Abstract
Humans’ endogenous testosterone concentrations vary over a number of temporal scales, with little known about variation longer than monthly cycles. Past studies of seasonal or circannual variation have principally used male participants and have produced inconsistent results. Thus, little is known about how testosterone concentrations fluctuate throughout the year, whether such variation differs between men and women, and whether there are influences of hormonal contraceptive use. The present study collected saliva samples from a large sample (N=718) of men and women, each collected at one time point within a relatively uniform distribution over a full calendar year. Both men and normally-cycling women displayed seasonal variation in salivary testosterone concentrations, such that testosterone concentrations are maximal in the fall and minimal in the summer. Notably, normally-cycling women had testosterone concentrations that were over 100% greater at their maximum in fall compared to their minimum in summer. Women using hormonal contraceptives not only had consistently lower endogenous testosterone concentrations, but also showed a flatter seasonal testosterone profile. The implications for studies of psychology and human behavioral endocrinology are discussed.
Keywords: Testosterone, Steroid, Circannual, Seasonal variation, Gender, Sex, Hormonal Contraceptive
Introduction
Seasonal and circannual rhythms of physiology and behavior exist in a wide variety of species. Recurring seasonal environmental changes (e.g., photoperiod, temperature, plant growth/food availability) commonly result in changes in body mass, reproduction, immune function, foraging and food intake (Prendergast et al., 2002; Prendergast et al., 2009). These seasonally-driven changes in behavior track changes in physiology that are often regulated by the endocrine system. Testosterone has been shown to follow a seasonal rhythm in many species – particularly in males (Bubenik et al., 1982; Courty & Dufaure, 1982; Dabbs, 1990a; Michael & Bonsall, 1977) – that can promote changes in behavior (e.g., aggression, mating) (Prendergast et al., 2009; Wingfield et al., 1990). Some evidence links seasonal variation in testosterone to seasonal changes in human behavior (Kimura & Hampson, 1994) and physiology, such as waist-to-hip ratio (Svartberg et al., 2003a; van Anders et al., 2006). Since a number of behaviors are linked to testosterone in humans (e.g., cognitive abilities, Moffat & Hampson, 1996; e.g., power and dominance competition, Stanton et al., 2009; Stanton & Schultheiss, 2009; e.g., relationship status and sexual intercourse, van Anders & Goldey, 2010; van Anders et al., 2007), accurately characterizing seasonal variation in humans’ testosterone concentrations would have important implications for understanding seasonal variation in human behavior.
Like many mammals, humans’ endogenous testosterone concentrations systematically vary over temporal scales ranging from days (Dabbs & de La Rue, 1991; Dabbs, 1990b), to months (Dabbs & de La Rue, 1991), to the lifespan (Dabbs, 1990a). While testosterone variation at short time scales (e.g., diurnal variation) is relatively well understood, the nature of long-time-scale variation (seasons and years) is much less well documented, particularly with regard to women. The few studies to be completed in humans have reported inconsistent findings and principally used male subjects. Not only have some found evidence of seasonal variation and some not, there are also inconsistencies in the peak and trough months of testosterone concentrations (Svartberg et al., 2003b). While the majority of studies report that testosterone concentrations peak in the fall for men (e.g., Moffat & Hampson, 2000; van Anders et al., 2006), other have reported peaks during other months of the year (e.g., Perry et al., 2000; Valero-Politi & Fuentes-Arderiu, 1998). Inconsistencies in prior findings may have resulted from the varied nature of different studies: some used atypical populations (e.g., prepubertal children, Bellastella et al., 1983; elderly men, Perry et al., 2000), used participants of only one sex (Dabbs, 1990a; Garde et al., 2000), or collected samples over less than a full year (Wisniewski & Nelson, 2000). Thus, the specific nature of the seasonal variation in humans’ testosterone concentrations remains poorly characterized.
Studies of women’s seasonal variation in testosterone are rare and have had conflicting findings. Considering the four existing studies of women: Garde and colleagues (2000) found that serum testosterone peaked in summer months, both van Anders and colleagues (2006) and Wisniewski and Nelson (2000) found salivary testosterone peaked in the fall, and Moffat and Hampson (2000) found no significant seasonal variation. Moreover, commonly-taken exogenous hormone treatments that affect sex steroid production and release, such as oral contraceptives, could be associated with alteration of seasonal testosterone variation in women. Hormonal contraceptive use is associated with suppression of women’s endogenous testosterone concentrations (Edwards & O’Neal, 2009; Schultheiss et al., 2005), as well as menstrual cyclicity of other steroids of ovarian origin, i.e., estradiol and progesterone (Johnson & Everitt, 2000).
Because of these effects of hormonal contraceptives on endogenous steroids, there is an ongoing debate within human behavioral endocrinology regarding the inclusion or exclusion of female human subjects on the basis of their use of exogenous hormonal contraception. Josephs (2009) argued that not only should all female participants be included in such studies, but that the field would benefit from trying to understand the effects of specific hormonal contraceptives on behavior and endocrine physiology. In contrast, since hormonal contraceptives are associated with reduced endogenous concentrations of steroid hormones including testosterone (Edwards & O’Neal, 2009; Schultheiss et al., 2005; Stanton & Edelstein, 2009), others researchers have argued that normally-cycling women and women taking hormonal contraceptives should be treated as fundamentally different and analyzed separately, or hormonal contraceptive users should be excluded entirely from analyses (Moffat & Hampson, 2000; van Anders & Goldey, 2010; van Anders et al., 2006). Some studies have found that hormonal contraceptive use is associated with alteration of the relationship between basal testosterone and behavior (Bancroft et al., 1991; Greco et al., 2007) and testosterone reactivity to social interactions (López et al., 2009) , while others have not (Edwards & O’Neal, 2009). The extent to which hormonal contraceptive use alters other aspects of systematic variation in testosterone, e.g., seasonal variation, will further inform research on the behavioral correlates of testosterone in women. Thus, seasonal variation in women – whether using or not using hormonal contraceptives – remains an open and important topic for study.
The present study aimed to describe the seasonal variability in endogenous testosterone for both men and women, and also aimed to document effects of hormonal contraceptive use on said variability in women. We hypothesized that both men and normally-cycling women would show seasonal variation in testosterone concentrations. Furthermore, we hypothesized that hormonal contraceptive use would not only be associated with a reduction in women’s endogenous testosterone concentrations, but would also be associated with a reduction in the peak-to-trough seasonal variation.
Methods
Participants
Seven hundred eighteen participants (299 men and 419 women; age range 18-66y; M = 22.7y, SD = 5.9y) were recruited via flyers and through online subject participation websites. All participants provided informed consent under a protocol approved by the Duke University Medical Center Institutional Review Board. Participants with testosterone concentrations greater than 3 SDs from the mean within sex (N = 13) or with testosterone measurement coefficients of variation (CV) greater than 50% were excluded from all analyses (N = 15). 3 participants had both CV > 50% and testosterone > 3SDs from the mean; i.e., 25 total participants were excluded for these 2 exclusionary criteria. 5 women did not report their hormonal contraceptive status and were omitted from the analyses. Thus, the total number of excluded participants was 30 and the final sample consisted of 688 participants (296 men, 262 normally-cycling women, and 130 women taking hormonal contraceptives).
Procedure
Our experiment employed a cross-sectional design in which participants were tested during a single session at a single time (time range of sessions: 13:00h to 18:00h). During an experimental session, participants first provided a saliva sample and then completed questionnaires and economic decision-making tasks unrelated to this report. Aspects of the behavioral data are reported elsewhere (Stanton et al., 2011). Female participants also provided information regarding whether or not they were currently using hormonal contraceptives.
Salivary sampling
For saliva collection, participants used a stick of sugar-free Trident original chewing gum to stimulate saliva flow and collected up to 7.5 mL of saliva in a sterile polypropylene vial (Dabbs, 1991; Schultheiss & Stanton, 2009). Participants discarded the chewing gum after saliva collection. Participants’ collection vials were sealed immediately and placed in frozen storage at the end of the session. Samples were freed from mucopolysaccarides and other residuals by three freeze-thaw cycles followed by centrifugation (see Schultheiss & Stanton, 2009).
Testosterone Assays
Salivary hormone concentrations were assessed with solid-phase Coat-A-Count125I radioimmunoassays for testosterone (TKTT) provided by Siemens Healthcare Diagnostics, Los Angeles, CA. To determine salivary hormone concentrations, we prepared water-based dilutions of all standards and controls. Four hundred μL of the saliva samples, standards, and controls were pipetted into antibody-coated tubes. All tubes were allowed to incubate overnight. One mL radio-labeled tracer was added to each tube following overnight incubation, and then all tubes were again incubated overnight. Finally, tubes were aspirated and counted for 3 minutes (see Schultheiss & Stanton, 2009 for details). Assay reliability was evaluated by including control samples in each assay (Bio-Rad Lyphochecks from Bio-Rad Laboratories, Hercules, CA). For samples of known concentration (45 pg/mL and 115 pg/mL), inter-assay CVs were 10.4% and 6.3%, respectively. Analytical sensitivity (B0 - 3 SD) was 1.34 pg/mL. All saliva samples were counted in duplicate and had a mean intra-assay CV of 9.97%.
Data Analysis
SYSTAT 12.0 statistical software was used for all statistical analyses. Descriptive statistics are shown as mean (± SD), unless otherwise noted.
Results
Table 1 reports the means, standard deviations and number of participants by month of endogenous testosterone concentrations for men, normally-cycling women, and women taking hormonal contraceptives. The grand means of testosterone were: Men (N = 296), M = 77.5 (± 25.0) pg/mL; Normally-cycling women (N = 262), M = 13.3 (± 5.6) pg/mL; Women taking hormonal contraceptives (N = 130), M = 8.5 (± 3.6) pg/mL.
Table 1.
Salivary testosterone (pg/mL) concentrations by month for men, normally-cycling women, and women using hormonal contraceptives
| Men | Normally-cycling women | Hormonal contraceptive women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | N | PW Comp. | Mean (SD) | N | PW Comp. | Mean (SD) | N | PW Comp. | |
| Month | |||||||||
| January | 91.3 (22.3) | 33 | Mr, Ap, S | 16.1 (5.8) | 18 | Ap | 8.9 (3.5) | 7 | |
| February | 79.0 (28.7) | 43 | 14.8 (5.8) | 41 | Ap. Ma | 10.3 (4.8) | 18 | Ap | |
| March | 69.5 (22.7) | 38 | Ja, N | 11.3 (6.1) | 24 | O | 8.5 (3.9) | 24 | |
| April | 65.7 (24.7) | 23 | Ja | 10.9 (4.0) | 38 | Ja, F, O | 6.2 (2.3) | 16 | F |
| May | 70.4 (18.5) | 17 | 9.6 (2.5) | 17 | O.N | 8.1 (0.4) | 3 | ||
| June | 71.2 (24.4) | 24 | 12.4 (4.9) | 24 | O | 7.5 (3.8) | 9 | ||
| July | 73.9 (21.9) | 36 | 13.0 (5.3) | 33 | O | 7.3 (3.3) | 18 | ||
| August | 86.1 (28.2) | 7 | 13.1 (5.2) | 16 | 10.1 (3.3) | 5 | |||
| September | 67.6 (17.8) | 20 | Ja | 14.3 (5.4) | 25 | 8.3 (2.5) | 13 | ||
| October | 86.8 (16.4) | 16 | 19.1 (6.9) | 11 | Mr, Ap, Ma, Jn, Ju | 9.5 (2.2) | 3 | ||
| November | 88.1 (22.8) | 33 | Mr | 16.3 (6.1) | 11 | Ma | 9.8 (2.9) | 8 | |
| December | 101.4 (39.8) | 6 | 15.9 (0.8) | 4 | 10.3 (3.2) | 6 | |||
SD = Standard Deviation, N = Number of subjects, PW Comp. = Significant pairwise comparison
Significant post-hoc pairwise comparisons (p < 0.05) using Tukey’s test are indicated with the following abbreviations of months in chronological order: Ja, F, Mr, Ap, My, Jn, Jl, Au, S, O, N, D
Likely due to the small window of time of day during which saliva was collected (Liening et al., 2010), testosterone was not related to time of saliva collection for either men, r(275) = 0.02, p = 0.80, or women, r(381) = -0.02, p = 0.70. For some participants, time of day for their saliva sample was not recorded. As a function of those missing time of day data, the degrees of freedom are slightly reduced for the reported correlations. This result stands in contrast to within-subjects designs in humans that have consistently shown that testosterone is subject to diurnal decline across the entire day (Brambilla et al., 2009; Brown et al., 2008; Dabbs & de La Rue, 1991; Dabbs, 1990b; Riad-Fahmy et al., 1982), which is consistent across many mammalian species. Due to our between-subjects design, it is likely that the large between-subjects variability of testosterone levels (Stanton, 2011) overshadowed the detection of diurnal decline. The time-of-day variable was omitted from all subsequent analyses due to a lack of significant effects.
First, we used month of the year as our independent variable to predict men’s testosterone concentrations. ANOVA showed a significant effect of month on testosterone concentrations, F(11, 284) = 4.23, p < 0.001 (Fig. 1; see Table 1 for pairwise comparisons between months). We then used progressive polynomial regression in an effort to characterize the nature of the effect of month on testosterone. Testing linear, quadratic, and cubic regression effects, the quadratic model showed the best fit of the data, F(2, 293) = 16.75, p < 0.001, and the effect of quadratic factor was, β = 1.38, t = 5.65, p < 0.001. This shows that testosterone concentrations were higher in the fall, peaked in December, and were lowest in the late winter and spring (Fig. 1). Following van Anders et al. (2006), seasons were based on solstices as follows: Fall (October-December), Winter (January-March), Spring (April-June), Summer (July-September).
Figure 1. Seasonal variation in salivary testosterone.
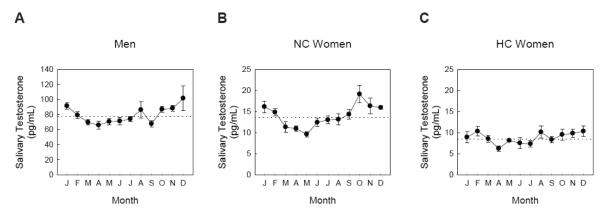
The means and standard errors of testosterone concentrations (in pg/mL) are depicted by month for men (Panel A), normally-cycling women (Panel B), and women taking hormonal contraceptives (Panel C). The horizontal, dotted lines represent the grand mean of testosterone within each panel. Month labels are in chronological order.
ANOVA was used to compare testosterone concentrations between normally-cycling women and women using hormonal contraceptives while collapsing across month. The use of hormonal contraceptives was associated with lower testosterone concentrations, F(1, 390) = 81.40, p < 0.001, as shown in past studies (Edwards & O’Neal, 2009; Schultheiss et al., 2005). We then separately tested the effects of month on testosterone for women who are normally-cycling and for those taking hormonal contraceptives.
For normally-cycling women, ANOVA showed a significant effect of month on testosterone concentrations, F(11, 250) = 4.42, p < 0.001 (see Table 1 for pairwise comparisons between months). Testing linear, quadratic, and cubic regression effects, the quadratic model showed the best fit of the data, F(2, 259) = 15.05, p < 0.001, and the effect of the quadratic factor was, β = 1.29, t = 5.21, p < 0.0011. In contrast, for women taking hormonal contraceptives, ANOVA showed only a trend effect of month on testosterone concentrations, F(11, 118) = 1.66, p = 0.09 (see Table 1 for pairwise comparisons between months), yet the quadratic model still showed a significant fit of the data, F(2, 127) = 4.73, p = 0.01, and the effect of quadratic factor was, β = 1.17, t = 3.06, p = 0.003. However, after collapsing over contraceptive use and adding second-order interactions to the regression model, the interaction of contraceptive use by quadratic effect of month did not reach significance, β = 1.18, t = 1.73, p = 0.08. This suggests, but does not demonstrate, a potential difference in the nature of the quadratic effect such that seasonal variation is more pronounced in normally-cycling women (Fig.1, Table 1); improved statistical power would aid in testing this difference in future studies. In contrast, the interaction of gender by quadratic effect of month for men and normally-cycling women (using testosterone values standardized within sex) was not significant, β = -0.03, t = -0.06, p = 0.95, which demonstrates that the nature of the quadratic effect was similar for normally-cycling women and men; i.e. those without exogenous hormone manipulation (Fig.1, Table 1).
Table 1 reveals that for women taking hormonal contraceptives, there are fewer months that show statistically significant differences in testosterone concentrations as compared to normally-cycling women, which reflects a flatter annual profile of testosterone. Our sample included fewer women on hormonal contraceptives than normally-cycling women, and this smaller sample size made the detection of statistically significant differences between months less likely in women using hormonal contraceptives as a function of reduced test power. Nevertheless, it is worth noting that the peak-to-trough amplitude (peak month testosterone concentration minus trough month testosterone concentration) for normally cycling women was 9.5 pg/mL, which is more than twice as large as the 4.1 pg/mL peak-to-trough amplitude for women taking hormonal contraceptives.
Discussion
Our first hypothesis that men and normally-cycling women would show significant seasonal variation in salivary testosterone concentrations was affirmed. Men had peak concentrations of testosterone in the fall and lowest concentrations of testosterone in the spring, which is generally consistent with past reports (Dabbs, 1990a; Svartberg et al., 2003b). Normally-cycling women showed a pattern of seasonal variation in testosterone that was similar to men, but they reached a peak annual testosterone concentration 2 months earlier (Oct.) than did men (Dec.). Similarly, van Anders and colleagues (2006) also found that normally-cycling women had peak testosterone concentrations in the fall when compared to the other seasons. Yet as a function of their smaller sample size, van Anders and colleagues (2006) were unable to characterize significant differences at a month-to-month concentration for normally-cycling women. In contrast, the present data are able to show that several spring months reflect significantly lower concentrations of testosterone than months in the fall and winter for normally-cycling women. Specifically, concentrations of testosterone were over 100% greater at peak (Oct.) compared to trough (Apr.) for normally-cycling women.
We found that the use of hormonal contraceptives was associated not only with lower endogenous testosterone concentrations (Edwards & O’Neal, 2009; Liening et al., 2010), but also that the use of hormonal contraceptives is associated with changes in the nature of the seasonal variation of testosterone in multiple ways. First, hormonal contraceptives are associated a reduction of the amplitude of peak to trough seasonal variation, much as they suppress endogenous variation in estradiol and progesterone over the menstrual cycle. Second, hormonal contraceptives appear to be associated with less consistent month-to-month variation, with more peaks and troughs2 within a year (Fig. 1). Together, the present data are the first to show that the seasonal profile of testosterone for women taking hormonal contraceptives is qualitatively different in multiple respects compared to that of normally-cycling women.
While our findings are statistically robust and novel, the present study is not without some limitations. First, using a longer data collection period of 2 or more full years would allow for the characterization of true circannual rhythm of testosterone via the inclusion of 2 full annual cycles. Animal studies of circannual rhythms have done this, with periods of data collection extending for several years (Prendergast et al., 2009), but such lengthy periods of data collection are exceptionally difficult and costly for studies of human subjects. Second, a longitudinal design (rather than the presently employed cross-sectional design) would provide greater control for inter-subject variability that adds noise to between-subjects designs. Additionally, in spite of our large sample size by human research standards, an even larger sample would further reduce variability in and add clarity to the true effects of season on testosterone concentrations, and this is particularly true with regard to increasing sample size for women using hormonal contraceptives. We will also note that our data were collected from principally younger adults (only 14 out of 688 subjects were over age 40) and in a climate that has significant seasonal variation in temperature. The extent to which our findings would generalize to more tropical climates and to all age ranges is unknown. Lastly, our participants were largely drawn from a community that has many individuals affiliated with local universities and schools (e.g., students, staff); such individuals may have different seasonal influences than those drawn from different populations (e.g., farming communities). Thus, future work on seasonal variability of testosterone in humans would benefit from larger sample sizes, collecting data longitudinally and over multiple years, as well as generalization to different populations and geographic regions.
The present findings have implications for future research on human behavioral endocrinology. First, accounting for seasonal variation in testosterone has the potential to reduce noise in data analysis thus clarifying relationships between testosterone and behavior. Second, the present study adds to a growing literature that demonstrates how hormonal contraceptive use is associated with alteration of natural variation of testosterone along multiple temporal scales, which may have implications for relationships between testosterone and behavior. Third, it is plausible that certain behaviors or aspects of physiology would vary over the course of the year, as a function of the activational effects of increasing or decreasing concentrations of testosterone. Thus, a conclusive characterization of the multiple rhythms of testosterone is essential. There are several other behaviors that are linked to testosterone and have also been demonstrated to follow patterns of seasonal variation, yet few have demonstrated mediation of the behavioral effects by variation in testosterone. For example, mating and conception has been shown to follow a seasonal rhythm in humans (Rizzi & Dalla-Zuanna, 2007), but this has not been shown to be conclusively mediated by seasonal variation in testosterone. The extent to which seasonal variation in humans’ testosterone concentrations might account for seasonal variation in other behaviors (e.g., aggression and dominance), cognitive processes (e.g., Kimura & Hampson, 1994), or aspects of psychopathology (e.g., seasonal affective disorder) is an open and important question for future research.
Highlights.
- Humans’ endogenous testosterone concentrations vary over a number of temporal scales, with little known about variation longer than monthly cycles.
- The present data show that men and normally-cycling women displayed seasonal variation in salivary testosterone concentrations, such that testosterone concentrations are maximal in the fall and minimal in the summer.
- Women using hormonal contraceptives not only had consistently lower endogenous testosterone concentrations than normally-cycling women, but also showed a flatter seasonal testosterone profile.
Acknowledgments
This research was supported by a Duke Institute for Brain Sciences Incubator Award (to S.A.H.), NIMH grant RC1-088680 (to S.A.H.), and the McClelland Postdoctoral Fellowship from the Hay Group (to S.J.S.). We thank R. Edward McLaurin and Rosie Phillips for their assistance with testing participants.
Footnotes
To rule out any effect of menopause, we repeated the analyses without the 9 female participants over the age of 40. The reported effects did not significantly change.
Peaks are defined as months for which testosterone concentrations are lower both the month before and month after and troughs are defined as months during which testosterone concentrations are higher both the month before and month after.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bancroft J, Sherwin BB, Alexander GM, Davidson DW, Walker A. Oral contraceptives, androgens, and the sexuality of young women: II. The role of androgens. Archives of Sexual Behavior. 1991;20(2):121–135. doi: 10.1007/BF01541939. [DOI] [PubMed] [Google Scholar]
- Bellastella A, Criscuolo T, Mango A, Perrone L, Sinisi AA, Faggiano M. Circannual rhythms of plasma luteinizing hormone, follicle-stimulating hormone, testosterone, prolactin and cortisol in prepuberty. Clin Endocrinol (Oxf) 1983;19(4):453–459. doi: 10.1111/j.1365-2265.1983.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94(3):907–913. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GL, McGarvey EL, Shirtcliff EA, Keller A, Granger DA, Flavin K. Salivary cortisol, dehydroepiandrosterone, and testosterone interrelationships in healthy young males: A pilot study with implications for studies of aggressive behavior. Psychiatry Research. 2008;159(1-2):67–76. doi: 10.1016/j.psychres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Bubenik GA, Morris JM, Schams D, Claus A. Photoperiodicity and circannual levels of LH, FSH, and testosterone in normal and castrated male, white-tailed deer. Can J Physiol Pharmacol. 1982;60(6):788–793. doi: 10.1139/y82-110. [DOI] [PubMed] [Google Scholar]
- Courty Y, Dufaure JP. Circannual testosterone, dihydrotestosterone and androstanediols in plasma and testis of lacerta vivipara, a seasonaly breeding viviparous lizard. Steroids. 1982;39(5):517–529. doi: 10.1016/0039-128x(82)90052-6. [DOI] [PubMed] [Google Scholar]
- Dabbs J, de La Rue D. Salivary testosterone measurements among women: Relative magnitude of circadian and menstrual cycles. Hormone Research. 1991;35:182–184. doi: 10.1159/000181899. [DOI] [PubMed] [Google Scholar]
- Dabbs JM., Jr. Age and seasonal variation in serum testosterone concentration among men. Chronobiol Int. 1990a;7(3):245–249. doi: 10.3109/07420529009056982. [DOI] [PubMed] [Google Scholar]
- Dabbs JM., Jr. Salivary testosterone measurements: reliability across hours, days, and weeks. Physiol Behav. 1990b;48(1):83–86. doi: 10.1016/0031-9384(90)90265-6. [DOI] [PubMed] [Google Scholar]
- Dabbs JM., Jr. Salivary testosterone measurements: collecting, storing, and mailing saliva samples. Physiol Behav. 1991;49(4):815–817. doi: 10.1016/0031-9384(91)90323-g. [DOI] [PubMed] [Google Scholar]
- Edwards DA, O’Neal JL. Oral contraceptives decrease saliva testosterone but do not affect the rise in testosterone associated with athletic competition. Hormones and Behavior. 2009;56(2):195–198. doi: 10.1016/j.yhbeh.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Garde AH, Hansen AM, Skovgaard LT, Christensen JM. Seasonal and biological variation of blood concentrations of total cholesterol, dehydroepiandrosterone sulfate, hemoglobin A(1c), IgA, prolactin, and free testosterone in healthy women. Clin Chem. 2000;46(4):551–559. [PubMed] [Google Scholar]
- Greco T, Graham CA, Bancroft J, Tanner A, Doll HA. The effects of oral contraceptives on androgen levels and their relevance to premenstrual mood and sexual interest: a comparison of two triphasic formulations containing norgestimate and either 35 or 25 μg of ethinyl estradiol. Contraception. 2007;76(1):8–17. doi: 10.1016/j.contraception.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Everitt B. Essential Reproduction. Wiley-Blackwell; Oxford: 2000. [Google Scholar]
- Josephs RA. Moving beyond dichotomies in research on oral contraceptives: a comment on Edwards and O’Neal. Horm Behav. 2009;56(2):193–194. doi: 10.1016/j.yhbeh.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Kimura D, Hampson E. Cognitive Pattern in Men and Women Is Influenced by Fluctuations in Sex-Hormones. Current Directions in Psychological Science. 1994;3(2):57–61. [Google Scholar]
- Liening SH, Stanton SJ, Saini EK, Schultheiss OC. Salivary testosterone, cortisol, and progesterone: Two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiology & Behavior. 2010;99(1):8–16. doi: 10.1016/j.physbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- López HH, Hay AC, Conklin PH. Attractive men induce testosterone and cortisol release in women. Hormones and Behavior. 2009;56(1):84–92. doi: 10.1016/j.yhbeh.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Michael RP, Bonsall RW. A 3-year study of an annual rhythm in plasma androgen levels in male rhesus monkeys (Macaca mulatta) in a constant laboratory environment. J Reprod Fertil. 1977;49(1):129–131. doi: 10.1530/jrf.0.0490129. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E. A curvilinear relationship between testosterone and spatial cognition in humans: possible influence of hand preference. Psychoneuroendocrinology. 1996;21(3):323–337. doi: 10.1016/0306-4530(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E. Salivary testosterone concentrations in left-handers: an association with cerebral language lateralization? Neuropsychology. 2000;14(1):71–81. [PubMed] [Google Scholar]
- Perry HM, Miller DK, Patrick P, Morley JE. Testosterone and leptin in older African-American men: Relationship to age, strength, function, and season. Metabolism. 2000;49(8):1085–1091. doi: 10.1053/meta.2000.7710. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: Behavior and neuroendocrine substrates. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain, and Behavior. Academic Press; San Diego, CA: 2002. pp. 93–156. [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: Behavior and neuroendocrine substrates. In: Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2nd ed Vol. 1. Academic Press; San Diego: 2009. pp. 507–538. [Google Scholar]
- Riad-Fahmy D, Read GF, Walker RF, Griffiths K. Steroids in saliva for assessing endocrine function. Endocr Rev. 1982;3(4):367–395. doi: 10.1210/edrv-3-4-367. [DOI] [PubMed] [Google Scholar]
- Rizzi EL, Dalla-Zuanna G. The Seasonality of Conception. Demography. 2007;44(4):705–728. doi: 10.1353/dem.2007.0040. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Stanton SJ. Assessment of salivary hormones. In: Harmon-Jones E, Beer JS, editors. Methods in social neuroscience. NY Guilford Press; New York: 2009. pp. 17–44. [Google Scholar]
- Schultheiss OC, Wirth MM, Torges CM, Pang JS, Villacorta MA, Welsh KM. Effects of implicit power motivation on men’s and women’s implicit learning and testosterone changes after social victory or defeat. J Pers Soc Psychol. 2005;88(1):174–188. doi: 10.1037/0022-3514.88.1.174. [DOI] [PubMed] [Google Scholar]
- Stanton SJ. The essential implications of gender in human behavioral endocrinology studies. Frontiers in Behavioral Neuroscience. 2011;5:9. doi: 10.3389/fnbeh.2011.00009. doi:10.3389/fnbeh.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, Beehner JC, Saini EK, Kuhn CM, LaBar KS. Dominance, Politics, and Physiology: Voters’ Testosterone Changes on the Night of the 2008 United States Presidential Election. PLoS One. 2009;4(10) doi: 10.1371/journal.pone.0007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, Edelstein RS. The physiology of women’s power motive: Implicit power motivation is positively associated with estradiol levels in women. Journal of Research in Personality. 2009;43(6):1109–1113. [Google Scholar]
- Stanton SJ, Mullette-Gillman ODA, McLaurin RE, Kuhn CM, LaBar KS, Platt ML, Huettel SA. Low- and High-Testosterone Individuals Exhibit Decreased Aversion to Economic Risk. Psychological Science. 2011;22(4):447–453. doi: 10.1177/0956797611401752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, Schultheiss OC. The hormonal correlates of implicit power motivation. Journal of Research in Personality. 2009;43(5):942–949. doi: 10.1016/j.jrp.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svartberg J, Jorde R, Sundsfjord J, Bonaa KH, Barrett-Connor E. Seasonal Variation of Testosterone and Waist to Hip Ratio in Men: The Tromso Study. J Clin Endocrinol Metab. 2003a;88(7):3099–3104. doi: 10.1210/jc.2002-021878. [DOI] [PubMed] [Google Scholar]
- Svartberg J, Jorde R, Sundsfjord J, Bonaa KH, Barrett-Connor E. Seasonal variation of testosterone and waist to hip ratio in men: the Tromso study. J Clin Endocrinol Metab. 2003b;88(7):3099–3104. doi: 10.1210/jc.2002-021878. [DOI] [PubMed] [Google Scholar]
- Valero-Politi J, Fuentes-Arderiu X. Annual rhythmic variations of follitropin, lutropin, testosterone and sex-hormone-binding globulin in men. Clinica Chimica Acta. 1998;271(1):57–71. doi: 10.1016/s0009-8981(97)00239-8. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Goldey KL. Testosterone and partnering are linked via relationship status for women and [‘]relationship orientation’ for men. Hormones and Behavior. 2010;58(5):820–826. doi: 10.1016/j.yhbeh.2010.08.005. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Hamilton LD, Schmidt N, Watson NV. Associations between testosterone secretion and sexual activity in women. Horm Behav. 2007;51(4):477–482. doi: 10.1016/j.yhbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Hampson E, Watson NV. Seasonality, waist-to-hip ratio, and salivary testosterone. Psychoneuroendocrinology. 2006;31(7):895–899. doi: 10.1016/j.psyneuen.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Jr., Ball GF. The “Challenge Hypothesis”: Theoretical Implications for Patterns of Testosterone Secretion, Mating Systems, and Breeding Strategies. American Naturalist. 1990;136(6):829–846. [Google Scholar]
- Wisniewski AB, Nelson RJ. Seasonal variation in human functional cerebral lateralization and free testosterone concentrations. Brain Cogn. 2000;43(1-3):429–438. [PubMed] [Google Scholar]


