Abstract
When given to pigeons, the direct-acting dopamine agonist apomorphine elicits pecking. The response has been likened to foraging pecking because it bears remarkable similarity to foraging behavior, and it is enhanced by food deprivation. On the other hand, other data suggest the response is not related to foraging behavior and may even interfere with food ingestion. Although elicited pecking interferes with food capture, it may selectively alter procurement phases of feeding, which can be isolated in operant preparations. To explore the relation between operant and elicited pecking, we provided pigeons the opportunity to earn different reinforcer magnitudes during experimental sessions. During signaled components, each of 4 pigeons could earn 2-, 4-, or 8-s access to grain for a single peck made at the end of a 5-min interval. In general, responding increased as a function of reinforcer magnitude. Apomorphine increased pecking for 2 pigeons and decreased pecking for the other 2. In both cases, apomorphine was more potent under the component providing the smallest reinforcer magnitude. Analysis of the pattern of pecking across the interval indicated that behavior lost its temporal organization as dose increased. Because apomorphine-induced pecking varied inversely with reinforcer magnitude, we conclude that elicited pecks are not functionally related to food procurement. The data are consistent with the literature on behavioral resistance to change and suggest that the effects of apomorphine may be modulated by prevailing stimulus–reinforcer relationships.
Keywords: apomorphine, foraging, reinforcer magnitude, resistance to change, pigeon, key peck
When given to pigeons, the nonselective, direct-acting dopamine agonist apomorphine elicits prolonged bouts of pecking (Abelson & Woods, 1980; Goodman, 1981; Lindenblatt & Delius, 1988; Pinkston, Madden, & Fowler, 2008). Pecking emerges within minutes of administration and lasts for about an hour; the behavioral response is robust, several thousand pecks may be recorded following a single administration. As equally remarkable as the behavioral output is the specificity; in birds, apomorphine elicits pecking and has few if any other behavioral effects (see Delius, 1985; Godoy & Delius, 1999). Pecks are most often directed to contrasting stimuli in the environment (e.g., spots, screw heads, joints in the floor or wall of the observation chamber). The functional significance of the elicited pecking is not completely understood. We take a view similar to others that simply labeling the behavior “stereotypy” or “drug-induced movements” will not bring us far in understanding the nature of the response (e.g., Brunelli, Magni, Moruzzi, & Musumeci, 1975).
Early research suggested that elicited pecks may be due to enhanced motivational effects (Brunelli et al., 1975). Formally, the pecking response bears striking similarities to foraging pecking (e.g., Delius, 1985; Deshpande, Sharma, Kherdikar, & Grewal, 1961), suggesting the pecking response may be related to foraging and feeding behavior. Such a conclusion is supported by the fact that food deprivation potentiates apomorphine's effects on pecking (see Deviche, 1984 and Wynne & Delius, 1995 for discussions). Lesions of forebrain structures that impair pigeons' ability to target food items selectively attenuate apomorphine-elicited pecking (Wynne & Delius, 1996). On the other hand, apomorphine-induced pecking often is not directed at available food and may even decrease food capture and ingestion (Cheng & Long, 1974; Deviche, 1984).
Still, foraging pecking is a complex sequence of several coordinated phases involving, among others, approach, procurement, and ingestive responses (Siemann & Delius, 1992; Zeilger, Levitt, & Levine, 1980), and it may be that apomorphine selectively increases only certain phases of the sequence. Operant preparations provide a means to separate procurement responses from consummatory responses, which are an advantage over simple observations of the drug's effects on freely feeding birds. As the research cited above seems to have established that the drug does not enhance food capture and ingestion, the present study was an analysis of the effects of apomorphine on operant procurement behavior. Previous work has shown that when the apomorphine-elicited pecks are directed at the operandum, operant response rate increases; when these pecks are directed at other parts of the apparatus, rate decreases (Abelson & Woods, 1980). It is likely such differences are topographical only and depend on the animal's individual history; for example, it has been shown that the characteristics of the operandum (key or treadle) can alter the topography of elicited pecking (Graeff & De Oliveira, 1975).
The primary rationale of the present experiment was to evaluate if apomorphine selectively alters food-procurement behavior. We established different rates of behavior by arranging and signaling different reinforcer magnitudes in different parts of the session (e.g., Osborne, 1978). If apomorphine-induced pecking is related to food procurement behavior, then its effects should be exacerbated during those portions of the session that promote and facilitate such behavior.
A secondary rationale was to conduct a follow-up study to that reported by Carey (1982), one of the very few studies that have examined how reinforcer magnitude modulates apomorphine's effects on operant behavior. Carey signaled three different magnitudes of intercranial stimulation (currents were determined individually for each animal) in different parts of the session. Increasing doses of apomorphine (0.125 – 0.5 mg/kg) progressively eliminated responding maintained by the lowest current but had little effect on pressing maintained by the highest current. Carey suggested the highest current had a “protective” effect on operant lever pressing, making behavior less susceptible to reduction by apomorphine. It must be noted, however, that there are several differences between preparations using more conventional reinforcers (e.g., food) and intercranial stimulation preparations (Lenzer, 1972), not the least of which is lack of clarity between procurement and consummatory behavior (unless special arrangements are made, see Hawkins & Pliskoff, 1964). Thus, a secondary goal of the present project was to replicate systematically the experiment by Carey using a more conventional stimulus as the reinforcer.
METHOD
Subjects
Four male White Carneau pigeons were used. All of the pigeons had experience with the experimental task and with several different compounds prior to this experiment (see Lamb & Ginsburg, 2008), but no pigeon had experienced any drug within several weeks preceding the present study or any experience with apomorphine. Each pigeon was housed individually and maintained at 80% of its laboratory free-feeding weight in a humidity- and temperature-controlled colony room; lights in the colony room were programmed on a 12:12 light:dark cycle. Water was continuously available in the home cage. Supplemental food was given postsession as needed to maintain body weight. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Apparatus
Experimental sessions were conducted in commercial pigeon chambers (Gerbrands Model G7410S). One wall of each chamber was constructed of brushed aluminum and housed three horizontally aligned response keys; only the center key was used. Response keys could be transilluminated white, green, or red by 24-V lamps mounted behind the key. Near the floor of the chamber, below the center key, a square aperture provided access to a standard diet (Purina Checkers, Purina Mills, St Louis, MO, USA) via a solenoid-operated food hopper. A 24-V white lamp mounted near the ceiling opposite the response panel provided general illumination. Each chamber was enclosed in a light- and sound- attenuating shell (Gerbrands, G7211) that was equipped with an exhaust fan. Experimental events and data recording were accomplished by a computer running Med-PC IVTM (Med Associates, Georgia, VT).
Procedure
Because pigeons had prior experience with the experimental task, no pretraining was required. Each session began with the onset of the house lamp. During the session, each pigeon pecked on a three-component multiple fixed-interval (FI) 5-min schedule of grain presentation, so in each component the food hopper was raised after the first response following 5 min. The duration of food access varied across components. Illumination of the center key by green, white, or red light resulted in 2-, 4-, or 8-s access to food, respectively. A single food presentation could be obtained in each multiple-schedule component. A limited hold was programmed that terminated the component if food was not collected within 60 s of the elapse of the FI; any food not collected was cancelled. Each session was composed of four blocks of three components. During each block, the order of components was determined randomly. Components were separated by a 60-s blackout, where the chamber and response keys were darkened and responding had no programmed consequences. Experimental sessions were conducted Monday-Friday.
Once responding stabilized on the schedule, each pigeon was tested with doses of 0.1, 0.32, 0.56, 1.0, and 3.2 mg/kg or the saline vehicle. Tests occurred on Tuesdays and Fridays. Each point on the dose-response function was determined twice. The order of the first determination was random; the order was reversed for the second determination. Every Thursday tests of the saline vehicle were made and served as control data.
Several weeks following tests with apomorphine, we conducted a prefeeding test; the rationale for this manipulation rests in the fact that our apomorphine tests produced differences more in line with current theories of behavioral persistence (e.g., Nevin & Grace, 2000), so we conducted a common test from that literature to determine if our procedure would yield similar outcomes. The test lasted one week. From Monday to Friday, each pigeon was given 0 (control), 20, 40, 60, and 80 g of food in an increasing series. Food was placed in the pigeon's home-cage food cup 30 min prior to the experimental session.
Drug preparation
Apomorphine HCL (Sigma, St. Louis, MO) was dissolved in physiological saline to an injection volume of 1 ml/kg. New solutions were prepared on the day of each test. Injections were given im into the pectoral muscle 10 min prior to the start of the session. All doses are expressed in terms of the salt.
RESULTS
Figure 1 shows average rate of responding observed on control tests made each Thursday during dose-response determinations. Response rates increased with increases in reinforcer magnitude. Repeated-measures ANOVA revealed a significant effect of magnitude on response rate (F(2,6) = 254.1, p < .001). Follow-up paired t-test comparisons revealed that response rates were significantly different for all comparisons (t-values ranged from 10.3 – 22.5, p < .001 in all cases).
Fig. 1.
Control rates of responding. The y-axis shows the rate of responding as a function of the reinforcer magnitude available at the end of the fixed interval. Points represent averages of the data collected following saline administrations every Thursday during dose-response determinations.
For the analysis of apomorphine's effects, rates obtained on test days were expressed as a proportion of control values. We first examined the data for systematic differences between the first and second determinations of the dose-response curve as apomorphine may induce sensitization with repeated treatment (e.g., Acerbo & Delius, 2004; Keller, Delius, & Acerbo, 2002). Data from both determinations were examined using repeated-measures ANOVA; there was no effect of determination order so the data from corresponding determinations were averaged together. The final dose-response functions are shown in Figure 2. For 2 pigeons, (P9 and P29) apomorphine increased pecking, while pecking was decreased for the other 2 pigeons (P5 and P17). Additionally, the data hint that different maximum effects were obtained as a function of reinforcer magnitude when apomorphine increased rate. Observations made during the sessions revealed that both pigeons pecked at the floor grate when apomorphine decreased pecking. Despite differences in the topographical characteristics of pecking, Figure 2 shows that pecking was affected similarly by reinforcer magnitude. Specifically, the potency of both apomorphine's rate-increasing and rate-decreasing effects varied inversely with magnitude.
Fig. 2.
Effects of apomorphine on key pecking. The y-axis shows the rate of key pecking expressed as a proportion of control rates (data from Thursdays) plotted against dose of apomorphine on the x-axis for each pigeon. Points above “S” indicate the effects of the saline vehicle. Apomorphine's rate-increasing effects are plotted in the left column; rate-decreasing effects are plotted in the right column. Note, both axes are log-spaced.
To quantify the effects of apomorphine on pecking, we calculated ED50 values for each curve. The ED (Effective Dose) 50 is defined as the dose that produces 50% of the maximum effect. For the rate-decreasing effects, this value was 0.5. Calculations for the rate-increasing effects were somewhat more complicated. Within each pigeon, we identified the maximum behavioral effect observed during the experiment and calculated the dose based on 50% of that value. Note, all empirical functions encompassed the identified value, which could then be calculated as the dose that increased behavior to the half-maximal value.
The ED50 was calculated by taking the two points lying immediately above and below the point where the dose–response function crossed the half-maximal value and estimated via linear interpolation using the logarithm of the doses. Table 1 shows the ED50 values for each pigeon. A repeated-measures ANOVA on the logarithm of the dose identified a significant effect of ED50 values as a function of reinforcer magnitude (F(2,6) = 13.54, p < .006). Follow-up paired t-tests indicated that the ED50 obtained under the smallest magnitude was reduced compared to the middle (t = 4.51, p < .025) and largest values (t = 3.67, p < .035); the latter two values were not different from each other.
Table 1.
ED50 (mg/kg) for each reinforcer magnitude.

|
Reinforcer Magnitude (s) |
Pigeon |
|||
|
P5 |
P9 |
P17 |
P29 |
|
| 2 | 0.26 | 0.82 | 0.44 | 0.91 |
| 4 | 0.48 | 1.56 | 1.91 | 1.81 |
| 8 | 0.45 | 1.27 | 1.90 | 2.40 |
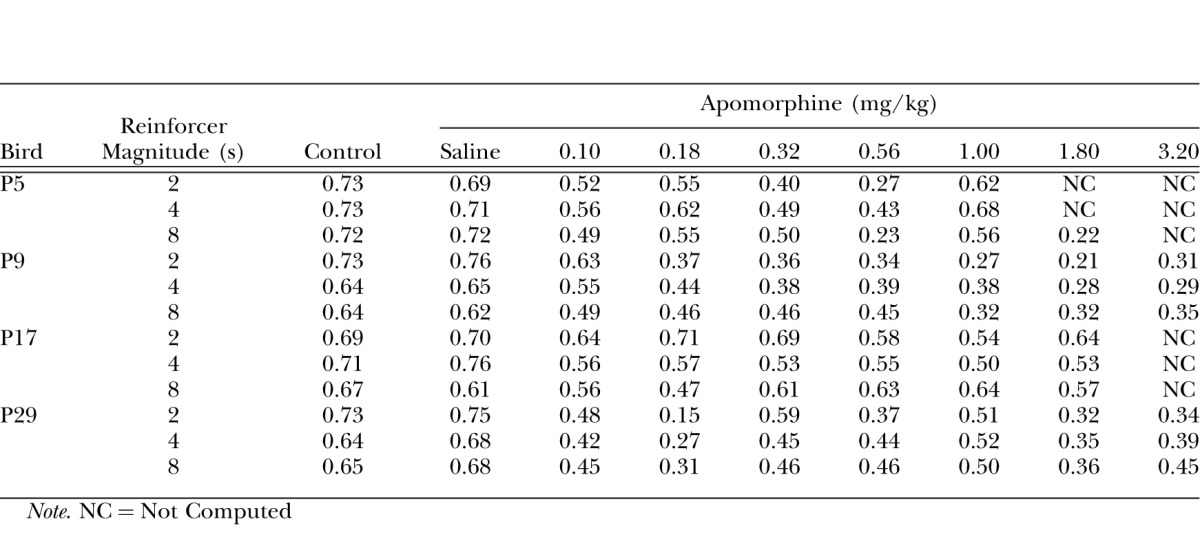
In addition to apomoprhine's effects on pecking rate, we examined its effects on the temporal pattern of behavior during the interval. Pattern was quantified by quarter-life (Herrnstein & Morse, 1957) which indicates the portion of the interval at which 25% of the responses have been emitted. Values greater than .25 indicate a positively increasing function across the interval and values less than .25 indicate a negatively increasing function. When quarter life equals .25, responding is approximately constant across the interval. Quarter life was calculated by dividing the fixed interval into 10 equal bins and summing the number of responses falling into each bin over the session for each component. The bin containing the 25th percentile was identified and quarter life was computed by linear interpolation. Quarter life was not computed if response rate fell below 10% of control values. Data from individual determinations were averaged and are shown in Table 2. Recall that tests with the saline vehicle were made each Thursday (control) and also were given as part of the dosing sequence (saline).
Table 2.
Quarter life values as a function of apomorphine dose.
|
Bird |
Reinforcer Magnitude (s) |
Control |
Apomorphine (mg/kg) |
|||||||
|
Saline |
0.10 |
0.18 |
0.32 |
0.56 |
1.00 |
1.80 |
3.20 |
|||
| P5 | 2 | 0.73 | 0.69 | 0.52 | 0.55 | 0.40 | 0.27 | 0.62 | NC | NC |
| 4 | 0.73 | 0.71 | 0.56 | 0.62 | 0.49 | 0.43 | 0.68 | NC | NC | |
| 8 | 0.72 | 0.72 | 0.49 | 0.55 | 0.50 | 0.23 | 0.56 | 0.22 | NC | |
| P9 | 2 | 0.73 | 0.76 | 0.63 | 0.37 | 0.36 | 0.34 | 0.27 | 0.21 | 0.31 |
| 4 | 0.64 | 0.65 | 0.55 | 0.44 | 0.38 | 0.39 | 0.38 | 0.28 | 0.29 | |
| 8 | 0.64 | 0.62 | 0.49 | 0.46 | 0.46 | 0.45 | 0.32 | 0.32 | 0.35 | |
| P17 | 2 | 0.69 | 0.70 | 0.64 | 0.71 | 0.69 | 0.58 | 0.54 | 0.64 | NC |
| 4 | 0.71 | 0.76 | 0.56 | 0.57 | 0.53 | 0.55 | 0.50 | 0.53 | NC | |
| 8 | 0.67 | 0.61 | 0.56 | 0.47 | 0.61 | 0.63 | 0.64 | 0.57 | NC | |
| P29 | 2 | 0.73 | 0.75 | 0.48 | 0.15 | 0.59 | 0.37 | 0.51 | 0.32 | 0.34 |
| 4 | 0.64 | 0.68 | 0.42 | 0.27 | 0.45 | 0.44 | 0.52 | 0.35 | 0.39 | |
| 8 | 0.65 | 0.68 | 0.45 | 0.31 | 0.46 | 0.46 | 0.50 | 0.36 | 0.45 | |
Note. NC = Not Computed
In general, apomorphine disrupted the temporal control over behavior. Similar effects were observed when the drug increased or decreased key pecking rates, suggesting topographical differences were not a factor in determining the extent of temporal disruption. For example, instances of near constant rates of responding may be observed when the drug increased key pecking (e.g., for P9 following 1.0 and 1.8 mg/kg). Constancy across the interval can also be seen when the drug decreased pecking (for P5 following 0.56 mg/kg). The effects on the temporal organization of pecking were not affected by reinforcer magnitude in an obvious way. The missing data points complicated statistical analysis of the data. When we omitted doses from analysis that were not represented by all subjects, the ANOVA revealed a significant effect of dose on quarter life (F(6,18) = 6.29, p < .001), but there was no significant effect of reinforcer magnitude or the interaction term.
Tests with apomorphine replicated the “protective” effect of larger reinforcer magnitudes reported by Carey (1982). Previous research has shown the conditions correlated with larger magnitudes can make behavior more persistent to environmental challenges (e.g., Nevin, 1974; Nevin & Grace, 2000; Podlesnick & Shahan, 2008), which may account for the “protective” effect. To determine whether those variables were operating in our procedure as others have reported, we conducted a week-long prefeeding test, which is common to studies on behavioral persistence. The data are shown in Figure 3. Response rates following prefeedings of 20–80 g (Tuesday – Friday) have been expressed as a proportion of control values obtained on Monday. The figure shows that behavior was disrupted more easily by prefeeding when pecking was maintained by the smaller reinforcer magnitudes. Repeated-measures ANOVA on the log-transformed data identified a significant effect of prefeeding amount (F(3,9) = 5.8, p < .02) and a significant interaction between prefeeding amount and reinforcer magnitude (F(6, 18) = 2.8, p < .04).
Fig. 3.
Effects of prefeeding on key pecking. The y-axis shows the rate of key pecking expressed as a proportion of control. The test lasted one week. Data from Monday served as the control; increasing amounts of food were given presession on Tuesdays-Fridays. Note the y-axis is log-spaced. Other details are as in Figure 2.
Consistent with previous findings, operant behavior maintained by increased reinforcer magnitude was more persistent in the face of partial satiation prior to the session (Nevin & Grace, 2000; Nevin & Shahan, 2011; Podlesnick & Shahan, 2008). The effects of prefeeding on temporal patterning during the fixed-interval are shown in Table 3. Unlike the effects on response rate, temporal patterning was not differentially affected by reinforcer magnitude. Only P9's data appeared consistently ordered with respect to magnitude; quarter life was largely unchanged for the remaining 3 pigeons. As with the effects of apomorphine, missing data complicated statistical analyses, and conditions with missing data were excluded. Repeated-measures ANOVA found no significant main effect or interaction between reinforcer magnitude and the pre-fed amount.
Table 3.
Quarter life values as a function of prefeeding amount.

|
Bird |
Reinforcer Magnitude (s) |
Amount (g) |
||||
|
0 |
20 |
40 |
60 |
80 |
||
| P5 | 2 | 0.81 | 0.60 | 0.69 | 0.64 | NC |
| 4 | 0.67 | 0.67 | 0.75 | 0.64 | 0.63 | |
| 8 | 0.64 | 0.70 | 0.51 | 0.43 | 0.61 | |
| P9 | 2 | 0.82 | 0.47 | 0.74 | 0.66 | 0.45 |
| 4 | 0.60 | 0.56 | 0.48 | 0.54 | 0.54 | |
| 8 | 0.80 | 0.39 | 0.75 | 0.81 | 0.66 | |
| P17 | 2 | 0.76 | 0.82 | 0.83 | 0.75 | 0.58 |
| 4 | 0.69 | 0.63 | 0.59 | 0.64 | 0.62 | |
| 8 | 0.72 | 0.78 | 0.65 | 0.60 | 0.58 | |
| P29 | 2 | 0.68 | 0.68 | 0.73 | NC | NC |
| 4 | 0.67 | 0.62 | 0.59 | NC | NC | |
| 8 | 0.60 | 0.70 | 0.64 | 0.73 | 0.69 | |
Note. NC = Not Computed
DISCUSSION
Earlier work suggested that apomorphine-elicited pecking reflected enhanced motivation of foraging behavior (Brunelli et al., 1975). Later work, however, indicated that elicited pecking actually interferes with food capture and ingestion (Cheng & Long, 1974; Deviche, 1984). We speculated that apomorphine selectively facilitates only certain aspects of feeding, specifically procurement behavior, and we arranged an operant situation where such responses could be isolated. When different reinforcer magnitudes were available according to a fixed-interval schedule, response output was an increasing function of magnitude. Following apomorphine tests, 2 pigeons showed rate-increasing effects and 2 pigeons showed rate-decreasing effects. Overall, however, all 4 pigeons showed that reinforcer magnitude was inversely related to apomorphine's potency to increase or decrease responding. The data are incongruent with the idea that apomorphine selectively enhances food-procurement behavior because circumstances that appeared to make such behavior more likely were less sensitive to the drug's effects. Prior data has shown that the drug impairs the ability of pigeons to capture and ingest food (Cheng & Long, 1974; Deviche, 1984). Thus, if elicited pecks are related to foraging, they must reflect an aspect of the behavior different from procurement and consummatory responses.
Although there were similar changes in potency across birds, there were apparent differences in the maximum effects of apomorphine. When apomorphine increased responding, the maximum effect appeared to be an inverse function of reinforcer magnitude. We did not test higher doses as the maximum effects obtained have been reported to be within the range of 2 – 3.2 mg/kg (Ableson & Woods, 1980; Godoy & Delius, 1999), so it is not clear from our data if the functions represent real maximum differences or horizontal shifts. The rate-decreasing effects did not show differences in maximum effects, likely because there was a floor beyond which no further decrease in operant behavior could be observed, while there was no ceiling for increasing effects. To our knowledge, these are the first data showing that reinforcer magnitude may alter the maximum effect of apomorphine-elicited pecking. Likely, this was serendipitous for us, as the rate increases are the rarer outcome following apomorphine. Both pigeons, however, revealed similar changes in maximum effect; this suggests further study on the interactions between apomorphine and reinforcer magnitude is warranted.
It is worth further comment that 2 of our pigeons revealed rate increases and 2 revealed rate decreases. One may speculate that these effects are functionally different, and this may play a role in the differences in the maximum behavioral effects observed. In contrast, our data show consistency across all pigeons with respect to reinforcer magnitude in terms of the potency of apomorphine's effects, which argue that differences in the location of the elicited pecks are simply topographical, and as just discussed, difference in maxima are due to the absence of a ceiling when pecks are directed toward the key. The literature has indicated that rate-increasing and rate-decreasing effects reflect the direction of the elicited pecks on or off the response key, respectively (Ableson & Woods, 1980; Graeff & Oliveira, 1975), and also suggest behavioral differences reflect distinct topographies, not functions.
Yet, the possibility that the different topographies of elicited pecks reflect functional differences has not received experimental attention, and it remains an important question for future research. Perhaps the greatest barrier to asking questions of function is that there is little data on why elicited pecking is directed on the key for some pigeons and off the key for others. Reinforcement history may play a role in determining the topography of apomorphine-elicited pecking (Graeff & De Oliveira, 1975), although all of our pigeons had similar histories, suggesting other factors may be important. In an unpublished study, we examined the onset of elicited pecking in 2 pigeons. Following the first administration of apomorphine, 1 pigeon pecked a spot on the floor and one pigeon pecked a screw on the wall. The forms observed on the first exposure persisted across several further determinations of apomorphine. Our observations are only anecdotal, but they suggested to us that the factors controlling topography may be subtle and local, such as what the pigeon happens to be observing during the onset of the first exposure to the drug. Once different topographies can be produced experimentally, questions of additional functional differences can be addressed.
In terms of temporal patterning, apomorphine consistently decreased quarter life for all pigeons. Similarly, Ableson & Woods (1980) reported that apomorphine decreased quarter life. The present study additionally shows that the ability of apomorphine to disrupt temporal pattern is not affected by reinforcer magnitude in the same way as response output. Prior work from our lab showed that some compounds may alter the temporal pattern of fixed-interval behavior as revealed by rate-dependency analyses (Dews & Wenger, 1977). Lamb & Ginsburg (2008) showed that the rate-dependent effects of desipramine and fluvoxamine were attenuated by increases in reinforcer magnitude with a procedure identical to the one used here. We re-analyzed the present data in terms of rate-dependency, but we did not generally find evidence that increased reinforcer magnitude attenuated the effects. Rate-dependent effects were observed for apomorphine, consistent with other work (Robbins, Roberts, & Koob, 1983), but neither the slopes nor intercepts varied systematically with reinforcer magnitude. In that regard, it is notable that another study from our lab showed the rate-dependent effects of d-amphetamine were not modulated by reinforcer magnitude, but the rate-dependent effects of cocaine were (Ginsburg, Pinkston, & Lamb, 2011); along with the present data, the pattern of results suggests that the ability of reinforcer magnitude to modulate the pattern-altering effects of dopaminergic compounds on operant behavior is under the influence of impulse-dependent transmitter release (see Ginsburg et al.).
Our data showed that reinforcer magnitude attenuated elicited pecking, and the results systematically replicate the effects reported by Carey (1982) showing that increased magnitude has a “protective” action on apomorphine's behavioral effects, extending those findings to operant behavior maintained by more conventional reinforcers (i.e., food) and to pigeons. In accounting for this fact, we note that the results are consistent with current theories of behavioral persistence (Nevin & Grace, 2000; Nevin & Shahan, 2011; Podlesnick & Shahan, 2008), which suggest that stimuli correlated with higher rates or magnitudes of reinforcement increase the resistance of behavior to environmental pertubations, which we show were operative in our procedure (see Figure 3). Extending work on persistence to the present case, the data indicate that operant behavior maintained by larger reinforcer magnitudes was less disrupted by elicited pecking. Additionally, the present results extend the scope of the current model. For 2 pigeons, the behavioral effect of the drug was to increase responding. To date, common ways of perturbing behavior generally decrease responding (e.g., satiation, extinction). The present data show that the predictions of the theory hold when the change in behavior is an increasing function. Such a prediction seems implicit in the theory's formulation as “resistance to change” broadly stated should describe departures from baseline regardless of direction. Thus, apomorphine-elicited pecking may represent an important avenue for future exploration of persistence of behavior in response to variables that increase response output.
Acknowledgments
This work was supported by Grant AA012337. The authors thank Gerardo Martinez for his assistance. Questions regarding this manuscript should be directed to Jonathan Pinkston who is now at the University of North Texas, Department of Behavior Analysis, Denton, TX, 76205 (E-mail: jonathan.pinkston@unt.edu).
REFERENCES
- Abelson J. S., Woods J. H. Effects of apomorphine on elicited and operant pecking in pigeons. Psychopharmacology. (1980);71:237–241. doi: 10.1007/BF00433057. [DOI] [PubMed] [Google Scholar]
- Acerbo M. J., Delius J. D. Behavioral sensitization to apomorphine in pigeons (Columba livia): blockade by the D1 dopamine antagonist SCH-23390. Behavioral Neuroscience. (2004);118:1080–1088. doi: 10.1037/0735-7044.118.5.1080. [DOI] [PubMed] [Google Scholar]
- Brunelli M., Magni F., Moruzzi G., Musumeci D. Apomorphine pecking in the pigeon. Archives of Italian Biology. (1975);113:303–325. [PubMed] [Google Scholar]
- Carey R. J. Rate dependent inhibition of self-stimulation by apomorphine. Pharmacology, Biochemistry, & Behavior. (1982);16:859–861. doi: 10.1016/0091-3057(82)90250-7. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Long J. P. Dopaminergic nature of apomorphine-induced pecking in pigeons. European Journal of Pharmacology. (1974);26:313–320. doi: 10.1016/0014-2999(74)90242-8. [DOI] [PubMed] [Google Scholar]
- Delius J.D. The peck of the pigeon: free for all. In: Lowe C.F., Richelle M., Blackman D.E., Bradshaw C.M., editors. Behaviour analysis and contemporary psychology. New York: Erlbaum; (1985). pp. 53–81. In. (Eds.) pp. [Google Scholar]
- Deshpande V. R. M., Sharma M. L., Kherdikar P. R., Grewal R. S. Some observations on pecking in pigeons. British Journal of Pharmacology. (1961);17:7–11. doi: 10.1111/j.1476-5381.1961.tb01098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deviche P. Administration of small doses of apomorphine attenuates feeding in non-deprived pigeons. Physiology & Behavior. (1984);33:581–585. doi: 10.1016/0031-9384(84)90375-5. [DOI] [PubMed] [Google Scholar]
- Dews P. B., Wenger G. R. Rate-dependency of the behavioral effects of amphetamine. In: Thompson T., Dews P. B., editors. Advances in behavioral pharmacology, Vol 1. New York: Academic Press; (1977). pp. 167–227. In. (Eds) pp. [Google Scholar]
- Ginsburg B. C., Pinkston J. W., Lamb R. J. Reinforcement magnitude modulation of rate dependent effects in pigeons and rats. Experimental & Clinical Psychopharmacology. (2011);19:285–294. doi: 10.1037/a0024311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy A. M., Delius J. D. Sensitization to apomorphine in pigeons is due to conditioning, subject to generalization but resistant to extinction. Behavioural Pharmacology. (1999);10:367–378. doi: 10.1097/00008877-199907000-00004. [DOI] [PubMed] [Google Scholar]
- Goodman I. J. Apomorphine and amphetamine induced stereotyped behavior in adult pigeons. Pharmacology, Biochemistry, & Behavior. (1981);15:701–704. doi: 10.1016/0091-3057(81)90008-3. [DOI] [PubMed] [Google Scholar]
- Graeff F. G., De Oliveira L. Influence of response topography on the effect of apomorphine and amphetamine on operant behavior of pigeons. Psychopharmacologia. (1975);41:127–132. doi: 10.1007/BF00421069. [DOI] [PubMed] [Google Scholar]
- Hawkins T. D., Pliskoff S. S. Brain-stimulation intensity, rate of self-stimulation, and reinforcement strength: An analysis through chaining. Journal of the Experimental Analysis of Behavior. (1964);7:285–288. doi: 10.1901/jeab.1964.7-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernstein R. J., Morse W. H. Effects of pentobarbital on intermittently reinforced behavior. Science. (1957);125:929–931. doi: 10.1126/science.125.3254.929-a. [DOI] [PubMed] [Google Scholar]
- Keller S., Delius J. D., Acerbo M. J. Apomorphine sensitization: evoking conditions, context dependence, effect persistence and conditioned nature. Behavioural Pharmacology. (2002);13:189–201. doi: 10.1097/00008877-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Lamb R. J., Ginsburg B. C. Reinforcement magnitude modulates the rate-dependent effects of fluvoxamine and desipramine on fixed-interval responding in the pigeon. Behavioural Pharmacology. (2008);19:51–60. doi: 10.1097/FBP.0b013e3282f3d093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzer I. I. Differences between behavior reinforced by electrical stimulation of the brain and conventionally reinforced behavior. Psychological Bulletin. (1972);78:103–118. doi: 10.1037/h0032975. [DOI] [PubMed] [Google Scholar]
- Lindenblatt U., Delius J. D. Nucleus basalis prosencephali, a substrate of pomorphine-induced pecking in pigeons. Brain Research. (1998);453:1–8. doi: 10.1016/0006-8993(88)90137-0. [DOI] [PubMed] [Google Scholar]
- Nevin J. A. Response strength in multiple schedules. Journal of the Experimental Analysis of Behavior. (1974);21:389–408. doi: 10.1901/jeab.1974.21-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin J. A., Grace R. C. Behavior momentum and the law of effect. The Behavior & Brain Sciences. (2000);23:73–90. doi: 10.1017/s0140525x00002405. [DOI] [PubMed] [Google Scholar]
- Nevin J. A., Shahan T. A. Behavioral momentum theory: Equations and applications. Journal of Applied Behavior Analysis. (2011);44:877–895. doi: 10.1901/jaba.2011.44-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne S. R. A quantitative analysis of the effects of amount of reinforcement on two response classes. Journal of Experimental Psychology: Animal Behavior Processes. (1978);4:297–317. [Google Scholar]
- Pinkston J. W., Madden G. J., Fowler S. C. Effects of white and infrared lighting on apomorphine-induced pecking in pigeons. Behavioural Pharmacology. (2008);19:347–352. doi: 10.1097/FBP.0b013e32830990ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlesnik C. A., Shahan T. A. Response-reinforcer relations and resistance to change. Behavioural Processes. (2008);77:109–125. doi: 10.1016/j.beproc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Robbins T. W., Roberts D. C., Koob G. F. Effects of d-amphetamine and apomorphine upon operant behavior and schedule-induced licking in rats with 6-hydroxydopamine-induced lesions of the nucleus accumbens. Journal of Pharmacology & Experimental Therapeutics. (1983);224:662–673. [PubMed] [Google Scholar]
- Siemann M., Delius J. D. Variability of forage pecking in pigeons. Ethology. (1992);92:29–50. [Google Scholar]
- Wynne B., Delius J. D. Sensitization to apomorphine in pigeons: unaffected by latent inhibition but still due to classical conditioning. Psychopharmacology. (1995);119:414–420. doi: 10.1007/BF02245857. [DOI] [PubMed] [Google Scholar]
- Wynne B., Delius J. D. Frontal forebrain lesions: effects on the foraging and apomorphine pecking of pigeons. Physiology & Behavior. (1996);59:757–762. doi: 10.1016/0031-9384(95)02160-4. [DOI] [PubMed] [Google Scholar]
- Zeilger H. P., Levitt P. W., Levine R. R. Movement patterns, stereotypy, and stimulus control. Journal of Comparative and Physiological Psychology. (1980);94:783–794. [Google Scholar]





