Abstract
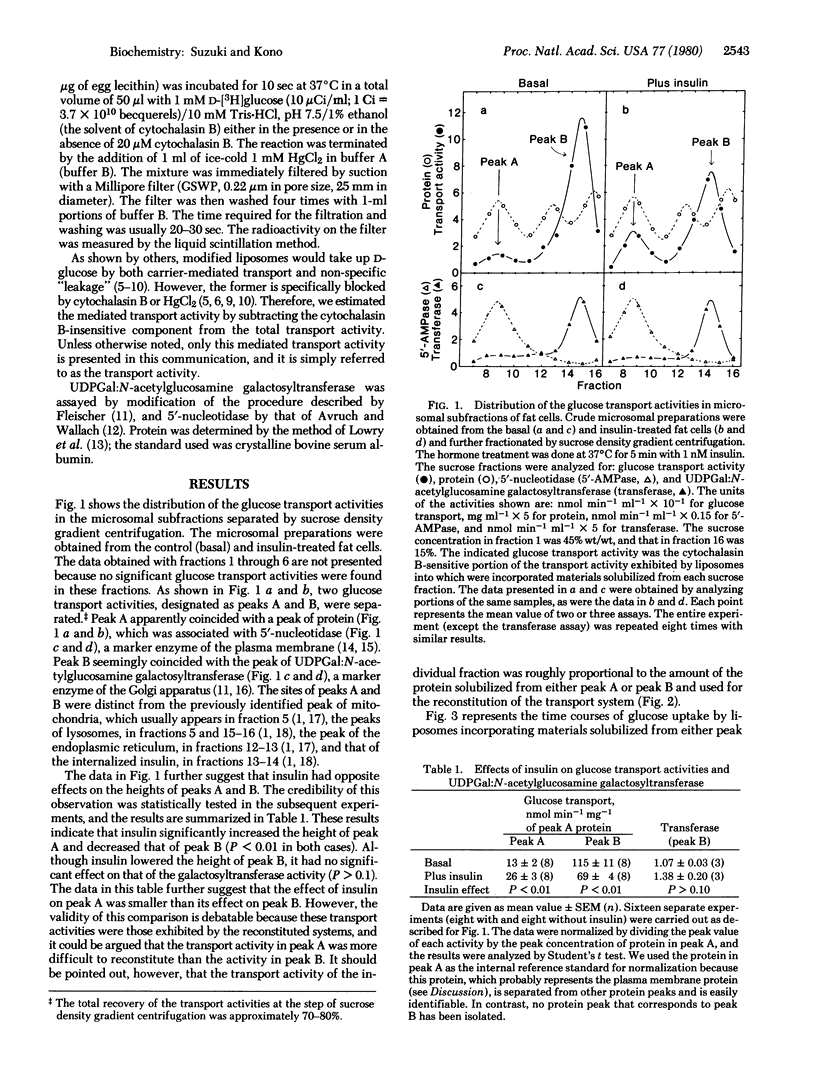
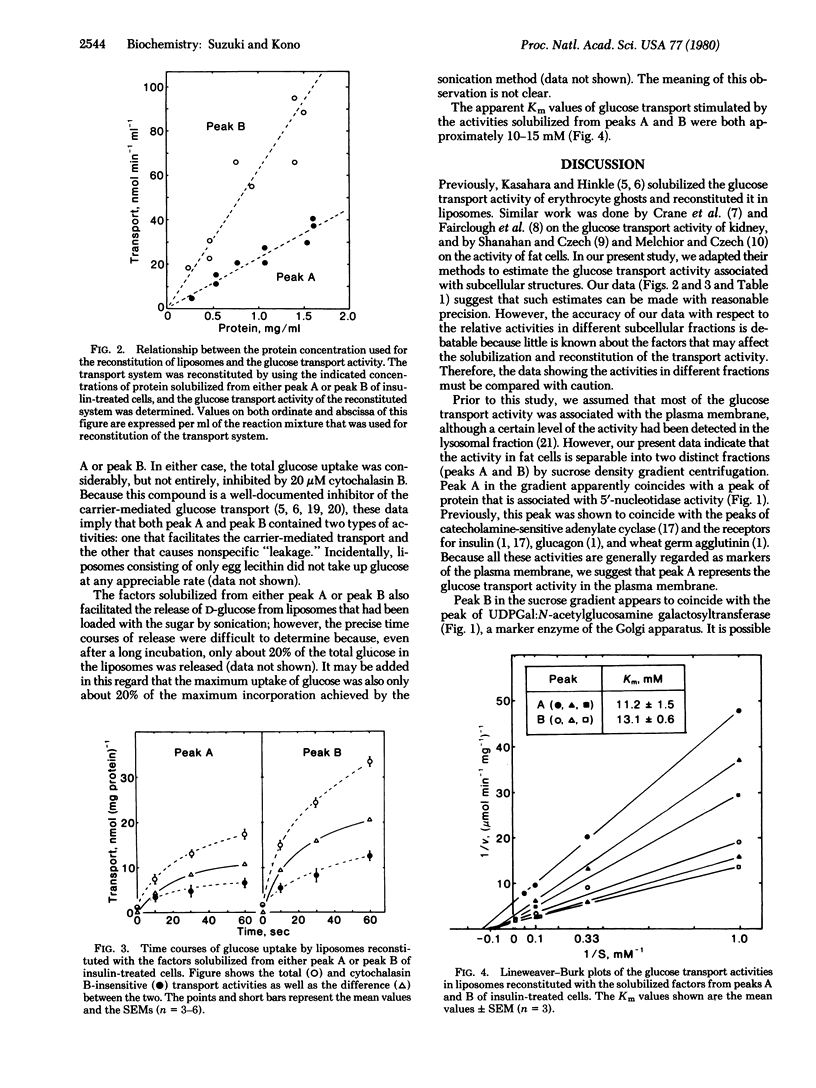
The glucose transport activity of fat cells was assayed in a cell-free system. The activity was solubilized and incorporated into egg-lecithin liposomes. The carrier-mediated glucose transport activity was estimated by subtracting the cytochalasin B-insensitive component from the total glucose uptake activity of the modified liposomes. When a crude microsomal preparation from fat cells was fractionated by sucrose density gradient centrifugation, two transport activities (peaks A and B) were separated. Peak A coincided with the peak of 5'-nucleotidase, a marker of the plasma membrane. Peak B appeared to coincide with the peak of UDPGal:N-acetylglucosamine galactosyltransferase, a marker of the Golgi apparatus. Peak A was considerably smaller than peak B under basal conditions. When cells were exposed to 1 nM insulin for 5 min before homogenization, the height of peak A increased whereas that of peak B decreased. Insulin had no significant effect on the galactosyltransferase activity. The Km values of glucose transport facilitated by the activities in peaks A and B were both approximately 10-15 mM. These results imply that insulin facilitates translocation of the transport activity from an intracellular storage site to the plasma membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Chandramouli V., Milligan M., Carter J. R., Jr Insulin stimulation of glucose transport in adipose cells. An energy-dependent process. Biochemistry. 1977 Mar 22;16(6):1151–1158. doi: 10.1021/bi00625a019. [DOI] [PubMed] [Google Scholar]
- Crane R. K., Malathi P., Preiser H. Reconstitution of specific Na+-dependent D-glucose transport in liposomes by Triton X-100-extracted proteins from purified brush border membranes of rabbit kidney cortex. FEBS Lett. 1976 Aug 15;67(2):214–216. doi: 10.1016/0014-5793(76)80369-9. [DOI] [PubMed] [Google Scholar]
- Czech M. P. Characterization of (3H)cytochalasin B binding to the fat cell plasma membrane. J Biol Chem. 1976 May 25;251(10):2905–2910. [PubMed] [Google Scholar]
- Docherty K., Brenchley G. V., Hales C. N. The permeability of rat liver lysosomes to sugars. Evidence for carrier-mediated facilitated diffusion. Biochem J. 1979 Feb 15;178(2):361–366. doi: 10.1042/bj1780361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- Fairclough P., Malathi P., Preiser H., Crane R. K. Reconstitution into liposomes of glucose active transport from the rabbit renal proximal tubule. Characteristics of the system. Biochim Biophys Acta. 1979 May 17;553(2):295–306. doi: 10.1016/0005-2736(79)90233-5. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Fleischer S., Ozawa H. Isolation and characterization of Golgi membranes from bovine liver. J Cell Biol. 1969 Oct;43(1):59–79. doi: 10.1083/jcb.43.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B. Isolation and characterization of Golgi apparatus and membranes from rat liver. Methods Enzymol. 1974;31:180–191. doi: 10.1016/0076-6879(74)31020-8. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution of D-glucose transport catalyzed by a protein fraction from human erythrocytes in sonicated liposomes. Proc Natl Acad Sci U S A. 1976 Feb;73(2):396–400. doi: 10.1073/pnas.73.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. The inhibition of sugar transport in chick embryo fibroblasts by cytochalasin B. Evidence for a membrane-specific effect. J Biol Chem. 1973 Jan 25;248(2):711–719. [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- Kono T. Destruction and restoration of the insulin effector system of isolated fat cells. J Biol Chem. 1969 Nov 10;244(21):5777–5784. [PubMed] [Google Scholar]
- Kono T., Robinson F. W., Sarver J. A. Insulin-sensitive phosphodiesterase. Its localization, hormonal stimulation, and oxidative stabilization. J Biol Chem. 1975 Oct 10;250(19):7826–7835. [PubMed] [Google Scholar]
- Kono T., Robinson F. W., Sarver J. A., Vega F. V., Pointer R. H. Actions of insulin in fat cells. Effects of low temperature, uncouplers of oxidative phosphorylation, and respiratory inhibitors. J Biol Chem. 1977 Apr 10;252(7):2226–2233. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loten E. G., Regen D. M., Park C. R. Transport of D-allose by isolated fat-cells: an effect of adenosine triphosphate on insulin stimulated transport. J Cell Physiol. 1976 Dec;89(4):651–660. doi: 10.1002/jcp.1040890423. [DOI] [PubMed] [Google Scholar]
- Melchior D. L., Czech M. P. Sensitivity of the adipocyte D-glucose transport system to membrane fluidity in reconstituted vesicles. J Biol Chem. 1979 Sep 25;254(18):8744–8747. [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Shanahan M. F., Czech M. P. Purification and reconstitution of the adipocyte plasma membrane D-glucose transport system. J Biol Chem. 1977 Dec 10;252(23):8341–8343. [PubMed] [Google Scholar]
- Shnitka T. K., Seligman A. M. Ultrastructural localization of enzymes. Annu Rev Biochem. 1971;40:375–396. doi: 10.1146/annurev.bi.40.070171.002111. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Internalization and degradation of fat cell-bound insulin. Separation and partial characterization of subcellular vesicles associated with iodoinsulin. J Biol Chem. 1979 Oct 10;254(19):9786–9794. [PubMed] [Google Scholar]
- Vega F. V., Kono T. Sugar transport in fat cells: effects of mechanical agitation, cell-bound insulin, and temperature. Arch Biochem Biophys. 1979 Jan;192(1):120–127. doi: 10.1016/0003-9861(79)90077-8. [DOI] [PubMed] [Google Scholar]
- Vinten J., Gliemann J., Osterlind K. Exchange of 3-O-methylglucose in isolated fat cells. Concentration dependence and effect of insulin. J Biol Chem. 1976 Feb 10;251(3):794–800. [PubMed] [Google Scholar]
- Wardzala L. J., Cushman S. W., Salans L. B. Mechanism of insulin action on glucose transport in the isolated rat adipose cell. Enhancement of the number of functional transport systems. J Biol Chem. 1978 Nov 25;253(22):8002–8005. [PubMed] [Google Scholar]
- Whitesell R. R., Gliemann J. Kinetic parameters of transport of 3-O-methylglucose and glucose in adipocytes. J Biol Chem. 1979 Jun 25;254(12):5276–5283. [PubMed] [Google Scholar]
- Yu K. T., Gould M. K. Permissive effect of ATP on insulin-stimulated sugar transport by rat soleus muscle. Am J Physiol. 1978 Apr;234(4):E407–E416. doi: 10.1152/ajpendo.1978.234.4.E407. [DOI] [PubMed] [Google Scholar]


