Abstract
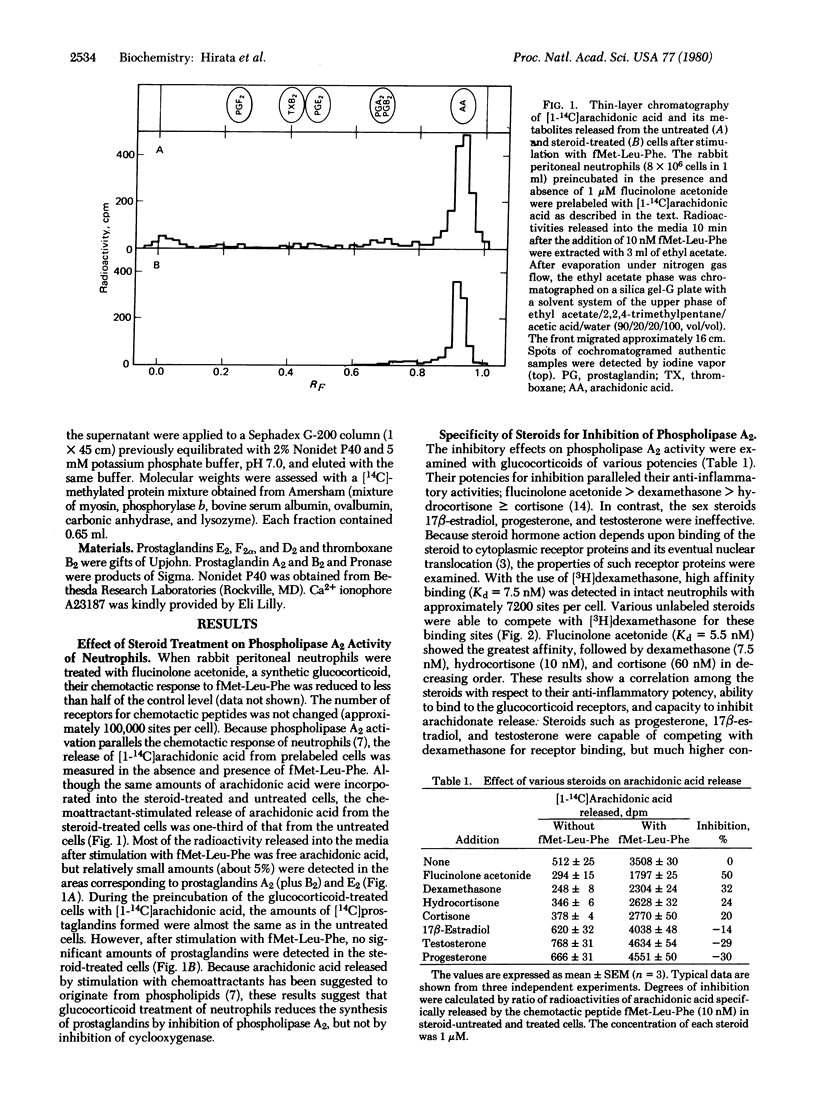
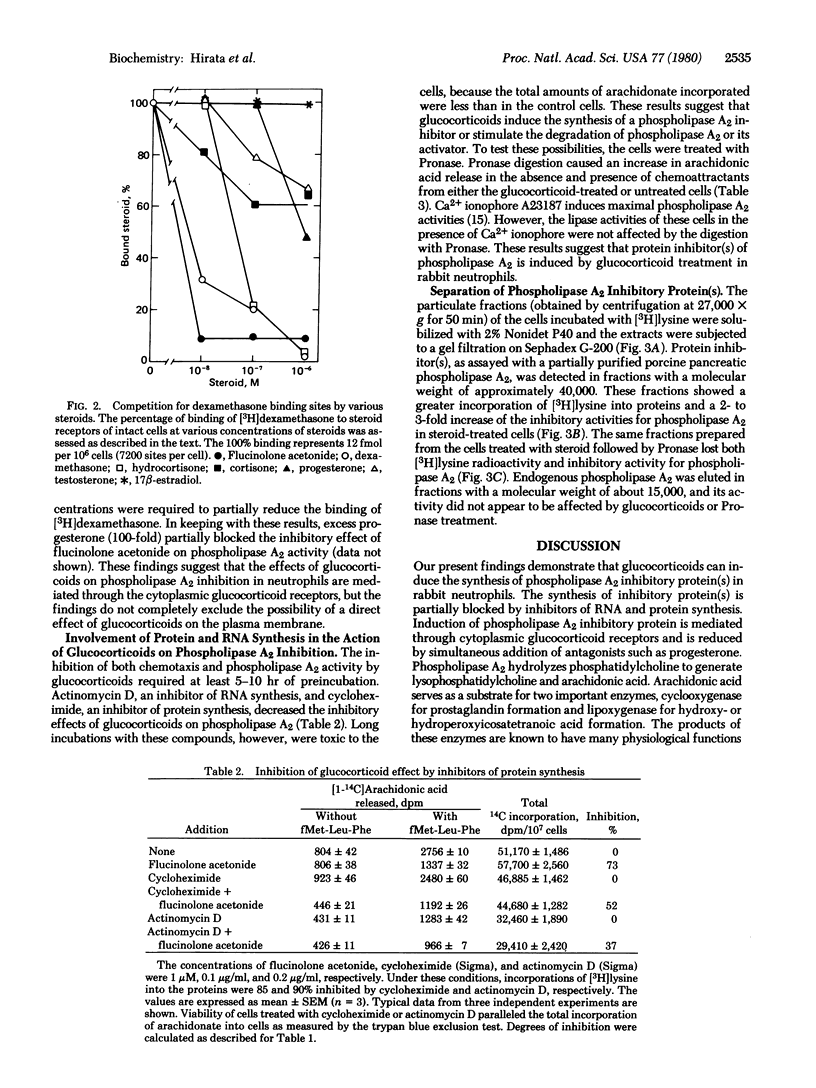
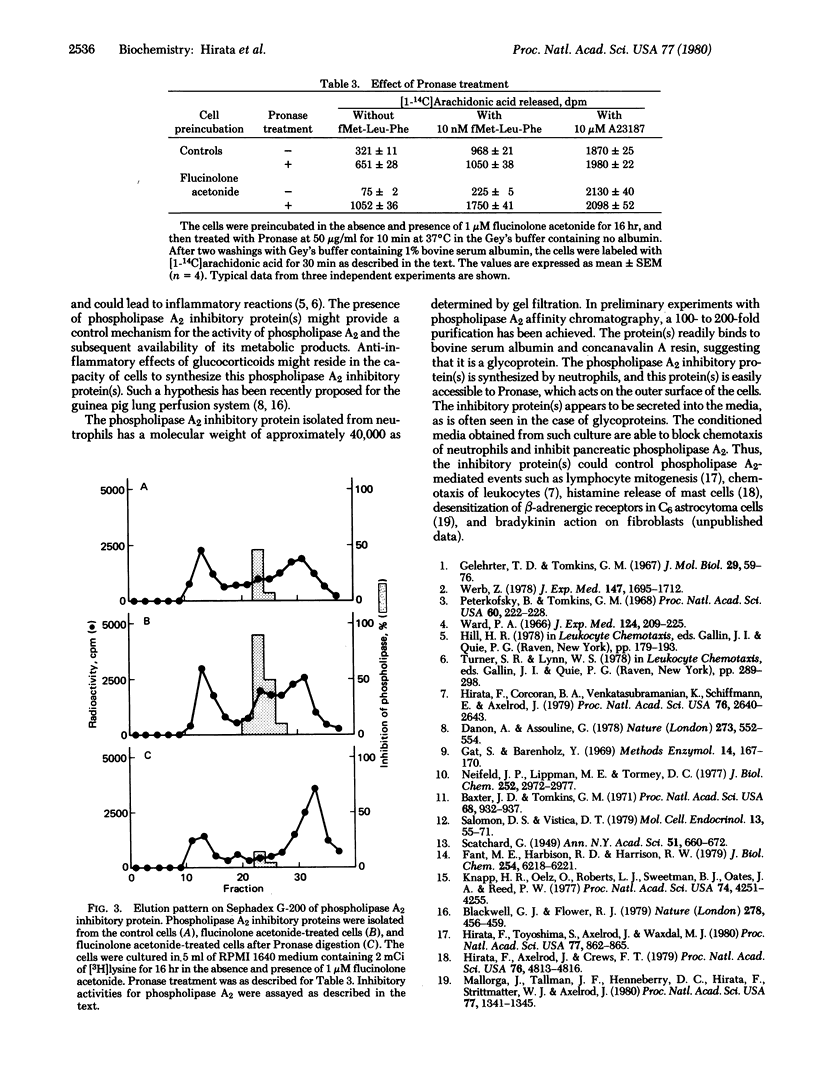
When rabbit peritoneal neutrophils were treated with glucocorticoids, their chemotactic response to stimulation by the chemoattractant fMet-Leu-Phe was markedly reduced. Preincubation of cells with glucocorticoids also decreased phospholipase A2 (phosphatide 2-acylhydrolase, EC 3.1.1.4) activity in situ as measured by the release of [1-14C]arachidonic acid previously incorporated into phospholipids. The inhibitory potencies of glucocorticoids on phospholipase A2 activity correlated well with their anti-inflammatory activities and their abilities to bind to glucocorticoid receptors. Inhibitors of RNA and protein synthesis suppressed the inhibitory effect of glucocorticoids on phospholipase A2 activity. Digestion of the glucocorticoid-treated cells by Pronase overcame the inhibitory activity. Phospholipase A2 activity induced by Ca2+ ionophore A23187 was not affected by Pronase treatment. Gel filtration of proteins from neutrophil membranes labeled with [3H]lysine showed an induction of protein(s) (about 40,000 daltons) after glucocorticoid treatment. This protein inhibited a partially purified pancreatic phospholipase A2 and reduced the peptide-initiated chemotactic response of neutrophils.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. D., Tomkins G. M. Specific cytoplasmic glucocorticoid hormone receptors in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1971 May;68(5):932–937. doi: 10.1073/pnas.68.5.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A., Assouline G. Inhibition of prostaglandin biosynthesis by corticosteroids requires RNA and protein synthesis. Nature. 1978 Jun 15;273(5663):552–554. doi: 10.1038/273552a0. [DOI] [PubMed] [Google Scholar]
- Fant M. E., Harbison R. D., Harrison R. W. Glucocorticoid uptake into human placental membrane vesicles. J Biol Chem. 1979 Jul 25;254(14):6218–6221. [PubMed] [Google Scholar]
- Flower R. J., Blackwell G. J. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979 Mar 29;278(5703):456–459. doi: 10.1038/278456a0. [DOI] [PubMed] [Google Scholar]
- Gelehrter T. D., Tomkins G. M. The role of RNA in the hormonal induction of tyrosine aminotransferase in mammalian cells in tissue culture. J Mol Biol. 1967 Oct 14;29(1):59–76. doi: 10.1016/0022-2836(67)90181-7. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J., Crews F. T. Concanavalin A stimulates phospholipid methylation and phosphatidylserine decarboxylation in rat mast cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4813–4816. doi: 10.1073/pnas.76.10.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Toyoshima S., Axelrod J., Waxdal M. J. Phospholipid methylation: a biochemical signal modulating lymphocyte mitogenesis. Proc Natl Acad Sci U S A. 1980 Feb;77(2):862–865. doi: 10.1073/pnas.77.2.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp H. R., Oelz O., Roberts L. J., Sweetman B. J., Oates J. A., Reed P. W. Ionophores stimulate prostaglandin and thromboxane biosynthesis. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4251–4255. doi: 10.1073/pnas.74.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallorga P., Tallman J. F., Henneberry R. C., Hirata F., Strittmatter W. T., Axelrod J. Mepacrine blocks beta-adrenergic agonist-induced desensitization in astrocytoma cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1341–1345. doi: 10.1073/pnas.77.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neifeld J. P., Lippman M. E., Tormey D. C. Steroid hormone receptors in normal human lymphocytes. Induction of glucocorticoid receptor activity by phytohemagglutinin stimulation. J Biol Chem. 1977 May 10;252(9):2972–2977. [PubMed] [Google Scholar]
- Peterkofsky B., Tomkins G. M. Evidence for the steroid-induced accumulation of tyrosine-aminotransferase messenger RNA in the absence of protein synthesis. Proc Natl Acad Sci U S A. 1968 May;60(1):222–228. doi: 10.1073/pnas.60.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D. S., Vistica D. T. Steroid receptors and steroid response in cultured L1210 murine leukemia cells. Mol Cell Endocrinol. 1979 Jan;13(1):55–71. doi: 10.1016/0303-7207(79)90076-5. [DOI] [PubMed] [Google Scholar]
- Ward P. A. The chemosuppression of chemotaxis. J Exp Med. 1966 Aug 1;124(2):209–226. doi: 10.1084/jem.124.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. Biochemical actions of glucocorticoids on macrophages in culture. Specific inhibition of elastase, collagenase, and plasminogen activator secretion and effects on other metabolic functions. J Exp Med. 1978 Jun 1;147(6):1695–1712. doi: 10.1084/jem.147.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]



