INTRODUCTION
The ultimate goal for tissue engineering and regenerative medicine is to develop therapies to restore lost, damaged, or aging tissues using engineered or regenerated products derived from either donor or autologous cells. Various approaches have been considered in tissue engineering and regenerative medicine, but currently the most common is to use a biodegradable scaffold in the shape of the new tissue that is seeded with either stem cells or autologous cells from biopsies of damaged tissues.1,2 The scaffold provides an environment that allows the implanted cells to proliferate, differentiate, and form the desired tissue or organ. Several biomimetic scaffold materials have been used for this purpose, including naturally occurring macromolecules such as collagen, alginate, agarose, hyaluronic acid derivatives, chitosan, and fibrin,3 and man-made polymers such as polyglycolic acid (PGA), polylactic acid (PLA), poly(caprolactone) (PCL), poly(dioxanone), poly(methyl methacrylate) (PMMA), and poly(glycerolsebacate).4–8
The approach of combining adult stem cells with biomimetic scaffolds and bioactive molecules is in varying stages of development for the treatment of disorders such as diabetes, arthritis, Parkinson disease, Alzheimer disease, atherosclerosis, cancer, and heart disease. This article focuses on dental diseases such as caries and periodontitis, which are pandemic, cause a permanent loss of tissues and functions, and affect the health of populations in all age groups worldwide.
PAST AND PRESENT APPROACHES IN TISSUE REGENERATION
Over the past few decades, new technologies in tissue engineering such as microfabrication, self-assembled biomimetic peptides, and 3-dimensional (3D) printing have rapidly developed. These technologies have enabled the building of simple tissues such as skin epithelium and production of composite tissues such as bone, kidney, and bladder.9–14
Regeneration of Nondental Tissues
The first tissue-based therapies for skin grafting were developed in India around 3000 BCE, but the synthesis of substitute materials for skin and various grafting techniques (eg, autologous and allografts) were not developed until the eighteenth century.15 The first engineered skin tissues were generated by Howard Green and colleagues in 1975.16,17 This product, which contained only a few layers of cells and did not contain dermis, led to the development of the first commercial skin product, named Epicel (Genzyme, Cambridge, MA, USA), which contains sheets of autologous keratinocytes. Another engineered product for skin was generated using bovine type I collagen and shark chondroitin 6-sulfate.18,19 These compounds were crosslinked and packed into a porous matrix with a silicone sheet attached onto one side as a temporary epidermis-like barrier. A composite product of reconstituted dermis and epidermis has led to the development of a commercial skin graft product called Apligraf (Organogenesis, Canton, MA, USA).20,21 The strategy of combining cells and extracellular matrix in skin-graft products was also used to successfully produce cartilage-graft materials. Cell-based cartilage repair techniques were first described in 1994.22 This technology led to the development of the first commercial product for cartilage grafts, called Carticel (Genzyme). Since 2008, significant advances in tissue engineering have been made for other tissues such as bone, kidney, bladder, blood vessels, and liver.9–14,23–26
Unlike other tissues, the skin and cartilage do not require an extensive vascular supply.23 An important challenge in organ regeneration is the acquisition of a functional vascular supply for the engineered organ. Endothelial cells and the paracrine factors that regulate them, such as vascular endothelial growth factor, were shown to induce angiogenesis and facilitate the integration of transplanted tissues/organs into the host. This finding led to a new treatment strategy in regenerative medicine by using peripheral blood-derived or bone marrow–derived endothelial progenitor cells to induce de novo vessel formation in regenerated organs.27–29 Vascular endothelial cells can also be generated from human embryonic stem (ES) cells. These cells can integrate into the host and form chimeric vasculature.30 Vascular endothelial cells can facilitate the differentiation of ES cells into various cell types such as pancreatic insulin-producing cells,31 cardiomyocytes,32 neurons, and glial cells.33 3D cardiac tissues with endothelial cell networks have been created and implanted onto infarcted rat hearts, which regain function after the surgery. The improvement of cardiac function was dependent on the endothelial cell densities within the engineered cardiac tissues. The number of capillaries in the transplanted tissues with the endothelial cell network is also greater than those without the endothelial cells.34
Regeneration of Dental Tissues and Supporting Structures
The regeneration of periodontium was the first tissue-engineering technology in dentistry, and was invented by Nyman and colleagues35 in 1982. This procedure, termed guided tissue regeneration (GTR), involves inserting a barrier membrane under the periodontal tissue flap to prevent the ingrowth of gingival epithelium and connective tissue, while creating a space on the root surface for progenitor cells from the periodontal ligament including cementoblasts, fibroblasts, and osteoblasts to migrate in and form new periodontal structures including cementum, periodontal ligament, and alveolar bone. Various types of bonegraft materials such as autogenous grafts, allografts, alloplasts, or xenografts have been placed in the space above root surfaces to facilitate bone formation.36–38
There are 2 main types of barrier membranes, resorbable and nonresorbable. The nonresorbable membranes require a second surgical procedure to remove the membranes at 4 to 6 weeks after the initial surgery. Two types of commonly used non-resorbable GTR barrier membranes include expanded polytetrafluoroethylene (ePTFE), also known as Gore-Tex, and nonexpanded polytetrafluoroethylene (nPTFE). The resorbable barrier materials were more recently developed and are available in 2 formats, synthetic polymers and natural barrier materials. The synthetic polymer GTR materials consist of a lactide/glycolide copolymer or PLA blended with a citric acid ester. The natural barrier membranes include those made from collagen, calcium sulfate, or enamel matrix proteins.38–40
The regeneration of periodontium with these products requires the presence of at least one bony wall at the treatment site, most likely to provide progenitor cells and vascular supply, allowing the repair and regeneration of the periodontal tissues. To improve on the limited level of success, strategies using exogenous growth factors and stem cells have been studied and await translational application to clinical practice. Potential growth factors for periodontal regeneration include bone morphogenetic proteins, platelet-derived growth factor, amelogenin proteins, and fibroblast growth factors.38,41–46
Current therapeutic approaches involve replacing the missing tooth structure with artificial materials as the capacity of adult human dental tissues to regenerate is virtually nonexistent, particularly for enamel, due to the absence of ameloblasts in formed teeth. The regeneration and repair of inner-tooth dentin can be obtained only if the healthy dental pulp tissue is still present and if bacterial contamination is completely removed.47,48 Typically, mechanical removal of decayed enamel and dentin is completed and artificial materials are used to fill in the prepared cavity, to prevent bacterial contamination and induce the formation of reparative dentin onto the dentinal floor of the cavity.
The regeneration of dentin is usually not possible in necrotic teeth. However, in children with incompletely formed teeth with wide-open root apices, pulp tissue can be regenerated through the opened root apices. Findings from prior revascularization studies of traumatized teeth showed that the success of pulp-tissue regeneration in replanted avulsed teeth depends on the diameter of the opening of root apices.49–51 A diameter of 1 mm (1000 μm) of the opening of root apices has been suggested as a minimum requirement to allow new tissues with neural and vascular structures to regrow into the tooth.49 Because diameters of the neural, vascular, and cellular structures are less than 100 μm (ie, 10–30-μm diameters for eukaryotic animal and human cells; 0.2–20-μm diameters for nerve fibers; and <100-μm diameters for most arteries in the dental pulp),52–54 theoretically the regeneration of pulp tissues may not need as much as a 1000-μm–diameter opening. However, the positive correlation of clinical success in revascularization of the replanted teeth and a 1-mm minimum apical opening requirement may be due to the existence of stem cells or progenitor cells in the apical area. Further studies are needed to test this notion.
Several case series showing clinical success of pulp-tissue regeneration in immature necrotic teeth led to the growing recognition of the regenerative potential of tissues at the apical end of these immature teeth.55–61 The recent identification of adult mesenchymal stem cells in these tissues also suggests that this cell population regrows into the tooth and regenerates the dentin-pulp complex of such immature necrotic teeth. However, the exact mechanisms by which such precursor cells contribute to clinical outcomes remain unknown.
USES OF STEM CELLS IN TISSUE REGENERATION
Cell-based therapies are the most common approaches in regenerative medicine. Challenges in applying this approach clinically are to acquire the appropriate source of cells, to identify methodologies to induce cell proliferation and differentiation, to maintain cell survival, and to remove unwanted cells.
As stem cells possess a remarkable potential to proliferate and develop into many different cell types to form the desired organ, these cells hold great promise for regenerative therapy. The progeny of stem cells may remain as unspecialized progenitors to serve as an internal source of repair and replenishment, or may differentiate into specialized cells to form the desired tissue. The most commonly used and studied stem cells are (1) ES cells, (2) somatic or adult stem cells, and (3) induced pluripotent stem cells.
Embryonic Stem Cells
ES cells are derived from the inner cell mass of early embryos, called blastocysts. ES cells were first isolated from mouse embryos in 1981.62,63 The success of this work led to the derivation of human ES cells from in vitro fertilized human blastocysts in 1998.64 ES cells are capable of dividing and renewing themselves for long periods without differentiating, whereas most somatic or adult stem cells cannot. In the appropriate environment, these cells can acquire epigenetic marks in their DNA to modulate their gene expression, allowing them to differentiate into any specialized cells. Various types of specialized cells derived from ES cells65 include retina cells,66,67 cardiomyocytes,32,68 neurons,69,70 hematopoietic cells,71,72 hepatic cells,73–75 trophoblasts,76,77 pancreatic insulin-producing cells,31 vascular endothelial cells,30,78,79 pituitary hormone–producing cells,30 and osteoblasts.80,81
Somatic or Adult Stem Cells
In general, the regenerative capacity of adult tissues depends on tissue-specific stem-cell populations that maintain stable numbers by self-renewal and possess the ability to differentiate into distinct cell lineages. Regeneration and renewal in adult mammals has been studied in several organs, including blood, mammary glands, gut, brain, skin, muscle, and hair. These tissues contain adult stem cells such as hematopoietic, endothelial, mammary, intestinal, neural, skin, muscle, and hair-follicle stem cells. Similarly, teeth and supporting structures contain multiple lineages of somatic stem cells, including:
mesenchymal stem cells isolated from the dental pulp of permanent teeth, termed Dental Pulp Stem Cells (DPSC)82 and from the dental pulp of exfoliated deciduous teeth termed Stem cells from Human Exfoliated Deciduous teeth (SHED)83
mesenchymal stem cells isolated from the periodontal ligament84
mesenchymal stem cells isolated from the apical end of developing tooth roots, termed Stem Cells from the Apical Papilla (SCAP),85–87 and
epithelial stem cells isolated from the labial cervical loop of rodent incisors.88–91
In primates, incisors cease growth once their roots are completely formed, whereas in rodents the incisors continue to grow throughout postnatal life because of the presence of epithelial and mesenchymal stem cells that have the capacity to self-renew and differentiate into all of the cell types of adult teeth, including ameloblasts, odontoblasts, and the stratum intermedium (SI). Thus, rodent incisors provide a model for determination of signaling mechanisms that coordinate cell-fate decisions, stem cell self-renewal, and maintenance. The labial cervical loop (CL), but not the lingual CL, of rodent incisors contains stem cells that give rise to ameloblasts and the SI (Fig. 1).92 Labeling experiments demonstrated that cells in the dental epithelium move in a proximal to distal direction.93 In the labial CL, the stem-cell progeny contribute to a population of transit-amplifying (T-A) cells (see Fig. 1A, B). T-A cells undergo several rounds of cell division before they move distally and differentiate into ameloblasts. The incisor epithelia seem to function as a conveyor belt, moving cells from a proximal, undifferentiated source to regularly repopulate the tooth with specialized cell types.
Fig. 1.
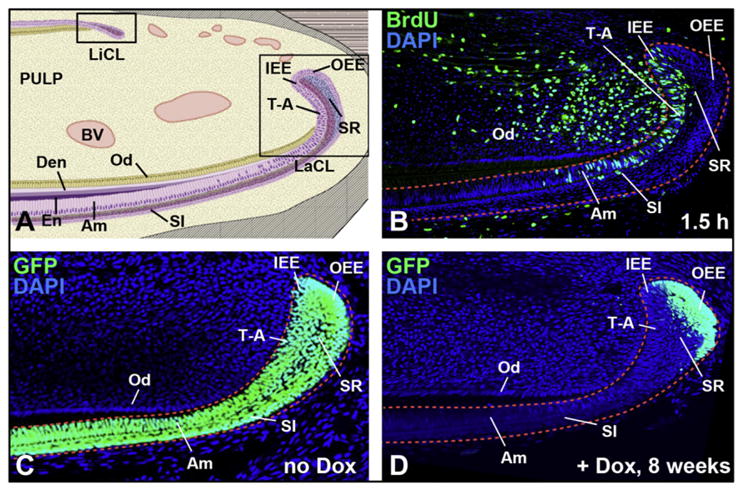
Mouse epithelial cervical loop stem cells. (A) Sagittal view of mandibular mouse incisors shows 2 stem-cell compartments in the lingual (liCL) and labial (laCL) cervical loops. The transit-amplifying (T-A) cells and ameloblasts (Am) arise from inner enamel epithelium (IEE), whereas the outer enamel epithelium (OEE) houses the label-retaining cells (LRCs) in the laCL. The LRCs are putative dental epithelial stem cells. D, dentin; E, enamel; Od, odontoblasts; SI, stratum intermedium; SR, stellate reticulum. (B) BrdU labeling (1.5 hours) of rapidly proliferating cells in the T-A region of laCL epithelium and adjacent mesenchyme. (C, D) Images from incisors of Krt5-tTA; H2B-GFP mice. In the absence of doxycycline (no Dox; C), GFP is present in all CL epithelial cells expressing Krt5 including OEE, IEE, SR, SI, and Am. In the presence of doxycycline (+Dox; D) for 8 weeks, H2B-GFP expression was turned off, leading to the retention of GFP in the slowly proliferating LRCs of the OEE.
Identification of organ-/tissue-specific adult stem-cell populations can be challenging, because stem cells often reside in heterogeneous niches intermingled with support cells. A useful characteristic of stem cells that has aided in their identification in vivo is the relatively slow cell-division kinetics of many stem cells relative to surrounding tissue.94 Slow-cycling cell populations have largely been identified through label-retention experiments, traditionally using 5-bromo-2′-deoxyuridine (BrdU) incorporation, because cells that divide slowly do not dilute the BrdU label as quickly as their rapidly dividing neighbors. Using this technique, BrdU label-retaining cells (LRCs) were identified in the labial CL of cultured perinatal incisors and in adult incisors in situ. Another approach to label retention is the use of transgenic mice harboring a tetracycline-sensitive, histone H2B conjugated with a green fluorescent protein cassette (H2B-GFP) under the control of a tissue-specific transactivator (see Fig. 1C, D).95 Expression of H2B-GFP is initially activated in all cells of the tissue of interest followed by a “chase” period when the transgene is repressed by exposure of the animal to doxycycline, such that dividing cells dilute the label. This technique was used to identify LRCs in the outer enamel epithelium of the adult labial CL.91 The LRCs of the dental epithelium expressed Gli1, a target gene of sonic hedgehog (SHH) signaling, and lineage-tracing experiments demonstrated that the Gli1-expressing cells were indeed stem cells.91
Understanding the regulation of adult stem-cell populations is key to the future use of such cells for clinical therapies. How stem cells are maintained at the appropriate number, what signals regulate their differentiation, and how they are established within the context of the developing organism are important questions in stem-cell research. Many signaling molecules and pathways are implicated in the development and homeostasis of stem cells. These signaling components include SHH, WNT, NOTCH, bone morphogenetic protein (BMP), and fibroblast growth factor (FGF) superfamily proteins that are important regulators of stem cell self-renewal and differentiation.88,90,92,96–98 During the development of epithelial-mesenchymal-derived organs such as teeth, these proteins mediate critical interactions between epithelial and mesenchymal cells that lead to the various stages of tooth development (Fig. 2). Gene-expression data on growth factors and bioactive molecules at each stage of tooth development can be found at www.biteit.helsinki.fi. An extensive review of dental stem cells and growth factors is provided in articles elsewhere in this issue.
Fig. 2.
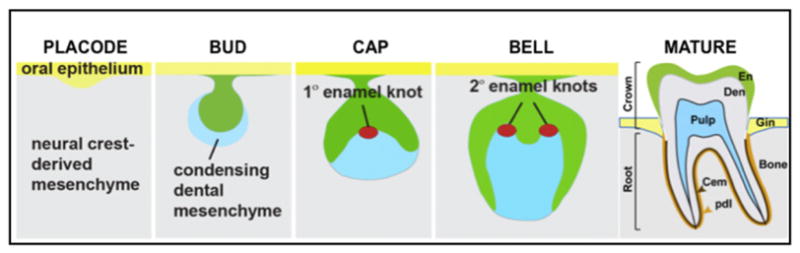
Schematic of molar development. (1) Placode stage: thickening of oral epithelium and invagination into the neural crest–derived mesenchyme. (2) Bud stage: neural crest–derived mesenchymal cells condense around the epithelial bud. (3) Cap stage: the primary enamel knot, a signaling center, is formed in the epithelial cap. (4) Bell stage. The secondary enamel knots, the future sites of cusps, are present. At the tip of the future cusps, the dental papilla mesenchymal cells, adjacent to the inner enamel epithelium, differentiate into dentin-producing cells, odontoblasts. Once the odontoblasts lay down the dentin matrix, the inner enamel epithelial cells, adjacent to odontoblasts, differentiate into enamel-producing cells (ameloblasts) and secrete enamel matrix. This process continues until the tooth crown is completely formed. (5) Mature tooth: once the crown is completely formed with mineralized enamel and dentin, the tooth erupts while the roots and periodontal supporting structures are continuously formed until the closure of the apical end of the tooth roots.
CELL-REPROGRAMMING TECHNIQUES
ES cells possess the capacity to multiply indefinitely. Under an appropriate microenvironment, these cells can differentiate into any cell types and are very useful in research and clinical applications in tissue engineering and regenerative medicine. However, undifferentiated ES cells have the potential to form tumors.99–101 The derivation of human ES cells with matched immunogenotypes from fertilized human embryos also raises ethical issues. By contrast, somatic stem cells or adult stem cells have limited applications. These cells are capable of generating cell types of the tissue in which the cells reside but not cells of a very different origin. For example, hematopoietic stem cells are blood-forming adult stem cells that give rise to various blood cells but not cells of different tissues.
The challenges of working with human ES cells and somatic stem cells in part led to the development of new techniques for obtaining stem cells. Two such techniques are transdifferentiation and induced pluripotent stem cells.
Transdifferentiation
This process converts a given cell type directly into another specialized cell type without bringing the cells back to a pluripotent state. The success of this approach was shown for the conversion between two closely related cell types. For example, a transcription factor, MyoD, was used to convert dermal fibroblasts, chondroblasts, gizzard smooth muscle cells, and pigmented retinal epithelial cells into elongated postmitotic mononucleated striated myoblasts.102 Similarly, adult mesenchymal stem cells from teeth and bone marrow were shown to normally differentiate only into other mesenchymal cell types such as chondrocytes and adipocytes.82,103 However, a combination of 3 neural transcription factors, ASCL1, BRN2, and MYT1L, converted mouse embryonic and postnatal fibroblasts into functional neurons in vitro.104 Furthermore, the transcription factor OCT4 and cytokine treatment converts human dermal fibroblasts into granulocytic, monocytic, megakaryocytic, and erythroid lineage cells.105 Another set of transcription factors, C/EBPβ and C/EBPα, were used to convert fibroblasts into macrophages.106 Even so, the transdifferentiation approach remains an area of great debate.
Induced Pluripotent Stem Cells
This technique was developed using a quartet of transcription factors, OCT3/4 (Pou5f1), SOX2, KLF4, and c-MYC, to reprogram somatic cells into pluripotent stem cells.107–109 The first induced pluripotent stem (iPS) cells were developed from adult mouse cells by Yamanaka and Takahashi in 2006107,108 and from adult human cells by the same group in 2007.110 This breakthrough discovery provided a new way to dedifferentiate cells while maintaining donor-specific immunocharacteristics necessary to prevent rejection by the immune system. The iPS cells possess almost identical properties to the ES cells in that they can multiply almost indefinitely without losing their potential to differentiate into any cells of the 3 germ layers: endoderm, mesoderm, and ectoderm.110,111
iPS cells can be produced from both normal and diseased tissues. For example, iPS cells were derived from human amniotic fluid cells collected for diagnosis from patients with β-thalassemia112 and those from cystic fibrosis lung removed from patients.113 Instead of transdifferentiation, this approach is also useful to reprogram adult stem cells to generate specialized cells of different origins. For example, endothelial CD34+ progenitor cells were derived from the iPS cells of bone marrow,114 and functional neurons were produced from the iPS cells of skin dermal fibroblasts.115
STRATEGIES FOR SELECTIVE REMOVAL OF UNDIFFERENTIATED STEM CELLS
Challenges in the clinical application of stem-cell–based therapies are not only to differentiate the cells into the desired specialized cell types but also to establish strategies to remove residual undifferentiated cells to prevent tumor formation. Both positive and negative selection systems have been proposed, including:
Engineered human ES cells to express herpes simplex virus thymidine kinase so that the ES cells can be killed by ganciclovir at concentrations that are nonlethal to other cell types116–118
Magnetic activated cell sorting using antibodies for differentiation markers for positive selection and/or a selective killing of residual undifferentiated cells by the cytotoxic monoclonal antibody mAb 84.119–121
SCAFFOLDS IN TISSUE ENGINEERING
The type of scaffolding material that stem cells will require to generate specific tissues is an area of great interest. The 2 basic methods for tissue engineering are a top-down approach and a bottom-up approach. The more traditional method is the top-down approach, whereby cells are seeded in a preformed 3D scaffold made from polymer, natural porous materials, or decellularized native extracellular matrix. In the bottom-up approach, various methods have been used to aggregate cells to form distinct subunits that could eventually be used as building blocks to engineer whole organs. Examples of these methods are cell printing, microwells, cell sheets, and self-assembled hydrogels. This section describes the various types of scaffold materials used in tissue engineering, the types of methods in which these materials are used (top-down vs bottom-up), and a few examples of how these materials are being applied to dental pulp regeneration. An extensive review of scaffolds in dental tissue generation is provided in an article elsewhere in this issue.
Scaffold Materials
Whether the approach is top-down or bottom-up, the role of a scaffold is to provide support for delivering cells and/or growth factors to the proposed site of tissue regeneration. Toward these goals, there are important features to consider in scaffold selection, including the physical and mechanical aspects of the material, its biocompatibility, and its degradation timeline. These physical aspects of a 3D scaffold include the porosity (pore volume fraction of the scaffold), pore size (pore diameter), pore structure (shape), and all aspects that can influence how well the cells adhere to the material.122–124 Hydrogels are polymeric structures that are crosslinked and swell in water. For a hydrogel, important aspects include swelling behavior and diffusivity of the hydrogel.
Important mechanical properties of a scaffold material include the viscoelasticity and the tensile strength. For dental regeneration purposes, the tensile strength may be not as important as the viscoelastic properties of the scaffold materials. In general, scaffold materials should reflect the microenvironment of target tissues/organs to facilitate cell growth and ultimately integration to the host. A beneficial clinical feature for dental pulp regeneration would be if the scaffold is injectable, as are some of the natural scaffold materials and hydrogels. In these cases, the gelation time would need to be taken in to consideration when seeding cells in a scaffold for implantation into a host.
An essential clinical feature for scaffold selection is biocompatibility. Naturally derived scaffold materials have the advantage that they are generally well tolerated, do not lead to immunogenic response, and do not involve the use of harsh chemicals during processing. However, a major drawback is the lack of control over the pore size and heterogeneity of the scaffold.
The degradation process of the scaffold is important, and should closely follow the rate of tissue regeneration. When using synthetic polymers, the release of acidic degradation products must be taken into consideration, as well as the resulting drop in pH in the surrounding microenvironment and how that affects the immune response, surrounding tissue, and other factors. Ceramics and bioactive glasses have only recently been studied in terms of how their dissolution products affect cell behavior, and further research is needed to completely understand the mechanism by which the cells and these by-products react.125
Synthetic Scaffolds
Biodegradable scaffold materials
Polyglycolic acid (PGA) is a simple, linear, aliphatic polyester that was first used as a biodegradable suture. The PGA suture was brought to market under the trade name Dexon. PGA in scaffolds was first introduced in the 1980s, alone as a mesh to investigate renal injury,126 and blended with Dacron (polyethylene terephthalate), to study tendon and ligament repair.127–129 Large-scale production of fibrous PGA scaffolds with consistent porosity was achieved in the early 1990s, which was used to regenerate cartilaginous tissue.130 The degradation rate was studied in vitro, whereby only 30% of the polymer remained after 8 weeks.
The first copolymer mixture to gain approval from the Food and Drug Administration was the mixture of PGA with a more hydrophobic polymer, polylactic acid (PLA). This copolymer, poly(lactic-co-glycolic acid) (PLGA) was first available as a suture material under the trade name Vicryl in 1974. PLGA scaffolds were used in the early 1990s toward engineering bone131 and liver,132 and were famously used in the tissue engineering of cartilage in the shape of a human ear.133 PLGA in a 50:50 mixture has a degradation time of about 8 weeks.134 PLGA can also be blended with other polymers as well as natural materials, such as gelatin,131 which was used to study trabecular bone regeneration.
PLA is another biodegradable aliphatic polyester, more hydrophobic than PGA. There are 2 racemic isoforms, poly-L-lactic acid (PLLA) and poly-D-lactic acid (PDLA). The racemic mixture can be termed poly-D,L-lactic acid (PDLLA) or simply PLA, without indication of which chiral form is present. PLA in scaffolds is usually found in a copolymer mixture (see above), although a few early studies looked at the use of PLA scaffolds for cartilage repair135 and nerve regeneration.136 PLLA fibrous scaffolds maintained integrity for a 42-day period, during which PDLLA fibrous scaffolds shrunk significantly after only 3 days.137
Poly(ε-caprolactone) (PCL) is a slowly degrading polymer that was first tested as a bulk material for dermal fibroblast growth.138 PCL scaffolds have been used toward tissue engineering efforts in bone, either alone139,140 or combined with hydroxyapatite (HA).141 PCL scaffolds are attractive for the longer term, as it degrades over 2 years.142,143
Non-biodegradable scaffold materials
In addition to biodegradable scaffolds, nonbiodegradable scaffolds have also been investigated for tissue-engineering purposes. These materials in some cases can be osseointegrated and are well tolerated by the body.
Polymethyl methacrylate (PMMA) is biocompatible and has been studied for its potential in drug delivery144 and dermal fillers,145 but a few studies have been done on its potential as a 3D scaffold,146 toward bone and cartilage repair,145,147 as well as a template for nerve regereration.148
Polytetrafluoroethylene (PTFE), more commonly known by its commercial name Teflon, is a polymer made up of repeating carbon and fluorine subunits. It has been extensively studied for its use in vascular grafts.149 Tissue-engineering efforts using this material outside of vascular work have been sparse, although successful culture of adipocytes150 and cartilage from the temporomandibular joint have been reported.151
Polydimethylsiloxane (PDMS) is a silicon-based polymer, most commonly used in soft lithography processing of microfluidic devices. PDMS scaffolds have been used in tissue engineering of the heart,152,153 bone,154,155 liver,156 and muscle.157
Other synthetic scaffold materials
Polyethylene glycol (PEG)-based scaffolds are the most widely used hydrogels. These scaffolds have been synthesized as copolymer solutions and come in variable weights. Poly(2-hydroxyethyl methacrylate) has been used since the late 1960s as a contact-lens material.158 Studies using the material as a hydrogel for cell encapsulation/tissue engineering began in the 1980s and have been used toward the regeneration of several tissues, including spinal cord/nerve,159 cardiac tissue,160 bone,161 and skin.162 There have been a few published studies of this material and composites in dental applications, discussed in the section on dental pulp regeneration. Polyvinyl alcohol has long been used to investigate islet encapsulation,163–166 and has been used as a drug-delivery material167 and in tissue engineering of the cornea168 and cartilage.169
Naturally Derived Materials
Naturally derived scaffolds
There are several naturally derived materials used as either a coating, alone as a hydrogel, or in combination with synthetic materials.
Alginate is a hydrogel comprising 1,4-linked β-D-mannuronic acid and α-L-guluronic acid, typically derived from brown seaweed and also bacteria.170 The advantages of alginate are its biocompatibility, low toxicity, and slow gelling time (20–60 minutes), depending on the concentration and temperature.171 Disadvantages of the material are the inability to control its degradation rate in vivo and its low viscoelasticity, although this can be improved by increased crosslinking or addition of other substances, such as HA.172 Several studies using alginate and alginate/HA mixtures have been performed in bone and cartilage tissue engineering.173,174
Agarose is well known for its use in nucleic acid electrophoresis, but it is also a useful hydrogel for cell encapsulation. It has been used in neuronal148,175 and cartilage176,177 tissue engineering, as well as in composites for engineering of bone173 and cornea178 with HA and fibrin, respectively.
Chitosan is a polymer derived from the deacetylation of chitin, the major component of crustacean exoskeletons. It can be formulated into an injectable hydrogel, and has been used in the study of epithelial wound healing,179 repair after myocardial infarction,180 and for intestinal181 and central nervous system182 tissue engineering. Chitosan is also used as a copolymer with other natural materials183–185 and synthetic materials.186,187
Collagen, fibrin, gelatin, hyaluronic acid, and pectin have been used as natural materials in conjunction with one of the other materials described previously, and are discussed here only regarding their contributions toward dental tissue engineering.
Bioceramics and Metals
Bioceramics and metals have long been used as implant materials for joint and tooth replacement. HA is a natural bioceramic constituting various hard tissues such as bone, dentin, and enamel.188 The HA-based materials have been widely used for dental tissue and bone engineering141,145,173,174 and are often used in conjunction with tricalcium phosphate (TCP).189–191
Titanium is the most widely used metal for implants because of its biocompatibility and a capacity to osteointegrate, a beneficial feature for dental implants. Titanium can be coated with various polymers.
3D Organ Printing
3D organ printing involves 3 sequential steps: (1) preprocessing or development of blueprints for organs, (2) processing or actual organ printing, and (3) postprocessing or organ conditioning and accelerated organ maturation. The 3D cell printers can print single cells or cell aggregates onto the previously printed successive layers of thermo-sensitive gels in a layer-by-layer fashion. These sequential layers are assembled to create the 3D organ.192–194 Recently, a new technique termed micro-masonry was introduced for the formation of engineered tissues or organs in 3 dimensions. The shape-controlled PEG microgels are mixed in a prepolymer solution and spread onto the surface of a template made from PDMS. The microgels assemble and closely pack to form a brick-wall–like structure on the surface of the template. The microgels are then illuminated to crosslink the polymer and create a 3D replica of the PDMS template. Cells can be incorporated into the prepolymer solution with a high survival rate (83.1% ± 2.3%).195
SCAFFOLDS IN DENTAL PULP TISSUE ENGINEERING
Humans have long used both natural and synthetic materials as replacements for lost teeth. The earliest known dental implant was made of iron and found in a Roman male, believed to be dated around 200 CE.196 The first tooth made from a natural material was found in a Mayan woman, estimated around 600 CE, and was made of nacre, or mother of pearl, from sea shells.197 Although dental implants continue to be used today, more recently tissue engineering has been used to recreate dental tissues. Not surprisingly, both natural and synthetic materials have been explored for this use, each with encouraging results. In dental tissue engineering, a wide variety of biomaterials have been used such as human bone derivatives, natural porous materials, bioceramics, and synthetic polymers.
Synthetic Scaffolds
The most extensively studied scaffold system for dental tooth regeneration is the use of biodegradable PGA scaffolds. The first reported studies maintained human adult dental pulp on a PGA scaffold for more than 60 days in culture.198 Follow-up studies used PGA scaffolds with human dental pulp and found upregulation of type I collagen, fibronectin, and several BMPs and their receptors, suggesting the capacity of this scaffold to maintain cell vitality and support the differentiation of human dental pulp cells.199
More recently, mixtures of PGA with both synthetic copolymers and other macromolecules were used for dental tissue engineering. PLGA scaffolds have 2 different pore sizes: 150 to 180 μm and 180 to 300 μm. These scaffolds were evaluated in rabbits using autologous DPSCs and were shown to induce osteodentin formation after subcutaneous implantation for 2 and 6 weeks.200
PGA scaffolds were compared with β-tricalcium phosphate (B-TCP), fibrin, and collagen scaffolds for their capacity to grow dental structures when seeded with tooth germs from 6-month-old minipigs.201 On fibrin and collagen gels, the porcine third molar tooth bud maintains its epithelial structure, resembling tooth buds, whereas on PGA and B-TCP, the implanted tooth buds produce more dentin-like material.
The mixtures of PGA fiber mesh scaffolds with porous or nonporous HA/B-TCP were used to seed porcine dental pulp–derived cells and were implanted subcutaneously for 6 weeks. Newly-formed hard tissues were observed in all implants but the dentin-like structure with expression of dentin sialoprotein (DSP), collagen type I, osteonectin, and bone sialoprotein (BSP) was only seen in the PGA-cell implants with porous HA/beta-tricalcium phosphate.202
PGA/PLLA and PLGA scaffolds were used in pioneering work in which scaffolds were formed in tooth molds, seeded with porcine third molar dissociated tooth buds, and allowed to grow in the omenta of athymic rats. After 20, 25, and 30 weeks, tooth-like structures containing pulp, dentin, and enamel were observed, with surrounding cells expressing BSP and amelogenin.203 Similar results were obtained by seeding rat tooth bud cells on both PGA and PLGA scaffolds for 12 weeks in the omentum204 or rat jaw.205
Studies comparing PLGA with HA, B-TCP, or calcium carbonate hydroxyapatite found that human DPSCs proliferated best on PLGA with B-TCP and were able to form mineralized structures. After 4 to 5 weeks, the rat tooth bud cells differentiated and expressed DSP.189
PCL scaffolds were used for the regeneration of various mineralized tissues such as bone, cartilage, and dentin.206–209 The PCL scaffolds support adhesion, proliferation, and odontoblastic differentiation. The incorporation of HA into PCL scaffolds enhances odontoblastic differentiation of human DPSCs.209
PEG is also known as polyethylene oxide or polyoxyethylene. These scaffold materials can support cell growth and differentiation as well as decelerate the degradation of fibrin, thus creating a new hybrid material, PEGylated fibrin gel, for cell delivery.210
Naturally Derived Materials
Naturally derived scaffolds
Alginate has been used in dental engineering to deliver cells and/or growth factors. The alginate hydrogel with either transforming growth factor (TGF)-β1 or acid treatment was applied to slices of human teeth with vital dentin-pulp complex tissues and maintained in culture. Hydrogel with TGF-β1 or acid treatment, but not the untreated control hydrogel, induced dentin matrix secretion and formation of new odontoblast-like cells in the human tooth slices.211
Collagens, particularly type I collagen, are major constituents of dentin and have been used to provide a 3D culture environment for various types of cells, including stem cells from the dental pulp.212 Compared with other natural scaffold products including gelatin and chitosan, the dental pulp cells cultured in the type I and III collagen gel exhibited a higher degree of odontoblastic differentiation as shown by alkaline phosphatase activity and expression of osteocalcin, dentin sialophosphoprotein (DSPP), and dentin matrix protein 1 (DMP1).212–216 Collagen gel can be used alone or in combination with growth factors (eg, TGF-β1, BMP4, FGF2)217 and other scaffold materials such as chitosan.218
Chitosan/HA blend (polyelectrolyte complex) was used for compatibility studies with mesenchymal stem cells. In a 2:1 blend (HA/chitosan), cells were viable for 72 hours and no cytotoxicity was apparent.185 The same group used chitosan/pectin scaffolds for bone regeneration with similarly positive results.184 Chitosan/collagen scaffolds adsorbed with BMP7 were seeded with human adult dental pulp cells and stained positive for dentin matrix proteins DSPP and DMP1, whereas scaffolds without BMP7 were negative.218
Hyaluronic acid sponges were used as 3D scaffolds for the regeneration of dental pulp. In comparison with the collagen sponge, the hyaluronic acid sponge can support cell growth in culture and in vivo from the amputated dental pulp of rat molars, with fewer immunologic reactions as shown by expression of inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin-6, as well as leukocyte infiltration.219 However, when used as an injectable hyaluronic acid gel for soft-tissue augmentation, adverse hypersensitivity reactions were reported, due to impurities and bacterial contamination.220
Fibrin consists of the blood proteins fibrinogen and thrombin, which are produced naturally in the body after injury to establish hemostasis and enhance wound healing. Because of these properties, fibrin glue, fibrin sealant, and fibrin in other forms were produced to aid bleeding control, speed wound healing, cover holes instead of sutures, and provide slow-release delivery of antibiotics or other drugs. Because of their biocompatibility, biodegradability, simple preparation, and manipulation, fibrin scaffolds have been used for multiple purposes (eg, filling in bone cavities, vascular graft, and repairing injuries to urinary tract, liver, and lung) and are also available as mixtures with other polymers such as fibrin-PEG blend.210,221–225 Fibrin hydrogel allows the incorporation of growth factors and bioactive molecules via a heparin-binding delivery system, cell seeding through inkjet printing, and self-assembly through a magnetically influenced technique.225 Blood clots have been used as natural scaffolds for bone healing in the tooth-extraction socket as well as for dental pulp regeneration/revascularization in immature necrotic teeth. Fibrin glue and platelet-rich fibrin can be prepared from whole blood before surgery. The mixture of these 2 components was used as a scaffold for reassembly of porcine tooth bud cells implanted in the extraction socket. After 36 weeks, these implants developed into a complete tooth or an unerupted tooth crown.226 The mixtures of fibrin and other polymers such as PEGylated fibrin scaffold aid in handling the material. The PEGylated fibrin scaffold is injectable, tunable, degradable, and compatible with dental stem cells. It induces osteoblastic and odontoblastic differentiation as well as the formation of dentin-like collagenous matrix and vascularized pulp-like structure after transplantation in vivo.226
Nanostructured Films and Self-assembled Peptides
Recently, investigators have been examining scaffold microtopography and nanotopography as a determinant for successful dentin regeneration. Scaffold nanotopography and molecular self-assembly offer new directions for the fabrication of tissues with similar cell and matrix organization to the native tissues at the nanoscale.227 This nanotechnology can be used not only for tissue engineering but also for the delivery of antimicrobial and/or anti-inflammatory drugs, which will be beneficial for endodontic regeneration. For example, the PGA scaffold was incorporated with an anti-inflammatory peptide, α-melanocortin (α-MSH). The PGA/α-MSH scaffold promotes the adhesion and proliferation of human pulp fibroblasts while inhibiting inflammatory responses.228 In another study, the nanostructured, self-assembling peptides were used as a carrier for the anti-inflammatory drug K5, which inhibits the production of inflammatory cytokines TNF-α and prostaglandin E2 from macrophages.229 More detailed studies are needed to evaluate the effects of these peptides on various cell types in the dental pulp and the formation of dentin in a more clinical relevant setting.
SUMMARY
The emergence of tissue engineering and regenerative medicine shed new light on the treatment of patients with degenerative disorders. These approaches combine tools from a variety of fields such as stem-cell biology, biomaterials, and developmental biology. Whereas regenerative medicine places more emphasis on cell-based therapy, particularly stem cells, to repair or replace damaged tissues/organs, tissue engineering focuses on using biomaterials with or without cells to make bioartificial tissues or organs. Various sources of stem cells have been identified and used to generate the desired specialized cell or tissue types. These stem cells are classified into 3 main types: ES cells, somatic/adult stem cells, and induced pluripotent stem cells. In addition, endothelial cells and their paracrine factors such as vascular endothelial growth factor were shown to play important roles in mediating angiogenesis to nurture engineered tissues or organs and facilitate host integration. Other growth factors and bioactive molecules such as those included in the FGF, BMP, Shh, Wnt, and Notch signaling pathways dictate various aspects of tooth morphogenesis and maturation, and thus can be used to guide the formation of engineered tooth tissues/organs in the manner recapitulating development. These key components are summarized in Fig. 3.
Fig. 3.
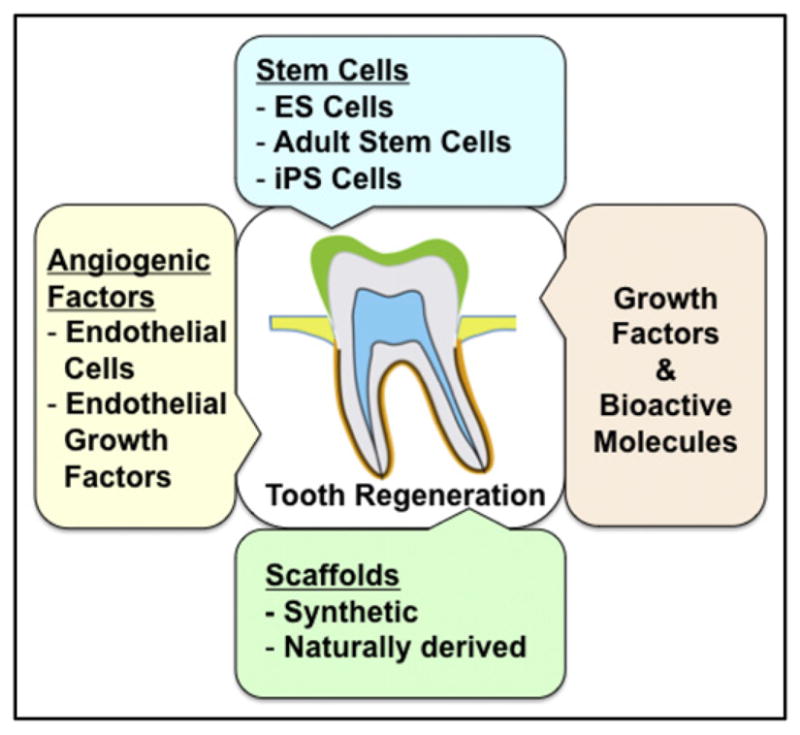
Key components of organ/tissue engineering. Key components include (1) stem cells (ie, embryonic stem cells, ES cells; somatic/adult stem cells; induced pluripotent stem cells, iPS cells) or any cells with the capacity to form the desired tissue/organ; (2) angiogenic factors to enhance vascularization of the engineered tissue/organ; (3) growth factors and/or bioactive molecules such as those in the fibroblast growth factor, bone morphogenetic protein, sonic hedgehog, Wnt, and Notch signaling pathways; and (4) scaffold to deliver cells and drugs. Together with embedded growth factors and/or bioactive molecules, the scaffold provides a microenvironment that supports the development and differentiation of stem cells into specialized cells to form the target tissue/organ.
Finally, in order to apply stem-cell–based therapies to the treatment of diseases, the appropriate microenvironment must be identified to guide the development of stem cells through the following 6 steps:
To increase survival of stem cells in the recipient/transplant.
To integrate the transplanted cells into the surrounding tissue without harming the recipient; research strategies must be created to avoid the problem of immune rejection without long-term use of immunosuppressive drugs.
To increase the proliferation of stem cells to generate sufficient amounts of tissue.
To induce the differentiation of stem cells into the desired cell type(s).
To maintain the differentiated cells and retain their functions throughout the recipient’s life.
To remove unwanted cells.
KEY POINTS.
Dental caries and periodontal disease are the most common diseases resulting in tissue loss. To replace or regenerate new tissues, various types of stem cells have been identified, including embryonic, somatic/adult, and induced pluripotent stem cells. Somatic and induced pluripotent stem cells can be obtained from teeth and periodontium.
Endothelial cells and their paracrine factors mediate the formation of vasculature into engineered tissues or organs.
Growth factors and bioactive molecules dictate various aspects of tooth morphogenesis and maturation and thus can be used to guide the formation of engineered tooth tissues in the manner recapitulating development.
Various biomaterials can be chosen when designing a scaffold, including synthetic, natural, degradable and non-degradable materials.
Advances in biomaterial sciences including microfabrication, self-assembled biomimetic peptides, and three-dimensional printing hold great promise for whole organ or partial tissue regeneration to replace teeth and periodontium.
Acknowledgments
Funding: The authors are supported by the NIH/NIDCR.
The authors thank Dr Kerstin Seidel for help with figures, and members of the Klein and Desai laboratories for helpful discussions.
References
- 1.Kim BS, Nikolovski J, Bonadio J, et al. Engineered smooth muscle tissues: regulating cell phenotype with the scaffold. Exp Cell Res. 1999;251:318–28. doi: 10.1006/excr.1999.4595. [DOI] [PubMed] [Google Scholar]
- 2.Stock UA, Vacanti JP. Tissue engineering: current state and prospects. Annu Rev Med. 2001;52:443–51. doi: 10.1146/annurev.med.52.1.443. [DOI] [PubMed] [Google Scholar]
- 3.Hutmacher DW, Goh JC, Teoh SH. An introduction to biodegradable materials for tissue engineering applications. Ann Acad Med Singapore. 2001;30:183–91. [PubMed] [Google Scholar]
- 4.Gloria A, De Santis R, Ambrosio L. Polymer-based composite scaffolds for tissue engineering. J Appl Biomater Biomech. 2010;8:57–67. [PubMed] [Google Scholar]
- 5.Novotny L, Crha M, Rauser P, et al. Novel biodegradable polydioxanone stents in a rabbit airway model. J Thorac Cardiovasc Surg. 2012;143:437–44. doi: 10.1016/j.jtcvs.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Neeley WL, Redenti S, Klassen H, et al. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials. 2008;29:418–26. doi: 10.1016/j.biomaterials.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao S, Young C, Redenti S, et al. Survival, migration and differentiation of retinal progenitor cells transplanted on micro-machined poly(methyl methacrylate) scaffolds to the subretinal space. Lab Chip. 2007;7:695–701. doi: 10.1039/b618583e. [DOI] [PubMed] [Google Scholar]
- 8.Tao SL, Desai TA. Aligned arrays of biodegradable poly(epsilon-caprolactone) nanowires and nanofibers by template synthesis. Nano Lett. 2007;7:1463–8. doi: 10.1021/nl0700346. [DOI] [PubMed] [Google Scholar]
- 9.Horst M, Madduri S, Gobet R, et al. Engineering functional bladder tissues. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.547. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Mhashilkar A, Atala A. Editorial: effective bio-economic approaches for stem cell therapy and regenerative medicine. Curr Stem Cell Res Ther. 2012;7:1. doi: 10.2174/157488812798483430. [DOI] [PubMed] [Google Scholar]
- 11.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 12.Guarino V, Causa F, Netti PA, et al. The role of hydroxyapatite as solid signal on performance of PCL porous scaffolds for bone tissue regeneration. J Biomed Mater Res B Appl Biomater. 2008;86:548–57. doi: 10.1002/jbm.b.31055. [DOI] [PubMed] [Google Scholar]
- 13.Yokoo T, Fukui A, Kobayashi E. Application of regenerative medicine for kidney diseases. Organogenesis. 2007;3:34–43. doi: 10.4161/org.3.1.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolf AS, Palmer SJ, Snow ML, et al. Creation of a functioning chimeric mammalian kidney. Kidney Int. 1990;38:991–7. doi: 10.1038/ki.1990.303. [DOI] [PubMed] [Google Scholar]
- 15.Herman AR. The history of skin grafts. J Drugs Dermatol. 2002;1:298–301. [PubMed] [Google Scholar]
- 16.Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979;76:5665–8. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981;1:75–8. [PubMed] [Google Scholar]
- 18.Burke JF, Yannas IV, Quinby WC, Jr, et al. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194:413–28. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yannas IV, Burke JF, Orgill DP, et al. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science. 1982;215:174–6. doi: 10.1126/science.7031899. [DOI] [PubMed] [Google Scholar]
- 20.Bell E, Ehrlich HP, Buttle DJ, et al. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981;211:1052–4. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- 21.Bell E, Ehrlich HP, Sher S, et al. Development and use of a living skin equivalent. Plast Reconstr Surg. 1981;67:386–92. doi: 10.1097/00006534-198103000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 23.Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2:403–30. doi: 10.1146/annurev-chembioeng-061010-114257. [DOI] [PubMed] [Google Scholar]
- 24.Norotte C, Marga FS, Niklason LE, et al. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–7. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uygun BE, Soto-Gutierrez A, Yagi H, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shito M, Tilles AW, Tompkins RG, et al. Efficacy of an extracorporeal flat-plate bioartificial liver in treating fulminant hepatic failure. J Surg Res. 2003;111:53–62. doi: 10.1016/s0022-4804(03)00048-9. [DOI] [PubMed] [Google Scholar]
- 27.McGuigan AP, Sefton MV. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials. 2007;28:2547–71. doi: 10.1016/j.biomaterials.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 29.Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res. 2003;58:390–8. doi: 10.1016/s0008-6363(02)00785-x. [DOI] [PubMed] [Google Scholar]
- 30.Zeng Y, Liu YS. Vascular endothelial cells and pituitary hormone producing cells derived from embryonic stem cells therapy for hypopituitarism. Med Hypotheses. 2011;77:680–1. doi: 10.1016/j.mehy.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Jaramillo M, Banerjee I. Endothelial cell co-culture mediates maturation of human embryonic stem cell to pancreatic insulin producing cells in a directed differentiation approach. J Vis Exp. 2012;61:e3759. doi: 10.3791/3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kado M, Lee JK, Hidaka K, et al. Paracrine factors of vascular endothelial cells facilitate cardiomyocyte differentiation of mouse embryonic stem cells. Biochem Biophys Res Commun. 2008;377:413–8. doi: 10.1016/j.bbrc.2008.09.160. [DOI] [PubMed] [Google Scholar]
- 33.Sun J, Zhou W, Ma D, et al. Endothelial cells promote neural stem cell proliferation and differentiation associated with VEGF activated Notch and Pten signaling. Dev Dyn. 2010;239:2345–53. doi: 10.1002/dvdy.22377. [DOI] [PubMed] [Google Scholar]
- 34.Sekine H, Shimizu T, Hobo K, et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:S145–52. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 35.Nyman S, Lindhe J, Karring T, et al. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9:290–6. doi: 10.1111/j.1600-051x.1982.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 36.Al Ghamdi AS, Shibly O, Ciancio SG. Osseous grafting part I: autografts and allografts for periodontal regeneration—a literature review. J Int Acad Periodontol. 2010;12:34–8. [PubMed] [Google Scholar]
- 37.AlGhamdi AS, Shibly O, Ciancio SG. Osseous grafting part II: xenografts and alloplasts for periodontal regeneration—a literature review. J Int Acad Periodontol. 2010;12:39–44. [PubMed] [Google Scholar]
- 38.Izumi Y, Aoki A, Yamada Y, et al. Current and future periodontal tissue engineering. Periodontol 2000. 2011;56:166–87. doi: 10.1111/j.1600-0757.2010.00366.x. [DOI] [PubMed] [Google Scholar]
- 39.Villar CC, Cochran DL. Regeneration of periodontal tissues: guided tissue regeneration. Dent Clin North Am. 2010;54:73–92. doi: 10.1016/j.cden.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Darby I. Periodontal materials. Aust Dent J. 2011;56(Suppl 1):107–18. doi: 10.1111/j.1834-7819.2010.01301.x. [DOI] [PubMed] [Google Scholar]
- 41.Javed F, Al-Askar M, Al-Rasheed A, et al. Significance of the platelet-derived growth factor in periodontal tissue regeneration. Arch Oral Biol. 2011;56:1476–84. doi: 10.1016/j.archoralbio.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Murakami S. Periodontal tissue regeneration by signaling molecule(s): what role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol 2000. 2011;56:188–208. doi: 10.1111/j.1600-0757.2010.00365.x. [DOI] [PubMed] [Google Scholar]
- 43.Kitamura M, Akamatsu M, Machigashira M, et al. FGF-2 stimulates periodontal regeneration: results of a multi-center randomized clinical trial. J Dent Res. 2011;90:35–40. doi: 10.1177/0022034510384616. [DOI] [PubMed] [Google Scholar]
- 44.Hughes FJ, Ghuman M, Talal A. Periodontal regeneration: a challenge for the tissue engineer? Proc Inst Mech Eng H. 2010;224:1345–58. doi: 10.1243/09544119JEIM820. [DOI] [PubMed] [Google Scholar]
- 45.Amin HD, Olsen I, Knowles JC, et al. Differential effect of amelogenin peptides on osteogenic differentiation in vitro: identification of possible new drugs for bone repair and regeneration. Tissue Eng Part A. 2012;18(11–12):1193–202. doi: 10.1089/ten.TEA.2011.0375. [DOI] [PubMed] [Google Scholar]
- 46.Nokhbehsaim M, Deschner B, Winter J, et al. Interactions of regenerative, inflammatory and biomechanical signals on bone morphogenetic protein-2 in periodontal ligament cells. J Periodontal Res. 2011;46:374–81. doi: 10.1111/j.1600-0765.2011.01357.x. [DOI] [PubMed] [Google Scholar]
- 47.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–9. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 48.Cox CF, Bergenholtz G, Heys DR, et al. Pulp capping of dental pulp mechanically exposed to oral microflora: a 1–2 year observation of wound healing in the monkey. J Oral Pathol. 1985;14:156–68. doi: 10.1111/j.1600-0714.1985.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 49.Kling M, Cvek M, Mejare I. Rate and predictability of pulp revascularization in therapeutically reimplanted permanent incisors. Endod Dent Traumatol. 1986;2:83–9. doi: 10.1111/j.1600-9657.1986.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 50.Ohman A. Healing and sensitivity to pain in young replanted human teeth. An experimental, clinical and histological study. Odontol Tidskr. 1965;73:166–227. [PubMed] [Google Scholar]
- 51.Andreasen JO, Borum MK, Jacobsen HL, et al. Replantation of 400 avulsed permanent incisors. 2. Factors related to pulpal healing. Endod Dent Traumatol. 1995;11:59–68. doi: 10.1111/j.1600-9657.1995.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 52.Provenza DV. The blood vascular supply of the dental pulp with emphasis on capillary circulation. Circ Res. 1958;6:213–8. doi: 10.1161/01.res.6.2.213. [DOI] [PubMed] [Google Scholar]
- 53.Matthews JI, Dorman HL, Bishop JG. Fine structures of the dental pulp. J Dent Res. 1959;38:940–6. [Google Scholar]
- 54.Cell biology/introduction/cell size. WIKIBOOKS; [Accessed May 31, 2012]. Available at: http://en.wikibooks.org/wiki/Cell_Biology/Introduction/Cell_size. [Google Scholar]
- 55.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008;34:876–87. doi: 10.1016/j.joen.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 57.Cotti E, Mereu M, Lusso D. Regenerative treatment of an immature, traumatized tooth with apical periodontitis: report of a case. J Endod. 2008;34:611–6. doi: 10.1016/j.joen.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 58.Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr Dent. 2007;29:47–50. [PubMed] [Google Scholar]
- 59.Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35:1343–9. doi: 10.1016/j.joen.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 60.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185–7. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 61.Reynolds K, Johnson JD, Cohenca N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: a case report. Int Endod J. 2009;42:84–92. doi: 10.1111/j.1365-2591.2008.01467.x. [DOI] [PubMed] [Google Scholar]
- 62.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 63.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 65.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 66.Klimanskaya I, Hipp J, Rezai KA, et al. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–45. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- 67.Lund RD, Wang S, Klimanskaya I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–99. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- 68.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 69.Kriks S, Shim JW, Piao J, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–51. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–21. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ledran MH, Krassowska A, Armstrong L, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Cai J, Zhao Y, Liu Y, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–39. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- 74.Lavon N, Yanuka O, Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004;72:230–8. doi: 10.1111/j.1432-0436.2004.07205002.x. [DOI] [PubMed] [Google Scholar]
- 75.Takayama K, Inamura M, Kawabata K, et al. Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4alpha transduction. Mol Ther. 2012;20:127–37. doi: 10.1038/mt.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerami-Naini B, Dovzhenko OV, Durning M, et al. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology. 2004;145:1517–24. doi: 10.1210/en.2003-1241. [DOI] [PubMed] [Google Scholar]
- 77.Chen G, Ye Z, Yu X, et al. Trophoblast differentiation defect in human embryonic stem cells lacking PIG-A and GPI-anchored cell-surface proteins. Cell Stem Cell. 2008;2:345–55. doi: 10.1016/j.stem.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang ZZ, Au P, Chen T, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–8. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 79.Levenberg S, Golub JS, Amit M, et al. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–6. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arpornmaeklong P, Wang Z, Pressler MJ, et al. Expansion and characterization of human embryonic stem cell-derived osteoblast-like cells. Cell Reprogram. 2010;12:377–89. doi: 10.1089/cell.2009.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee KW, Yook JY, Son MY, et al. Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells Dev. 2010;19:557–68. doi: 10.1089/scd.2009.0147. [DOI] [PubMed] [Google Scholar]
- 82.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 85.Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sonoyama W, Liu Y, Yamaza T, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang GT, Sonoyama W, Liu Y, et al. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645–51. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang XP, Suomalainen M, Felszeghy S, et al. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 2007;5:e159. doi: 10.1371/journal.pbio.0050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thesleff I, Wang XP, Suomalainen M. Regulation of epithelial stem cells in tooth regeneration. C R Biol. 2007;330:561–4. doi: 10.1016/j.crvi.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 90.Klein OD, Lyons DB, Balooch G, et al. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–85. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seidel K, Ahn CP, Lyons D, et al. Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development. 2010;137:3753–61. doi: 10.1242/dev.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harada H, Kettunen P, Jung HS, et al. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147:105–20. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith CE, Warshawsky H. Cellular renewal in the enamel organ and the odontoblast layer of the rat incisor as followed by radioautography using 3H-thymidine. Anat Rec. 1975;183:523–61. doi: 10.1002/ar.1091830405. [DOI] [PubMed] [Google Scholar]
- 94.Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–9. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 97.Blanpain C, Lowry WE, Pasolli HA, et al. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–35. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–73. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arnhold S, Klein H, Semkova I, et al. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251–5. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 100.Asano T, Sasaki K, Kitano Y, et al. In vivo tumor formation from primate embryonic stem cells. Methods Mol Biol. 2006;329:459–67. doi: 10.1385/1-59745-037-5:459. [DOI] [PubMed] [Google Scholar]
- 101.Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–57. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 102.Choi J, Costa ML, Mermelstein CS, et al. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci U S A. 1990;87:7988–92. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Janebodin K, Horst OV, Ieronimakis N, et al. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS One. 2011;6:e27526. doi: 10.1371/journal.pone.0027526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vierbuchen T, Ostermeier A, Pang ZP, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Szabo E, Rampalli S, Risueno RM, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–6. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 106.Feng R, Desbordes SC, Xie H, et al. PU. 1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proc Natl Acad Sci U S A. 2008;105:6057–62. doi: 10.1073/pnas.0711961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamanaka S, Takahashi K. Induction of pluripotent stem cells from mouse fibroblast cultures. Tanpakushitsu Kakusan Koso. 2006;51:2346–51. [in Japanese] [PubMed] [Google Scholar]
- 108.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 109.Takahashi K, Okita K, Nakagawa M, et al. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–9. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 110.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 111.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 112.Fan Y, Luo Y, Chen X, et al. Generation of human beta-thalassemia induced pluripotent stem cells from amniotic fluid cells using a single excisable lentiviral stem cell cassette. J Reprod Dev. 2012 doi: 10.1262/jrd.2011-046. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 113.Mou H, Zhao R, Sherwood R, et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10:385–97. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu Y, Liu L, Zhang L, et al. Efficient commitment to functional CD34+ progenitor cells from human bone marrow mesenchymal stem-cell-derived induced pluripotent stem cells. PLoS One. 2012;7:e34321. doi: 10.1371/journal.pone.0034321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oki K, Tatarishvili J, Woods J, et al. Human Induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30(6):1120–33. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 116.Schuldiner M, Itskovitz-Eldor J, Benvenisty N. Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cells. 2003;21:257–65. doi: 10.1634/stemcells.21-3-257. [DOI] [PubMed] [Google Scholar]
- 117.Naujok O, Kaldrack J, Taivankhuu T, et al. Selective removal of undifferentiated embryonic stem cells from differentiation cultures through HSV1 thymidine kinase and ganciclovir treatment. Stem Cell Rev. 2010;6:450–61. doi: 10.1007/s12015-010-9148-z. [DOI] [PubMed] [Google Scholar]
- 118.Hara A, Aoki H, Taguchi A, et al. Neuron-like differentiation and selective ablation of undifferentiated embryonic stem cells containing suicide gene with Oct-4 promoter. Stem Cells Dev. 2008;17:619–27. doi: 10.1089/scd.2007.0235. [DOI] [PubMed] [Google Scholar]
- 119.Schriebl K, Satianegara G, Hwang A, et al. Selective removal of undifferentiated human embryonic stem cells using magnetic activated cell sorting followed by a cytotoxic antibody. Tissue Eng Part A. 2012;18:899–909. doi: 10.1089/ten.TEA.2011.0311. [DOI] [PubMed] [Google Scholar]
- 120.Choo AB, Tan HL, Ang SN, et al. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells. 2008;26:1454–63. doi: 10.1634/stemcells.2007-0576. [DOI] [PubMed] [Google Scholar]
- 121.Lim DY, Ng YH, Lee J, et al. Cytotoxic antibody fragments for eliminating undifferentiated human embryonic stem cells. J Biotechnol. 2011;153:77–85. doi: 10.1016/j.jbiotec.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 122.Goldstein AS, Zhu G, Morris GE, et al. Effect of osteoblastic culture conditions on the structure of poly(DL-lactic-co-glycolic acid) foam scaffolds. Tissue Eng. 1999;5:421–34. doi: 10.1089/ten.1999.5.421. [DOI] [PubMed] [Google Scholar]
- 123.O’Brien FJ, Harley BA, Yannas IV, et al. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433–41. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 124.Zeltinger J, Sherwood JK, Graham DA, et al. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7:557–72. doi: 10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]
- 125.Hoppe A, Guldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–74. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 126.Mounzer AM, McAninch JW, Schmidt RA. Polyglycolic acid mesh in repair of renal injury. Urology. 1986;28:127–30. doi: 10.1016/0090-4295(86)90103-2. [DOI] [PubMed] [Google Scholar]
- 127.Cabaud HE, Feagin JA, Rodkey WG. Acute anterior cruciate ligament injury and repair reinforced with a biodegradable intraarticular ligament. Experimental studies. Am J Sports Med. 1982;10:259–65. doi: 10.1177/036354658201000501. [DOI] [PubMed] [Google Scholar]
- 128.Rodkey WG, Cabaud HE, Feagin JA, et al. A partially biodegradable material device for repair and reconstruction of injured tendons. Experimental studies. Am J Sports Med. 1985;13:242–7. doi: 10.1177/036354658501300405. [DOI] [PubMed] [Google Scholar]
- 129.Townley CO, Fumich RM, Shall LM. The free synovial graft as a shield for collagen ingrowth in cruciate ligament repair. Clin Orthop Relat Res. 1985;(197):266–71. [PubMed] [Google Scholar]
- 130.Freed LE, Vunjak-Novakovic G, Biron RJ, et al. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (N Y) 1994;12:689–93. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 131.Thomson RC, Yaszemski MJ, Powers JM, et al. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J Biomater Sci Polym Ed. 1995;7:23–38. doi: 10.1163/156856295x00805. [DOI] [PubMed] [Google Scholar]
- 132.Wintermantel E, Cima L, Schloo B, et al. Angiopolarity: a new design parameter for cell transplantation devices and its application to degradable systems. ASAIO Trans. 1991;37:M334–6. [PubMed] [Google Scholar]
- 133.Cao Y, Vacanti JP, Paige KT, et al. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg. 1997;100:297–302. doi: 10.1097/00006534-199708000-00001. [discussion: 303–4] [DOI] [PubMed] [Google Scholar]
- 134.Singhal AR, Agrawal CM, Athanasiou KA. Salient degradation features of a 50:50 PLA/PGA scaffold for tissue engineering. Tissue Eng. 1996;2:197–207. doi: 10.1089/ten.1996.2.197. [DOI] [PubMed] [Google Scholar]
- 135.Chu CR, Coutts RD, Yoshioka M, et al. Articular cartilage repair using allogeneic perichondrocyte-seeded biodegradable porous polylactic acid (PLA): a tissue-engineering study. J Biomed Mater Res. 1995;29:1147–54. doi: 10.1002/jbm.820290915. [DOI] [PubMed] [Google Scholar]
- 136.Evans GR, Brandt K, Widmer MS, et al. In vivo evaluation of poly(L-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials. 1999;20:1109–15. doi: 10.1016/s0142-9612(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 137.Li WJ, Cooper JA, Jr, Mauck RL, et al. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2:377–85. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 138.Doyle V, Pearson R, Lee D, et al. An investigation of the growth of human dermal fibroblasts on poly-L-lactic acid in vitro. J Mater Sci Mater Med. 1996;7:381–5. [Google Scholar]
- 139.Corden TJ, Jones IA, Rudd CD, et al. Physical and biocompatibility properties of poly-epsilon-caprolactone produced using in situ polymerisation: a novel manufacturing technique for long-fibre composite materials. Biomaterials. 2000;21:713–24. doi: 10.1016/s0142-9612(99)00236-7. [DOI] [PubMed] [Google Scholar]
- 140.Calvert JW, Marra KG, Cook L, et al. Characterization of osteoblast-like behavior of cultured bone marrow stromal cells on various polymer surfaces. J Biomed Mater Res. 2000;52:279–84. doi: 10.1002/1097-4636(200011)52:2<279::aid-jbm6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 141.Marra KG, Szem JW, Kumta PN, et al. In vitro analysis of biodegradable polymer blend/hydroxyapatite composites for bone tissue engineering. J Biomed Mater Res. 1999;47:324–35. doi: 10.1002/(sici)1097-4636(19991205)47:3<324::aid-jbm6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 142.Pitt CG, Chasalow FI, Hibionada YM, et al. Aliphatic polyesters. 1. The degradation of poly(epsilon-caprolactone) in vivo. J Appl Polym Sci. 1981;26:3779–87. [Google Scholar]
- 143.Pitt CG, Gratzl MM, Kimmel GL, et al. Aliphatic polyesters. 2. The degradation of poly(DL-lactide), poly(epsilon-caprolactone), and their copolymers in vivo. Biomaterials. 1981;2:215–20. doi: 10.1016/0142-9612(81)90060-0. [DOI] [PubMed] [Google Scholar]
- 144.Bettencourt A, Almeida AJ. Poly(methyl methacrylate) particulate carriers in drug delivery. J Microencapsul. 2012;29(4):353–67. doi: 10.3109/02652048.2011.651500. [DOI] [PubMed] [Google Scholar]
- 145.Rogers-Foy JM, Powers DL, Brosnan DA, et al. Hydroxyapatite composites designed for antibiotic drug delivery and bone reconstruction: a caprine model. J Invest Surg. 1999;12:263–75. doi: 10.1080/089419399272386. [DOI] [PubMed] [Google Scholar]
- 146.Liu Y, Ji Y, Ghosh K, et al. Effects of fiber orientation and diameter on the behavior of human dermal fibroblasts on electrospun PMMA scaffolds. J Biomed Mater Res A. 2009;90:1092–106. doi: 10.1002/jbm.a.32165. [DOI] [PubMed] [Google Scholar]
- 147.Arevalo-Silva CA, Eavey RD, Cao Y, et al. Internal support of tissue-engineered cartilage. Arch Otolaryngol Head Neck Surg. 2000;126:1448–52. doi: 10.1001/archotol.126.12.1448. [DOI] [PubMed] [Google Scholar]
- 148.Lynam D, Bednark B, Peterson C, et al. Precision microchannel scaffolds for central and peripheral nervous system repair. J Mater Sci Mater Med. 2011;22:2119–30. doi: 10.1007/s10856-011-4387-3. [DOI] [PubMed] [Google Scholar]
- 149.Peck M, Gebhart D, Dusserre N, et al. The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs. 2012;195:144–58. doi: 10.1159/000331406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kral JG, Crandall DL. Development of a human adipocyte synthetic polymer scaffold. Plast Reconstr Surg. 1999;104:1732–8. doi: 10.1097/00006534-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 151.Springer IN, Fleiner B, Jepsen S, et al. Culture of cells gained from temporomandibular joint cartilage on non-absorbable scaffolds. Biomaterials. 2001;22:2569–77. doi: 10.1016/s0142-9612(01)00148-x. [DOI] [PubMed] [Google Scholar]
- 152.Patel AA, Desai TA, Kumar S. Microtopographical assembly of cardiomyocytes. Integr Biol (Camb) 2011;3:1011–9. doi: 10.1039/c1ib00024a. [DOI] [PubMed] [Google Scholar]
- 153.Ayala P, Desai TA. Integrin alpha3 blockade enhances microtopographical down-regulation of alpha-smooth muscle actin: role of microtopography in ECM regulation. Integr Biol (Camb) 2011;3:733–41. doi: 10.1039/c1ib00012h. [DOI] [PubMed] [Google Scholar]
- 154.Kim EJ, Boehm CA, Mata A, et al. Post microtextures accelerate cell proliferation and osteogenesis. Acta Biomater. 2010;6:160–9. doi: 10.1016/j.actbio.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mata A, Kim EJ, Boehm CA, et al. A three-dimensional scaffold with precise micro-architecture and surface micro-textures. Biomaterials. 2009;30:4610–7. doi: 10.1016/j.biomaterials.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leclerc E, Sakai Y, Fujii T. Microfluidic PDMS (polydimethylsiloxane) bioreactor for large-scale culture of hepatocytes. Biotechnol Prog. 2004;20:750–5. doi: 10.1021/bp0300568. [DOI] [PubMed] [Google Scholar]
- 157.Fujita H, Shimizu K, Nagamori E. Novel method for fabrication of skeletal muscle construct from the C2C12 myoblast cell line using serum-free medium AIM-V. Biotechnol Bioeng. 2009;103:1034–41. doi: 10.1002/bit.22318. [DOI] [PubMed] [Google Scholar]
- 158.Refojo MF. Contact lens materials. Int Ophthalmol Clin. 1973;13:263–77. doi: 10.1097/00004397-197301310-00020. [DOI] [PubMed] [Google Scholar]
- 159.Hejcl A, Lesny P, Pradny M, et al. Biocompatible hydrogels in spinal cord injury repair. Physiol Res. 2008;57(Suppl 3):S121–32. doi: 10.33549/physiolres.931606. [DOI] [PubMed] [Google Scholar]
- 160.Madden LR, Mortisen DJ, Sussman EM, et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A. 2010;107:15211–6. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Netti PA, Shelton JC, Revell PA, et al. Hydrogels as an interface between bone and an implant. Biomaterials. 1993;14:1098–104. doi: 10.1016/0142-9612(93)90211-j. [DOI] [PubMed] [Google Scholar]
- 162.Young CD, Wu JR, Tsou TL. High-strength, ultra-thin and fiber-reinforced pHE-MA artificial skin. Biomaterials. 1998;19:1745–52. doi: 10.1016/s0142-9612(98)00083-0. [DOI] [PubMed] [Google Scholar]
- 163.Aung T, Inoue K, Kogire M, et al. Comparison of various gels for immobilization of islets in bioartificial pancreas using a mesh-reinforced polyvinyl alcohol hydrogel tube. Transplant Proc. 1995;27:619–21. [PubMed] [Google Scholar]
- 164.Aung T, Inoue K, Kogire M, et al. Improved insulin release from a bioartificial pancreas using mesh-reinforced polyvinyl alcohol hydrogel tube: immobilization of islets in agarose gel. Transplant Proc. 1994;26:790–1. [PubMed] [Google Scholar]
- 165.Qi Z, Shen Y, Yanai G, et al. The in vivo performance of polyvinyl alcohol macro-encapsulated islets. Biomaterials. 2010;31:4026–31. doi: 10.1016/j.biomaterials.2010.01.088. [DOI] [PubMed] [Google Scholar]
- 166.Qi M, Gu Y, Sakata N, et al. PVA hydrogel sheet macroencapsulation for the bioartificial pancreas. Biomaterials. 2004;25:5885–92. doi: 10.1016/j.biomaterials.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 167.Taheri A, Atyabi F, Dinarvnd R. Temperature-responsive and biodegradable PVA: PVP k30:poloxamer 407 hydrogel for controlled delivery of human growth hormone (hGH) J Pediatr Endocrinol Metab. 2011;24:175–9. doi: 10.1515/jpem.2011.079. [DOI] [PubMed] [Google Scholar]
- 168.Bakhshandeh H, Soleimani M, Hosseini SS, et al. Poly (epsilon-caprolactone) nanofibrous ring surrounding a polyvinyl alcohol hydrogel for the development of a biocompatible two-part artificial cornea. Int J Nanomedicine. 2011;6:1509–15. doi: 10.2147/IJN.S19011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bodugoz-Senturk H, Macias CE, Kung JH, et al. Poly(vinyl alcohol)-acrylamide hydrogels as load-bearing cartilage substitute. Biomaterials. 2009;30:589–96. doi: 10.1016/j.biomaterials.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 170.Smidsrod O, Skjak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–8. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 171.Drury JL, Dennis RG, Mooney DJ. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187–99. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 172.Yuan Z, Nie H, Wang S, et al. Biomaterial selection for tooth regeneration. Tissue Eng Part B Rev. 2011;17:373–88. doi: 10.1089/ten.teb.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Khanarian NT, Haney NM, Burga RA, et al. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials. 2012;33(21):5247–58. doi: 10.1016/j.biomaterials.2012.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Khanarian NT, Jiang J, Wan LQ, et al. A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng Part A. 2012;18:533–45. doi: 10.1089/ten.tea.2011.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Balgude AP, Yu X, Szymanski A, et al. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–84. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 176.Rotter N, Aigner J, Naumann A, et al. Behavior of tissue-engineered human cartilage after transplantation into nude mice. J Mater Sci Mater Med. 1999;10:689–93. doi: 10.1023/a:1008912514271. [DOI] [PubMed] [Google Scholar]
- 177.Hung CT, Lima EG, Mauck RL, et al. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech. 2003;36:1853–64. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 178.Alaminos M, Del Carmen Sanchez-Quevedo M, Munoz-Avila JI, et al. Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Invest Ophthalmol Vis Sci. 2006;47:3311–7. doi: 10.1167/iovs.05-1647. [DOI] [PubMed] [Google Scholar]
- 179.Obara K, Ishihara M, Ishizuka T, et al. Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates wound healing in healing-impaired db/db mice. Biomaterials. 2003;24:3437–44. doi: 10.1016/s0142-9612(03)00220-5. [DOI] [PubMed] [Google Scholar]
- 180.Liu ZQ, Wang HB, Wang Y, et al. The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment. Biomaterials. 2012;33:3093–106. doi: 10.1016/j.biomaterials.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 181.Zakhem E, Raghavan S, Gilmont RR, et al. Chitosan-based scaffolds for the support of smooth muscle constructs in intestinal tissue engineering. Biomaterials. 2012;33:4810–7. doi: 10.1016/j.biomaterials.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pakulska MM, Ballios BG, Shoichet MS. Injectable hydrogels for central nervous system therapy. Biomed Mater. 2012;7:024101. doi: 10.1088/1748-6041/7/2/024101. [DOI] [PubMed] [Google Scholar]
- 183.Hong Y, Gong Y, Gao C, et al. Collagen-coated polylactide microcarriers/chitosan hydrogel composite: injectable scaffold for cartilage regeneration. J Biomed Mater Res A. 2008;85:628–37. doi: 10.1002/jbm.a.31603. [DOI] [PubMed] [Google Scholar]
- 184.Coimbra P, Ferreira P, de Sousa HC, et al. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int J Biol Macromol. 2011;48:112–8. doi: 10.1016/j.ijbiomac.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 185.Coimbra P, Alves P, Valente TA, et al. Sodium hyaluronate/chitosan polyelectrolyte complex scaffolds for dental pulp regeneration: synthesis and characterization. Int J Biol Macromol. 2011;49:573–9. doi: 10.1016/j.ijbiomac.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 186.Sahoo SK, Panda AK, Labhasetwar V. Characterization of porous PLGA/PLA microparticles as a scaffold for three dimensional growth of breast cancer cells. Biomacromolecules. 2005;6:1132–9. doi: 10.1021/bm0492632. [DOI] [PubMed] [Google Scholar]
- 187.Li C, Wang L, Yang Z, et al. A viscoelastic chitosan-modified three-dimensional porous poly(L-lactide-co-epsilon-caprolactone) scaffold for cartilage tissue engineering. J Biomater Sci Polym Ed. 2012;23:405–24. doi: 10.1163/092050610X551970. [DOI] [PubMed] [Google Scholar]
- 188.Eastoe JE. Organic matrix of tooth enamel. Nature. 1960;187:411–2. doi: 10.1038/187411b0. [DOI] [PubMed] [Google Scholar]
- 189.Zheng L, Yang F, Shen H, et al. The effect of composition of calcium phosphate composite scaffolds on the formation of tooth tissue from human dental pulp stem cells. Biomaterials. 2011;32:7053–9. doi: 10.1016/j.biomaterials.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 190.Ebrahimian-Hosseinabadi M, Ashrafizadeh F, Etemadifar M, et al. Evaluating and modeling the mechanical properties of the prepared PLGA/nano-BCP composite scaffolds for bone tissue engineering. J Mater Sci Tech. 2011;27:1105–12. [Google Scholar]
- 191.Arinzeh TL, Tran T, McAlary J, et al. A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials. 2005;26:3631–8. doi: 10.1016/j.biomaterials.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 192.Mironov V, Boland T, Trusk T, et al. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21:157–61. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 193.Boland T, Mironov V, Gutowska A, et al. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:497–502. doi: 10.1002/ar.a.10059. [DOI] [PubMed] [Google Scholar]
- 194.Jakab K, Norotte C, Marga F, et al. Tissue engineering by self-assembly and bioprinting of living cells. Biofabrication. 2010;2:022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fernandez JG, Khademhosseini A. Micro-masonry: construction of 3D structures by microscale self-assembly. Adv Mater. 2010;22:2538–41. doi: 10.1002/adma.200903893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Crubezy E, Murail P, Girard L, et al. False teeth of the Roman world. Nature. 1998;391:29. doi: 10.1038/34067. [DOI] [PubMed] [Google Scholar]
- 197.Bobbio A. The first endosseous alloplastic implant in the history of man. Bull Hist Dent. 1972;20:1–6. [PubMed] [Google Scholar]
- 198.Mooney DJ, Powell C, Piana J, et al. Engineering dental pulp-like tissue in vitro. Biotechnol Prog. 1996;12:865–8. doi: 10.1021/bp960073f. [DOI] [PubMed] [Google Scholar]
- 199.Buurma B, Gu K, Rutherford RB. Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds. Eur J Oral Sci. 1999;107:282–9. doi: 10.1046/j.0909-8836.1999.eos107408.x. [DOI] [PubMed] [Google Scholar]
- 200.El-Backly RM, Massoud AG, El-Badry AM, et al. Regeneration of dentine/pulp-like tissue using a dental pulp stem cell/poly(lactic-co-glycolic) acid scaffold construct in New Zealand white rabbits. Aust Endod J. 2008;34:52–67. doi: 10.1111/j.1747-4477.2008.00139.x. [DOI] [PubMed] [Google Scholar]
- 201.Ohara T, Itaya T, Usami K, et al. Evaluation of scaffold materials for tooth tissue engineering. J Biomed Mater Res A. 2010;94:800–5. doi: 10.1002/jbm.a.32749. [DOI] [PubMed] [Google Scholar]
- 202.Tonomura A, Mizuno D, Hisada A, et al. Differential effect of scaffold shape on dentin regeneration. Ann Biomed Eng. 2010;38:1664–71. doi: 10.1007/s10439-010-9910-z. [DOI] [PubMed] [Google Scholar]
- 203.Young CS, Terada S, Vacanti JP, et al. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81:695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- 204.Duailibi MT, Duailibi SE, Young CS, et al. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523–8. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 205.Duailibi SE, Duailibi MT, Zhang W, et al. Bioengineered dental tissues grown in the rat jaw. J Dent Res. 2008;87:745–50. doi: 10.1177/154405910808700811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Savarino L, Baldini N, Greco M, et al. The performance of poly-epsilon-caprolactone scaffolds in a rabbit femur model with and without autologous stromal cells and BMP4. Biomaterials. 2007;28:3101–9. doi: 10.1016/j.biomaterials.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 207.Williams JM, Adewunmi A, Schek RM, et al. Bone tissue engineering using poly-caprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–27. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 208.Li WJ, Tuli R, Okafor C, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 209.Yang X, Yang F, Walboomers XF, et al. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J Biomed Mater Res A. 2010;93:247–57. doi: 10.1002/jbm.a.32535. [DOI] [PubMed] [Google Scholar]
- 210.Galler KM, Cavender AC, Koeklue U, et al. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen Med. 2011;6:191–200. doi: 10.2217/rme.11.3. [DOI] [PubMed] [Google Scholar]
- 211.Dobie K, Smith G, Sloan AJ, et al. Effects of alginate hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Res. 2002;43:387–90. doi: 10.1080/03008200290000574. [DOI] [PubMed] [Google Scholar]
- 212.Kim NR, Lee DH, Chung PH, et al. Distinct differentiation properties of human dental pulp cells on collagen, gelatin, and chitosan scaffolds. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e94–100. doi: 10.1016/j.tripleo.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 213.Mizuno M, Miyamoto T, Wada K, et al. Type I collagen regulated dentin matrix protein-1 (Dmp-1) and osteocalcin (OCN) gene expression of rat dental pulp cells. J Cell Biochem. 2003;88:1112–9. doi: 10.1002/jcb.10466. [DOI] [PubMed] [Google Scholar]
- 214.Srisuwan T, Tilkorn DJ, Al-Benna S, et al. Revascularization and tissue regeneration of an empty root canal space is enhanced by a direct blood supply and stem cells. Dent Traumatol. 2012 doi: 10.1111/j.1600-9657.2012.01136.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 215.Yamauchi N, Yamauchi S, Nagaoka H, et al. Tissue engineering strategies for immature teeth with apical periodontitis. J Endod. 2011;37:390–7. doi: 10.1016/j.joen.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 216.Yamauchi N, Nagaoka H, Yamauchi S, et al. Immunohistological characterization of newly formed tissues after regenerative procedure in immature dog teeth. J Endod. 2011;37:1636–41. doi: 10.1016/j.joen.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 217.Srisuwan T, Tilkorn DJ, Al-Benna S, et al. Survival of rat functional dental pulp cells in vascularized tissue engineering chambers. Tissue Cell. 2012;44:111–21. doi: 10.1016/j.tice.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 218.Yang X, Han G, Pang X, et al. Chitosan/collagen scaffold containing bone morphogenetic protein-7 DNA supports dental pulp stem cell differentiation in vitro and in vivo. J Biomed Mater Res A. 2012 doi: 10.1002/jbm.a.34064. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 219.Inuyama Y, Kitamura C, Nishihara T, et al. Effects of hyaluronic acid sponge as a scaffold on odontoblastic cell line and amputated dental pulp. J Biomed Mater Res B Appl Biomater. 2010;92:120–8. doi: 10.1002/jbm.b.31497. [DOI] [PubMed] [Google Scholar]
- 220.Friedman PM, Mafong EA, Kauvar AN, et al. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg. 2002;28:491–4. doi: 10.1046/j.1524-4725.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- 221.Atrah HI. Fibrin glue. BMJ. 1994;308:933–4. doi: 10.1136/bmj.308.6934.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Evans LA, Ferguson KH, Foley JP, et al. Fibrin sealant for the management of genitourinary injuries, fistulas and surgical complications. J Urol. 2003;169:1360–2. doi: 10.1097/01.ju.0000052663.84060.ea. [DOI] [PubMed] [Google Scholar]
- 223.Feinstein AJ, Varela JE, Cohn SM, et al. Fibrin glue eliminates the need for packing after complex liver injuries. Yale J Biol Med. 2001;74:315–21. [PMC free article] [PubMed] [Google Scholar]
- 224.Bastarache JA. The complex role of fibrin in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;296:L275–6. doi: 10.1152/ajplung.90633.2008. [DOI] [PubMed] [Google Scholar]
- 225.Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 226.Yang KC, Wang CH, Chang HH, et al. Fibrin glue mixed with platelet-rich fibrin as a scaffold seeded with dental bud cells for tooth regeneration. J Tissue Eng Regen Med. 2011 doi: 10.1002/term.483. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 227.Reches M, Gazit E. Controlled patterning of aligned self-assembled peptide nanotubes. Nat Nanotechnol. 2006;1:195–200. doi: 10.1038/nnano.2006.139. [DOI] [PubMed] [Google Scholar]
- 228.Fioretti F, Mendoza-Palomares C, Helms M, et al. Nanostructured assemblies for dental application. ACS Nano. 2010;4:3277–87. doi: 10.1021/nn100713m. [DOI] [PubMed] [Google Scholar]
- 229.Yang WS, Park YC, Kim JH, et al. Nanostructured, self-assembling peptide K5 blocks TNF-alpha and PGE(2) production by suppression of the AP-1/p38 pathway. Mediators Inflamm. 2012;2012:489810. doi: 10.1155/2012/489810. [DOI] [PMC free article] [PubMed] [Google Scholar]


