Abstract
In this issue of Immunity, Hegazy et al. (2010) report that in response to lymphocytic choriomeningitis virus (LCMV) infection, fully differentiated virus-specific Th2 cells can be reprogrammed into GATA-3+T-bet+ cells capable of producing both interleukin-4 and interferon-γ.
CD4+ T cells, also know as T helper (Th) cells, play critical roles in orchestrating adaptive immune responses to a variety of infectious pathogens, allergens, and self-antigens. Based on their functions and their patterns of cytokine expression, activated Th cells were initially classified into two lineages, Th1 and Th2 cells (Mosmann and Coffman, 1989). Th1 cells produce interferon-γ (IFN-γ) and Th2 cells produce interleukin-4 (IL-4), IL-5, and IL-13 as their signature cytokines. The Th1-Th2 cell paradigm dominated the field for almost two decades, until Th17 (IL-17-producing Th) cells and iTreg (inducible regulatory T) cells that are also differentiated from naive CD4+ T cells were reported (for review, see Zhu and Paul, 2008). With the discovery of these unique CD4+ T lineages, the relationship among these different Th cell types has become an important question. Many reports have shown that although the phenotypes of differentiated Th1 and Th2 cells are relatively stable, Th17 and Treg cells, including natural-occurring regulatory T (nTreg) and iTreg cells, are plastic (for review, see Zhou et al., 2009). In this issue of Immunity, Hegazy et al. (2010) show that well-differentiated Th2 cells can also be “taught” by appropriate stimuli to produce IFN-γ both in vivo and in vitro.
Early studies of Th1 and Th2 cell clones implied that these cells are terminally differentiated (Mosmann and Coffman, 1989). In long-term mouse CD4+ T cell clones, the expression of IFN-γ and IL-4 was mutually exclusive. Both IFN-γ and IL-12, two cytokines that are important for inducing Th1 cells, also inhibit Th2 cell differentiation (Zhu and Paul, 2008). Similarly, IL-4, the critical cytokine driving Th2 cell differentiation, especially in vitro, suppresses the development of Th1 cells. The master transcription factors, T-bet and GATA-3, are also preferentially expressed in Th1 and Th2 cells, respectively. Indeed, differentiated Th1 cells fail to produce IL-4 either when they are reactivated in the presence of IL-4 or when GATA-3 expression is enforced in these cells by retroviral-mediated transduction. It is also difficult to induce IFN-γ production in fully differentiated Th2 cells with Th1 cell-inducing cytokines, IL-12 and IFN-γ, although enforced T-bet expression in Th2 cells induces IFN-γ production.
A recent study using genome-wide analysis to compare patterns of epigenetic modifications in a variety of CD4+ cells, including naive, nTreg, differentiated Th1, Th2, Th17, and iTreg cells, demonstrates that bivalent permissive and suppressive forms of histone modifications are found at Tbx21, the gene locus encoding T-bet, in all non-Th1 cells, suggesting that these differentiated cells may retain their capacity to express T-bet (Wei et al., 2009). This was also true for histone modifications at the Gata3 locus in all non-Th2 cells. Although both Th17 and nTreg cells have been shown to express T-bet and IFN-γ when they are stimulated through their T cell receptor (TCR) in the presence of IL-12 (Lee et al., 2009; Wei et al., 2009), our unpublished data suggest that in contrast to fully differentiated Th17 and Treg cells that can turn on T-bet expression, fully differentiated Th2 cells fail to upregulate T-bet and to acquire IFN-γ-producing capacity in response to IL-12 during reactivation. Therefore, it seemed curious that bivalent modifications at the Tbx21 locus were found in Th2 cells. Hegazy et al. (2010) now solve this enigma by showing that differentiated Th2 cells can also be stimulated to express T-bet and to acquire IFN-γ-producing capability when stimulated appropriately. Therefore, the bivalent epigenetic modifications at Tbx21 locus found in all non-Th1 cells appear to underlie the mechanism of plasticity of these cells to become IFN-γ-producing cells when needed.
Hegazy et al. (2010) show that both antigen-specific TCR stimulation and an inflammatory environment that would normally induce a Th1 cell response are required for causing Th2 cells to produce IFN-γ in vivo and that such IFN-γ production completely depends on T-bet upregulation (Figure 1). Seven to ten days after LCMV infection, transferred Th2 cells, bearing TCR specific for the LCMV epitope GP61–68, that have been differentiated for 2–3 weeks in vitro, upregulated T-bet and acquired IFN-γ-producing capacity. However, these Th2 cells failed to produce IFN-γ in mice infected with a mutant LCMV strain lacking GP61–68, indicating the importance of TCR stimulation for alteration in cytokine-producing potential. The cytokines IL-12, IFN-γ, and type I interferons (IFN-α and -β), which are induced by LCMV infection, contributed to the conversion of Th2 cells in vivo. Antigen-specific Th2 cells transferred into IL-12p40-deficient recipients produced half as much IFN-γ as those transferred into wild-type mice upon LCMV infection. IFN-γR1-deficient and IFN-αR1-deficient Th2 cells almost completely lost their ability to acquire IFN-γ-producing capacity in response to LCMV. Therefore, both TCR stimulation and cytokine-signaling elicited by IL-12, IFN-γ, and type I interferons are required for upregulating T-bet expression and inducing IFN-γ in differentiated Th2 cells in vivo.
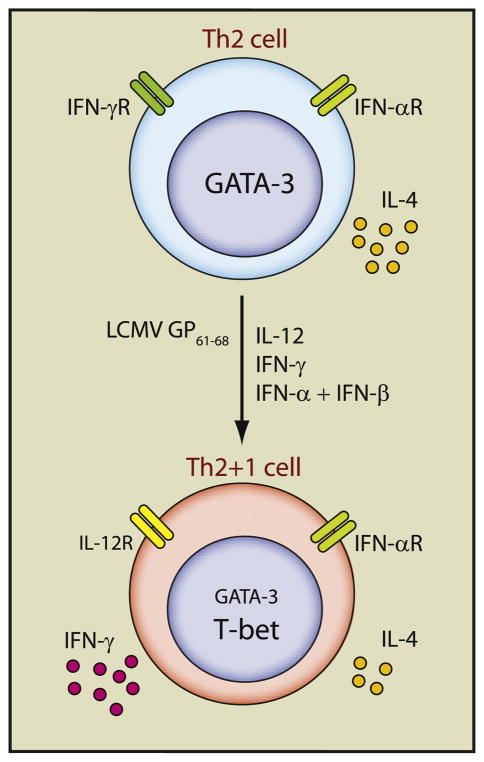
Figure 1. Reprogramming of Th2 Cells into GATA-3+T-bet+ Cells.
Antigen-specific TCR stimulation and a Th1 cytokine inflammatory environment are required for inducing T-bet-dependent IFN-γ production by fully differentiated Th2 cells. GP61–68-specific Th2 cells that had been differentiated in vitro for 2–3 weeks express high amounts of GATA-3 and produce IL-4 upon stimulation. They can respond to IFN-γ and type I interferons (IFN-α and -β), which induce STAT1 and STAT4 activation. In response to LCMV infection after transfer, these Th2 cells are activated by the GP61–68 epitope in the presence of the inflammatory cytokines, IL-12, IFN-γ, and type I interferons. All these stimuli are required for inducing GATA-3+T-bet+ “Th2+1” cells capable of producing both IL-4 and IFN-γ. Like Th1 cells, the “Th2+1” cells upregulate IL-12Rβ2 and down-regulate IFN-γR expression but these cells continue to express intermediate levels of GATA-3 and IL-4, which is distinct from classic Th1 cells. The “Th2+1” cells persist and retain their phenotype for more than 60 days after LCMV infection. T-bet-deficient Th2 cells fail to be converted to “Th2+1” phenotype; they are unable to produce IFN-γ but continue to express high amounts of GATA-3 and IL-4 in response to LCMV infection, resulting in fatal immunopathology.
Hegazy et al. (2010) also show that cytokine-signaling pathways triggered by IL-12, IFN-γ, and type I interferons are necessary and sufficient for inducing IFN-γ production by Th2 cells in vitro. IL-12 and IFN-γ, the two cytokines that are generally used for in vitro Th1 cell differentiation of naive CD4+ T cells, failed to convert Th2 cells to IFN-γ producers. This could be accounted for the lack of IL-12Rβ2 expression on Th2 cells and the resultant failure of IL-12 to activate STAT4 in these cells, despite the capacity of Th2 cells to activate STAT1 in response to IFN-γ. Type I interferons activate both STAT1 and STAT4 in Th2 cells; type I interferons and IFN-γ have an additive effect on STAT1 activation. It seems likely that combined stimulation of Th2 cells by IFN-γ and type I interferons results in expression of IL-12Rβ2 on these cells and their acquisition of responsiveness to IL-12. Therefore, the combination of IL-12, IFN-γ, and type I interferons is the key for inducing T-bet expression and IFN-γ production in Th2 cells. The Th2 cells used for the in vivo conversion experiments had been differentiated for 2–3 weeks in vitro prior to transferring to the mice that were then infected with LCMV. By contrast, the Th2 cells used in the in vitro conversion experiments had been cultured for only 5 days and thus may have not been fully differentiated. It remains to be tested whether additional stimuli besides IL-12, IFN-γ, and type I interferons may be needed for converting Th2 cells that had been differentiated for 2–3 weeks into T-bet-expressing cells in vitro.
Hegazy et al. (2010) demonstrate that when T-bet is upregulated in differentiated Th2 cells both in vivo, by LCMV infection, and in vitro, by IL-12, IFN-γ, and type I interferons, the expression of GATA-3 is only modestly suppressed, resulting in GATA-3+T-bet+ “Th2+1” cells. It has already been shown that some transcription factors of different lineages can be coexpressed. For example, Foxp3+ RORγt+ cells (Zhou et al., 2008) and Foxp3+T-bet+ cells (Koch et al., 2009) have recently been found in vivo. The discovery of GATA-3+T-bet+ cells raises the question of the existence of other transcription factor coexpressers, such as GATA-3+Bcl-6+ Th2-like follicular helper T cells and GATA-3+Foxp3+ Th2-related regulatory T cells.
The GATA-3+T-bet+ cells are able to produce both IL-4 and IFN-γ, an interesting feature also displayed by NKT cells that have been reported earlier to coexpress GATA-3 and T-bet. In fact, almost all the IL-4-producing cells found after LCMV infection also coexpress IFN-γ. These GATA-3+T-bet+ cells can persist in animals for more than 60 days after LCMV infection. However, the physiological function of these cells remains to be carefully tested. For example, it is unknown that whether these cells will give protection to LCMV-infected mice when endogenous Th1 cells are absent. Hegazy et al. (2010) do show that if these virus-specific Th2 cells fail to convert into “Th2+1” cells (i.e., if they are derived from T-bet-deficient donors), they can induce fatal immunopathology during LCMV infection. However, this result needs to be interpreted with care because 1–3 million T-bet-deficient LCMV-specific Th2 cells were transferred in these experiments. Assuming a transfer efficiency of 10%, these mice will have 100,000 or more highly polarized, recently primed LCMV-specific Th2 cells, which should be quite different in both number and degree of activation than the number of Th2 memory cells that might exist in a mouse previously primed under Th2 cell-inducing conditions and then challenged with a Th1 cell-inducing infectious agent with a cross-reactive epitope. Furthermore, will cotransfer of wild-type and T-bet-deficient virus-specific Th2 cells still induce such fatal immunopathology?
Despite these unresolved issues, this report is quite important. It dramatically changes our current view on classic Th1 and Th2 cells and extends the recent finding of CD4+ T cell plasticity from Th17 and Treg cells to Th2 cells. It also provokes many important future questions. Are in vivo-generated Th2 cells also plastic? Can one identify “Th2+1” cells in humans? Can these “Th2+1” cells efficiently exert Th1 or Th2 functions without having cross-inhibitory effects between IL-4 and IFN-γ? Can Th2 cells be induced to express IL-17? Because bivalent histone H3 modifications were found at the Gata3 locus in non-Th2 cells similar to the bivalent histone H3 modifications at the Tbx21 locus in non-Th1 cells, can fully differentiated Th1 cells be made to express Th2 cytokines?
The plasticity of Th cells in general may become particularly important when the naive CD4+ T cell pool is depleted with age and thus cross-reactivity of memory cells contributes to the development of immune responses to newly encountered pathogens. Under these circumstances, cross-reactivity and plasticity of the memory CD4+ T cells may play an important role in host defense.
As a general issue, “plasticity” often means that cells of a given differentiated type acquire the capacity to produce a new set of cytokines while retaining the capacity to produce the cytokines characteristic of their initial differentiated state. This could mean that the number of physiologic Th populations with distinctive cytokine-producing potential is much larger than previously thought. If so, this would appear to pose a complex problem for the immune system in the sensing of and differentiation between distinct types of microbial threats and determining the appropriate response, both in type and magnitude.
References
- Hegazy AN, Peine M, Helmstetter C, Panse I, Fröhlich A, Bergthaler A, Flatz L, Pinschewer DD, Radbruch A, Löhning M. Immunity. 2010;32:116–128. doi: 10.1016/j.immuni.2009.12.004. this issue. [DOI] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Kille-brew JR, Urdahl KB, Campbell DJ. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zhu J, Paul WE. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]