Abstract
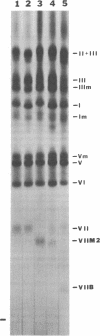
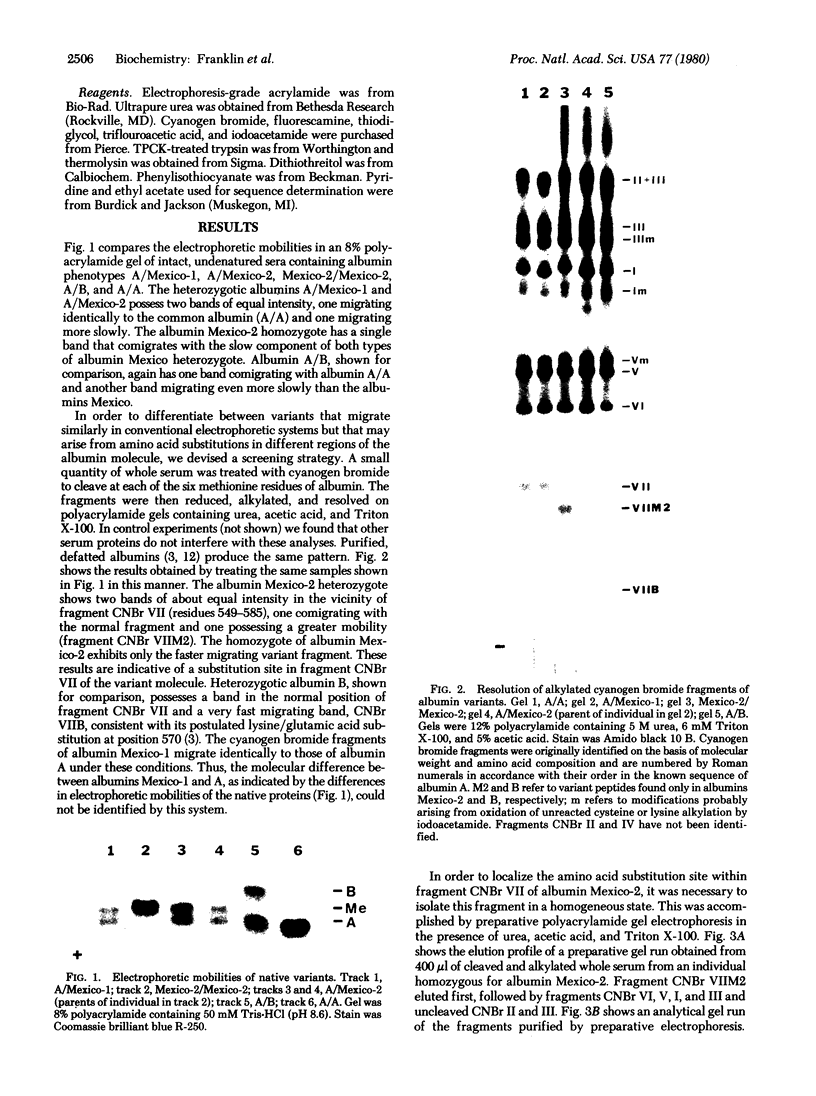
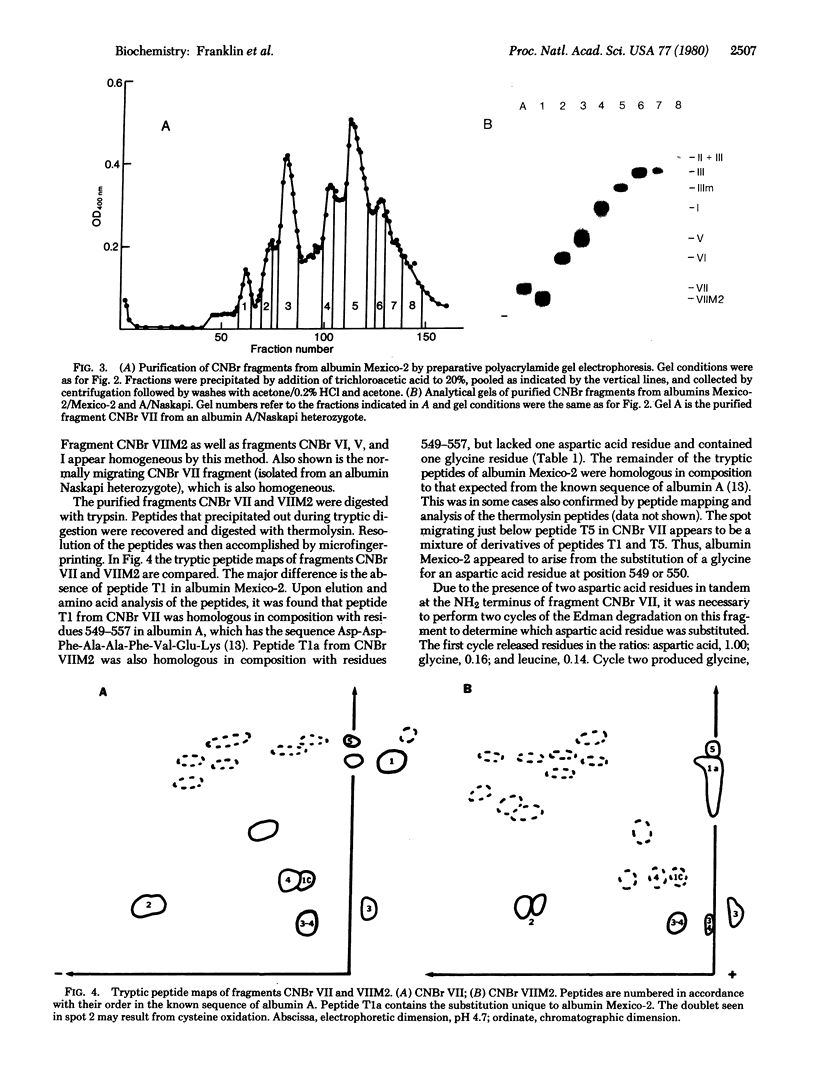
Using an electrophoretic screening procedure, we have discovered that two species of human serum albumin Mexico occur that are indistinguishable by conventional electrophoretic methods. We suggest that these species be referred to as albumins Mexico-1 and Mexico-2. Isolation and determination of the partial sequence of the cyanogen bromide fragment of albumin Mexico-2 that differs from the corresponding fragment of the common albumin A revealed this variant to arise from at least a glycine/aspartic acid substitution at position 550. This region of the albumin molecule is involved in the binding of the fatty acid, palmitate.
Keywords: albumin Mexico-1, variant screening, peptide mapping, Edman degradation
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P. H., Rushforth N. B., Miller M., LeCompte P. M. Epidemiologic studies of diabetes in the Pima Indians. Recent Prog Horm Res. 1976;32:333–376. doi: 10.1016/b978-0-12-571132-6.50021-x. [DOI] [PubMed] [Google Scholar]
- Blankstein L. A., Stollar B. D., Franklin S. G., Zweidler A., Levy S. B. Biochemical and immunological characterization of two distinct variants of histone H2A in Friend leukemia. Biochemistry. 1977 Oct 18;16(21):4557–4562. doi: 10.1021/bi00640a003. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Doyen N., Lapresle C. Partial non-cleavage by cyanogen bromide of a methionine--cystine bond from human serum albumin and bovine alpha-lactalbumin. Biochem J. 1979 Jan 1;177(1):251–254. doi: 10.1042/bj1770251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin S. G., Zweidler A. Non-allelic variants of histones 2a, 2b and 3 in mammals. Nature. 1977 Mar 17;266(5599):273–275. doi: 10.1038/266273a0. [DOI] [PubMed] [Google Scholar]
- Johnston F. E., Blumberg B. S., Agarwal S. S., Melartin L., Burch T. A. Alloalbuminemia in southwestern U.S. indians: polymorphism of albumin Naskapi and albumin Mexico. Hum Biol. 1969 May;41(2):263–270. [PubMed] [Google Scholar]
- Melartin L., Blumberg B. S., Lisker R. Albumin Mexico, a new variant of serum albumin. Nature. 1967 Sep 16;215(5107):1288–1289. doi: 10.1038/2151288a0. [DOI] [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Regeneration of amino acids from thiazolinones formed in the Edman degradation. Anal Biochem. 1975 Sep;68(1):47–53. doi: 10.1016/0003-2697(75)90677-6. [DOI] [PubMed] [Google Scholar]
- PEACOCK A. C., BUNTING S. L., QUEEN K. G. SERUM PROTEIN ELECTROPHORESIS IN ACRYLAMIDE GEL: PATTERNS FROM NORMAL HUMAN SUBJECTS. Science. 1965 Mar 19;147(3664):1451–1453. doi: 10.1126/science.147.3664.1451. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr, Feldhoff R. C. Fragments of bovine serum albumin produced by limited proteolysis. Isolation and characterization of tryptic fragments. Biochemistry. 1975 Jul 29;14(15):3384–3391. doi: 10.1021/bi00686a015. [DOI] [PubMed] [Google Scholar]
- Polesky H. F., Rokala D. A., Burch T. A. Serum albumin polymorphism in Indians of the southwestern United States. Nature. 1968 Oct 12;220(5163):175–176. doi: 10.1038/220175a0. [DOI] [PubMed] [Google Scholar]
- Urban M. K., Franklin S. G., Zweidler A. Isolation and characterization of the histone variants in chicken erythrocytes. Biochemistry. 1979 Sep 4;18(18):3952–3960. doi: 10.1021/bi00585a017. [DOI] [PubMed] [Google Scholar]
- Weitkamp L. R., Salzano F. M., Neel J. V., Porta F., Geerdink R. A., Tárnoky A. L. Human serum albumin: twenty-three genetic variants and their population distribution. Ann Hum Genet. 1973 Apr;36(4):381–392. doi: 10.1111/j.1469-1809.1973.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Wilding G., Blumberg E. S., Vesell E. S. Reduced warfarin binding of albumin variants. Science. 1977 Mar 11;195(4282):991–994. doi: 10.1126/science.841323. [DOI] [PubMed] [Google Scholar]
- Winter W. P., Weitkamp L. R., Rucknagel D. L. Amino acid substitution in two identical inherited human serum albumin variants: albumin Oliphant and albumin Ann Arbor. Biochemistry. 1972 Feb 29;11(5):889–896. doi: 10.1021/bi00755a031. [DOI] [PubMed] [Google Scholar]