Abstract
Background
An increasing number of women are utilizing fertility treatments, but little is known about their relation to autism spectrum disorders (ASD).
Methods
To determine the association between maternal fertility therapy use and risk of having a child with ASD, we conducted a nested case-control study within the Nurses’ Health Study II (n = 116,430). Maternally reported diagnoses of ASD were confirmed through a supplementary questionnaire and, in a subgroup, the Autism Diagnostic Interview-Revised. Controls were randomly selected by frequency matching to case children’s year of birth. Associations were examined by self-reported infertility and type of therapy using conditional logistic regression.
Results
In all, 9% of the 507 cases and 7% of 2,529 controls indicated fertility therapy use for the index pregnancy. No significant associations with self-reported fertility therapies or history of infertility were seen in primary analyses. In subgroup analyses of women with maternal age ≥35 years (n = 1,020), artificial insemination was significantly associated with ASD; ovulation inducing drug (OID) use was significantly associated in crude but not adjusted analyses (odds ratio 1.81, 95% CI 0.96–3.42). Results were similar by diagnostic subgroup, though within the advanced maternal age group, OID and artificial insemination were significantly associated with Asperger syndrome and pervasive developmental disorder not-otherwise specified, but not autistic disorder.
Conculsion
Assisted reproductive therapy and history of infertility did not increase risk of having a child with ASD in this study. However, the associations observed with OID and artificial insemination among older mothers, for whom these exposures are more common, warrant further investigation.
Keywords: autism, infertility, fertility treatments, assisted reproductive technology (ART), ovulation-inducing drugs
Autism, a serious neurodevelopmental disorder affecting social behaviours and language, is known to be strongly influenced by genetics. However, evidence from studies of pre- and perinatal factors suggests the fetal environment may also have an important role.1–3 Recent work has suggested that fertility therapies might be associated with autism and related conditions, but specific associations are understudied and results are conflicting. It is known that fertility therapies influence risk of multiple births, preterm delivery, and birthweight,4,5 all of which have also been associated with autism.6,7 Investigations report either no risk or a moderately increased risk following the use of assisted reproductive therapies (ART) for imprinting disorders, cerebral palsy, developmental disabilities and neural tube defects.4,5,8–11 For autism specifically, the majority of available studies have assessed ART or in vitro fertilisation (IVF), have not provided autism spectrum disorder (ASD)-specific adjusted results due to small case numbers, and reports of associations across these studies have been conflicting.12–17 In the few studies with adequate data, one study reported a lower risk of autism among women who used ART,18 while one reported no association with assisted conception overall.19 Few studies have assessed underlying infertility itself in association with autism. Two small studies, each with limitations, reported an increased risk of having a child with ASD among parents with a history of infertility.20,21 A large cohort study did not see associations with different types of infertility, although information on infertility diagnosis was not available for all participants. With the exception of one recent study conducted in Denmark,19 prior work has been limited by small sample sizes, lack of appropriate adjustment for confounding factors, and inability to investigate a range of therapies in association with ASD specifically. The most recent and well-conducted investigation of fertility therapies and ASD found potential associations in a subgroup analysis for specific hormones used in fertility medications,19 but there are no large studies on the topic in the United States (US) at this time.
As the prior work shows, questions remain regarding developmental effects of specific types of fertility therapies, including not just ART subtypes but also use of ovulation-inducing drugs (OID). As OID are often the first line of treatment in infertility,22 it is important to investigate the potential effects of these drugs on the offspring. Only one other study has specifically examined the relationship between OID use and ASD, and did not find a significant association in primary analyses after adjustment for potentially confounding factors, but did see significant relationships in certain subgroups.19 In addition, a recent report found that children born after OID use had poorer perinatal health and more episodes of hospitalisation than control children.23 The lack of research on these topics, particularly in the US, as well as the inconsistency of research findings, highlights the need for further investigation of infertility, fertility therapies and their potential effects on risk for ASD.
We therefore examined the relationship between history of infertility, use of fertility therapies and risk of having a child with an ASD by conducting a nested case–control study that consisted of participants from a large, well-established cohort, the Nurses’ Health Study II (NHS II).
Methods
Participants
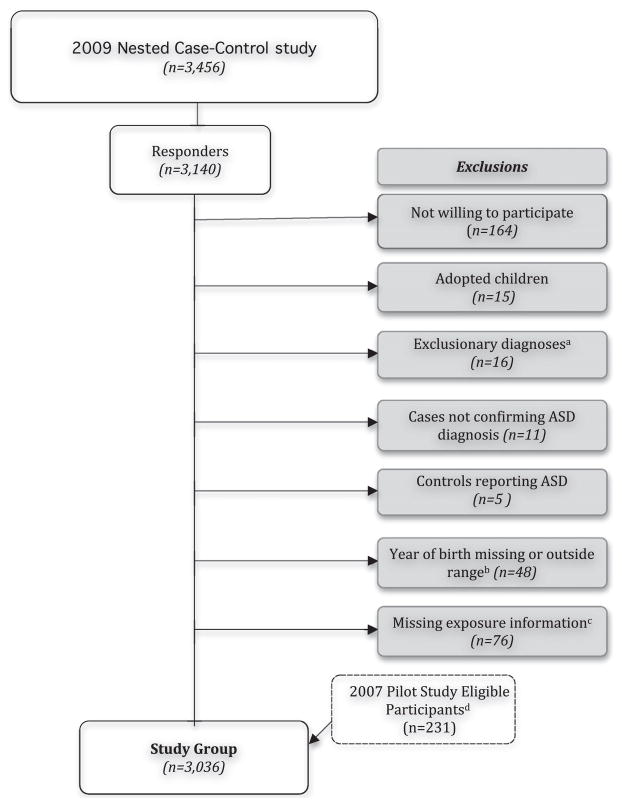
The study population is drawn from participants in the NHS II, a prospective cohort of 116 430 female nurses aged 25–43 when recruited in 1989 and followed by biennial mailed questionnaires to assess the incidence of cancer and other chronic diseases. The Partners Health Care Institutional Review Board has reviewed and approved the methods of this study, and completion and return of questionnaires sent by US mail constitutes implied consent. In 2005, participants in the NHS II were asked to report about disorders in their children, including autism, Asperger syndrome and ‘other autism spectrum.’ In 2009 we initiated a nested case–control follow-up study in order to learn more about these women. Controls were randomly selected and oversampled at a ratio of 4:1 using frequency matching for years in which case mothers reported births (as at the time no information on case year of birth was available). Figure 1 shows the details of this follow-up study mailing of 656 cases and 2800 controls and outlines the study exclusions. Following exclusions, a total of 3036 individuals were included in this analysis.
Figure 1.
Nurses’ Health Study II autism nested case–control study. Case numbers as follows: 656 cases in nested case–control mailing; 582 cases responded; 51 cases not willing to participate; 9 cases adopted; 12 cases with exclusionary diagnoses; 1 case missing year of birth; 41 cases missing exposure information; 51 cases from pilot study; 507 cases in the study group. aExclusionary diagnoses were the following genetic disorders associated with autism spectrum disorders (ASD): fragile X syndrome, Down’s syndrome, tuberous sclerosis, trisomy 18, XXY, Jacobsen syndrome (11q deletion), Rett’s disorder. bControls with year of birth outside range of case births were not included in these analyses. cIndividuals missing/skipping the fertility therapy question specific to index birth on our follow-up study questionnaire were excluded. dA small pilot study conducted shortly before the full nested case–control follow-up study collected identical exposure information. Only individuals from the pilot study meeting all inclusion criteria were included.
Case definition
Cases were defined as individuals with both maternal report of ASD on the 2005 questionnaire and maternal report of an autistic disorder, Asperger syndrome, pervasive developmental disorder (PDD), or pervasive developmental disorder not otherwise specified (PDD-NOS) diagnosis by a medical professional on our follow-up questionnaire.
The ASD diagnosis was validated by a trained professional who administered the Autism Diagnostic Interview-Revised (ADI-R)24 over the phone to 50 randomly selected case mothers who indicated willingness to complete the interview. Of these, 43 (86%) met ADI-R cut-offs for a diagnosis of autism; the remaining individuals either missed the diagnostic cut-off for full autism by one point on one domain (n = 6) or met the cut-off for one domain (n = 1) and came close to cut-offs for the remaining domains. Reliability of telephone administration of the ADI-R has been previously demonstrated.25
Exposure information
All exposures in this study were self-reported by nurse participants. Information on history of infertility (defined as trying to get pregnant for 12 months or more without success) and OID use was previously collected among all cohort participants on NHS II questionnaires (all NHS II questionnaires are available online at http://www.channing.harvard.edu/nhs/questionnaires/index.shtml). Types of ovulation drugs and infertility diagnoses were also assessed on these questionnaires at multiple time points. This information was augmented by the follow-up study questionnaire sent to the nested case–control study participants. The follow-up questionnaire asked participants about diagnostic and pregnancy information for the index child. Specifically, participants were asked whether they had used any of the following fertility therapies for the index birth: IVF, intracytoplasmic sperm injection (ICSI), gamete intrafallopian transfer (GIFT), zygote intrafallopian transfer (ZIFT), OID (as pills or injections), use of sperm donor, egg donor, or frozen embryos, or ‘other’, with the request to specify the type of other fertility therapy.
Statistical analyses
Univariate relationships and basic characteristics of infertility and fertility therapies were assessed by comparing frequencies and descriptive statistics. Consistent with the Center for Disease Control definition, fertility therapies involving manipulations of both the sperm and the egg (including IVF, ICSI, GIFT, ZIFT, and use of donor or frozen eggs, sperm or embryos) were grouped under the category of ART. The relationship between ASD and infertility and fertility therapy use, as a group and by types with sufficient numbers (those with at least five exposed cases – this included in primary analysis OID, OID subtypes, ART and artificial insemination), was assessed by conditional logistic regression, stratified by child year of birth, with and without adjustment for potential confounders. To assess the effect of different types of infertility, self-report of tubal, spousal, ovulatory and cervical infertility, as well as of endometriosis or polycystic ovarian syndrome, was examined when infertility was reported prospectively from NHS II questionnaires prior to the index birth. Potential confounders in multivariate analyses included maternal and paternal age (as continuous variables in years; assessed on follow-up questionnaire), race (as binary white/other – as 93% of the NHS II are Caucasian), income (in five levels of household income in US dollars), spouse education (in four categories: high school or less, 2 year college, 4 year college, and graduate), birth order (as a continuous variable; assessed on the follow-up questionnaire), pre-pregnancy smoking status (as a binary variable), pre-pregnancy body mass index (BMI) (in four categories: <18.5, 18.5–<25, 25–<30, 30+), prior miscarriages (binary) and prior induced abortions (binary). These covariates were assessed on the NHS II questionnaires unless noted. Each potential confounder was assessed in association with both the exposure and outcome and compared in models with and without the variable. As prior miscarriages, prior abortions, pre-pregnancy BMI, pre-pregnancy smoking status and spouse education were not associated with both the exposure and outcome, these variables were not included in fully adjusted models (addition of these factors did not alter results). Race and income were also not significantly associated with both exposure and outcome but were included in adjusted models for face validity (removal of these factors from models did not alter results).
In order to assess whether individual therapies may have different associations, we utilised separate models for each type of therapy, in addition to creating indicator variables for certain combinations of therapies. Specifically, for OID, separate groups were created for individuals reporting OID use only, OID and another fertility therapy, or only another fertility therapy (but no OID), and each of these groups was compared with the referent group of no fertility therapy use. Likewise, to assess the effect of ART in comparison with other therapies, individuals with ART and individuals with other (non-ART) fertility therapies were compared with those with none (no category for ART and other fertility therapy was created, as it is common procedure for individuals undergoing ART procedures such as IVF and ICSI to receive OID injections and/or medications).
For all analyses, crude (adjusted for child year of birth only), maternal-age adjusted, parental-age adjusted, and multivariate models were compared. In adjusting for maternal and paternal age, we also examined the possibly non-linear relationship between these factors and ASD non-parametrically with restricted cubic splines, a method that provides greater flexibility in modelling the relationships between outcome and predictor variables.26,27 Tests for non-linearity of these factors used the likelihood ratio test, comparing the model with the linear version of the term to the model with the linear and cubic spline terms. As there was no evidence for non-linearity in the effect of parental age, results were adjusted for these two factors as continuous variables in years.
The missing indicator method28 was used to handle the missing covariate data (we also examined effects of using the complete case and imputed median value methods, but no material differences according to these methods were noted). Missingness was <5% for race, <10% for paternal education and paternal age and approximately 20% for income; missingness was similar by case status.
Subgroup and sensitivity analyses
We assessed relationships in the subgroup of women aged 35 or older at time of index birth (the ‘advanced maternal age subgroup’); in this group exposures are more common. We also examined associations according to diagnostic subgroup: autistic disorder, Asperger syndrome, or PDD/PDD-NOS. For analyses with limited power, we collapsed the Asperger and PDD groups to create a ‘mild ASD’ category. Associations by diagnostic subgroup were also assessed among mothers with advanced maternal age.
A number of sensitivity analyses were also utilised to test the robustness of results. We conducted the analyses in later birth cohorts in order to assess the extent to which increased prevalence of fertility therapy use over time may have affected ability to observe associations. We also conducted an analysis of the association of OID (the only fertility therapy with previously collected information from previous NHS II questionnaires) with ASD using only previously collected data from the biennial questionnaires, in order to compare prospectively collected data to the retrospective reporting from the follow-up questionnaires and examine the potential for recall bias. This analysis of OID, as well as the analysis of infertility (which was also previously collected on biennial questionnaire) was conducted not only within our study group with follow-up information but also within the full eligible NHS II population.
Results
A total of 507 cases and 2529 controls were included in the primary analyses. Individuals participating in our follow-up study did not differ from those not willing to participate on demographic factors. Among our study group, fertility therapy use was slightly more common among cases (9%) than controls (7%) in crude comparisons, although the chi-squared test was not significant. Table 1 displays basic characteristics of the study group; case mothers were slightly older at baseline than control mothers and were more likely to have had pregnancy complications. Further, there was a higher proportion of male, first-born and multiple births among case children. Distributions of other factors, including maternal and paternal age, were similar between the groups. Comparing fertility therapy use, numbers were small when broken out by type of therapy, but overall frequencies were similar for different therapies in cases and controls in the full study group (Table 2). Only ‘other’ fertility therapy was noted more frequently among cases (P = 0.01), and this was primarily driven by reports of artificial insemination (including intrauterine and intracervical insemination). While fertility therapy use was much higher among the multiple births group, the proportion using therapies did not differ between cases and controls. Examining previously collected NHS II information on OID and infertility, no crude differences in frequency of reporting were seen. Further, cases and controls had nearly identical frequencies of self-reported types of infertility prior to index birth.
Table 1.
Basic characteristics of the study group (n = 3036)
| Variable | ASD mothers (n = 507) n (%) or mean (std) |
Control mothers (n = 2529) n (%) or mean (std) |
P-valuea |
|---|---|---|---|
| Demographic characteristics | |||
| Nurse’s age at baseline (years) | |||
| 24–29 | 154 (30%) | 941 (37%) | 0.004** |
| 30–35 | 233 (46%) | 1115 (44%) | |
| 36–42 | 120 (24%) | 473 (19%) | |
| Mean age at baseline | 32.4 (4.4) | 31.6 (4.3) | 0.0002*** |
| Raceb | |||
| Caucasian | 483 (95%) | 2369 (95%) | 0.93 |
| Other | 23 (5%) | 115 (5%) | |
| Income ($) | |||
| <40 000 | 21 (4%) | 114 (5%) | 0.32 |
| 40 000–74 000 | 142 (28%) | 644 (25%) | |
| 75 000–99 000 | 96 (19%) | 426 (17%) | |
| 100 000–149 000 | 88 (17%) | 441 (17%) | |
| ≥150 000 | 51 (10%) | 324 (13%) | |
| Spouse’s education | |||
| High school or less | 62 (12%) | 363 (14%) | 0.22 |
| 2 year college | 87 (17%) | 388 (15%) | |
| 4 year college | 152 (30%) | 854 (34%) | |
| Graduate school | 161 (32%) | 756 (30%) | |
| Reproductive and pregnancy characteristics | |||
| Average number of children | 2.6 (1.1) | 2.7 (1.1) | 0.03* |
| Prior miscarriage or stillbirth | 140 (28%) | 779 (31%) | 0.17 |
| Prior induced abortion | 103 (20%) | 409 (16%) | 0.02* |
| History of infertility | 111 (22%) | 571 (23%) | 0.72 |
| Pregnancy complicationsc | 87 (17%) | 244 (10%) | <0.0001**** |
| Maternal age (years) | |||
| <20–24 | 28 (6%) | 120 (5%) | 0.24 |
| 25–29 | 122 (24%) | 519 (21%) | |
| 30–34 | 192 (38%) | 1034 (41%) | |
| ≥35 | 165 (33%) | 856 (34%) | |
| Paternal age (years) | |||
| <20–24 | 18 (4%) | 60 (2%) | 0.08 |
| 25–29 | 93 (18%) | 385 (15%) | |
| 30–34 | 162 (32%) | 880 (35%) | |
| ≥35 | 219 (44%) | 1167 (46%) | |
| Index child characteristics | |||
| Mean year of birth | 1989 (6.1) | 1990 (6.1) | 0.0003*** |
| First-born | 222 (44%) | 865 (34%) | <0.0001**** |
| Multiple birth | 22 (4%) | 44 (2%) | 00.0002*** |
| Male child | 423 (83%) | 1285 (51%) | <0.0001**** |
Significance values for all tables:
P < 0.05,
P < 0.01,
P < 0.001,
P < 0.0001.
P-values from chi-squared tests for categorical variables and t-test for continuous variables; calculated among those not missing on the variable of interest.
Numbers missing on variables above as follows: race 46 (1 case); income 689 (109 cases); paternal education 213 (45 cases); prior miscarriage 15 (4 cases); pregnancy complications 7 (3 cases); paternal age 52 (15 cases); multiple births 2 (1 case).
Includes gestational diabetes, preeclampsia, or pregnancy induced hypertension. When also including pregnancy complications listed under ‘other’ on our questionnaire (such as bleeding, placenta previa and preterm labour), there were 180 cases and 484 controls.
ASD, autism spectrum disorders; std, standard deviation.
Table 2.
Self-reported fertility therapy use and infertility according to case status in the study population (n = 3036)
| Type of therapy | ASD mothers (n = 507) n (%) |
Control mothers (n = 2529) n (%) |
P-value |
|---|---|---|---|
| Any fertility therapy | 44 (9%) | 173 (7%) | 0.14 |
| Ovulation-inducing drugs | 32 (6%) | 117 (5%) | 0.11 |
| Pills | 20 (4%) | 74 (3%) | 0.23 |
| Injections | 11 (2%) | 42 (2%) | 0.43 |
| OID without ARTa | 26 (5%) | 100 (4%) | 0.23 |
| Any ART | 12 (2%) | 55 (2%) | 0.79 |
| IVF | 3 (0.6%) | 26 (1%) | 0.36 |
| ICSI | 1 (0.2%) | 12 (0.5%) | 0.38 |
| Egg donor | 2 (0.2%) | 0 (0%) | 0.002** |
| Sperm donor | 6 (1%) | 23 (1%) | 0.57 |
| Frozen embryo | 0 (0%) | 1 (0%) | 0.65 |
| GIFT or ZIFT | 3 (0.6%) | 5 (0.2%) | 0.11 |
| Other fertility therapy | 13 (3%) | 28 (1%) | 0.01* |
| Artificial Insemination | 8 (2%) | 16 (0.6%) | 0.03* |
| Father procedures | 2 (0.4%) | 2 (0.1%) | 0.07 |
| Surgical procedures | 2 (0.4%) | 4 (0.2%) | 0.28 |
| Other | 1 (0.2%) | 5 (0.2%) | 0.99 |
| NHS II questionnaire information (previously collected) | |||
| Prior infertilityb | 112 (22%) | 575 (23%) | 0.75 |
| Tubal infertility | 9 (2%) | 50 (2%) | 0.76 |
| Ovulatory infertility | 33 (7%) | 201 (8%) | 0.27 |
| Spousal infertility | 26 (5%) | 109 (4%) | 0.41 |
| Cervical infertility | 8 (2%) | 51 (2%) | 0.51 |
| Endometriosis | 16 (3%) | 75 (3%) | 0.82 |
| Other infertility | 19 (4%) | 99 (4%) | 0.86 |
| Ever reported infertilityc | 137 (27%) | 687 (27%) | 0.95 |
| Prior OID use | 62 (12%) | 305 (12%) | 0.92 |
| Recent OID used | 53 (10%) | 265 (10%) | 0.98 |
| Ever reported OID use | 82 (16%) | 374 (15%) | 0.43 |
Includes pills or injections, but excluding those used as part of an ART cycle. Significance information:
P < 0.05,
P < 0.01.
Infertility prior to index birth. Reported types of infertility listed are for those individuals with infertility prior to index birth; types may not sum to total with prior infertility due to lack of information on infertility type or reporting of multiple types (these was no difference in frequency of reporting of multiple types of infertility in cases and controls).
Through 2005; includes infertility after index birth.
Defined as use only on the most recent NHS II questionnaire prior to child’s birth, within 0–2 years. Prior OID use is use at any time prior to the child’s birth, and ever reported use includes reports after the child’s birth.
Self-reported information. Abbreviations as follows: ART, assisted reproductive technology; GIFT, gamete intrafallopian transfer; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilisation; OID, ovulation-inducing drugs; ZIFT, zygote intrafallopian transfer.
ASD, autism spectrum disorders; NHS II; Nurses’ Health Study II.
Crude and adjusted analyses for the primary study group are shown in Table 3. In adjusted analyses, no significant associations with ASD for infertility or fertility therapy use overall or by different types were seen (Table 3; infertility types not shown but all were non-significant). Combination indicator models accounting for use of other therapies for ART and OID also did not demonstrate significant associations with ASD in the primary analyses. Artificial insemination was significantly associated with ASD in crude analysis, as was the combination of OID and another fertility therapy in the combination therapy model, but after adjustment for parental age and birth order, these associations were not significant.
Table 3.
Risk of autism spectrum disorders according to self-reported fertility therapy use and infertility in the study population (n = 3036)
| Type of therapy | Case exposed n (%) |
Crude (YOB)a OR [95% CI] |
Parental age adjustedb OR [95% CI] |
Fully adjustedc OR [95% CI] |
|---|---|---|---|---|
| Infertility | 112 (22%) | 1.06 [0.84, 1.34] | 1.00 [0.79, 1.28] | 0.93 [0.73, 1.19] |
| Any fertility therapy | 44 (9%) | 1.41 [0.99, 2.00] | 1.27 [0.88, 1.83] | 1.11 [0.77, 1.62] |
| Any ART | 12 (2%) | 1.28 [0.67, 2.42] | 0.83 [0.39, 1.74] | 0.70 [0.33, 1.47] |
| Ovulation drugs (OID) | 32 (6%) | 1.49 [0.99, 2.25] | 1.46 [0.96, 2.20] | 1.32 [0.87, 2.01] |
| OID pills | 20 (4%) | 1.42 [0.85, 2.37] | 1.43 [0.86, 2.38] | 1.32 [0.79, 2.21] |
| OID injections | 11 (2%) | 1.45 [0.74, 2.86] | 1.31 [0.66, 2.61] | 1.18 [0.59, 2.36] |
| Artificial insemination | 8 (2%) | 2.83 [1.19, 6.75] | 2.62 [1.09, 6.27] | 2.17 [0.90, 5.21] |
| Models assessing combinations of fertility therapies | ||||
| No fertility therapies | 463 (92%) | Reference | Reference | Reference |
| OID only | 22 (4%) | 1.30 [0.80, 2.11] | 1.32 [0.81, 2.13] | 1.20 [0.73, 1.95] |
| OID + other fertility therapy | 10 (2%) | 2.25 [1.06, 4.75] | 1.90 [0.88, 4.01] | 1.66 [0.76, 3.60] |
| Other (non-OID) fertility therapy | 12 (2%) | 1.20 [0.64, 2.28] | 0.91 [0.46, 1.81] | 0.76 [0.38, 1.51] |
| No fertility therapies | 463 (92%) | Reference | Reference | Reference |
| ART | 12 (2%) | 1.31 [0.69, 2.48] | 0.85 [0.40, 1.78] | 0.72 [0.34, 1.51] |
| Other (non-ART) fertility therapy | 32 (6%) | 1.45 [0.96, 2.18] | 1.45 [0.96, 2.18] | 1.29 [0.85, 1.96] |
Odds ratios and their 95% confidence intervals are shown.
All models were conditional logistic regression models stratified by child year of birth.
In models adjusting for maternal age only, results were very similar to crude (YOB adjusted) models.
Fully adjusted models included maternal and paternal age, race, income, and birth order. Results did not change when additionally adjusting for prior miscarriages or stillbirths, prior induced abortions, or paternal education, nor when additionally adjusting for other types of therapies in OID, ART, and AI models. Results in analyses restricted to singleton births only were materially unchanged.
ART, assisted reproductive technology; OID, ovulation-inducing drugs; YOB, year of birth.
Subgroup results
In diagnostic subgroup analyses, which examined risk of different reported ASD diagnoses, no significant associations were seen in the primary study group (data not shown). In the advanced maternal age subgroup, which included only mothers aged 35 or older at time of the child’s birth, fertility therapies were more common among case mothers than control mothers; overall fertility therapy use was 14.6% in cases compared with 8.6% in controls (P = 0.008). Examining types of therapies, OID and artificial insemination were more common among case mothers, but there was no difference in prevalence of IVF or ART overall between advanced maternal age cases and controls. Reported history of infertility was high in this group (35%), but did not differ between cases and controls. After adjustment for potential confounders, only the association with artificial insemination remained significant [Table 4; odds ratio (OR) = 3.73 [95% confidence interval (CI) 1.38, 10.1], P = 0.009] when assessing therapies in individual models. However, estimates for any fertility therapy, OID and OID pills approached significance. In models examining combinations of therapies, individuals using non-ART fertility therapies had nearly a doubling in risk of ASD compared with those using no fertility therapies (OR = 1.92 [95% CI 1.03, 3.60], P = 0.04). The majority of individuals in this category used either OID alone or OID and artificial insemination.
Table 4.
Risk of autism spectrum disorders according to self-reported fertility therapy use and infertility in the advanced maternal age subgroup (n = 1021)a
| Type of therapy | Case exposed n (%) |
Crude (YOB) OR [95% CI] |
Fully adjustedb OR [95% CI] |
|---|---|---|---|
| Infertility | 61 (37%) | 1.12 [0.79, 1.59] | 0.97 [0.67, 1.40] |
| Any fertility therapy | 25 (15%) | 2.00 [1.22, 3.30] | 1.58 [0.91, 2.75] |
| Any ART | 8 (5%) | 1.49 [0.67, 3.34] | 0.85 [0.32, 2.23] |
| Ovulation drugs (OID) | 16 (10%) | 2.08 [1.13, 3.82] | 1.81 [0.96, 3.42] |
| OID-pills | 9 (6%) | 2.41 [1.07, 5.43] | 2.18 [0.95, 5.00] |
| OID-injections | 8 (5%) | 2.32 [0.99, 5.45] | 1.79 [0.73, 4.36] |
| Artificial Insemination | 8 (5%) | 4.38 [1.67, 11.47] | 3.73 [1.38, 10.1]** |
| Models assessing combinations of fertility therapies | |||
| No fertility therapy | 140 (85%) | Reference | Reference |
| OID only | 9 (6%) | 1.78 [0.81, 3.88] | 1.63 [0.73, 3.61] |
| OID + other fertility therapy | 7 (4%) | 2.93 [1.14, 7.53] | 2.30 [0.85, 6.20] |
| Other (non-OID) fertility therapy | 9 (5%) | 1.79 [0.82, 3.90] | 1.20 [0.50, 2.87] |
| No fertility therapy | 140 (85%) | Reference | Reference |
| ART | 8 (5%) | 1.38 [0.59, 3.23] | 0.94 [0.36, 2.48] |
| Other (non-ART) fertility therapy | 17 (10%) | 2.28 [1.25, 4.18] | 1.98 [1.06, 3.70]* |
| By diagnostic subgroup | |||
| Autistic disorder cases (45 cases, 856 controls) | |||
| Infertility | 19 (42%) | 1.35 [0.73, 2.50] | 1.18 [0.62, 2.25] |
| Any fertility therapy | 6 (13%) | 1.64 [0.66, 4.04] | 0.88 [0.28, 2.78] |
| Asperger syndrome and PDD-NOS cases (120 cases, 856 controls) | |||
| Infertility | 42 (35%) | 1.03 [0.69, 1.56] | 0.89 [0.58, 1.37] |
| Any fertility therapy | 19 (16%) | 2.18 [1.25, 3.81] | 1.82 [0.99, 3.34] |
| OID | 13 (11%) | 2.38 [1.23, 4.63] | 2.12 [1.06, 4.23]* |
| Artificial insemination | 6 (5%) | 5.64 [1.91, 16.6] | 4.60 [1.51, 14.0]** |
| Models assessing combinations of fertility therapies | |||
| No fertility therapies | 101 (85%) | Reference | Reference |
| OID only | 9 (8%) | 2.41 [1.10, 5.30] | 2.20 [0.98, 4.94] |
| OID + other fertility therapy | 4 (3%) | 2.56 [0.80, 8.18] | 2.08 [0.62, 6.95] |
| Other (non-OID) fertility therapy | 6 (5%) | 1.76 [0.71, 4.40] | 1.27 [0.46, 3.49] |
| No fertility therapies | 101 (85%) | Reference | Reference |
| ART | 5 (4%) | 1.47 [0.55, 3.92] | 1.01 [0.34, 3.09] |
| Other (non-ART) fertility therapy | 14 (12%) | 2.65 [1.38, 5.10] | 2.29 [1.16, 4.49]* |
Advanced maternal age (≥35) sub-group included 164 autism spectrum disorders cases. Average year of birth in this subgroup was 1994. Fertility therapies with <5 exposed cases are not shown in the table; no significant associations for such therapies were seen.
Adjusted as in Table 3.
ART, assisted reproductive technology; FT, fertility therapy; OID, ovulation-inducing drugs; PDD-NOS, pervasive developmental disorder not otherwise specified; YOB, year of birth.
When examining diagnostic subgroups within the advanced maternal age group, no significant associations were seen for autism cases only. Because of limited power to assess individual diagnoses, we combined Asperger syndrome and PDD/PDD-NOS cases to examine associations with ‘mild ASD’. Both OID use and artificial insemination were significantly associated with mild ASD in fully adjusted models, although confidence intervals were wide (OR for OID: 2.12 [95% CI 1.06, 4.23], P = 0.03; and for artificial insemination: 4.52 [95% CI 1.48, 13.9], P = 0.008). Consistent with these results, non-ART therapy use was also significantly associated with mild ASD in the advanced maternal age combination of therapy use model (OR = 2.23 [95% CI 1.13, 4.40]). Point estimates were similar to those of the combined mild ASD group when PDD-NOS and Asperger syndrome were examined separately, although confidence intervals were wide due to small numbers of individuals.
Sensitivity analysis results
Sensitivity analyses assessing only those born later during follow-up to account for time trends in therapy use did not alter results. In separate sensitivity analyses comparing OID reporting from the NHS II questionnaire with our follow-up study questionnaire, agreement was very high for those with both sources of information. Of those reporting OID use on the follow-up questionnaire, 93% reported OID use on an NHS II questionnaire, and 84% had use reported prior to the index birth; the majority of the remainder (n = 14) gave birth to their child prior to collection of OID information on NHS II questionnaires. Results using the previous reports of OID were similar to results from the follow-up report, and no significant associations were found. Further, when examining whether the reasons for infertility and type of OID were different between women reporting a child with ASD and those without (information which was provided on the prospectively collected biennial questionnaires), no significant differences were found. When examining these factors in the full eligible NHS II cohort, OID was significantly more common among case mothers in crude comparisons, but after adjusting for maternal age, birth order and year of birth, this association did not persist.
Comments
Findings from this cohort of nurses with nested case–control follow-up study information did not show any strong associations with self-reported fertility therapies or infertility overall. However, among mothers aged 35 or older, we saw significant associations with ovulation drugs and artificial insemination, particularly when assessing risk of milder ASD (reported diagnoses of Asperger syndrome or PDD/PDD-NOS).
This study has a number of strengths, including a large national sample, the ability to adjust for confounding by many maternal characteristics that may influence risk of ASD, and information on a range of fertility therapies not previously assessed in association with ASD. However, a number of limitations should be noted when considering our results. Our results may not be generalisable to other racial or socio-economic groups, which were underrepresented in our study. We relied primarily on maternal report for both exposure and outcome definition. Results in our diagnostic validation subgroup, however, suggest a high degree of accuracy of maternal reporting of diagnosis, consistent with other studies that have demonstrated the reliability of maternal report.29,30 We were not able to see children in person and conduct measures such as the Autism Diagnostic Observation Schedule or confirm diagnostic subgroups through in-person measures; however, the ADI-R has high validity and is considered the ‘gold standard’ for autism diagnosis.24,31 For exposures, we did not have records from fertility clinics to validate the therapies used, and misclassification of therapies and infertility is possible. Further, there is potential for false-positive reports in fertility therapies utilised. Specifically, although our follow-up questionnaire asked about therapies used for the index pregnancy, we cannot rule out the potential for reporting of treatments outside the time of conception. Such reporting could bias results towards the null. However, the extent of such biases may be minimised in this study population of nurses. It has been demonstrated that the reliability of reporting of medical conditions and general information is very high in these women.32,33 A separate validation study conducted within this cohort of reported ovulatory infertility demonstrated high accuracy (95% concordance with medical records) and suggests a low degree of such misclassification within this study group.34
We did not have sufficient numbers to examine all types of therapies, such as ICSI, GIFT, ZIFT or IVF specifically, in adjusted or subgroup analyses. Our study was limited by fairly wide confidence intervals and uncertain estimates for a number of analyses. An extremely large study will be required to examine rare therapies in association with ASD. However, a large cohort study from Denmark did not see an association between IVF and ASD,19 nor have three other studies,14–16 although two had a very small number of ASD cases. Two additional studies also did not find associations with IVF and developmental delay more broadly.12,17
Our results from the advanced maternal age analyses are suggestive of an association between OID and ASD. Associations with any fertility therapy, OID, and OID pills approached statistical significance and may have been hampered by limited power. However, as significant associations were only seen in subgroups, results should be interpreted with caution. In secondary analyses utilising only previously collected OID information from NHS II questionnaires, we did not see significant associations after adjusting for potential confounders in either the primary study or the advanced maternal age subgroup. However, that previously collected information is limited by the lack of specificity to the index birth, in that we cannot be sure the OID reported on the biennial questionnaire was used in the appropriate cycle actually resulting in the index birth. Our analysis of previously collected OID information assessed ever report of OID, OID reports at any time prior to the index pregnancy, as well as OID reported on only the questionnaire prior to the index birth. As questionnaires are answered every 2 years, even OID reported on the prior questionnaire may not be in the relevant conception window, and thus there may actually be a greater degree of misclassification in the previously collected cohort information. Thus, the results from our follow-up study assessing fertility therapy use specific to the index pregnancy are likely more accurate than those using the biennial questionnaire information.
The largest magnitude of association seen in this study was with artificial insemination in the subgroup of women with advanced maternal age, although confidence intervals were wide due to small numbers of women in this subgroup. Consistent with associations seen for individual therapies in this subgroup, we also saw a significant result when comparing individuals using non-ART fertility therapies (which, in our study group, was primarily accounted for by OID and artificial insemination combined) when compared with individuals not using any fertility therapies. However, the finding with artificial insemination should be considered preliminary not only due to the small numbers and imprecise point estimates, but also due to the fact that our questionnaire did not have a specific check box for artificial insemination. Rather, the questionnaire had an ‘other’ category, and participants wrote in artificial insemination, intrauterine insemination or intracervical insemination to be included in this exposure category. We cannot rule out the potential for biased recall particularly for this open-ended question; however, among those checking off the ‘other’ box, cases and controls recorded the type of therapy used in equal proportions.
Our findings of associations with artificial insemination and OID are consistent with the largest study to date of assisted conception and ASD.19 Specifically, in Hvidtjørn and colleagues’ cohort study utilising data from national registries in Denmark, OID was defined as use with or without artificial insemination. That study found a significant association with use of follicle stimulating hormone, a specific hormone included in some preparations of OID and targeted in others, as well as a significant crude association with OID. The overall adjusted OR for OID in their work was 1.20 [95% CI 0.99, 1.44]; however, because the point estimate was adjusted for downstream consequences of therapy use it may be falsely attenuated. A subgroup analysis of OID use in mothers who gave birth to female offspring was significantly associated with ASD in adjusted analyses. In our study, we did not adjust for factors downstream of exposure in final models, as doing so may introduce bias.36 However, in secondary analyses we did explore the effects of excluding those with multiple births, gestational diabetes or low birthweight; results were materially unchanged. Stratification by sex also produced similar results. More sophisticated methods, such as Marginal Structural Models, are required for analyses of effects of potential modifiers,37,38 and such models should be considered in future analyses with adequate sample sizes.
Prior to this study and Hvidtjørn et al.’s work, no study has investigated OID independently, and only one study reported adjusted results of the specific association between ASD and ART.18 In Maimburg and Vaeth’s study of the ASD–ART association, conducted in Denmark and including 461 cases, risk of autism was lower among women who used IVF and ICSI compared with non-users.18 Their result may have been due to chance or low statistical power as only 10 cases were identified as exposed; or, again, adjustment for factors that are a result of fertility therapies may have falsely attenuated the association. Two other studies did find increased risk of more broadly defined developmental disturbances following ART, which may be consistent with our subgroup results.13,17
The significant associations we saw with OID and artificial insemination in the advanced maternal age subgroup only could be due to chance. It is also possible that recall was improved in this subgroup, because of the fact that the lag time between index birth and follow-up questionnaire was shorter within this group (by about 4–5 years). However, when comparing agreement between previous biennial OID reports and follow-up OID reports, agreement was similar and high for all age groups, suggesting difference in recall does not fully account for differences seen by maternal age. An alternate explanation for associations seen with OID in the advanced maternal age group is that utilisation of ovulation drugs could stimulate the release of suboptimal oocytes that would not otherwise have gone on to implantation, and this effect could be stronger among older women for whom oocyte quality may already be reduced. Or, it may be that factors causing infertility or the biology of infertility itself may underlie the associations seen, such as hormonal imbalances leading to the indication for ovulation drugs, or structural, male factor, or other problems such as issues with implantation leading to use of artificial insemination. We do not have the ability in these data to assess which of these potential pathways is most likely, although we did not see an association with infertility itself, or with different types of infertility prior to index birth. These findings are consistent with Hvidtjørn and colleagues’ results. In other work, an early pilot study found infertility was associated with a significant increase in risk of schizophrenia and autism,20 while a case–control study found that autism case parents reported infertility requiring medical intervention about twice as often as healthy control parents,21 although numbers were small and the difference was not statistically significant. Doornbos and colleagues9 found that infertility was three times higher in families with children affected by imprinting disorders, which share some of the behavioural traits of ASD. Further work investigating potential underlying pathways, and using large samples, would be useful in attempting to explain associations seen with different fertility therapies.
In summary, this study did not find a significant increase in risk of ASD following maternal self-reported infertility or overall fertility therapy use. The results from this and other currently available studies suggest there is no strong risk of autism associated with ART specifically, but continued research is needed to examine different types of treatments. In particular, our results suggest women with advanced maternal age, independent of age itself, may be at moderately increased risk of ASD with certain fertility therapies such as ovulation drugs and artificial insemination. Considering the frequency of use of OID and the impact of ASD on the affected individual and the family, more detailed investigations to clarify these associations are warranted.
Acknowledgments
The work reported in this manuscript was funded in part by CA50385, the main NHS II grant, #1788 from the Autism Speaks Foundation, and A-14917 from the US Army Medical Research and Material Command (USAMRMC). The first author, Dr Lyall, is currently affiliated with the M.I.N.D. Institute, University of California, Davis.
References
- 1.Gardener H, Spiegelman D, Buka S. Prenatal risk factors for autism: comprehensive meta-analysis. The British Journal of Psychiatry. 2009;195:7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology. 2005;161:916–925. doi: 10.1093/aje/kwi123. Discussion 926–918. [DOI] [PubMed] [Google Scholar]
- 3.Newschaffer CJ, Fallin D, Lee NL. Heritable and nonheritable risk factors for autism spectrum disorders. Epidemiologic Reviews. 2002;24:137–153. doi: 10.1093/epirev/mxf010. [DOI] [PubMed] [Google Scholar]
- 4.Reddy UM, Wapner RJ, Rebar RW, Tasca RJ. Infertility, assisted reproductive technology, and adverse pregnancy outcomes: executive summary of a National Institute of Child Health and Human Development workshop. Obstetrics and Gynecology. 2007;109:967–977. doi: 10.1097/01.AOG.0000259316.04136.30. [DOI] [PubMed] [Google Scholar]
- 5.Schieve L, Rasmussen SA, Buck GM, Schendel DE, Reynolds MA, Wright VC. Are children born after assisted reproductive therapy at increased risk for adverse health outcomes? Obstetrics and Gynecology. 2004;103:1154–1163. doi: 10.1097/01.AOG.0000124571.04890.67. [DOI] [PubMed] [Google Scholar]
- 6.Croen L, Grether JK, Selvin S. Descriptive epidemiology of autism in a California population: who is at risk? Journal of Autism and Developmental Disorders. 2002;32:217–224. doi: 10.1023/a:1015405914950. [DOI] [PubMed] [Google Scholar]
- 7.Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Archives of Pediatrics and Adolescent Medicine. 2007;161:326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- 8.Badawy A, Shokeir T, Allam AF, Abdelhady H. Pregnancy outcome after ovulation induction with aromatase inhibitors or clomiphene citrate in unexplained infertility. Acta Obstetricia et Gynecologica Scandinavica. 2009;88:187–191. doi: 10.1080/00016340802638199. [DOI] [PubMed] [Google Scholar]
- 9.Doornbos ME, Maas SM, McDonnell J, Vermeiden JP, Hennekam RC. Infertility, assisted reproduction technologies and imprinting disturbances: a Dutch study. Human Reproduction. 2007;22:2476–2480. doi: 10.1093/humrep/dem172. [DOI] [PubMed] [Google Scholar]
- 10.Elizur SE, Tulandi T. Drugs in infertility and fetal safety. Fertility and Sterility. 2008;89:1595–1602. doi: 10.1016/j.fertnstert.2008.02.092. [DOI] [PubMed] [Google Scholar]
- 11.Hvidtjørn D, Schieve L, Schendel D, Jacobsson B, Sværke C, Thorsen P. Cerebral palsy, autism spectrum disorders, and developmental delay in children born after assisted conception: a systematic review and meta-analysis. Archives of Pediatrics and Adolescent Medicine. 2009;163:72–83. doi: 10.1001/archpediatrics.2008.507. [DOI] [PubMed] [Google Scholar]
- 12.Ericson A, Nygren KG, Olausson PO, Källén B. Hospital care utilization of infants born after IVF. Human Reproduction. 2002;17:929–932. doi: 10.1093/humrep/17.4.929. [DOI] [PubMed] [Google Scholar]
- 13.Klemetti R, Sevón T, Gissler M, Hemminki E. Health of children born as a result of in vitro fertilization. Pediatrics. 2006;118:1819–1827. doi: 10.1542/peds.2006-0735. [DOI] [PubMed] [Google Scholar]
- 14.Lidegaard O, Pinborg A, Andersen AN. Imprinting diseases and IVF: Danish National IVF cohort study. Human Reproduction. 2005;20:950–954. doi: 10.1093/humrep/deh714. [DOI] [PubMed] [Google Scholar]
- 15.Pinborg A, Loft A, Schmidt L, Andersen AN. Morbidity in a Danish national cohort of 472 IVF/ICSI twins, 1132 non-IVF/ICSI twins and 634 IVF/ICSI singletons: health-related and social implications for the children and their families. Human Reproduction. 2003;18:1234–1243. doi: 10.1093/humrep/deg257. [DOI] [PubMed] [Google Scholar]
- 16.Pinborg A, Loft A, Schmidt L, Greisen G, Rasmussen S, Andersen AN. Neurological sequelae in twins born after assisted conception: controlled national cohort study. BMJ (Clinical Research Ed) 2004;329:311. doi: 10.1136/bmj.38156.715694.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strömberg B, Dahlquist G, Ericson A, Finnström O, Köster M, Stjernqvist K. Neurological sequelae in children born after in-vitro fertilisation: a population-based study. Lancet. 2002;359:461–465. doi: 10.1016/S0140-6736(02)07674-2. [DOI] [PubMed] [Google Scholar]
- 18.Maimburg RD, Vaeth M. Do children born after assisted conception have less risk of developing infantile autism? Human Reproduction. 2007;22:1841–1843. doi: 10.1093/humrep/dem082. [DOI] [PubMed] [Google Scholar]
- 19.Hvidtjørn D, Grove J, Schendel D, Schieve LA, Sværke C, Ernst E, et al. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. Journal of Epidemiology and Community Health. 2011;65(6):497–502. doi: 10.1136/jech.2009.093823. [DOI] [PubMed] [Google Scholar]
- 20.Funderburk SJ, Carter J, Tanguay P, Freeman BJ, Westlake JR. Parental reproductive problems and gestational hormonal exposure in autistic and schizophrenic children. Journal of Autism and Developmental Disorders. 1983;13:325–332. doi: 10.1007/BF01531570. [DOI] [PubMed] [Google Scholar]
- 21.Stein D, Weizman A, Ring A, Barak Y. Obstetric complications in individuals diagnosed with autism and in healthy controls. Comprehensive Psychiatry. 2006;47:69–75. doi: 10.1016/j.comppsych.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Homburg R, Insler V. Ovulation induction in perspective. Human Reproduction Update. 2002;8:449–462. doi: 10.1093/humupd/8.5.449. [DOI] [PubMed] [Google Scholar]
- 23.Klemetti R, Sevón T, Gissler M, Hemminki E. Health of children born after ovulation induction. Fertility and Sterility. 2010;93(4):1157–1168. doi: 10.1016/j.fertnstert.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 25.Ward-King J, Cohen IL, Penning H, Holden JJ. Brief report: telephone administration of the autism diagnostic interview–revised: reliability and suitability for use in research. Journal of Autism and Developmental Disorders. 2010;40:1285–1290. doi: 10.1007/s10803-010-0987-x. [DOI] [PubMed] [Google Scholar]
- 26.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in Medicine. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 27.Govindarajulu U, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Statistics in Medicine. 2007;26:3735–3752. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 28.Miettinen OS. Theoretical Epidemiology: Principles of Cccurrence Research in Medicine. New York: Wiley & Sons; 1985. Regression Analysis; pp. 231–233. [Google Scholar]
- 29.CDC. Mental health in the United States: parental report of diagnosed autism in children aged 4–17 years – United States, 2003–2004. MMWR Morbidity and Mortality Weekly Report. 2006;55:481–486. [PubMed] [Google Scholar]
- 30.Faraone SV, Biederman J, Milberger S. How reliable are maternal reports of their children’s psychopathology? One-year recall of psychiatric diagnoses of ADHD children. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:1001–1008. doi: 10.1097/00004583-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, et al. Diagnosing autism: analyses of data from the Autism Diagnostic Interview. Journal of Autism and Developmental Disorders. 1997;27:501–517. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- 32.Barr RG, Herbstman J, Speizer FE, Camargo CA., Jr Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. American Journal of Epidemiology. 2002;155:965–971. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]
- 33.Colditz G, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. American Journal of Epidemiology. 1986:894–900. 123. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 34.Rich-Edwards J, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, et al. Adolescent body mass index and infertility caused by ovulatory disorder. American Journal of Obstetrics and Gynecology. 1994;171:171–177. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 35.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia, PA: Lippincott-Raven Publishers; 1998. [Google Scholar]
- 36.Peterson M, Sinisi SE, van der Lann MJ. Estimation of direct causal effects. Epidemiology. 2006;17:276–284. doi: 10.1097/01.ede.0000208475.99429.2d. [DOI] [PubMed] [Google Scholar]
- 37.Van Der Weele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20:18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]