Abstract
Background and Objective
Current treatments of port-wine stain birthmarks typically involve use of a pulsed dye laser (PDL) combined with cooling of the skin. Currently, PDL therapy protocols result in varied success, as some patients experience complete blanching, while others do not. Over the past decade, we have studied the use of photodynamic therapy (PDT) as either a replacement or adjuvant treatment option to photocoagulate both small and large vasculature. The objective of the current study was to evaluate a PDT protocol that involves use of an alternate intravascular photosensitizer mono-L-aspartylchlorin-e6 (NPe6) activated by an array of low-cost light emitting diodes.
Study Design/Materials and Methods
To monitor the microvasculature, a dorsal window chamber model was installed on 22 adult male mice. The light source consisted of a custom-built LED array that emitted 10 W at a center wavelength of 664 nm (FWHM = 20 nm). The light source was positioned at a fixed distance from the window chamber to achieve a fixed irradiance of 127 mW/cm2. A retroorbital injection of NPe6 (5 mg/kg) was performed to deliver the drug into the bloodstream. Laser irradiation was initiated immediately after injection. To monitor blood-flow dynamics in response to PDT, we used laser speckle imaging. We employed a dose–response experimental design to evaluate the efficacy of NPe6-mediated PDT.
Results
We observed three general hemodynamic responses to PDT: (1) At low radiant exposures, we did not observe any persistent vascular shutdown; (2) at intermediate radiant exposures, we observed an acute decrease in blood flow followed by gradual restoration of blood flow over the 7-day monitoring period; and (3) at high radiant exposures, we observed acute vascular shutdown that persisted during the entire 7-day monitoring period. Dose–response analysis enabled identification of 85 J/cm2 as a characteristic radiant exposure required to achieve persistent vascular shutdown at Day 7 following PDT.
Conclusion
The experimental data suggest that NPe6-mediated PDT can achieve persistent vascular shutdown of normal microvasculature.
Keywords: light-activated drug therapy, dose–response, persistent vascular shutdown, dorsal window chamber, laser speckle imaging
INTRODUCTION
Port wine stain (PWS) is a congenital vascular malformation commonly found on the face and neck regions. Current treatments typically involve use of a pulsed dye laser (PDL) combined with cooling of the skin [1]. Yellow light, in the 585–595 nm wavelength range, is strongly absorbed by hemoglobin and can photocoagulate the targeted vasculature.
Currently, PDL therapy protocols result in varied success, as some patients experience complete blanching, while others do not [2]. Many factors contribute to less than optimal PWS blanching, including reperfusion, angiogenesis, small-diameter vessels (<20 μm) that are resistant to conventional PDL-based protocols, vascular density and depth. The limited efficacy of PDL treatments has led us to believe that a new protocol is needed to tackle especially the problem of resistant small vasculature.
Over the past decade, we have studied the use of photodynamic therapy (PDT) as either a replacement or adjuvant treatment option to photocoagulate both small and large vasculature [3–5]. We previously used benzoporphyrin derivative monoacid ring A (BPD) as the photosensitizer, and studied treatment protocols involving PDT alone, PDL alone or the combination of photodynamic and PDL therapies. Our preliminary preclinical and clinical data suggest that the combination protocol is a superior method to induce persistent shutdown of the vasculature. Unfortunately, our implementation of BPD-mediated PDT is limited due to the high cost of BPD and the lack of availability of 576-nm light sources.
In our current study, we devised a protocol that involves use of an alternate intravascular photosensitizer mono-L-aspartylchlorin-e6 (NPe6) activated by an array of low-cost light emitting diodes (wavelength = 664 ± 20 nm) [6]. Previous studies have demonstrated that NPe6 is an effective photosensitizer for mediating light-induced changes to the microvasculature [7,8]. To assess therapeutic efficacy, we used the mouse dorsal window chamber model and laser speckle imaging to monitor blood-flow dynamics. In multiple published studies [3,5,9–13], we have used this combination to monitor the hemodynamic response of the vasculature to phototherapeutic protocols.
MATERIALS AND METHODS
Rodent Dorsal Window Chamber Model
To monitor the microvasculature, a dorsal window chamber model was used [10]. Twenty-two adult male mice (25–30 g, C3H strain) were used in accordance with a protocol approved by the Institutional Animal Care and Use Committee at University of California, Irvine. The window chamber model was prepared for convenient, longitudinal observation of the vascular network on the sub-dermal side of the mouse, and is described in detail by Moy et al. [10]. Animals were anesthetized with a combination of ketamine and xylazine (2:1 ratio, 0.1/100 g body weight) administered through intraperitoneal injection. Two titanium window chamber frames were implanted on the back of each animal using screws, spacers, and nuts. Sutures were used to restrict movement of the skin within the chamber, preserving the location of the vasculature over the 7-day evaluation period. A full thickness of skin was removed, revealing the subdermal layer of vasculature. To prevent dehydration, isotonic saline solution was applied onto the exposed skin, followed by placement of a glass cover slip and retention ring to hold the solution in place. The animal was allowed to recover for ~24 hour prior to PDT.
Light Source
The light source consisted of a custom-built LED array, provided by Light Sciences Oncology, that emitted 10 W at a center wavelength of 664 nm (FWHM = 20 nm). Light output was controlled with a variable-current power supply.
PDT Protocol
To perform PDT, a custom light-tight box was developed. The animal was anesthetized with isoflurane (5%) delivered with a nose cone placed over its snout. An aperture was used to isolate the window chamber and avoid irradiation of surrounding tissue regions. The light source was positioned at a fixed distance from the window chamber to achieve a fixed irradiance of 127 mW/cm2. The irradiation time was varied as described below.
NPe6 was reconstituted using 4 ml of double deionized water in a 10 ml vial, to create a stock solution of 25 mg/ml. A retroorbital injection of NPe6 (5 mg/kg) was performed to deliver the drug into the bloodstream. Laser irradiation was initiated immediately after injection.
Laser Speckle Imaging (LSI)
To monitor blood-flow dynamics in response to PDT, we used laser speckle imaging (LSI). The imaging instrument was nearly identical to that described by Bui et al. [11]. Briefly, the animal was anesthetized with isoflurane (5%) delivered with a nose cone placed over its snout. The animal was positioned with the subdermal side of the window chamber imaged directly by a camera (Nuance, Caliper Life Sciences, Hopkinton, MA) equipped with a 5× zoom lens. A HeNe laser (λ = 633 nm, 30 mW) was used to irradiate the epidermal side of the window chamber and the transmitted speckle pattern imaged with the camera. A beam expander and ground-glass diffuser was used to homogenize the incident laser beam. An image exposure time of T = 10 milliseconds was used to achieve a linear response range of the instrument for the expected blood-flow speeds in the arterioles and venules [12]. Custom software written in LabVIEW (Version 8.6, National Instruments, Austin, TX) and MATLAB (The Mathworks, Natick, MA) was used to acquire and process images.
We collected LSI data on Days 0 (i.e., day on which PDT was performed), 1, 2, 3, and 7. On Day 0, LSI data were collected immediately prior to and after PDT was completed. For each time point, a sequence of 10 raw speckle images was collected. Each image was converted to a speckle contrast image using a 7 × 7 sliding window. At each window position, the mean intensity (〈I〉) and standard deviation (σ) were computed, and the speckle contrast (K) of the center pixel in the window was calculated using the following expression:
A simplified speckle imaging equation [14] was used to convert each speckle contrast image to a speckle flow index (SFI) image. The equation is SFI = (2TK2)−1, where SFI is the reciprocal of the speckle decorrelation time.
Dose–Response Experimental Design
We set out to determine if NPe6-mediated PDT is capable of achieving persistent vascular shutdown and to estimate a characteristic radiant exposure required to achieve shutdown. To this end we employed a dose–response experimental design, typically used in pharmaceutical studies and also used previously in laser-tissue interaction studies [15–17]. Our independent variable was radiant exposure (0–1,000 J/cm2), which we varied in a logarithmic fashion. Since irradiance was fixed at 127 mW/cm2, we essentially varied the irradiation time to control radiant exposure. A single experiment was performed at each of the 20 radiant exposures, in addition to two control experiments. Additional negative control experiments were performed using either (a) the maximum radiant exposure (1,000 J/cm2) without any NPe6 administered, or (b) the minimum radiant exposure (0 J/cm2) with NPe6 administered.
Using Prism software (Version 5.0d, GraphPad Software, San Diego, CA), we performed a dose–response analysis to determine the RE50/7, defined as the characteristic radiant exposure at which complete vascular shutdown was achieved through Day 7. Complete vascular shutdown was defined as no flow seen in the SFI image on Day 7, when compared to the baseline SFI image on Day 0, pre-NPe6-mediated PDT. We also determined the 95% confidence interval of the RE50/7 value.
Two authors (W.J.M. and B.C.) independently reviewed the SFI images from each of the 22 experiments and graded each one as either a “0” (lack of complete vascular shutdown at Day 7) or a “1” (complete vascular shutdown at Day 7).
RESULTS
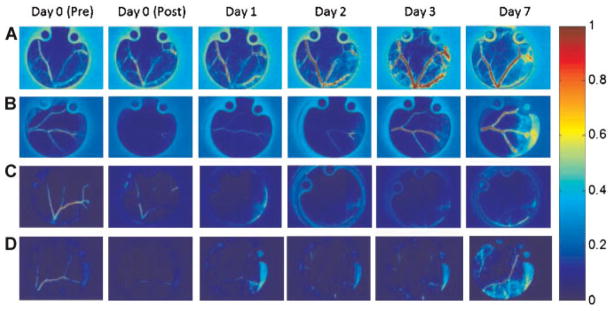
With LSI and the dorsal window chamber, we observed three general hemodynamic responses to PDT (Fig. 1). At low radiant exposures, we did not observe any or persistent vascular shutdown (Fig. 1A). The 0 J/cm2 radiant energy exposure and the two control experiments exhibited this pattern, as was expected. At intermediate radiant exposures, we observed an acute decrease in blood flow followed by gradual restoration of blood flow over the 7-day monitoring period (Fig. 1B). At high radiant exposures, we observed acute vascular shutdown that persisted during the entire 7-day monitoring period.
Fig. 1.
Representative SFI images of the microvasculature following irradiation. A: No acute shutdown, no persistent shutdown on Day 7. Experiment: 0 J/cm2 radiant exposure. B: Acute shutdown post-experiment, but no persistent shutdown on Day 7. Experiment: 60 J/cm2 radiant exposure. C: Acute shutdown and followed by persistent shutdown. Experiment: 600 J/cm2 radiant exposure. D: Acute shutdown post-experiment, but no persistent shutdown on Day 7. Experiment: 90 J/cm2 radiant exposure. For each experiment, the SFI values for each image was normalized with respect to the Day 0 (Pre) baseline.
Following the graded system explained above, we determined that 9 out of the 20 experimental conditions produced a “0” result, with the remaining 11 experiments producing a “1” result at Day 7. Radiant exposures of 80 and 100–1,000 J/cm2 produced a “1” result, while radiant exposures of 0–70 and 90 J/cm2 resulted in a “0”. Additional negative control experiments were conducted using either (a) retroorbital administration of NPe6 and no light, or (b) no NPe6 administration and a radiant exposure of 1,000 J/cm2. Both of these experiments resulted in a lack of acute or persistent vascular shutdown.
Dose–response analysis enabled identification of 85 J/cm2 as the RE50/7 value for NPe6, to achieve persistent vascular shutdown at Day 7 following PDT (Fig. 2). A fairly sharp demarcation between a “0” response and a “1” response existed, resulting in a fairly narrow 95% confidence interval of 72–95 J/cm2 for the RE50/7 value.
Fig. 2.
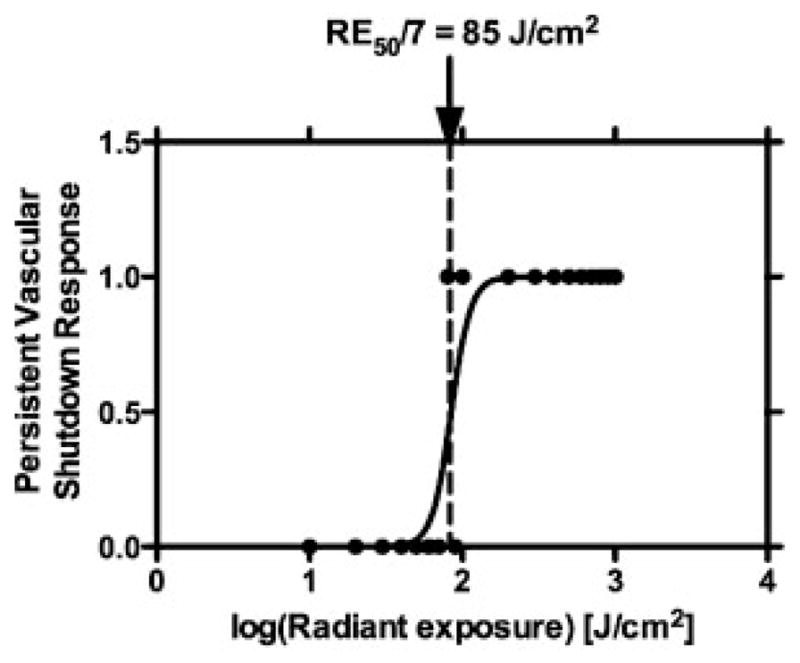
Dose–response analysis of persistent vascular shutdown response at specific radiant exposures. RE50/7 of 85 J/cm2 was determined from the data.
DISCUSSION
The experimental data (Figs. 1 and 2) of this study suggest that NPe6-mediated PDT can achieve persistent vascular shutdown at doses greater than 90 J/cm2. Given this observed efficacy of NPe6-mediated PDT, we propose that this approach is a potential treatment for PWS and other cutaneous vascular lesions in humans, and further preclinical evaluation is warranted.
Examination of SFI data from high radiant exposures (>90 J/cm2) revealed the desired outcome of persistent vascular shutdown continuing through Day 7. The shutdown was very apparent from inspection of the SFI images, as both small and large diameter vessels showed a complete absence of blood flow. Hence, NPe6-mediated PDT can achieve shutdown of smaller-diameter vessels that otherwise may be resistant to PDL therapy.
In our previous studies involving BPD-mediated PDT, we found that persistent vascular shutdown was achieved during a 16 minutes irradiation time [3–5]. During our clinical study [4], we found that patient acceptance of this irradiation time was not an issue. In our current study, we determined a RE50/7 value (85 J/cm2) associated with an irradiation time of ~10 minutes. Hence, we expect that NPe6-mediated PDT also would be tolerated by patients, at least from a time perspective. Additionally, the low cost of the LED-based light source components is expected to make the treatment more accessible and easier to adopt in the clinic.
At low radiant exposures (<80 J/cm2), we observed an acute reduction in blood flow that gradually returned by Day 7 (Fig. 1B). These data suggest the onset of three biological response mechanisms: (a) restoration of the vascular network by reperfusion of incompletely photocoagulated microvasculature, (b) development of new blood vessels separate from existing vasculature, and (c) angiogenesis and its regenerative effects on existing vasculature. Our results of reperfusion following PDT with low radiant exposures are consistent with those from previous pulsed-laser studies that have demonstrated the difficulty of achieving persistent vascular shutdown [11,13]. Our current study and these previous studies collectively demonstrate the critical need to perform longitudinal monitoring of blood flow in response to phototherapies, since immediate vascular responses (<24 hours) may differ greatly from those observed several days past the therapeutic intervention.
Our past work with BPD [3,13] demonstrates that translation of PDT results from rodents to humans requires additional considerations. Light doses required for the clinical response in humans were different. In addition, we frequently observed ulceration in the rodent window model due to lack of deep vasculature (a result of the surgery) that did not occur in the human studies. Thus, the primary value of the preliminary animal experiments is to confirm that persistent vascular shut down can be achieved; however, dosing information and side effect profiles must be determined in future clinical experiments.
Current literature does include some information about dosing and side effect profiles that will be useful for our planned clinical experiments. With respect to the safety concerns in humans of NPe6-mediated PDT, we refer to the study conducted by Chan et al. [18] in which NPe6 was administered to 14 patients with basal cell carcinoma, squamous cell carcinoma or papillary carcinoma. Patients received varying drug dosages (0.5–3.5 mg/kg) and light dosages (argon pumped dye laser at 664 nm; 25–200 J/cm2; 50–400 mW/cm2). Ninety percent of the patients exhibited a complete response, which was defined as no clinical or histopathological evidence of tumor remaining after PDT treatment. Adverse effects were pain at the treatment site (two patients) and erythema and edema of the treatment site (one patient). Three patients experienced pruritus, facial edema, erythema or tingling, all of which were not clearly related to treatment. Photosensitivity tested with a solar stimulator was not evident 1 week post-treatment in 12/14 subjects. One patient had erythema with the solar simulator until 3 weeks post-treatment and one patient was not tested. In Usuda et al. [19], 29 patients with 38 lesions of centrally located early lung cancer received PDT with a 40 mg/m2 dosage of NPe6, followed by 664 nm wavelength light (100 J/cm2; 150 mW/cm2). They achieved complete remission in 35/38 lesions (92.1%). Recurrence occurred in seven lesions. Patients were told to avoid light for 2 weeks post-treatment. The study did not report any indications of extensive necrotic damage of the tumors and no other adverse effects were noted by the authors.
Previous studies of NPe6-mediated PDT have demonstrated promising outcomes for tumor treatment [6–8,18–20]. Our current study of NPe6-mediated PDT treatment highlights the potential role of this therapy in treatment plans targeting unwanted cutaneous vasculature. Our future work will study the role of additional independent variables (irradiance, drug dosage, fractionation, combination protocols, etc.) on our ability to achieve persistent vascular shutdown.
Acknowledgments
The work was supported in part by grants obtained from the National Institutes of Health (HD065536), the National Institutes of Health Laser Microbeam and Medical Program (LAMMP, a P41 Biotechnology Resource, project number RR001192), and the Arnold and Mabel Beckman Foundation. The authors thank Liz Bromley and Steven Daly of Light Sciences Oncology for their generosity in providing us with NPe6 and the light source. The authors thank Dr. Tom Foster (University of Rochester) for discussions involving NPe6-mediated PDT; and Sean White and Austin Moy (both at University of California, Irvine) for assistance in preparing this manuscript.
References
- 1.Chapas AM, Eickhorst K, Geronemus RG. Efficacy of early treatment of facial port wine stains in newborns: A review of 49 cases. Lasers Surg Med. 2007;39(7):563–568. doi: 10.1002/lsm.20529. [DOI] [PubMed] [Google Scholar]
- 2.Vallee JA, Kelly KM, Rohrer TE, Arndt KA, Dover JS. Lasers in the treatment of vascular lesions. In: Kaminer MS, Arndt KA, Dover JS, Rohrer TE, Zachary CB, editors. Atlas of cosmetic surgery. Philadelphia: W.B. Saunders; 2009. [Google Scholar]
- 3.Channual J, Choi B, Osann K, Pattanachinda D, Lotfi J, Kell KM. Vascular effects of photodynamic therapy and pulsed dye laser therapy protocols. Lasers Surg Med. 2008;40:644–650. doi: 10.1002/lsm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tournas JA, Lai J, Truitt A, Huang YC, Osann KE, Choi B, Kelly KM. Combined benzoporphyrin derivative monoacid ring A photodynamic therapy and pulsed dye laser for port wine stain birthmarks. Photodiag Photodynamic Ther. 2009;6:195–199. doi: 10.1016/j.pdpdt.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith TK, Choi B, Ramirez-San-Juan JC, Nelson JS, Osann K, Kelly KM. Microvascular blood flow dynamics associated with photodynamic therapy, pulsed dye laser irradiation and combined regimens. Lasers Surg Med. 2006;38:532–539. doi: 10.1002/lsm.20335. [DOI] [PubMed] [Google Scholar]
- 6.Mitra S, Foster TH. In vivo confocal fluorescence imaging of the intratumor distribution of the photosensitizer mono-L-aspartylchlorin-e6. Neoplasia. 2008;10:429–438. doi: 10.1593/neo.08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fingar VH, Wieman TJ, Wiehle SA, Cerrito PB. The role of microvascular damage in photodynamic therapy: The effect of treatment on vessel constriction, permeability, and leukocyte adhesion. Cancer Res. 1992;52:4914–4921. [PubMed] [Google Scholar]
- 8.McMahon KS, Wieman TJ, Moore PH, Fingar VG. Effects of photodynamic therapy using mono-L-aspartyl chlorin e6 on vessel constriction, vessel leakage, and tumor response. Cancer Res. 1994;54:5374–5379. [PubMed] [Google Scholar]
- 9.Choi B, Kang NM, Nelson JS. Laser speckle imaging for monitoring blood flow dynamics in the in vivo rodent dorsal skin fold model. Microvasc Res. 2004;68:143–146. doi: 10.1016/j.mvr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Moy AJ, White SW, Indrawan ES, Lotfi J, Nudelman MJ, Costantini SJ, Agarwal N, Jia W, Kelly KM, Sorg BS, Choi B. Wide-field functional imaging of blood flow and hemoglobin oxygen saturation in the rodent dorsal window chamber. Microvasc Res. 2011;82:199–209. doi: 10.1016/j.mvr.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bui AK, Teves KM, Indrawan ES, Jia W, Choi B. Longitudinal, multimodal functional imaging of microvascular response to photothermal therapy. Opt Lett. 2010;35:3216–3218. doi: 10.1364/OL.35.003216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi B, Ramirez-San-Juan JC, Lotfi J, Nelson JS. Linear response range characterization and in vivo application of laser speckle imaging of blood flow dynamics. J Biomed Opt. 2006;11:4. doi: 10.1117/1.2341196. [DOI] [PubMed] [Google Scholar]
- 13.Choi B, Jia W, Channual J, Kelly KM, Lotfi J. The importance of long-term monitoring to evaluate the microvascular response to light-based therapies. J Invest Dermatol. 2008;128(2):485–488. doi: 10.1038/sj.jid.5700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez-San-Juan JC, Ramos-García R, Guizar-Iturbide I, Martínez-Niconoff G, Choi B. Impact of velocity distribution assumption on simplified laser speckle imaging equation. Opt Express. 2008;16(5):3197–3203. doi: 10.1364/oe.16.003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barton JK, Hammer DX, Pfefer TJ, Lund DJ, Stuck BE, Welch AJ. Simultaneous irradiation and imaging of blood vessels during pulsed laser delivery. Lasers Surg Med. 1999;24(3):236–243. doi: 10.1002/(sici)1096-9101(1999)24:3<236::aid-lsm9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Chan KF, Hammer DX, Choi B, Teichman JM, McGuff HS, Pratisto H, Jansen ED, Welch AJ. Free electron laser lithotripsy: Threshold radiant exposures. J Endourol. 2000;14(2):161–167. doi: 10.1089/end.2000.14.161. [DOI] [PubMed] [Google Scholar]
- 17.Vargas G, Barton JK, Welch AJ. Use of hyperosmotic chemical agent to improve the laser treatment of cutaneous vascular lesions. J Biomed Opt. 2008;13(2):021114. doi: 10.1117/1.2907327. [DOI] [PubMed] [Google Scholar]
- 18.Chan AL, Juarez M, Allen R, Volz W, Albertson T. Pharmacokinetics and clinical effects of mono-L-aspartyl chlorin e6 (NPe6) photodynamic therapy in adult patients with primary or secondary cancer of the skin and mucosal surfaces. Photodermatol Photoimmunol Photomed. 2005;21:72–78. doi: 10.1111/j.1600-0781.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- 19.Usuda J, Tsutsui H, Honda H, Ichinose S, Ishizumi T, Hirata T, Inoue T, Ohtani K, Maehara S, Imai K, Tsunoda Y, Kubota M, Ikeda N, Furukawa K, Okunaka T, Kato H. Photodynamic therapy for lung cancers based on novel photodynamic diagonosis using talaporfin sodium (NPe6) and autofluorescence bronchoscopy. Lung Cancer. 2007;58(3):317–323. doi: 10.1016/j.lungcan.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Gomer CJ, Ferrario A. Tissue distribution and photosensitizing properties of mono-L-aspartyl chlorin e6 in a mouse tumor model. Cancer Res. 1990;50:3985–3990. [PubMed] [Google Scholar]