Abstract
Background
Modulation of nicotinic acetylcholine receptors (nAChRs), specifically α4β2 subunit containing nAChRs, may be effective in the treatment of patients with major depressive disorder (MDD). Using [123I] 5-I-A-85380 single photon emission computed tomography (SPECT), we studied β2 subunit containing nAChR (β2*-nAChR) availability in patients with MDD. In order to understand the molecular basis of the change in receptor availability, we also studied β2*-nAChR binding in postmortem samples of human brains of MDD subjects.
Methods
23 medication-free, early-onset, non-smoking subjects with familial MDD (8 acutely depressed (aMDD), 15 euthymic, recovered MDD subjects (rMDD)), and 23 age- and gender-matched, non-smoking controls had one [123I] 5-I-A-85380 SPECT scan and a magnetic resonance imaging (MRI) scan. β2*-nAChR availability was quantified as VT/fP. β2*-nAChR binding was analyzed in postmortem samples of the prefrontal cortex in 14 subjects with MDD and age-matched controls with [125I] 5-I-A-85380.
Results
β2*-nAChR availability in aMDD and rMDD subjects was significantly lower across all brain regions than in respective controls and lower in aMDD subjects than in rMDD subjects. MDD patients showed significant correlations between β2*-nAChR availability and lifetime number of depressive episodes, trauma and anxiety scores. There were no differences in β2*-nAChR number between groups in the human postmortem study.
Conclusion
β2*-nAChR availability is decreased in patients with MDD. The difference between β2*-nAChR availability in vivo and in postmortem samples may be analogous to data with dopaminergic PET ligands and dopamine receptor availability; lower receptor availability for the SPECT ligand could be caused by increased endogenous acetylcholine.
1. Introduction
Converging lines of evidence suggest that the cholinergic system may be a potential target for the development of novel molecular approaches for the treatment of depression(1). First, cholinergic hyperactivity plays a role in the pathophysiology of depression (2–4) and can exacerbate depression-like symptoms in patients and controls (4–6). Second, tobacco dependence is highly prevalent in MDD populations (7) and MDD patients have a higher prevalence of smokers (8–10). Population-based studies show that smokers are twice as likely as nonsmokers to have a lifetime history of major depression (11–13). Furthermore, nicotine binds to neuronal nicotinic acetylcholine receptors (nAChRs) and partial agonists and antagonists of nAChRs including nicotine (14), mecamylamine and dihydro-beta-erythroidine (DHβE) (15), cytisine (16) and varenicline (17), have antidepressant-like effects in preclinical studies. However, mice lacking the β2-nAChR subunit are insensitive to the antidepressant-like effects of mecamylamine (15) and amitriptyline (18). This suggests that β2 subunit containing (β2*) nAChRs may be important for the potential antidepressant like effect in preclinical models of depression. In addition to clinical studies of nicotine (19, 20), recent clinical trials have reported success with the use of nAChR modulators. Therefore mecamylamine (21), the s-enantiomer of mecamylamine (http://clinicaltrials.gov/ct2/show/NCT00593879 ClinicalTrials.gov Identifier NCT00593879; http://www.targacept.com/wt/page/tc_5214), and varenicline have been shown to have antidepressant effects in depression (22).
The recent development and use of [123I]5-I-A ([123I]-5-iodo-3-[2(S)-2-azetidinylmethoxy] pyridine) as a SPECT radioligand to quantify β2*-nAChRs in vivo in the human brain now allows unparalleled access to this system in vivo (23–25).
The objective of the study was to determine if there is a core dysfunction in the β2*-nAChR system in MDD using SPECT and the selective β2*-nAChR radioligand [123I]5-I-A. We chose to study subjects who were fully recovered from MDD to avoid the confounding effects of acute illness or antidepressant treatment (26). We hypothesized that patients with MDD would have decreased β2*-nAChR availability compared with healthy controls.
Analogous to findings from radioligand based studies of dopamine in humans, it is critically important to note that changes in receptor availability in vivo could be due to a change in receptor number or a change in receptor occupancy. It has been shown previously in nonhuman primates that elevated ACh levels induced by a high dose of intravenous physostigmine, an acetylcholinesterase inhibitor, can compete effectively in vivo with [123I]5-IA binding at β2*-nAChRs (27). Therefore, lower binding of [123I]5-IA to the β2*-nAChR could reflect one of two processes: an actual, absolute change in the amount of receptor or a change in synaptic ACh levels, such that an increased amount of ACh would prevent the radioligand from binding to the receptor, resulting in low binding of the radioligand.
We also quantified β2*-nAChR availability in postmortem samples of brains of patients with MDD compared with healthy controls. β2*-nAChR availability in the postmortem human samples, in the absence of endogenous acetylcholine, would help interpret the precise mechanism of potential changes in binding observed with [123I]5-IA in humans.
2. Materials and Methods
2. 1. Methods for SPECT Study
Participants
All prospective subjects had a an interview with an experienced psychiatrist who elicited a complete psychiatric and medical history, a Structured Clinical Interview for DSM-IV Disorders (SCID-I) (28), standardized psychiatric assessments, a physical examination, routine blood tests, pregnancy test, urine toxicology and EKG. Participants were administered the Beck Depression Inventory (BDI), the Center for Epidemiologic Studies Depression Scale (CES-D), the Spielberger State-Trait Anxiety Inventory (STAI), the NEO Personality Inventory (NEO-PI-R), the Childhood Trauma Questionnaire (CTQ) and the Hamilton Depression Rating Scale-21 item (HDRS) (29).
Twenty-three non-smoking, medication-free subjects with recurrent MDD (8 subjects with acute MDD (aMDD) and 15 fully recovered MDD subjects (rMDD)) and 23 age- and gender-matched healthy controls were included in this study. Subjects with a significant axis I diagnosis were excluded from the study and only subjects with a primary diagnosis of MDD were included in the study. In accordance with previous studies in these populations (30–32), all MDD subjects met the following criteria:
age of onset of first major depressive episode < 25 years, and
a lifetime history of at least two major depressive episodes, and
at least one first degree relative with a reported history of MDD or an axis I disorder.
MDD subjects were medication-free for at least 3 months, and aMDD subjects had to score >16 on the HDRS. Subjects were classified as rMDD based on four criteria: self-reported euthymia for >4 months following the last episode of major depression, clinician rated euthymia > 4 months based on a clinical interview, absence of criterion for a major depressive episode > 4 months as judged by the SCID, and an HDRS score of less than 8. Control non-smoking subjects were only included in the study if they had no lifetime/family (first degree relative) history of any axis I or axis II disorders as judged by the SCID-I (28).
None of the subjects recruited had smoked for at least 6 months and 14 of the MDD subjects (6 aMDDs and 8 rMDDs) reported no lifetime history of smoking. Non-smoking status was confirmed by plasma cotinine levels of <15 ng/mL, urine cotinine levels of <100 ng/mL (confirmed using a dipstick (NicAlert) on the day of the scan), and exhaled carbon monoxide levels of <11 ppm on the day of intake and on the day of the scan. Subjects were excluded if they had a positive pregnancy test at screening on the day of the SPECT scan or a history of prior radiation exposure within the past year such that participation this study would place them over FDA limits for annual radiation exposure.
This study was approved by the Yale University School of Medicine Human Investigation Committee and the Radiation Safety Committee. After complete description of the study to the subjects, written informed consent was obtained. The use of the radiotracer [123I]5-IA was approved by the US Food and Drug Administration.
[123I]5-I-A-85380 SPECT and Magnetic Resonance (MR) imaging
All subjects had one [123I]5-IA SPECT scan and one MRI scan. MRI was performed on a Signa 1.5T system (General Electric Co, Milwaukee, Wisconsin) as described previously (33). SPECT imaging protocol was conducted as described previously (34). In brief, [123I]5-IA was administered using a bolus plus constant infusion paradigm at a ratio of 7.0 h for 8 hours. There were no significant differences in the total injected dose, bolus dose, infusion dose, and bolus to infusion ratio between depressed and control groups (Table 1). Three 30-min emission and one 15-minute simultaneous transmission and emission protocol (STEP) scans were obtained between 6–8 hours of infusion on a Picker PRISM 3000 XP (Cleveland, OH) SPECT camera. Plasma samples were collected in the middle of the second scan to quantify total parent and free fraction (fp) of parent tracer in plasma (35) and to correct for individual differences in metabolism and protein binding of [123I]5-IA (36). A 57Co-distributed source was measured with each experiment to control for day-to-day variation in camera sensitivity.
Table 1.
Radiochemical parameters for [123I]5-IA in acutely depressed (aMDD) and recovered depressed (rMDD) subjects and their respective controls (C-aMDD, C-rMDD).
| Measure | Acutely Depressed (aMDD, N=8) | aMDD Controls (C-aMDD, N=8) | Recovered Depressed (rMDD, N=15) | rMDD Controls (C-rMDD, N=15) | Statistics |
|---|---|---|---|---|---|
| Total parent level, kBq/mL | 0.37 (0.07) | 0.34 (0.17) | 0.28 (0.10) | 0.38 (0.006) | F3,38=5.1, p=0.005 |
| Free parent level, kBq/mL | 0.13 (0.02) | 0.11 (0.06) | 0.13 (0.02) | 0.09 (0.03) | F3,38=5.5, p=0.003 |
| Free fraction level, kBq/mL.% | 34.1 (3.2) | 31.7 (3.4) | 35.4 (3.0) | 33.1 (4.8) | F3,38=1.1, p=0.4 |
| Total injected dose, MBq | 363.5 (10.3) | 326.3 (62.6) | 360.2 (36.0) | 344.2 (46.3) | F3,42=0.9, p=0.48 |
| Bolus dose, MBq | 157.7 (7.1) | 148.1 (26.6) | 157.4 (15.7) | 149.3 (20.7) | F3,42=0.8, p=0.49 |
| Infusion dose, MBq | 206 (5.5) | 190.2 (33.3) | 202.9 (20.4) | 194.9 (25.7) | F3,42=0.9, p=0.45 |
| Bolus to infusion ratio | 7.0 (0.02) | 7.0 (0.04) | 7.0 (0.03) | 7.0 (0.03) | F3,42=1.4, 0.26 |
Image Analysis and Outcome Measures
SPECT emission images were analyzed as described previously (25). Specifically, SPECT emission images were reconstructed using a filtered back projection algorithm with a ramp filter on a 128 × 128 matrix to obtain 50 slices with a pixel size of 2.06 × 2.06 × 3.56 mm in the x-, y-, and z-axes. A three-dimensional (3D) Butterworth filter (order 10, cutoff frequency 0.24 cycle/pixel) was applied post hoc. A co-registered MR image was used to guide the placement of standard 2-dimensional region of interest (ROI) templates using MEDx software (Medical Numerics, Inc.). A 3D volume of interest (VOI) was generated for each region and transferred to the co-registered SPECT image to determine regional radioactive densities. The chosen regions were those known to contain β2*-nAChRs and included the frontal, parietal, anterior cingulate, temporal, and occipital regions, the thalamus, the striatum (an average of caudate and putamen), hippocampus, amygdala, brainstem, and the cerebellum. Regional [123I]5-IA uptake was determined by VT/fp where VT is volume of distribution and fp is free plasma fraction. Each case was analyzed by two raters, and the mean of the two raters was used. Inter-rater variability for VT/fp was <10% across all regions, which was computed as percent difference between two raters via following equation [(VT/fp 1/VT/fp 2)−1]*100=% difference.
2. 2. VBM Analysis
In order to study any volumetric differences as previously reported in MDD populations (37, 38), a voxel-based morphometric analysis of the data was performed with SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK). A detailed description of the steps involved in processing data for voxel-based morphometry is available in the SPM5 manual (http://www.fil.ion.ucl.ac.uk/spm/). Prior to preprocessing, images were checked for movement artifacts and the origin of the image was set at the anterior commissure. Images were segmented, normalized and smoothed simultaneously using a unified segmentation algorithm (39). In contrast to optimized voxel-based morphometry, which was used in SPM2 and in which these steps were completed sequentially, this study used the unified segmentation algorithm in SPM5 to simultaneously calculate image registration, tissue classification and bias correction using our participants’ structural MR images combined with the tissue probability maps provided in this version of SPM. Structural MR images were segmented into grey matter, white matter and cerebrospinal fluid tissue classifications. The segmented and modulated normalized images were smoothed with an 8 mm full-width-half maximum filter. A two-sample t-test was then used to delineate any significant differences in grey matter volume between the depressed and healthy control groups (N=23 each), recovered depressed and matched control group (N=15 each), and acutely depressed and matched-control groups (N=8 each) separately. Given the small sample size and preliminary nature of this analysis, a threshold of p<.05, uncorrected, was used to view the results.
2.3. Methods for Postmortem Study
Human brain specimens from the prefrontal cortex (PFC) of patients with MDD and matched controls were obtained from the Dallas Brain Collection (40). Methods are described in the supplement.
2.4 Statistical analysis
All data were analyzed using SAS version 9.1 (Cary, NC). Prior to analysis, data were examined descriptively and tested for normality using normal probability plots and Kolmogorov test statistics. All data were approximately normal. Receptor binding data were analyzed using linear mixed models. These models included region as a within-subjects factor, diagnostic group (aMDD, rMDD, and respective controls) as a between-subjects factor, and the interaction between group and region. Based on the Bayesian information criterion, the best fitting variance-covariance structure was compound symmetry with heterogeneous variance – i.e., a constant correlation between regions and region-specific variances are estimated. Significant interactions were explained by appropriate post-hoc tests and graphical displays. Post-hoc tests were adjusted for 11 regional comparisons using the Bonferroni correction (i.e., 2-sided alpha=.0045 threshold). An omnibus model including the four diagnostic groups was first developed. After a significant group by region interaction was confirmed, separate models were developed for 1) all MDD vs. all controls, 2) acute MDD vs. respective controls, 3) recovered MDD vs. respective controls, and 4) acute MDD vs. recovered MDD. Inclusion of age and gender as co-variates did not alter results and were therefore dropped from the analysis. Potential associations between binding potential and crucial clinical variables of interest were assessed using correlation analysis. In order to control for multiple correlations, the correlation of clinical/personality variables with binding data was only reported where significant correlations were observed consistently in a region. Postmortem data were analyzed using ANOVA and correlations were analyzed using appropriate correlation coefficients. For these data, each cell was an average of three measurements performed for each brain region for each patient. Data were then analyzed by ANOVA with “group” (Control or MDD) as between-factor and “brain region” (gray or white matter) as within-factor. Alpha was set at 5%.
3. Results
Results for SPECT study
3.1. Clinical Characteristics
Clinical and demographic characteristics of participants in each group are shown in Table 2; the groups were well matched on most parameters studied though there were significant differences in ethnicity and lifetime smoking history between the groups. Consistent with the acute nature of their illness, subjects in the aMDD group had significantly higher scores in the CES-D, BDI, NEO-PI-R Neuroticism subscale, CTQ Total score and CTQ Physical Neglect subscale, and in STAI, and significantly lower scores in the NEO Conscientiousness subscale than the subjects in the rMDD group.
Table 2.
Demographic and clinical characteristics for acutely depressed (aMDD), recovered depressed (rMDD), and their matched control groups(C-aMDD, C-rMDD).
| Characteristics | Acutely Depressed (aMDD, N=8) | aMDD Controls (C-aMDD, N=8) | Recovered Depressed (rMDD, N=15) | rMDD Controls (C-rMDD, N=15) | Statistics |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 36.3 (13.8) | 37.3 (15.5) | 32.5 (10.6) | 31.8 (11.0) | F3,42= 0.51 p=0.68 |
| Gender (male), N (%) | 3 (37.5) | 3 (37.5) | 3 (20) | 3 (20) | X23 =1.7, p=0.65 |
| Race, N (%) | |||||
| Caucasian | 5(62.5) | 8(100) | 12(80) | 8(53.3) | X23 =7.9, p=0.048 |
| Non-Caucasian | 3(37.5) | - | 3 (20) | 7(46.7) | |
| CES-D | 29.1 (5.8) | 2.3 (3.3) | 4.7 (5.9) | 3.3 (3.6) | F3,39 =53.7, p=0.000 |
| BDI score, mean (SD) | 20.4 (4.2) | 2.0 (2.3) | 2.7 (4.8) | 1.1 (1.7) | F3,37 =28.4, p=0.000 |
| NEO-PI-R score, mean (SD) | |||||
| Neuroticism | 111.5 (17.5) | - | 76.4 (23.1) | - | F1,19 =11.1, p=0.03 |
| Extraversion | 89 (19.3) | - | 108.9 (19.2) | - | F1,19 =4.6, p=0.045 |
| Openness to experience | 125.8 (15.7) | - | 130.8 (18.7) | - | F1,19 = 0.3, p=0.57 |
| Agreeableness | 116.8 (14.5) | - | 132.1 (19.7) | - | F1,19 =3.0, p=0.1 |
| Concientiousness | 91.5 (9.1) | - | 123.9 (26.2) | - | F1,19 =8.6, p=0.09 |
| DAS score, mean (SD) | 163.4 (49.9) | - | 113.7 (27.4) | - | F1,21 =9.7, p=0.005 |
| CTQ total score, mean (SD) | 4.9 (3.3) | - | 2.1 (2.3) | - | F1,21 =5.7, p=0.03 |
| Emotional abuse | 1.4 (1.1) | - | 0.6 (0.7) | - | F1,20 =3.6, p=0.07 |
| Physical abuse | 0.6 (1.2) | - | 0.4 (0.9) | - | F1,20 =0.2, p=0.67 |
| Sexual abuse | 0.8 (1.4) | - | 0.2 (0.8) | - | F1,20 =1.5, p=0.26 |
| Emotional neglect | 1.4 (0.9) | - | 0.9 (1.1) | - | F1,20 =1.4, p=0.27 |
| Physical neglect | 0.8 (1.2) | - | 0.1 (0.3) | - | F1,20 =4.5, 0.047 |
| STAI-State score, mean (SD) | 53.6 (3.2) | 23 (4.2) N=2 | 28.3 (7.4) | 24.2 (7.4) N=6 | F3,21 =28.0, p=0.000 |
| STAI-Trait score, mean (SD) | 56.4 (6.4) N=5 | 24 (5.7) N=2 | 32.5 (8.8) N=12 | 24.8 (4.8) N=6 | F3,21 =19.4, p=0.000 |
| Age of onset, years, mean (SD) | 17.7 (4.9) | - | 18 (4.6) | - | F1,20 =0.02, p=0.90 |
| Lifetime number of depressive episodes, mean (SD) | 4.8 (3.8)a | - | 4.9 (6.2) | - | F1,17 =0.01, p=0.97 |
| Number of weeks depressed, mean (SD) | 28 (17.9)a | - | 50.6 (87.6) | - | F1,16 =0.21, p=0.65 |
| Number of euthymic months, mean (SD) | - | - | 26.3 (25.6) | - | |
| Past psychotropic medication use | |||||
| SSRIs | 5 | - | 12 | - | |
| SNRIs | 2 | - | 1 | - | |
| Mood stabilizers | 0 | - | 1 | - | |
| Antipsychotics | 0 | - | 1 | - | |
| Anxiolytics | 3 | - | 1 | - | |
| Medication naive | 3 | - | 2 | - | |
| Lifetime smoking history, N (%) | 2 (25) | 0 (0) | 8 (53.3) | 1 (6.6) | X26 =15.1 p=0.02 |
| CO level on scan day, ppm, mean (SD) | 2 (1.6) | 1.4 (1.9) | 1 (1.4) | 2.2 (2.1) | F3,42 =1.3, p=0.28 |
Subjects with episodic illness were included.
3.2. β2*-nAChR Availability in Major Depressive Disorder
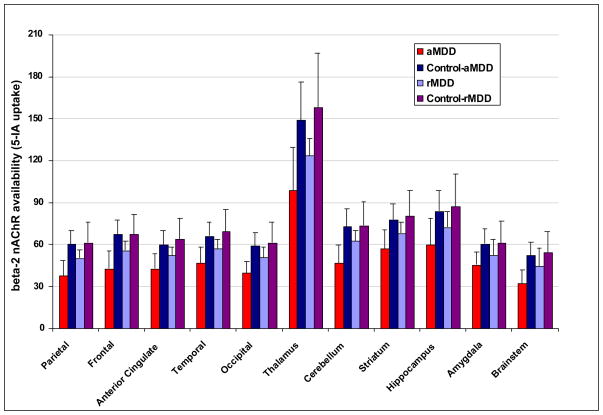
Radiochemical blood parameters are shown in Table 1. Results of the omnibus model including all four diagnostic groups revealed a significant effect of group (F(3,42)=8.65, p<.0001; see Figure 1) and a significant group by region interaction (F(30,420)=1.82, p=.006). Given the significant group by region interaction, we chose to disentangle group by region differences by developing independent models including two groups at a time.
Figure 1.
Comparison of β2*-nAChR availability throughout the brain in acutely depressed (n=8) and recovered depressed patients (n=15) and respective control subjects (n=8 and n=15 respectively). (Main effect of group: F 3,42=8.7, p= 0.0001; Group*region interaction: F30,420=0.00005). For acute MDD (N=8) and respective controls (N=8) main effect of group F1,14=15.8, p=0.0014; group*region interaction: F10,140=2.52, p=0.008. For recovered MDD (N=15) and respective controls (N=15) main effect of group F1, 28=7.9, p=.009 and group*region interaction F10, 280, p=.03. For acute MDD and recovered MDD main effect of group F1,21=10.0, p=.005, group*region interaction F10,210=1.34, p=0.21.
aMDD vs. controls
There was a significant main effect of group (F(1,14)=15.8, p=0.0014) and a significant interaction between group and region (F(10,140)=2.52, p=0.008). Post hoc comparison revealed decreased β2*-nAChR availability in aMDD subjects compared to healthy controls in nine of the eleven ROIs examined (except hippocampus and amygdala, all adjusted p<0.007).
rMDD vs. controls
There was a significant group effect (F(1, 28)=7.9, p=0.009) and group by region effect (F(10, 280, p=0.03). Post hoc comparisons showed lower β2*-nAChR availability among patients compared to controls in the frontal cortex (adjusted p=0.03), anterior cingulate (adjusted p=0.04), and thalamus (adjusted p=0.008).
aMDD vs. rMDD patients
There was a significant overall group effect (F(1,21)=10.0, p=0.005) with lower overall β2*-nAChR availability observed among acute MDD patients compared to recovered patients. However, the interaction between group and region was not significant (F(10,210)=1.34, p=0.21).
Correlation with clinical features
When aMDD and rMDD groups were combined, there was a significant positive correlation between lifetime number of episodes and β2*-nAChR availability in temporal cortex (r=0.46, p=0.049), occipital cortex (r=0.48, p=0.037), and striatum (r=0.49, p=0.034). This was also observed in the rMDD group alone (temporal cortex r=0.54, p=0.036, occipital cortex r=0.56, p=0.03, thalamus r=0.54, p=0.038 and striatum r=0.58, p=0.022).
The CTQ total score and emotional abuse subscale were negatively correlated with receptor availability in all ROIs studied except amygdala in the aMDD but not the rMDD group. The STAI Trait subscale score was negatively correlated with receptor availability in anterior cingulate (r=−0.65, p=0.027), temporal (r=−0.64, p=0.026) and, occipital (r=−0.65, p=0.021) regions and showed a trend for significance in striatum (r=−0.51, p=0.09) in the rMDD group but not in the aMDD group. NEO conscientiousness scores were significantly correlated with receptor availability in all ROIs studied except amygdala and brainstem (which showed a trend for significance) in the aMDD group but not the rMDD group.
The relationship between a history of past cigarette smoking and β2*-nAChR availability could not be studied because of the small and unbalanced number of subjects with a past history of cigarette smoking.
VBM analysis
An SPM analysis of brain volumes of all MDD subjects compared to all controls showed no significant changes in volume in this population even at the relatively generous threshold used in the analysis. Similarly, there were no significant changes in volume between the aMDD subjects and their respective controls, the rMDD subjects and their respective controls, or between the aMDD and rMDD subjects.
Results of [125I]5-I-A equilibrium binding in postmortem brain tissue
Demographic data for the sample of patients with a diagnosis of MDD and matched controls and results are provided in the supplement.
4. Discussion
We present a multimodal analysis of β2*-nAChR availability to show that MDD is associated with a significant dysfunction in the cholinergic system. From the in vivo data, we observed that acute and recovered patients with MDD have significantly lower β2*-nAChR availability than age- and gender-matched controls. Crucially, there were no differences in β2*-nAChR number in the postmortem sample of MDD subjects compared with controls under conditions that washed out any endogenous bound ACh. This suggests that, consistent with the cholinergic hypothesis of depression (4), the lower β2*-nAChR availability in vivo is likely better explained by increased levels of extracellular ACh in patients with MDD rather than by a decrease in the total number of receptors. Finally, we found that the lower β2*-nAChR availability in MDD patients had functional effects in that it was related to critical personality measures.
Use of [123I]5-IA to quantify β2*-nAChR availability
Our data demonstrate a persistent enduring deficit in cortical and subcortical β2*-nAChR availability in subjects with MDD. As aMDD patients had significantly lower β2*-nAChR availability than in rMDD subjects in most regions, it is possible that ACh levels may recover somewhat in patients with remitted MDD. The continued, significant decrease in β2*-nAChR availability in fully recovered, euthymic, medication free rMDD subjects suggests that the changes in MDD are not an epiphenomenon of treatment or illness but may be associated with trait vulnerability to depression as demonstrated by us previously for other targets (26, 41, 42). nAChRs have a ubiquitous role in the modulation of multiple neurotransmitter systems considered crucial in the pathophysiology of MDD such as serotonin, noradrenaline, glutamate and GABA (43) and the observed dysfunction may reflect a cause or effect of dysfunction in these other systems. Finally there were no differences in brain volume between the patients and controls suggesting that brain atrophy could not account for changes in receptor availability in this sample.
Postmortem studies
As described above, the decreased binding of the SPECT radioligand [123I]5-IA to β2*-nAChR could reflect an actual, absolute change in the amount of receptor or may reflect a change in synaptic ACh levels, with an increased amount of ACh preventing the radioligand from binding to the receptor (27). Consistent with this possibility, the postmortem samples show no change in receptor number in patients with MDD when compared with either controls. These results, together with the non-human primate study (27), suggest that when β2*-nAChR availability is measured in the absence of bound endogenous ACh, there is no evidence of a change in receptor number.
Functional consequences of lower β2*-nAChR availability
We observed a significant positive correlation between the number of depressive episodes and β2*-nAChR availability in the combined MDD sample and rMDD patients. Assuming that the lower β2*-nAChR availability represents increased extracellular ACh levels, the correlation implies that the greater the number of episodes, the greater the amount of extracellular ACh. Studies suggest that tobacco smokers have higher β2*-nAChR availability compared with non-smokers in non-psychiatric populations (34) which normalizes by 6–12 weeks of abstinence (44). However given the small and unbalanced number of subjects with a past history of cigarette smoking, we were not able to understand the effect of this variable on β2*-nAChR availability. Finally observations of the relationship between childhood trauma and β2*-nAChR availability replicates a relationship that has been observed in previous studies of the association between trauma and MDD (45).
Limitations
Notwithstanding the robust statistical significance of the SPECT binding results, the sample size of the population studied is relatively small and composed nearly entirely of subjects without a past history of cigarette smoking; only 11 of the 46 subjects studied had a past history of cigarette smoking. Future studies need to be performed to examine the relationship between past cigarette smoking and β2*-nAChR availability. The nature and logistics of using a SPECT radioligand, the absence of a quantifiable reference region and input function and the absence of a measurement of non-displaceable binding in patients with MDD, are issues that are beyond the scope of this paper but have been addressed in detail elsewhere (25, 27, 46). While the amygdala is a crucial structure in the pathophysiology of depression, SPECT has poor resolution for small brain regions and the findings for the amygdala should be interpreted with caution a. A PET study needs to be performed to confirm these results. Although past smoking status is a critical issue, it was not possible to analyse this effect adequately given the small and unequal number of subjects who have smoked in the past. The use of a population exclusively composed of current non-smokers while essential to interpretation of the results limits generalizability and should be examined in future studies.
In conclusion, patients with MDD show lower β2*-nAChR availability that persists beyond the cessation of treatment and into full recovery and further studies are needed to clarify the molecular underpinnings of decreased receptor availability. A similar study using a SPECT ligand specific to β4 containing nAChRs seems warranted considering the high density of these receptors in the habenula complex, a region increasingly implicated in depressed mood states (47).
Supplementary Material
Acknowledgments
We acknowledge support from the Clinical Neuroscience Research Unit at the Connecticut Mental Health Center and the Connecticut Department of Mental Health and Addictions Services.
This work was supported by research grants from the National Institutes of Health (MH077681 to MRP, MH077914 to Z.B), the National Alliance for Research on Schizophrenia and Depression (Z.B.), the Freedman Fellowship (AS) and the Clinical and Translational Science Award Grant UL1 RR024139 from the National Center for Research Resources to Yale University (Z.B.).
References
- 1.Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7(6):525–35. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- 2.Owens MJ, Overstreet DH, Knight DL, Rezvani AH, Ritchie JC, Bissette G, Janowsky DS, Nemeroff CB. Alterations in the hypothalamic-pituitary-adrenal axis in a proposed animal model of depression with genetic muscarinic supersensitivity. Neuropsychopharmacology. 1991;4(2):87–93. [PubMed] [Google Scholar]
- 3.Dilsaver SC. Pathophysiology of “cholinoceptor supersensitivity” in affective disorders. Biological Psychiatry. 1986;21(8–9):813–829. doi: 10.1016/0006-3223(86)90246-5. [DOI] [PubMed] [Google Scholar]
- 4.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632–5. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 5.Risch SC, Cohen RM, Janowsky DS, Kalin NH, Murphy DL. Mood and behavioral effects of physostigmine on humans are accompanied by elevations in plasma beta-endorphin and cortisol. Science. 1980;209(4464):1545–6. doi: 10.1126/science.7433977. [DOI] [PubMed] [Google Scholar]
- 6.Janowsky DS, Overstreet DH. Cholinergic dysfunction in depression. Pharmacol Toxicol. 1990;66 (Suppl 3):100–11. doi: 10.1111/j.1600-0773.1990.tb02077.x. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman M, Chelminski I, McDermut W. Major depressive disorder and axis I diagnostic comorbidity. J Clin Psychiatry. 2002;63(3):187–93. doi: 10.4088/jcp.v63n0303. [DOI] [PubMed] [Google Scholar]
- 8.Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143(8):993–7. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 9.Poirier MF, Canceil O, Bayle F, Millet B, Bourdel MC, Moatti C, Olie JP, Attar-Levy D. Prevalence of smoking in psychiatric patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):529–37. doi: 10.1016/s0278-5846(01)00304-9. [DOI] [PubMed] [Google Scholar]
- 10.Glassman AH. Cigarette smoking: implications for psychiatric illness. Am J Psychiatry. 1993;150(4):546–53. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- 11.Breslau N, Kilbey MM, Andreski P. Nicotine dependence and major depression. New evidence from a prospective investigation. Arch Gen Psychiatry. 1993;50(1):31–5. doi: 10.1001/archpsyc.1993.01820130033006. [DOI] [PubMed] [Google Scholar]
- 12.Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8(3):99–110. [PubMed] [Google Scholar]
- 13.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107–15. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 14.Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Jr, Janowsky DS, Kling MA. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 1999;142(2):193–9. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- 15.Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology (Berl) 2006;189(3):395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- 16.Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52(5):1256–62. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA, Picciotto MR. Varenicline has antidepressant-like activity in the forced swim test and augments sertraline’s effect. Eur J Pharmacol. 2009;605(1–3):114–6. doi: 10.1016/j.ejphar.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry. 2004;56(9):657–64. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Salin-Pascual RJ, de la Fuente JR, Galicia-Polo L, Drucker-Colin R. Effects of transderman nicotine on mood and sleep in nonsmoking major depressed patients. Psychopharmacology (Berl) 1995;121(4):476–9. doi: 10.1007/BF02246496. [DOI] [PubMed] [Google Scholar]
- 20.McClernon FJ, Hiott FB, Westman EC, Rose JE, Levin ED. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2006;189(1):125–33. doi: 10.1007/s00213-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 21.George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: a preliminary study. J Clin Psychopharmacol. 2008;28(3):340–4. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- 22.Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65(2):144–9. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musachio JL, Villemagne VL, Scheffel UA, Dannals RF, Dogan AS, Yokoi F, Wong DF. Synthesis of an I-123 analog of A-85380 and preliminary SPECT imaging of nicotinic receptors in baboon. Nucl Med Biol. 1999;26(2):201–7. doi: 10.1016/s0969-8051(98)00101-2. [DOI] [PubMed] [Google Scholar]
- 24.Fujita M, Tamagnan G, Zoghbi SS, Al-Tikriti MS, Baldwin RM, Seibyl JP, Innis RB. Measurement of alpha4beta2 nicotinic acetylcholine receptors with [123I]5-I-A-85380 SPECT. J Nucl Med. 2000;41(9):1552–60. [PubMed] [Google Scholar]
- 25.Staley JK, van Dyck CH, Weinzimmer D, Brenner E, Baldwin RM, Tamagnan GD, Riccardi P, Mitsis E, Seibyl JP. 123I-5-IA-85380 SPECT measurement of nicotinic acetylcholine receptors in human brain by the constant infusion paradigm: feasibility and reproducibility. J Nucl Med. 2005;46(9):1466–72. [PubMed] [Google Scholar]
- 26.Bhagwagar Z, Cowen PJ. ‘It’s not over when it’s over’: persistent neurobiological abnormalities in recovered depressed patients. Psychol Med. 2008;38(3):307–13. doi: 10.1017/s0033291707001250. [DOI] [PubMed] [Google Scholar]
- 27.Fujita M, Al-Tikriti MS, Tamagnan G, Zoghbi SS, Bozkurt A, Baldwin RM, Innis RB. Influence of acetylcholine levels on the binding of a SPECT nicotinic acetylcholine receptor ligand [123I]5-I-A-85380. Synapse. 2003;48(3):116–22. doi: 10.1002/syn.10194. [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders - Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 29.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased Brain GABA Concentrations Following Acute Administration of a Selective Serotonin Reuptake Inhibitor. Am J Psychiatry. 2004;161(2):368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- 31.Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9(4):386–92. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- 32.Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P. Increased 5-HT2A Receptor Binding in Euthymic, Medication-Free Patients Recovered From Depression: A Positron Emission Study With [11C]MDL 100,907. American Journal of Psychiatry. 2006;163(9):1580–1587. doi: 10.1176/ajp.2006.163.9.1580. [DOI] [PubMed] [Google Scholar]
- 33.Esterlis I, Cosgrove KP, Batis JC, Bois F, Stiklus SM, Perkins E, Seibyl JP, Carson RE, Staley JK. Quantification of smoking-induced occupancy of beta2-nicotinic acetylcholine receptors: estimation of nondisplaceable binding. J Nucl Med. 2010;51(8):1226–33. doi: 10.2967/jnumed.109.072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O’Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26(34):8707–14. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zoghbi SS, Tamagnan G, Baldwin MF, Al-Tikriti MS, Amici L, Seibyl JP, Innis RB. Measurement of plasma metabolites of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-IA-85380), a nicotinic acetylcholine receptor imaging agent, in nonhuman primates. Nucl Med Biol. 2001;28(1):91–6. doi: 10.1016/S0969-8051(00)00188-8. [DOI] [PubMed] [Google Scholar]
- 36.Cosgrove KP, Mitsis EM, Bois F, Frohlich E, Tamagnan GD, Krantzler E, Perry E, Maciejewski PK, Epperson CN, Allen S, O’Malley S, Mazure CM, Seibyl JP, van Dyck CH, Staley JK. 123I-5-IA-85380 SPECT Imaging of Nicotinic Acetylcholine Receptor Availability in Nonsmokers: Effects of Sex and Menstrual Phase. J Nucl Med. 2007;48(10):1633–40. doi: 10.2967/jnumed.107.042317. [DOI] [PubMed] [Google Scholar]
- 37.Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: A meta-analysis of voxel based morphometry studies. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 38.van Tol MJ, van der Wee NJ, van den Heuvel OA, Nielen MM, Demenescu LR, Aleman A, Renken R, van Buchem MA, Zitman FG, Veltman DJ. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry. 2010;67(10):1002–11. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- 39.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123(1):1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhagwagar Z, Murthy N, Selvaraj S, Hinz R, Taylor M, Fancy S, Grasby P, Cowen P. 5-HTT Binding in Recovered Depressed Patients and Healthy Volunteers: A Positron Emission Tomography Study With [11C]DASB. Am J Psychiatry. 2007;164(12):1858–1865. doi: 10.1176/appi.ajp.2007.06111933. [DOI] [PubMed] [Google Scholar]
- 42.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, PMM, PJC Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2008;11(2):255–60. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 43.Gotti C, Moretti M, Bohr I, Ziabreva I, Vailati S, Longhi R, Riganti L, Gaimarri A, McKeith IG, Perry RH, Aarsland D, Larsen JP, Sher E, Beattie R, Clementi F, Court JA. Selective nicotinic acetylcholine receptor subunit deficits identified in Alzheimer’s disease, Parkinson’s disease and dementia with Lewy bodies by immunoprecipitation. Neurobiol Dis. 2006;23(2):481–9. doi: 10.1016/j.nbd.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, Stiklus S, Krishnan-Sarin S, O’Malley S, Perry E, Tamagnan G, Seibyl JP, Staley JK. beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry. 2009;66(6):666–76. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, Vaupel DB, Pavlova O, Stein EA. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2–18F-FA-85380. J Nucl Med. 2008;49(10):1628–35. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shytle R, Sheehan D, Sanberg P, Arias H. Neuronal nicotinic receptors as therapeutic targets for mood disorders. In: Arias H, editor. Pharmacology of Nicotinic Acetylcholine Receptors from the Basic and Therapeutic Perspectives. India: Research Signpost; 2011. pp. 187–198. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.