Abstract
p53 is an important tumor suppressor, functioning as a transcriptional activator and repressor. Upon receiving signals from multiple stress related pathways, p53 regulates numerous activities such as cell cycle arrest, senescence, and cell death. When p53 activities are not required, the protein is held in check by interacting with 2 key homologous regulators, Mdm2 and MdmX, and a search for inhibitors of these interactions is well underway. However, it is now recognized that Mdm2 and MdmX function beyond simple inhibition of p53, and a complete understanding of Mdm2 and MdmX functions is ever more important. Indeed, increasing evidence suggests that Mdm2 and MdmX affect p53 target gene specificity and influence the activity of other transcription factors, and Mdm2 itself may even function as a transcription co-factor through post-translational modification of chromatin. Additionally, Mdm2 affects post-transcriptional activities such as mRNA stability and translation of a variety of transcripts. Thus, Mdm2 and MdmX influence the expression of many genes through a wide variety of mechanisms, which are discussed in this review.
Keywords: Hdm2, Mdm4, transcription, translation
Introduction
The RING domain proteins Mdm2 and MdmX inhibit the functions of the tumor suppressor p53 under unstressed conditions.1 In response to cellular stress, p53 functions as a transcription factor to promote multiple cellular outcomes.2 The importance of p53 in restraining tumorigenesis is evidenced by its being mutated in more than 50% of human cancers. In the remaining cancers that harbor wild-type p53, overexpression of Mdm2 and MdmX is common in some tumor types such as sarcomas.3-5
Multiple mechanisms exist by which Mdm2 inhibits p53. First, Mdm2 binds to the p53 transactivation domain and prevents recruitment of transcriptional co-activators to target promoters.6,7 Second, Mdm2, via its RING domain, polyubiquitinates p53, targeting it for degradation by the ubiquitin-proteasome pathway.8,9 Third, Mdm2 promotes monoubiquitination of p53, exposing a nuclear export signal, leading to its cytoplasmic translocation.10,11 Fourth, Mdm2 inhibits p53 interaction with DNA.12-15 Fifth, Mdm2 inhibits p53 mRNA translation indirectly through promoting the degradation of the ribosomal protein L26,16 a protein that augments p53 translation.17
MdmX, the structural homologue of Mdm2, also functions as a negative regulator of p53-mediated transcription.18,19 The RING domain of MdmX, however, does not possess detectable E3 ligase activity.19 Although there is generally less mechanistic information as to how MdmX regulates transcription functions of p53, it was shown to be a much less potent inhibitor of p53 interaction with DNA.14,15
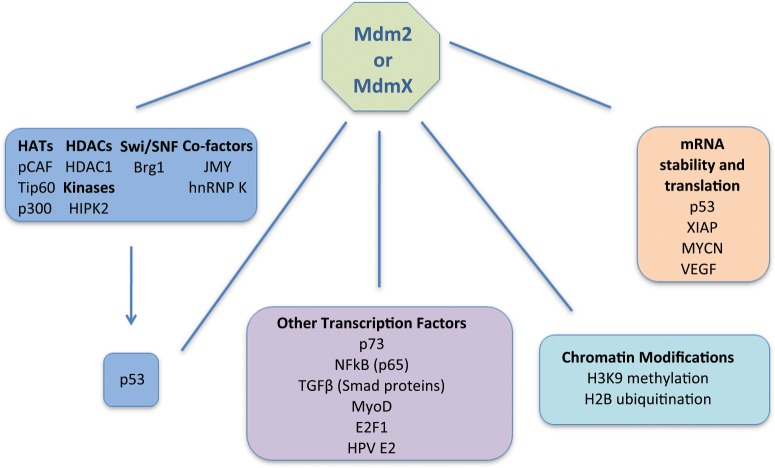
Extensive efforts have focused on developing inhibitors of Mdm2 and MdmX for cancer therapy,20 and it is therefore important that we understand how these 2 proteins function as fully as possible. Furthermore, accumulating evidence suggests the existence of p53-independent functions of Mdm2 and MdmX.1,3 In this review we focus on the more enigmatic aspects of Mdm2 and MdmX, that is, how the 2 proteins regulate gene expression. We discuss how they function both via affecting p53 target gene selectivity and through a wide array of interactions with proteins and mRNA. Figure 1 summarizes the different modes by which Mdm2 and MdmX regulate gene expression.
Figure 1.
Mdm2 and MdmX regulate gene expression by multiple modes. Mdm2 and MdmX affect p53-mediated target gene selectivity both directly and indirectly. First, they regulate enzymes that catalyze p53 post-translational modifications as well as p53-mediated chromatin remodeling and p53 interacting co-factors that influence target gene selectivity. Through direct binding to p53, they also regulate its activities. Second, Mdm2 and MdmX influence the activity of transcription factors other than p53. Third, Mdm2 also interacts with chromatin. where it influences chromatin modifications. Fourth, Mdm2 regulates the stability and translation of a number of mRNA transcripts.
Mdm2 and MdmX Regulate p53 Target Gene Selectivity
Insights derived from mouse models
It was initially assumed that Mdm2 and MdmX are nondiscriminatory inhibitors of p53 transactivation functions. Multiple lines of evidence now suggest that Mdm2 and MdmX affect target gene selectivity by p53 in a cell and tissue specific manner. It has only recently become possible to study these functions of Mdm2 and MdmX in mice, thanks to conditional mouse models in which p53 is reactivated in adult tissues of mice lacking Mdm2 and/or MdmX as well as p53 expression.
A hint about the differential effects of Mdm2 and MdmX on p53-mediated transcription is given by the phenotypes of the null mice. Mdm2 null mice die at 5.5 days post coitum (dpc).21,22 Bax deletion, although not suppressing the lethality of Mdm2 null mice, delays the death of the embryo, and the death is due to cell cycle arrest rather than apoptosis.23 MdmX null mice die later in development, at 7.5 to 12 dpc, due to proliferative arrest.24,25 Deletion of the cell cycle arrest gene p21 partially rescues the phenotype.23,26 These results suggest that p53 activity in the absence of Mdm2 or MdmX is qualitatively different, activating specific outcomes. However, these experiments do not answer whether the activation of cell cycle arrest leading to death in mouse embryos lacking both Mdm2 and Bax results from a timing delay in activation of p53 or is due to another reason. To be more specific, it may be that activation of p53 at 7.5 dpc preferentially promotes cell cycle arrest, whereas activation of p53 earlier in development will result in death by apoptosis.
A more careful examination of the effects of Mdm2 and MdmX loss on p53 activity was carried out in mouse embryo fibroblasts (MEFs) into which a temperature-sensitive (TS) p53 (p53A135V) was engineered.27 This analysis showed that the phenotypes discussed above are not due to the timing of p53 activation but rather are due to specific effects of Mdm2 and MdmX loss. p53A135V was introduced into MEFs that were isolated from mice lacking p53 and Mdm2 (TSΔ2), p53 and MdmX (TSΔX), or p53 alone (TS). In such mice, at the permissive temperature (32°C), p53 levels differ between the cell lines, with levels highest in TSΔ2, medium in TS, and lowest in TSΔX. The low p53 levels in TSΔX are due to Mdm2-mediated degradation, as cells engineered from the triple knock-outs (TSΔ2ΔX) do not exhibit low p53 levels. p53 reactivation in Mdm2 null MEFs results in apoptotic cell death, but not in MdmX nulls. TSΔ2ΔX behave likeTSΔ2, whereas TSΔX cells exhibit primarily G1 arrest following p53 reactivation despite the low p53 levels. An examination of p53 target gene mRNAs revealed that in the TSΔX cells, p53 activates Mdm2 and the cell cycle arrest gene p21, but not cyclin G1, Bax, Perp, and Noxa. TSΔ2, in contrast, activates the proapoptotic genes Perp and Noxa but not cyclin G1 and Bax, whereas p21 is only slightly elevated. Thus, Mdm2 and MdmX determine the outcome of p53 activation through affecting target gene selectivity by p53. A possible mechanistic explanation is that rather than directly influencing target gene activation, Mdm2 and MdmX simply affect p53 levels, and these, in turn, affect the outcome—cell cycle arrest when p53 levels are low versus apoptosis when p53 levels are high.
A recently published mouse model of inducible p53 in the background of either MdmX or Mdm2 knockout has enabled analysis of transcriptional responses to p53 activation in the absence of Mdm2 or MdmX in vivo. Knockin mice that express 4-hydroxy-tamoxifen (4-OHT)-dependent p53 (p53ERTAM) from its endogenous locus in response to 4-OHT were generated in wild-type (WT), MdmX null, or Mdm2 null backgrounds.28 Systemic reactivation of p53 in the adult MdmX null mice leads to activation of p53 target genes in a tissue specific manner. p21 is induced in all tested tissues except the small intestine, whereas the proapoptotic target Puma induction is restricted to the bone marrow, spleen, thymus and intestine—the classic radiosensitive tissues. Accordingly, tissues expressing Puma exhibit increased apoptosis. However, in spite of these responses to p53 activation, the mice are viable and recover well from 7-day sustained p53 activation. The intestines maintain function despite ongoing apoptosis, probably due to the continued proliferation resulting from lack of p21 activation in this tissue. This response is surprising in light of the previously published results of p53 activation in Mdm2 null background, where the mice cannot sustain a transient p53 restoration by 4-OHT administration and suffer severe loss of intestinal barrier function leading to death within 6 days.29 In this mouse model of p53 reactivation, restored p53 levels are higher in the absence of MdmX than in WT cells,28 in contrast to the model discussed above from Barboza et al.,27 where levels of TS p53 are lower in the absence of MdmX than in its presence. Thus, inherent differences between the experimental systems may influence the outcome of p53 activation, from which we can learn about the factors influencing the response to p53 activation in different contexts.
Studies with human cancer cells
Global alterations in gene expression following MdmX and Mdm2 knockdown in MCF7 breast cancer cells containing WT p53 have been analyzed.30,31 In this system, knockdown of either Mdm2 or MdmX leads to p21 induction and cell cycle arrest at G1 phase but does not increase cell death. Apoptosis is increased in cells harboring siRNAs directed against Mdm2 or MdmX in response to DNA damage with doxorubicin or cisplatin. The authors focused on genes that are commonly affected by the down-regulation of Mdm2 and MdmX, identifying 394 genes, of which 222 are up-regulated in response to the knockdown. The list of up-regulated genes is enriched for cell cycle arrest-promoting p53 target genes, including p21, BTG2, and ACTA2. The remaining 172 genes are down-regulated in the knockdown cells and are enriched for E2F1 target genes, consistent with repression of E2F1 activity by elevated p21. It would be interesting to analyze the microarray data for genes differentially affected in cells harboring siRNAs directed against Mdm2 versus MdmX.
Work in our laboratory has shown that levels of Mdm2 or MdmX can have profound impact on the p53 response. We examined the effect of excess Mdm2 on p53-mediated target gene activation in H1299 lung cancer cells with tetracycline-regulatable p53 that were engineered to constitutively express excess Mdm2.32 The excess Mdm2 does not affect p53 levels or localization but does affect target gene activation. Specifically, upon p53 induction in cells without excess Mdm2, expression of p21, Bax, 14-3-3σ, and PIG-3 genes is increased. In cells expressing extra Mdm2, however, 14-3-3σ and PIG-3 mRNA levels are markedly reduced, whereas p21 and Bax expression is unaffected. The outcome of p53 activation is thus altered by the excess Mdm2, from primarily G1 arrest with little G2 arrest in control cells to significantly increased G2 arrest in cells expressing excess Mdm2. Conversely, our recent study demonstrated that down-regulation of MdmX impairs stress-induced transcriptional activation of the p53 target genes Mdm2 and Wip1 but not several other p53 targets such as p21, 14-3-3σ, cyclin G1, Bax, PIG-3, and Noxa. 15 Mechanistically, down-regulation of MdmX in MCF7 cells results in impaired recruitment of p53 to the Mdm2 promoter.
Mdm2 regulates p53 post-translational modifications
Another way by which Mdm2 affects p53-regulated gene expression is through regulating the proteins that post-translationally modify p53. In fact, post-translational modifications of p53 have been well documented to influence its target gene activation.33-35 Complicating the issues here is the fact that in some cases modifiers of p53 are themselves targets of ubiquitination and degradation by Mdm2, such as the histone acetyl transferases PCAF36 and Tip60.37
Perhaps the best-studied interplay between Mdm2 and p53 modifiers involves histone acetyl transferases. Mdm2 inhibits p30038,39 and PCAF40-mediated acetylation of p53. Although Kobet et al. 38 concluded that Mdm2 binds to p300 and to p53 via separate domains, forming a ternary complex, Matt et al. 41 reported that there is no specific interaction between Mdm2 and p300. Teufel et al. 7 reconciled this dispute by describing a multidomain interaction between p300 and p53, mapping 4 regions on p300 that interact with TAD1 (residues 20-40) and TAD2 (residues 41-60), that comprise the 2 transcriptional activation domains of p53. Structural analysis supports a model of ternary complex formation with Mdm2 interacting with TAD1 and p300 interacting with TAD2.42 Further support for the multidomain interaction of p300 with p53 comes from the observation that whereas the interaction of Mdm2 with p53 is inhibited dramatically by phosphorylation of p53(T18), the interaction of p53 with p300 or CBP is inhibited in an additive fashion by phosphorylation of p53 on multiple residues.43,44
The inhibition of p53 acetylation by Mdm2 is affected by the protein SOX4, which is often up-regulated in cancers. SOX4 shifts the balance between ubiquitination and acetylation of p53. By binding to p53, SOX4 inhibits ubiquitination of p53 by Mdm2, whereas by interacting with p300/CBP it promotes p53 acetylation.45
Not only does Mdm2 inhibit p53 acetylation, it also promotes HDAC1-mediated p53 deacetylation via multiple mechanisms. Initially, Mdm2 was shown to associate with HDAC1 in cells and to promote p53 deacetylation and ubiquitination.46 It was later shown that other factors affect the Mdm2 and HDAC1-mediated p53 deacetylation. The matrix attachment region (MAR) binding protein SMAR1 promotes p53 deacetylation by forming a ternary complex with Mdm2 and S15-phosphorylated p53, leading to HDAC1 recruitment and deacetylation of p53.47 Kap1 is an adaptor protein that interacts with p53 in an Mdm2-dependent manner. Kap1 cooperates with Mdm2 both to promote HDAC1 interaction with p53, leading to p53 deacetylation, and to enhance Mdm2-mediated ubiquitination of p53.48 A subsequent study reported that Kap1 forms a ternary complex with p53 and Mdm2 following some forms of stress and thus influences p53 target gene selectivity.49
Acetylation is not the only p53 post-translational modification regulated by Mdm2. The protein kinase HIPK2, which phosphorylates p53 on S46, thus promoting apoptosis, is also a target of Mdm2. Overexpression of mutant Mdm2 that cannot bind to and degrade p53 but can still degrade HIPK2 results in lower p53 S46 phosphorylation and compromised cell death in response to lethal dose of doxorubicin.50 HIPK2 promotes apoptosis also by a p53-independent mechanism, through the inhibition of the transcriptional co-repressor CtBP.51 Therefore, by down-regulating HIPK2, Mdm2 may affect transcription by p53 as well as by CtBP.
Mdm2 inhibits p53-promoted chromatin remodeling
Chromatin remodeling is an essential step in transcriptional regulation and plays a key role in development and cancer.52 Human SWI/SNF chromatin remodeling complexes contain 1 of 2 ATPase subunits: Brahma (Brm) or Brahma-Related Gene 1 (Brg1).53 Both Brm- and Brg1-containing complexes differentially regulate p53 activity. Brg1-containing complexes are recruited by p53 to a subset of p53 target gene promoters, including the p21 promoter, but not to the Mdm2 promoter.54 Mdm2 overexpression in normal cells was shown to arrest cells in G1.55 This growth inhibitory function of Mdm2 requires both p53 and Brg1.56 Mdm2 protein can compete Brg1-containing complexes off p53, allowing the released Brg1-containing complexes to inhibit the expression of cyclin A and arrest the cell cycle. Thus, 2 epigenetic processes that are involved in p53-mediated gene regulation (histone post-translational modifications and chromatin remodeling) are influenced by Mdm2.
Binding partners that regulate p53 activity can themselves be Mdm2 targets
The E3 ligase activity of Mdm2 can also influence p53 transcriptional activity through targeting its cofactors. The first case involves Junction-Mediating and Regulatory protein (JMY), a cofactor of p300 that augments p53 mediated transcriptional activation and apoptosis.57 More recently, cytoplasmic JMY has been implicated in the nucleation of actin filaments promoting cell motility.58 Mdm2 interacts with JMY and targets it for proteasomal degradation, reducing the level of nuclear JMY. Following DNA damage, the Mdm2:JMY interaction is reduced and JMY accumulates in cells. This accumulation is enhanced in cells treated with the Mdm2:p53 inhibitor Nutlin-3. Accumulated JMY then functions in augmenting the apoptotic response, as demonstrated by down-regulation of JMY leading to decrease in apoptosis and increased G1 arrest in UV-treated cells.59
The second instance involves heterogeneous nuclear ribonucleoprotein K (hnRNP K), a poly-C binding protein that can interact with both DNA and RNA via its hnRNP K homology (KH) domain. hnRNP K is involved in transcription control in multiple ways, regulating the expression of c-Myc, SRC, and BRCA1, among others.60 hnRNP K accumulates in cells following DNA damage and is recruited to p53 target promoters in a p53-dependent manner. It is required for efficient recruitment of p53 to its target promoters and for induction of p53 target genes, and siRNA to hnRNP K severely impairs cell cycle arrest following DNA damage. hnRNP K is a target of Mdm2-mediated ubiquitination, and its stabilization following DNA damage requires inhibition of its ubiquitination.61 How hnRNP K functions in p53 recruitment and target gene activation is still unclear. It also remains to be determined whether hnRNP K is required for induction of all p53 targets or a specific subset.
Beyond p53: Mdm2 and MdmX Regulate Multiple Transcription Factors
Mice in which p53 is knocked out alone62 or together with Mdm221,22 or with MdmX24,25 have similarly increased incidence of cancers and early death. These elegant models indicate that if there are p53-independent functions of Mdm2 or MdmX, they are not significant to the life of the mouse. Nevertheless, other models suggest that the story may be not so simple. For example, a study of tumor onset and spectrum comparing p53 null mice with p53;Mdm2 double null or p53 null Mdm2 heterozygous mice showed that deletion of 1 (but not 2) Mdm2 allele alters the tumor onset and spectrum, increasing sarcoma incidence.63 An additional example is provided by a careful examination of the timing of tumor formation in mice lacking p53 and MdmX, or p53 alone, revealing an earlier onset of tumors in the p53;MdmX double knock-out mice. Analysis in MEFs showed that MdmX promotes bipolar mitoses and genetic stability in a p53-independent manner.64,65 This function is specific to MdmX, as Mdm2 co-deletion does not affect the growth of p53 null MEFs.66
Perhaps the most obvious candidate for Mdm2-mediated regulation is p73, the p53 homologue67,68 that, like p53, can promote cell cycle arrest and apoptosis.67,69 There are multiple isoforms of p73. Those expressed from its P1 promoter contain a transactivation domain (TAp73) and display functional similarities to p53.70 Mdm2 was shown to bind to p73 and inhibit its transcriptional activity without promoting its degradation.71-74 In another study, however, overexpressed Mdm2 was shown to stabilize p73 and enhance its transcriptional activity.75 A later report demonstrated that Mdm2 mediates the NEDD8 modification of TAp73, promoting its nuclear export and inhibiting its activity.76 Nutlin-3 disrupts the interaction between Mdm2 and TAp73, leading to stabilization and activation of p73.77 Mdm2 protects activated T cells from p73-mediated cell death.78 Upon T-cell activation, NF-κB signaling activates the Mdm2 P1 promoter. The induced Mdm2 interacts with p73 and inhibits the activation of its proapoptotic target gene Bim, thus preventing cell death.
But the story does not end with p73, as there are several examples of other p53-independent factors that are regulated by Mdm2. A study into the links between Mdm2 and NF-κB found that Mdm2 can both inhibit p53-mediated repression of p65 and directly activate p65 transcription via interaction with SP1 sites in cells lacking p53.79 An examination of Mdm2 overexpression in rhabdomyosarcoma cell lines revealed differential responses depending on the state of NF-κB.80 In Rh30 cells, Mdm2 promotes the expression of p65 as described above, enhancing cell growth. In RD cells, which have constitutive expression of p65, Mdm2 represses p65, inhibiting cell growth and promoting apoptosis.
Unlike p73 and NF-κB signaling that were shown to be affected by Mdm2, transforming growth factor β (TGFβ) signaling is regulated by both Mdm2 and MdmX. TGFβ is a family of 5 cytokines that play roles in cell proliferation, migration, differentiation, and apoptosis. All TGFβs activate the TGFβ Ser/Thr kinase receptors, which signal through phosphorylation of Receptor Smad proteins. Phosphorylated Receptor Smads translocate to the nucleus, bind Smad4 and other cofactors, and function as transcription factors, leading to both tumor promoting as well as suppressing outcomes, depending on the cellular context. Among the genes regulated by Smad proteins are the CDK inhibitors p21 and p27. 81
Mdm2 was initially shown to inhibit TGFβ signaling independent of p53 in a screen for TGFβ suppressor cDNAs.82 Further investigation revealed that in an overexpression system, Mdm2 suppresses the activity of Smads1, 2, 3, and 4. Moreover, both Mdm2 and MdmX exhibit inhibitory functions toward Smad4 through affecting its subcellular localization, leading to its exclusion from the nucleus. Curiously, neither Mdm2 nor MdmX forms a complex with Smad4, leaving the mechanism for cytoplasmic sequestration upon co-expression unsolved.83 A second study showed that MdmX but not Mdm2 inhibits co-expressed Smad3 and Smad4 transcriptional activity, although it should be noted that Mdm2 still exhibits some inhibitory effect on endogenous Smads.84 The authors did not see any effect of Mdm2 or MdmX on Smad localization. A possible explanation for the discrepancy is that Yam et al. 83 performed their analyses in HeLa cells, whereas Kadakia et al. 84 used H1299 cells. Overexpression of p300, a cofactor for Smad transcriptional activation, reverses the Smad inhibition exerted by overexpressed MdmX.84
Considering the tissue-specific effects of Mdm2 and MdmX knockout mice discussed above, it comes as no surprise that studies on specific cancers or cell types reveal additional unique roles for Mdm2 in the regulation of cell physiology.
Overexpression of Mdm2 in the myoblast cell line C2C12 induces adipocyte differentiation through inhibition of MyoD-dependent transcriptional activation,85 and overexpression of SP1 or pRb can rescue MyoD activity.86 Further analysis of the role of Mdm2 in adipogenesis revealed that Mdm2 is required for cAMP-regulatory element binding protein (CREB)-dependent transactivation during adipocyte differentiation, in a p53-independent way.87
In pancreatic cancer cells with activated K-Ras and mutant p53, Mdm2 is overexpressed due to the hyperactive Ras signaling and is essential for cell viability.88 Analysis of cell cycle regulating proteins revealed that Mdm2 is required for the expression of cyclin D1, c-Jun, and c-Myc in these cells, suggesting a potential mechanism for the p53-independent growth promoting activity of Mdm2.
One of the most studied transcription factors regulated by Mdm2 is E2F1. Unfortunately, as often the case in science (indeed epitomized by p53 itself), the more you learn about a topic, the more complex it becomes. E2F family transcription factors are regulators of cell cycle genes. E2Fs are primarily regulated by their inhibitory binding partner pRb (although some family members are pRb independent), which becomes phosphorylated by cyclin-dependent kinases (CDKs) leading to its dissociation from E2Fs, allowing them to transactivate their targets (reviewed in Chen et al. 89). The p53 target gene p21 encodes a CDK inhibitor and thus links p53 to the pRb pathway. However, the links between the 2 pathways are much more complex, with pRb, p53, Mdm2, and ARF forming a network of binding interactions affecting each other’s activities.90 Mdm2 interacts with E2F1 and stimulates the transcriptional activity of E2F1 and its binding partner DP1.91 Moreover, Mdm2 mediates E2F1 activation through interacting with the E2F1 inhibitor pRb, in a p53-independent manner,92,93 and targeting pRb for degradation.94 ARF overexpression leads to higher levels of pRb, suggesting that ARF inhibits Mdm2-mediated pRb degradation.94 Interestingly, Mdm2 was also shown to promote E2F1 degradation,95 under conditions in which E2F1 normally induces apoptosis. Casein kinase 1 (CK1) complexes with Mdm2 to promote the degradation of p53 and the stabilization of E2F1 in unstressed cells.96 Therefore, Mdm2 regulates E2F1 activity under different conditions both positively and negatively, directly and indirectly, by protein-protein interaction and E3 ligase activity.
Recently Mdm2 was reported to influence transcription by affecting protein folding. Mdm2 possesses an ATP binding activity through the P-loop motif within its RING domain.97,98 Mdm2 functions as a chaperone toward p53 and E2F1, and this activity is influenced by ATP binding, in which binding of the nucleotide to Mdm2 stimulates p53 folding into a DNA binding-competent form.99 E2F1, in contrast, is misfolded in response to Mdm2 ATP binding, and thus its DNA binding activity is reduced. Although E2F1 is an in vitro ubiquitination target of Mdm2, this activity of Mdm2 is not involved in the chaperone function.100
A surprising role for Mdm2 in the expression of human papillomavirus (HPV) genes has been described.101 Mdm2 with intact E3 ligase activity is required for the gene expression mediated by the HPV-16 protein E2. Mdm2 directly interacts with E2 and is recruited to HPV-16 type promoter DNA, where it promotes E2-mediated transcriptional activation.
In summary, Mdm2 and MdmX influence target gene activation both by p53 and by a variety of transcription factors and signaling cascades. With the growing number of Mdm2 targets being discovered, we are likely to discover additional links with pathways important for life and death of a cell.
Linking Mdm2 to Chromatin
Evidence collected over the past years suggests that Mdm2 itself can affect transcription by localizing to promoter DNA, in both p53-dependent and -independent manners. Early on it was shown in an in vitro transcription system that Mdm2 fused to a heterologous DNA binding domain represses transcription.102 A region between aa 50-222 is required for this inhibition and directly interacts with basal transcription machinery components (TFIIE and TBP) in a p53-independent manner. That study examined Mdm2 (aa 1-324) function on naked DNA. In subsequent study, a heterologous DNA binding domain (Gal4) fused to full-length Mdm2 confirmed repression of p53-independent transcription of a luciferase reporter in cells. However, in this case, Mdm2 RING domain deletion abolishes the repressive ability of Mdm2.103 These data suggest that Mdm2 can function as a co-repressor if recruited to a promoter via its interaction with p53 as well as with other transcription factors that may possess DNA binding activity and can interact with Mdm2. Taken together, these studies suggest that there may be 2 independent domains in Mdm2 that are involved in transcriptional repression: one that resides within amino acids 50-222 and another within the RING domain.
In more physiological settings, Mdm2 was indeed shown to interact with endogenous promoters. Chromatin immunoprecipitation (ChIP) in cells overexpressing Mdm232,104 as well as in cells with high103,105 or normal106 levels of endogenous Mdm2 demonstrated that Mdm2 can be detected on p53 target gene promoters. The association of endogenous Mdm2 with p53 response elements is p53 dependent, as Mdm2 was not detected on these gene promoters in cells lacking p53106. Further supporting this observation, Mdm2 was shown to co-localize with p53 on the p21 gene promoter in a sequential ChIP experiment.103 Following DNA damage, Mdm2 dissociates from p53 target gene promoters.104,106 A global Mdm2 ChIP, however, has not been reported. Hence, we cannot conclude whether Mdm2 localization to chromatin is solely a p53-dependent event.
Chromatin bound Mdm2 may suppress p53 through multiple mechanisms. As described above, the classic model for Mdm2 inhibition of p53 transcription activity is by inhibiting its transactivation domain from recruiting transcriptional co-activators such as p300/CBP. Additionally, Mdm2 can recruit transcriptional co-repressors as well as attach ubiquitin moieties to histones, modifying the chromatin state at its vicinity.
Mdm2 forms a complex with SUV39H1 and EHMT1 histone methyl transferase proteins that catalyze the repressive histone H3 Lys9 (H3K9) methylation.107 Mdm2 recruits SUV39H1 and EHMT1 to p53, forming a complex that possesses H3K9 methylation ability in vitro. Interestingly, H3K9 methylation of the p53 target promoters p21 and Puma is increased following activation of p53, and Mdm2 expression promotes this modification. This suggests that SUV39H1 and EHMT1-mediated H3K9 methylation may play a role in the Mdm2:p53 negative feedback loop. ARF interaction with Mdm2 inhibits interaction with SUV39H1 and EHMT1, suggesting that oncogenic stress regulates Mdm2-interaction with histone methyl transferases. It is interesting to note that MdmX does not interact with SUV39H1 and EHMT1.107 Whether Mdm2 associates with chromatin while performing this function needs to be directly tested.
A more direct function of Mdm2 in influencing gene expression is through monoubiquitination of histone H2B. Mdm2 localizes with p53 to its response element in the p21 gene, which promotes monoubiquitination of histone H2B.103 The function of this modification is not well understood. On one hand, Mdm2 tethered to DNA represses basal transcription from a luciferase reporter in a RING domain-dependent manner,103 suggesting a repressive role for this modification as has been reported in yeast.108 On the other hand, analysis of the genome-wide occurrence of monoubiquitinated histone H2B (ubH2B) showed correlation with actively transcribed genes. Analysis of ubH2B dynamics along the p21 gene revealed association throughout the whole coding region that is stimulated by p53 induction and quickly removed when p53 levels are reduced.109 Thus, Mdm2 may exert positive as well as negative effects on transcription, both mediated by the Mdm2 RING domain. It is important to note, however, that Mdm2 is not the primary E3 ligase for H2B. The RNF20/40 complex is thought to be the main modifier, although BRCA1 can also promote this modification.110
Mdm2 Regulates mRNA Stability and Translation
As if there were not enough complexity in the multiple roles Mdm2 plays in regulating both p53-dependent and -independent transcription, we now know there are additional aspects of gene expression that are affected by Mdm2. Notably, a growing number of mRNAs have been reported to be regulated by Mdm2 post-transcriptionally (Table 1). The Mdm2 RING domain was shown to specifically interact with RNA in vitro in 1996.111 However, we are just beginning to discover which mRNA transcripts Mdm2 interacts with in the cell and the biological consequences of such interactions.
Table 1.
Mdm2-Mediated Regulation of mRNA Translation and Stability
| mRNA | Mechanism | References |
|---|---|---|
| p53 | Mdm2 RING domain interacts with p53 mRNA and enhances its translation | Candeias et al. 112 |
| Gajjar et al.113 | ||
| p53 | Mdm2 polyubiquitinates the p53 translation enhancer, L26 | Ofir-Rosenfeld et al. 16 |
| XIAP | Mdm2 RING domain interacts with the 5′-UTR IRES sequence, enhancing XIAP translation | Gu et al. 123 |
| MYCN | Mdm2 RING domain interacts with AU-rich elements within the 3′-UTR of MYCN mRNA, enhancing its stability | Gu et al. 128 |
| VEGF | Mdm2 RING domain interacts with VEGF 3′-UTR and increases its stability | Zhou et al. 130 |
p53 mRNA was the first transcript discovered to be regulated by Mdm2. The Mdm2 binding domain-encoding sequence in the p53 mRNA interacts with Mdm2, leading to enhanced translation of p53 as well as inhibition of Mdm2 E3 ligase activity.112 The stimulation of p53 translation does not depend on Mdm2 E3 ligase activity since an inactive mutant, Mdm2C464A, enhances p53 translation almost as well as WT Mdm2. In a recent follow-up study, the p53 mRNA: Mdm2 interaction was shown to depend on ATM-mediated phosphorylation of Mdm2 at S395, which contributes to p53 induction following DNA damage.113 Its importance was exemplified by the discovery that whereas Mdm2 siRNA increases apoptosis in unstressed conditions, it actually protects cells from undergoing apoptosis following DNA damage in an ATM-dependent manner. Interestingly, the p53 mRNA sequence encoding its Mdm2 binding domain is the same sequence that interacts with Mdm2, whereas the Mdm2 domain that binds the p53 mRNA is the RING finger domain, which is responsible for ubiquitinating p53. This suggests that the 2 functions of Mdm2 in the regulation of p53 may have co-evolved in p53 and Mdm2.114
Mdm2 regulates p53 mRNA through an additional, indirect mechanism, mediated by nucleolin and the ribosomal protein L26. In unstressed cells, nucleolin interacts with a double-stranded RNA structure formed by the 5′- and 3′-UTRs of the p53 mRNA, thereby inhibiting translation, possibly through stabilizing the repressive RNA structure.17,115 Following DNA damage, L26 interacts with the same double-stranded p53 mRNA structure and augments its translation,17,116 at least in part by interacting with nucleolin and preventing its homodimerization.115 Under unstressed conditions, Mdm2 ubiquitinates L26 and targets it for degradation, allowing nucleolin to repress p53 translation. Furthermore, the Mdm2 acidic domain interacts with L26 and prevents it from binding and augmenting translation of p53 mRNA. Following DNA damage, this inhibition is relieved, and L26 is free to augment p53 translation.16
Another mRNA regulated by Mdm2 is the X-linked inhibitor-of-apoptosis protein, XIAP. XIAP inhibits caspases 3, 7, and 9,117-119 the caspases responsible for initiating apoptosis in response to cellular stress via the intrinsic pathway.120,121 Transfected Mdm2 leads to an increase in XIAP protein levels.79 The XIAP mRNA contains an internal ribosome entry site (IRES) sequence in its 5′-UTR.122 Mdm2 interacts with the XIAP IRES through its RING domain, thereby enhancing its translation. Importantly, cancer cells overexpressing Mdm2 are resistant to IR-induced apoptosis, which is dependent on the Mdm2:XIAP IRES interaction but independent of p53, as it was observed in p53 null cells.123
Another gene whose mRNA is regulated by Mdm2 is MYCN, a gene often amplified in neuroblastoma that is associated with poor prognosis.124,125 Mdm2 is also often amplified in neuroblastomas126 and was shown to be a direct transcriptional target of MYCN in this tumor type.127 Forming a positive feedback loop, Mdm2 interacts with the MYCN mRNA and leads to its stabilization.128 Mechanistically, the stabilization of MYCN mRNA is mediated by the Mdm2 RING domain interaction with AU-rich elements within the 3′-UTR of MYCN mRNA and correlates with increased translation of MYCN. In contrast to Mdm2 regulation of XIAP, however, Mdm2 does not regulate IRES-dependent translation of MYCN; rather the enhanced translation seems to be a result of increased MYCN mRNA stability. Knockdown of Mdm2 in neuroblastoma cells down-regulates MYCN and inhibits colony formation (while not activating p53) and also protects p53 null neuroblastoma cells from resistance to IR.128
Vascular endothelial growth factor (VEGF) is an important inducer of angiogenesis and is essential for solid tumor development.129 The Mdm2 RING domain interacts with the VEGF mRNA 3′-UTR and increases its stability, leading to increased levels of VEGF protein, similar to its regulation of MYCN. In a xenograft mouse model, knockdown of Mdm2 in p53-null neuroblastoma cells reduces tumor size and microvessel density.130 The possible involvement of Mdm2-mediated regulation of MYCN in that xenograft experiment was not addressed.
There are still many unanswered questions regarding regulation of post-transcriptional gene expression by Mdm2. The precise mechanisms of Mdm2-mediated mRNA stability and translation enhancement are not yet fully delineated. For example, it would be interesting determine the structure of the p53 double-stranded UTR complex and how nucleolin and L26 affect it. More globally, an anti-Mdm2 RNA immunoprecipitation followed by a microarray or RNA-seq analysis may give us a more extensive picture of the transcripts bound and potentially regulated by Mdm2.
MdmX has not been shown as of yet to regulate mRNA stability or translation, but a thorough examination has not been reported. The RING domain is the primary region by which Mdm2 regulates mRNAs. Considering the high degree of similarity between the Mdm2 and MdmX RING domains, it is possible that MdmX carries out similar functions. The fact that both RING domains bind nucleotides with similar affinity and specificity might suggest that they have similar or related interactions with RNA.97,98
In conclusion, Mdm2 and MdmX modulate gene expression by multiple pathways, with outcomes and mechanisms not fully elucidated. Both global approaches for studying the effects of these proteins on gene expression as well as biochemical mechanistic studies will help us answer the “what” and “how” questions. The answers are likely to be quite complex, as the Mdm proteins appear to have tissue-specific effects that may also depend on the developmental stage. Future research will very likely reveal new and interesting modes by which the 2 proteins regulate gene expression separately and together.
Acknowledgments
We thank Moshe Oren and Stephen Jones for thoughtful discussions and helpful suggestions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review was supported by NIH grants 58316 and 77742.
References
- 1. Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20:299-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell, 2009;137: 413-31 [DOI] [PubMed] [Google Scholar]
- 3. Manfredi JJ. The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev. 2010;24: 1580-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80-3 [DOI] [PubMed] [Google Scholar]
- 5. Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26:3453-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857-60 [DOI] [PubMed] [Google Scholar]
- 7. Teufel DP, Freund SM, Bycroft M, Fersht AR. Four domains of p300 each bind tightly to a sequence spanning both transactivation subdomains of p53. Proc Natl Acad Sci U S A. 2007;104:7009-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296-9 [DOI] [PubMed] [Google Scholar]
- 9. Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25-7 [DOI] [PubMed] [Google Scholar]
- 10. Carter S, Bischof O, Dejean A, Vousden KH. C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat Cell Biol. 2007;9:428-35 [DOI] [PubMed] [Google Scholar]
- 11. Lohrum MA, Woods DB, Ludwig RL, Balint E, Vousden KH. C-terminal ubiquitination of p53 contributes to nuclear export. Mol Cell Biol. 2001;21:8521-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zauberman A, Barak Y, Ragimov N, Levy N, Oren M. Sequence-specific DNA binding by p53: identification of target sites and lack of binding to p53 - MDM2 complexes. EMBO J. 1993;12:2799-808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poyurovsky MV, Katz C, Laptenko O, et al. The C terminus of p53 binds the N-terminal domain of MDM2. Nat Struct Mol Biol. 2010;17:982-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cross B, Chen L, Cheng Q, Li B, Yuan ZM, Chen J. Inhibition of p53 DNA Binding Function by the MDM2 Protein Acidic Domain. J Biol Chem. 2011;286:16018-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biderman L, Poyurovsky MV, Assia Y, Manley JL, Prives C. MdmX is required for p53 interaction with and full induction of the Mdm2 promoter after cellular stress. Mol Cell Biol. 2012;32:1214-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ofir-Rosenfeld Y, Boggs K, Michael D, Kastan MB, Oren M. Mdm2 regulates p53 mRNA translation through inhibitory interactions with ribosomal protein L26. Mol Cell. 2008;32:180-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takagi M, Absalon MJ, McLure KG, Kastan MB. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49-63 [DOI] [PubMed] [Google Scholar]
- 18. Shvarts A, Steegenga WT, Riteco N, et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 1996;15: 5349-57 [PMC free article] [PubMed] [Google Scholar]
- 19. Stad R, Ramos YF, Little N, et al. Hdmx stabilizes Mdm2 and p53. J Biol Chem. 2000;275:28039-44 [DOI] [PubMed] [Google Scholar]
- 20. Popowicz GM, Domling A, Holak TA. The structure-based design of Mdm2/Mdmx-p53 inhibitors gets serious. Angew Chem Int Ed Engl. 2011;50:2680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206-8 [DOI] [PubMed] [Google Scholar]
- 22. Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995; 378:203-6 [DOI] [PubMed] [Google Scholar]
- 23. Chavez-Reyes A, Parant JM, Amelse LL, de Oca Luna RM, Korsmeyer SJ, Lozano G. Switching mechanisms of cell death in mdm2- and mdm4-null mice by deletion of p53 downstream targets. Cancer Res. 2003;63:8664-9 [PubMed] [Google Scholar]
- 24. Parant J, Chavez-Reyes A, Little NA, et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat Genet. 2001;29:92-5 [DOI] [PubMed] [Google Scholar]
- 25. Migliorini D, Lazzerini Denchi E, Danovi D, et al. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol Cell Biol. 2002;22:5527-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steinman HA, Sluss HK, Sands AT, Pihan G, Jones SN. Absence of p21 partially rescues Mdm4 loss and uncovers an antiproliferative effect of Mdm4 on cell growth. Oncogene. 2004;23:303-6 [DOI] [PubMed] [Google Scholar]
- 27. Barboza JA, Iwakuma T, Terzian T, El-Naggar AK, Lozano G. Mdm2 and Mdm4 loss regulates distinct p53 activities. Mol Cancer Res. 2008; 6:947-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia D, Warr MR, Martins CP, Brown Swigart L, Passegue E, Evan GI. Validation of MdmX as a therapeutic target for reactivating p53 in tumors. Genes Dev. 2011;25:1746-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ringshausen I, O’Shea CC, Finch AJ, Swigart LB, Evan GI. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell. 2006;10:501-14 [DOI] [PubMed] [Google Scholar]
- 30. Heminger K, Markey M, Mpagi M, Berberich SJ. Alterations in gene expression and sensitivity to genotoxic stress following HdmX or Hdm2 knockdown in human tumor cells harboring wild-type p53. Aging (Albany NY). 2009;1:89-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berberich SJ. RNAi knockdown of HdmX or Hdm2 leads to new insights into p53 signaling. Cell Cycle. 2010;9:3640-1 [DOI] [PubMed] [Google Scholar]
- 32. Ohkubo S, Tanaka T, Taya Y, Kitazato K, Prives C. Excess HDM2 impacts cell cycle and apoptosis and has a selective effect on p53-dependent transcription. J Biol Chem. 2006;281:16943-50 [DOI] [PubMed] [Google Scholar]
- 33. Beckerman R, Prives C. Transcriptional regulation by p53. Cold Spring Harb Perspect Biol. 2010;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764-72 [DOI] [PubMed] [Google Scholar]
- 36. Jin Y, Zeng SX, Lee H, Lu H. MDM2 mediates p300/CREB-binding protein-associated factor ubiquitination and degradation. J Biol Chem. 2004;279:20035-43 [DOI] [PubMed] [Google Scholar]
- 37. Legube G, Linares LK, Lemercier C, Scheffner M, Khochbin S, Trouche D. Tip60 is targeted to proteasome-mediated degradation by Mdm2 and accumulates after UV irradiation. EMBO J. 2002;21:1704-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kobet E, Zeng X, Zhu Y, Keller D, Lu H. MDM2 inhibits p300-mediated p53 acetylation and activation by forming a ternary complex with the two proteins. Proc Natl Acad Sci U S A. 2000;97:12547-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ito A, Lai CH, Zhao X, et al. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 2001;20:1331-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jin Y, Zeng SX, Dai MS, Yang XJ, Lu H. MDM2 inhibits PCAF (p300/CREB-binding protein-associated factor)-mediated p53 acetylation. J Biol Chem. 2002;277:30838-43 [DOI] [PubMed] [Google Scholar]
- 41. Matt T, Martinez-Yamout MA, Dyson HJ, Wright PE. The CBP/p300 TAZ1 domain in its native state is not a binding partner of MDM2. Biochem J. 2004;381:685-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferreon JC, Lee CW, Arai M, Martinez-Yamout MA, Dyson HJ, Wright PE. Cooperative regulation of p53 by modulation of ternary complex formation with CBP/p300 and HDM2. Proc Natl Acad Sci U S A. 2009;106:6591-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee CW, Ferreon JC, Ferreon AC, Arai M, Wright PE. Graded enhancement of p53 binding to CREB-binding protein (CBP) by multisite phosphorylation. Proc Natl Acad Sci U S A. 2010;107:19290-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jenkins LM, Yamaguchi H, Hayashi R, et al. Two distinct motifs within the p53 transactivation domain bind to the Taz2 domain of p300 and are differentially affected by phosphorylation. Biochemistry. 2009;48:1244-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pan X, Zhao J, Zhang WN, et al. Induction of SOX4 by DNA damage is critical for p53 stabilization and function. Proc Natl Acad Sci U S A. 2009;106:3788-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ito A, Kawaguchi Y, Lai CH, et al. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 2002;21:6236-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pavithra L, Mukherjee S, Sreenath K, et al. SMAR1 forms a ternary complex with p53-MDM2 and negatively regulates p53-mediated transcription. J Mol Biol. 2009;388:691-702 [DOI] [PubMed] [Google Scholar]
- 48. Wang C, Ivanov A, Chen L, et al. MDM2 interaction with nuclear corepressor KAP1 contributes to p53 inactivation. EMBO J. 2005;24:3279-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Okamoto K, Kitabayashi I, Taya Y. KAP1 dictates p53 response induced by chemotherapeutic agents via Mdm2 interaction. Biochem Biophys Res Commun. 2006;351:216-22 [DOI] [PubMed] [Google Scholar]
- 50. Rinaldo C, Prodosmo A, Mancini F, et al. MDM2-regulated degradation of HIPK2 prevents p53Ser46 phosphorylation and DNA damage-induced apoptosis. Mol Cell. 2007;25:739-50 [DOI] [PubMed] [Google Scholar]
- 51. Zhang Q, Yoshimatsu Y, Hildebrand J, Frisch SM, Goodman RH. Homeodomain interacting protein kinase 2 promotes apoptosis by downregulating the transcriptional corepressor CtBP. Cell. 2003;115:177-86 [DOI] [PubMed] [Google Scholar]
- 52. Wu JI. Diverse functions of ATP-dependent chromatin remodeling complexes in development and cancer. Acta Biochim Biophys Sin (Shanghai). 2012;44:54-69 [DOI] [PubMed] [Google Scholar]
- 53. Mohrmann L, Verrijzer CP. Composition and functional specificity of SWI2/SNF2 class chromatin remodeling complexes. Biochim Biophys Acta. 2005;1681:59-73 [DOI] [PubMed] [Google Scholar]
- 54. Xu Y, Zhang J, Chen X. The activity of p53 is differentially regulated by Brm- and Brg1-containing SWI/SNF chromatin remodeling complexes. J Biol Chem. 2007;282:37429-35 [DOI] [PubMed] [Google Scholar]
- 55. Brown DR, Thomas CA, Deb SP. The human oncoprotein MDM2 arrests the cell cycle: elimination of its cell-cycle-inhibitory function induces tumorigenesis. EMBO J. 1998;17:2513-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Frum R, Ramamoorthy M, Mohanraj L, Deb S, Deb SP. MDM2 controls the timely expression of cyclin A to regulate the cell cycle. Mol Cancer Res. 2009;7:1253-67 [DOI] [PubMed] [Google Scholar]
- 57. Shikama N, Lee CW, France S, et al. A novel cofactor for p300 that regulates the p53 response. Mol Cell. 1999;4:365-76 [DOI] [PubMed] [Google Scholar]
- 58. Zuchero JB, Coutts AS, Quinlan ME, Thangue NB, Mullins RD. p53-cofactor JMY is a multifunctional actin nucleation factor. Nat Cell Biol. 2009;11:451-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coutts AS, Boulahbel H, Graham A, La Thangue NB. Mdm2 targets the p53 transcription cofactor JMY for degradation. EMBO Rep. 2007;8:84-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Choi HS, Hwang CK, Song KY, Law PY, Wei LN, Loh HH. Poly(C)-binding proteins as transcriptional regulators of gene expression. Biochem Biophys Res Commun. 2009;380:431-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moumen A, Masterson P, O’Connor MJ, Jackson SP. hnRNP K: an HDM2 target and transcriptional coactivator of p53 in response to DNA damage. Cell. 2005;123:1065-78 [DOI] [PubMed] [Google Scholar]
- 62. Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215-21 [DOI] [PubMed] [Google Scholar]
- 63. McDonnell TJ, Montes de Oca Luna R, Cho S, Amelse LL, Chavez-Reyes A, Lozano G. Loss of one but not two mdm2 null alleles alters the tumour spectrum in p53 null mice. J Pathol. 1999;188:322-8 [DOI] [PubMed] [Google Scholar]
- 64. Matijasevic Z, Steinman HA, Hoover K, Jones SN. MdmX promotes bipolar mitosis to suppress transformation and tumorigenesis in p53-deficient cells and mice. Mol Cell Biol. 2008;28:1265-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Matijasevic Z, Krzywicka-Racka A, Sluder G, Jones SN. MdmX regulates transformation and chromosomal stability in p53-deficient cells. Cell Cycle. 2008;7:2967-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jones SN, Sands AT, Hancock AR, et al. The tumorigenic potential and cell growth characteristics of p53-deficient cells are equivalent in the presence or absence of Mdm2. Proc Natl Acad Sci U S A. 1996;93:14106-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jost CA, Marin MC, Kaelin WG., Jr. p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature. 1997;389:191-4 [DOI] [PubMed] [Google Scholar]
- 68. Kaghad M, Bonnet H, Yang A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809-19 [DOI] [PubMed] [Google Scholar]
- 69. Melino G, Bernassola F, Ranalli M, et al. p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J Biol Chem. 2004;279:8076-83 [DOI] [PubMed] [Google Scholar]
- 70. Irwin MS, Kaelin WG. p53 family update: p73 and p63 develop their own identities. Cell Growth Differ. 2001;12:337-49 [PubMed] [Google Scholar]
- 71. Balint E, Bates S, Vousden KH. Mdm2 binds p73 alpha without targeting degradation. Oncogene. 1999;18:3923-9 [DOI] [PubMed] [Google Scholar]
- 72. Gu J, Nie L, Kawai H, Yuan ZM. Subcellular distribution of p53 and p73 are differentially regulated by MDM2. Cancer Res. 2001;61:6703-7 [PubMed] [Google Scholar]
- 73. Dobbelstein M, Wienzek S, Konig C, Roth J. Inactivation of the p53-homologue p73 by the mdm2-oncoprotein. Oncogene. 1999;18:2101-6 [DOI] [PubMed] [Google Scholar]
- 74. Zeng X, Chen L, Jost CA, et al. MDM2 suppresses p73 function without promoting p73 degradation. Mol Cell Biol. 1999;19:3257-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ongkeko WM, Wang XQ, Siu WY, et al. MDM2 and MDMX bind and stabilize the p53-related protein p73. Curr Biol. 1999;9:829-32 [DOI] [PubMed] [Google Scholar]
- 76. Watson IR, Blanch A, Lin DC, Ohh M, Irwin MS. Mdm2-mediated NEDD8 modification of TAp73 regulates its transactivation function. J Biol Chem. 2006;281:34096-103 [DOI] [PubMed] [Google Scholar]
- 77. Lau LM, Nugent JK, Zhao X, Irwin MS. HDM2 antagonist Nutlin-3 disrupts p73-HDM2 binding and enhances p73 function. Oncogene. 2008;27:997-1003 [DOI] [PubMed] [Google Scholar]
- 78. Busuttil V, Droin N, McCormick L, et al. NF-kappaB inhibits T-cell activation-induced, p73-dependent cell death by induction of MDM2. Proc Natl Acad Sci U S A. 2010;107:18061-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gu L, Findley HW, Zhou M. MDM2 induces NF-kappaB/p65 expression transcriptionally through Sp1-binding sites: a novel, p53-independent role of MDM2 in doxorubicin resistance in acute lymphoblastic leukemia. Blood. 2002;99:3367-75 [DOI] [PubMed] [Google Scholar]
- 80. Cheney MD, McKenzie PP, Volk EL, Fan L, Harris LC. MDM2 displays differential activities dependent upon the activation status of NFkappaB. Cancer Biol Ther. 2008;7:38-44 [DOI] [PubMed] [Google Scholar]
- 81. Massague J. TGFbeta in Cancer. Cell. 2008; 134:215-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sun P, Dong P, Dai K, Hannon GJ, Beach D. p53-independent role of MDM2 in TGF-beta1 resistance. Science. 1998;282:2270-2 [DOI] [PubMed] [Google Scholar]
- 83. Yam CH, Siu WY, Arooz T, et al. MDM2 and MDMX inhibit the transcriptional activity of ectopically expressed SMAD proteins. Cancer Res. 1999;59:5075-8 [PubMed] [Google Scholar]
- 84. Kadakia M, Brown TL, McGorry MM, Berberich SJ. MdmX inhibits Smad transactivation. Oncogene. 2002;21:8776-85 [DOI] [PubMed] [Google Scholar]
- 85. Fiddler TA, Smith L, Tapscott SJ, Thayer MJ. Amplification of MDM2 inhibits MyoD-mediated myogenesis. Mol Cell Biol. 1996;16:5048-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Guo CS, Degnin C, Fiddler TA, Stauffer D, Thayer MJ. Regulation of MyoD activity and muscle cell differentiation by MDM2, pRb, and Sp1. J Biol Chem. 2003;278:22615-22 [DOI] [PubMed] [Google Scholar]
- 87. Hallenborg P, Feddersen S, Francoz S, et al. Mdm2 controls CREB-dependent transactivation and initiation of adipocyte differentiation. Cell Death Differ. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sui X, Shin S, Zhang R, et al. Hdm2 is regulated by K-Ras and mediates p53-independent functions in pancreatic cancer cells. Oncogene. 2009;28:709-20 [DOI] [PubMed] [Google Scholar]
- 89. Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9:785-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9:738-48 [DOI] [PubMed] [Google Scholar]
- 91. Martin K, Trouche D, Hagemeier C, Sorensen TS, La Thangue NB, Kouzarides T. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature. 1995;375:691-4 [DOI] [PubMed] [Google Scholar]
- 92. Xiao ZX, Chen J, Levine AJ, et al. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature. 1995;375:694-8 [DOI] [PubMed] [Google Scholar]
- 93. Sdek P, Ying H, Zheng H, et al. The central acidic domain of MDM2 is critical in inhibition of retinoblastoma-mediated suppression of E2F and cell growth. J Biol Chem. 2004;279:53317-22 [DOI] [PubMed] [Google Scholar]
- 94. Sdek P, Ying H, Chang DL, et al. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol Cell. 2005;20:699-708 [DOI] [PubMed] [Google Scholar]
- 95. Loughran O, La Thangue NB. Apoptotic and growth-promoting activity of E2F modulated by MDM2. Mol Cell Biol. 2000;20:2186-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huart AS, MacLaine NJ, Meek DW, Hupp TR. CK1alpha plays a central role in mediating MDM2 control of p53 and E2F-1 protein stability. J Biol Chem. 2009;284:32384-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Priest C, Prives C, Poyurovsky MV. Deconstructing nucleotide binding activity of the Mdm2 RING domain. Nucleic Acids Res. 2010;38:7587-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Poyurovsky MV, Jacq X, Ma C, et al. Nucleotide binding by the Mdm2 RING domain facilitates Arf-independent Mdm2 nucleolar localization. Mol Cell. 2003;12:875-87 [DOI] [PubMed] [Google Scholar]
- 99. Wawrzynow B, Zylicz A, Wallace M, Hupp T, Zylicz M. MDM2 chaperones the p53 tumor suppressor. J Biol Chem. 2007;282:32603-12 [DOI] [PubMed] [Google Scholar]
- 100. Stevens C, Pettersson S, Wawrzynow B, et al. ATP stimulates MDM2-mediated inhibition of the DNA-binding function of E2F1. FEBS J. 2008;275:4875-86 [DOI] [PubMed] [Google Scholar]
- 101. Gammoh N, Gardiol D, Massimi P, Banks L. The Mdm2 ubiquitin ligase enhances transcriptional activity of human papillomavirus E2. J Virol. 2009;83:1538-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Thut CJ, Goodrich JA, Tjian R. Repression of p53-mediated transcription by MDM2: a dual mechanism. Genes Dev. 1997;11:1974-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Minsky N, Oren M. The RING domain of Mdm2 mediates histone ubiquitylation and transcriptional repression. Mol Cell. 2004;16: 631-9 [DOI] [PubMed] [Google Scholar]
- 104. Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133:612-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Arva NC, Gopen TR, Talbott KE, et al. A chromatin-associated and transcriptionally inactive p53-Mdm2 complex occurs in mdm2 SNP309 homozygous cells. J Biol Chem. 2005;280: 26776-87 [DOI] [PubMed] [Google Scholar]
- 106. White DE, Talbott KE, Arva NC, Bargonetti J. Mouse double minute 2 associates with chromatin in the presence of p53 and is released to facilitate activation of transcription. Cancer Res. 2006;66:3463-70 [DOI] [PubMed] [Google Scholar]
- 107. Chen L, Li Z, Zwolinska AK, et al. MDM2 recruitment of lysine methyltransferases regulates p53 transcriptional output. EMBO J. 2010;29:2538-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sun ZW, Allis CD. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature. 2002;418:104-8 [DOI] [PubMed] [Google Scholar]
- 109. Minsky N, Shema E, Field Y, Schuster M, Segal E, Oren M. Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat Cell Biol. 2008;10:483-8 [DOI] [PubMed] [Google Scholar]
- 110. Weake VM, Workman JL. Histone ubiquitination: triggering gene activity. Mol Cell. 2008;29:653-63 [DOI] [PubMed] [Google Scholar]
- 111. Elenbaas B, Dobbelstein M, Roth J, Shenk T, Levine AJ. The MDM2 oncoprotein binds specifically to RNA through its RING finger domain. Mol Med. 1996;2:439-51 [PMC free article] [PubMed] [Google Scholar]
- 112. Candeias MM, Malbert-Colas L, Powell DJ, et al. P53 mRNA controls p53 activity by managing Mdm2 functions. Nat Cell Biol. 2008;10:1098-105 [DOI] [PubMed] [Google Scholar]
- 113. Gajjar M, Candeias MM, Malbert-Colas L, et al. The p53 mRNA-Mdm2 interaction controls Mdm2 nuclear trafficking and is required for p53 activation following DNA damage. Cancer Cell. 2012;21:25-35 [DOI] [PubMed] [Google Scholar]
- 114. Naski N, Gajjar M, Bourougaa K, Malbert-Colas L, Fahraeus R, Candeias MM. The p53 mRNA-Mdm2 interaction. Cell Cycle. 2009;8:31-4 [DOI] [PubMed] [Google Scholar]
- 115. Chen J, Guo K, Kastan MB. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J Biol Chem. 2012;287:16467-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chen J, Kastan MB. 5′-3′-UTR interactions regulate p53 mRNA translation and provide a target for modulating p53 induction after DNA damage. Genes Dev. 2010;24:2146-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Riedl SJ, Renatus M, Schwarzenbacher R, et al. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791-800 [DOI] [PubMed] [Google Scholar]
- 118. Scott FL, Denault JB, Riedl SJ, Shin H, Renatus M, Salvesen GS. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 2005;24:645-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shiozaki EN, Chai J, Rigotti DJ, et al. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519-27 [DOI] [PubMed] [Google Scholar]
- 120. Kuida K, Haydar TF, Kuan CY, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325-37 [DOI] [PubMed] [Google Scholar]
- 121. Lakhani SA, Masud A, Kuida K, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat Cell Biol. 1999;1:190-2 [DOI] [PubMed] [Google Scholar]
- 123. Gu L, Zhu N, Zhang H, Durden DL, Feng Y, Zhou M. Regulation of XIAP translation and induction by MDM2 following irradiation. Cancer Cell. 2009;15:363-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121-4 [DOI] [PubMed] [Google Scholar]
- 125. Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111-6 [DOI] [PubMed] [Google Scholar]
- 126. Corvi R, Savelyeva L, Breit S, et al. Non-syntenic amplification of MDM2 and MYCN in human neuroblastoma. Oncogene. 1995;10:1081-6 [PubMed] [Google Scholar]
- 127. Slack A, Chen Z, Tonelli R, et al. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci U S A. 2005;102:731-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gu L, Zhang H, He J, Li J, Huang M, Zhou M. MDM2 regulates MYCN mRNA stabilization and translation in human neuroblastoma cells. Oncogene. 2012;31:1342-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841-4 [DOI] [PubMed] [Google Scholar]
- 130. Zhou S, Gu L, He J, Zhang H, Zhou M. MDM2 regulates vascular endothelial growth factor mRNA stabilization in hypoxia. Mol Cell Biol. 2011;31:4928-37 [DOI] [PMC free article] [PubMed] [Google Scholar]