and Philadelphia chromosome–positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 36.Peyton JD, Rodon Ahnert J, Burris H, Britten C, Chen LC, Tabernero J, Duval V, Rouyrre N, Silva A, Quadt C, Baselga J. A dose-escalation study with the novel formulation of the oral pan-class I PI3K inhibitor BEZ235, solid dispersion system (SDS) sachet, in patients with advanced solid tumors. J Clin Oncol. 2011 May 20;29(Supplement):abstract 3066. [Google Scholar]
- 37.Fan G, Jiang YP, Lu Z, Martin DW, Kelly DJ, Zuckerman JM, Ballou LM, Cohen IS, Lin RZ. A transgenic mouse model of heart failure using inducible Gαq. J Biol Chem. 2005;280:40337–40346. doi: 10.1074/jbc.M506810200. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Duff HJ. Developmental changes in transient outward current in mouse ventricle. Circ Res. 1997;81:120–127. doi: 10.1161/01.res.81.1.120. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274:H747–H751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 40.Ballou LM, Cross ME, Huang S, McReynolds EM, Zhang BX, Lin RZ. Differential regulation of the phosphatidylinositol 3-kinase/Akt and p70 S6 kinase pathways by the α1A-adrenergic receptor in rat-1 fibroblasts. J Biol Chem. 2000;275:4803–4809. doi: 10.1074/jbc.275.7.4803. [DOI] [PubMed] [Google Scholar]
- 41.Lau DH, Clausen C, Sosunov EA, Shlapakova IN, Anyukhovsky EP, Danilo P, Jr, Rosen TS, Kelly C, Duffy HS, Szabolcs MJ, Chen M, Robinson RB, Lu J, Kumari S, Cohen IS, Rosen MR. Epicardial border zone overexpression of skeletal muscle sodium channel SkM1 normalizes activation, preserves conduction, and suppresses ventricular arrhythmia: An in silico, in vivo, in vitro study. Circulation. 2009;119:19–27. doi: 10.1161/CIRCULATIONAHA.108.809301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.