Abstract
Background:
Rhabdomyosarcoma (RMS) is the commonest type of soft-tissue sarcoma in children. Patients with metastatic RMS continue to have very poor prognosis. Recently, several works have demonstrated a connection between Notch pathway activation and the regulation of cell motility and invasiveness. However, the molecular mechanisms of this possible relationship remain unclear.
Methods:
The Notch pathway was manipulated pharmacologically and genetically. The mRNA changes were analysed by quantitative PCR and protein variations by western blot and immunofluorescence. Finally, the capabilities of RMS cells to adhere, heal a wound and invade were assessed in the presence of neuronal cadherin (N-cadherin)- and α9-integrin-blocking antibodies.
Results:
Cells treated with γ-secretase inhibitor showed lower adhesion capability and downregulation of N-cadherin and α9-integrin. Genetic manipulation of the Notch pathway led to concomitant variations in N-cadherin and α9-integrin. Treatment with anti-N-cadherin-blocking antibody rendered marked inhibition of cell adhesion and motility, while anti-α9-integrin-blocking antibody exerted a remarkable effect on cell adhesion and invasiveness.
Conclusion:
Neuronal cadherin and α9-integrin are postulated as leading actors in the association between the Notch pathway and promotion of cell adhesion, motility and invasion, pointing to these proteins and the Notch pathway itself as interesting putative targets for new molecular therapies against metastases in RMS.
Keywords: rhabdomyosarcoma, Notch, NCAD, ITGA9, invasion, soft-tissue sarcomas
Rhabdomyosarcoma (RMS), a malignant tumour of early onset, is the most common type of soft-tissue sarcoma in children. Regarding histopathological criteria, RMS can be divided into two main subtypes: embryonal and alveolar (eRMS and aRMS, respectively). The majority of aRMS (80–85%) contain one of the reciprocal chromosomal translocations: t(2;13)(q35;q14) or t(1;13)(p36;q14). These translocations generate the novel fusion genes PAX3-FOXO1 and PAX7-FOXO1, respectively (Barr et al, 1993; Davis et al, 1994). However, no characteristic translocations have been described in eRMS. Patients with metastatic RMS have very poor prognosis and more intense therapies are thus indicated. Moreover, the major cause of death in these patients is the formation of distant metastases. The cellular components that control mobility, invasiveness and metastasis in RMS remain largely unknown. The molecules responsible for these processes should be identified before the development of targeted therapies focused on reducing metastasis in this neoplasia. Recently, we described the role of Notch pathway activation in controlling the migration and invasiveness processes in RMS (Roma et al, 2011). Similarly, the role of the Notch pathway in invasion and metastasis has also been reported in osteosarcoma (Zhang et al, 2008; Zhang et al, 2010; Engin et al, 2009) and some adult cancers, such as colon, prostate, cervical carcinoma and glioblastoma among others (Zayzafoon et al, 2004; Pang et al, 2010; Chigurupati et al, 2010; Christofori, 2011).
The acquisition of invasive properties by cancer cells during tumour progression is closely linked to changes in the expression of adhesion molecules that mediate the interaction of cancer cells with their surrounding cells and extracellular matrix. During tumour progression, malignant cells typically downregulate proteins that mediate adhesion to basal membrane and neighbouring cells and, at the same time, promote the expression of adhesion molecules that facilitate dynamic interaction with extracellular matrix components (Mariotti et al, 2007). A paradigmatic example of this phenomenon is the cadherin switch, frequently observed in the epithelial–mesenchymal transition of epithelial cancer cells (Cavallaro et al, 2002; Hazan et al, 2004). During this transition, E-cadherin expression is downregulated with concomitant enhancement of neuronal cadherin (N-cadherin) expression, which correlates with the acquisition of greater invasiveness and metastatic potential by malignant cells.
Neuronal cadherin (N-cadherin or cadherin-2) is a member of the classical type I cadherin family, which is critically involved in tissue development and maintenance. Like other classical type I cadherins, N-cadherin contains a large N-terminal extracellular domain composed of five tandem-repeated domains, a transmembrane segment and a cytoplasmatic C-terminal domain able to interact with catenins (Mariotti et al, 2007). Although the mechanism by which increased N-cadherin expression promotes malignancy is not fully understood, in a subset of primary tumour cells, it is thought to promote adhesion of malignant cells to N-cadherin-expressing stromal or endothelial cells, thereby facilitating invasion and migration of tumour cells to distant sites (Sandig et al, 1997; Qi et al, 2005). Several experiments have demonstrated that the expression of N-cadherin suffices to promote invasion and metastasis in some cancers (Nieman et al, 1999; Hazan et al, 2000; Li et al, 2009).
The superfamily of integrins includes several transmembrane cell adhesion proteins that bind to extracellular matrix, cell surface and soluble ligands. All integrins act as heterodimers composed of α- and β- subunits. The α- and β-polypeptidic chains are distinct with no detectable homology between them (Takada et al, 2007). The heterodimer α9β1 has been implicated in several processes that entail dynamic interaction between cells and extracellular matrix, such as re-epithelialisation during cutaneous wound healing (Singh et al, 2009), cell adhesion in medulloblastoma and in hematopoietic progenitor cells (Fiorilli et al, 2008; Schreiber et al, 2009) and finally, cell migration in neoplasic and non-neoplasic cells (Gupta and Vlahakis, 2009; Oommen et al, 2011).
The main objective of this work was to demonstrate the existence of a connection between Notch pathway activation and the regulation of mechanisms underlying cell motility and invasiveness in RMS. It has been previously suggested that Notch1 and Notch3 signalling may promote an upregulation of N-cadherin expression in primary melanoma (Liu et al, 2006; Wang et al, 2007); however, the molecular mechanisms of this possible relationship remain unclear. Moreover, as we demonstrated recently, the Notch pathway mediates RMS cell motility and invasiveness (Roma et al, 2011). The oncogenic potential of the Notch pathway was first described in acute T-cell lymphoblastic leukaemia in the late 1980s (Radtke et al, 1999). An abnormal upregulation of the Notch pathway has also been reported in ovarian (Park et al, 2006), breast (Glahan and Callahan, 1997) and other cancers (Nickoloff et al, 2003). With respect to paediatric malignancies, Notch signalling appears to contribute essentially to osteosarcoma metastasis (Zhang et al, 2008; Zhang et al, 2010) and proliferation (Tanaka et al, 2009); Notch signalling also promotes medulloblastoma cancer stem cell survival (Fan et al, 2006) and contributes to angiogenesis in neuroblastoma (Funahashi et al, 2008). The four Notch receptors are synthesised as precursor proteins that are processed by furin-like enzymes in the trans-Golgi compartment. This cleavage leads to the production of a heterodimeric Notch receptor, which is further processed at the cell surface in a ligand-dependent manner by α- and γ-secretase. The α-secretase cleavage occurs at an extracellular site and removes the Notch ectodomain, whereas γ-secretase cleavage occurs in the Notch transmembrane domain, leading to the release of the intracellular Notch fragment (NICD) that translocates to the nucleus where it binds to CSL (CBF1-Su(H)-Lag1) transcription repressors, converting them into transcriptional activators. The targets of these transcription factors in vertebrates are typified by Hes and Hey genes (Artavanis-Tsakonas et al, 1999; Luo et al, 2005). The possible demonstration of a connection between Notch signalling and the expression of adhesion molecules may shed light on the unknown molecular mechanism of the Notch-mediated invasive phenotype of RMS cells and allow us to adopt new therapeutic approaches to the fight against metastases in this neoplasia.
Materials and Methods
Cell culture, transfections and drug treatments
RH30, CW9019 and HTB82 RMS cell lines were grown in MEM medium with Earle’s Salts, supplemented with 10% FCS, 2 mℳℒ-glutamine, 1 mℳ sodium pyruvate, 1 × non-essential aminoacids, 100 U ml−1 penicillin and 0.1 mg ml−1 streptomycin (all culture reagents supplied by PAA Laboratories, Haidmannweg, Austria) and maintained at 37 °C in a 5% CO2 water-jacketed incubator. Full-length dnMAML1, Delta and Hes1 complementary DNAs (cDNAs) were generously provided by Dr Anna Bigas (Institut Municipal d’Investigació Mèdica, Barcelona, Spain). The full-length NICD cDNA was a kind gift from Dr Rosanna Paciucci (Institut de Recerca Hospital Vall d’Hebron, Barcelona, Spain). All cDNAs used were cloned into a pCDNA3.1 vector, except dnMAML1 that was cloned into a pEGFP vector. All cDNA transfections were carried out in parallel with the respective empty vector as a control. Transient transfections were performed using 8 μg of plasmid DNA and FuGENE 6 transfection reagent according to the manufacturer’s protocol (Roche, Basel, Switzerland). Transfection efficiency was approximately 70% for the three cell lines and was assessed by calculating the percentage of GFP-positive cells referred to the total of cells. Transfected cells were harvested between 48 and 72 h and processed for subsequent assays. Gamma-secretase inhibitor (GSI)-XXI (Calbiochem, San Diego, CA, USA) was diluted in DMSO and added to the culture medium for 3 days at a final concentration of 4 nℳ as previously described (Roma et al, 2011). Control plates were supplemented with an equivalent volume of DMSO.
Complementary DNA microarrays
Microarrays were carried out using the Affymetrix microarray platform and the Genechip Human Gene 1.0 ST Array; 100 ng of total RNA were used per sample. Two plates of RH30 cells previously treated with GSI were compared with their control (DMSO). Quality of total RNA was assessed by Bioanalyzer Assay (Agilent). Chips were processed on an Affymetrix GeneChip Fluidics Station 450 and Scanner 3000. Results were analysed using TMEV software (Saeed et al, 2006).
Western blotting
Cells were homogenised in lysis buffer (50 mℳ Tris-HCl, pH 7.4, 150 mℳ NaCl, 1 mℳ PMSF, 1 mℳ EDTA, 5 mg ml−1 aprotinin, 5 mg ml−1 leupeptin, 1% Triton X-100, 1% sodium deoxycholate and 0.1% SDS) and incubated for 4 min at 90 °C. The total protein content was measured using the DC assay kit (Bio-Rad Laboratories, Hercules, CA, USA), a 10% SDS–PAGE was performed and the resolved proteins were in turn transferred onto PVDF membranes (Bio-Rad Laboratories). Membranes were then incubated with anti-Hes1 polyclonal antibody AB5702 (Millipore, Billerica, MA, USA) diluted 1 : 200, anti-N-cadherin monoclonal antibody C2542 (Sigma, St Louis, MO, USA) diluted 1 : 1000, anti-α9-integrin monoclonal antibody clone 3E4 (Abnova, Aachen, Germany) diluted 1 : 250 and anti-Notch1 (C-20) sc-6014 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1 : 500. An anti-α-tubulin monoclonal antibody (Cell Signaling, Danvers, MA, USA) diluted 1 : 2000 was used as a loading control.
Immunofluorescence
Each well was plated with 3 × 105 cells on 12-mm round glass coverslips pre-coated with a 4% collagen-I solution (BD Biosciences, Erembodegem, Belgium). After 24 h, cells were transfected with the appropriate plasmids as described above. After 48 h, cells were washed with PBS, fixed in 4% paraformaldehyde, incubated with 50 mℳ NH4Cl and permeabilised with 0.1% Triton X-100. Samples were then blocked with 5% FCS prior to incubation with the following primary antibodies: anti-Hes1 AB5702 (Millipore) diluted 1 : 50, anti-N-cadherin C2542 (Sigma) diluted 1 : 250 and anti-α9-integrin clone 3E4 (Abnova) diluted 1 : 125. Cells were then incubated with suitable secondary antibodies labelled with AlexaFluor-647 or AlexaFluor-488 (Molecular probes, Eugene, OR, USA). Nuclei were stained with 5 ng ml−1 of Hoechst33342 (Sigma). Fluorescence was visualised in a FV1000 confocal microscope (Olympus, Hamburg, Germany) and quantified with Olympus Fluorview software. A minimum of 100 cells obtained from random selected fields was evaluated.
Immunohistochemistry
Patient samples (n=9) were obtained from the Vall d’Hebron Hospital Pathology Department (kindly provided by Dr Nuria Toran). Paraffin-embedded tissues were sliced, deparaffinised and rehydrated, followed by antigen retrieval in 10 mℳ citrate buffer (DAKO). Endogenous peroxidase activity was quenched using 1% hydrogen peroxide. Samples were blocked and incubated with antibodies against Hes1 (AB5702, Millipore), N-cadherin (C2542, Sigma) and α9-integrin (3E4, Abnova), all diluted 1 : 200. After incubation with peroxidase-conjugated secondary antibody and peroxidase substrate, samples were counterstained with hematoxylin for 10 s, dehydrated and mounted. Labelling of each antibody was evaluated using a semiquantitative method regarding the percentage of positive cells (⩽25%, 26–50%, 51–75% and >75% corresponding to a score of 1, 2, 3 and 4 respectively) and intensity of the labelling (0–3). The assigned final score (1–7) corresponds to the sum of the labelling and percentage of positive cell scores.
RNA isolation, retrotranscription and real-time PCR
RNA was isolated using the Quick-prep micro RNA isolation kit following the manufacturer’s instructions (Qiagen, Valencia, CA, USA). Samples of 2 μg of total RNA were reverse transcripted using random primers (Invitrogen, Carlsbad, CA, USA). The reaction mixture was incubated for 1 h at 37 °C with 200 U of Moloney murine leukaemia virus reverse transcriptase (Promega, Madison, WI, USA). A 40-cycle PCR based on the TaqMan assay (Applied Biosystems, Foster City, CA, USA) was performed to detect N-cadherin and α9-integrin (Hs00983056_m1 and Hs00979865_m1 TaqMan assays, respectively). The downstream Notch effector Hes1 (assay Hs00172878_m1) was used to assess Notch activation. The housekeeping gene TBP (assay Hs00172424_m1) was used as internal control. Quantification of relative levels of each mRNA analysed was performed by the method of (Livak and Schmittgen, 2001). All samples were tested in triplicate.
Fibronectin and collagen adhesion assay
A total of 3 × 105 GSI-pre-treated cells per well was seeded in a 24-well plate previously coated with collagen or fibronectin. Cells were then incubated at 37 °C for 15 min (RH30), 30 min (CW9019) or 1 h (HTB82), respectively, fixed with 4% paraformaldehyde and stained with 0.2% crystal violet. Cells were then lysed in 10% acetic acid and the absorbance at 590 nm was determined as a value proportional to the number of cells adhered to the substrate. To establish the specificity of the assay for N-cadherin and α9-integrin, some wells were treated with 20 μg ml−1 of either N-cadherin- (GC-4, Sigma) or α9-integrin-blocking antibodies (Y9A2, Chemicon International, Temecula, CA, USA). The control wells were treated with an anti-α-tubulin monoclonal antibody (Cell Signaling) at the same concentration, as isotypic control. All measurements were taken in triplicate.
Transwell assay
Cells were trypsinised, seeded at 105 cells per well and incubated in the presence or absence of 20 μg ml−1 of either N-cadherin or α9-integrin-blocking antibodies for 30 min on ice. Following the incubation, cells were plated in the upper chamber previously coated with BD Matrigel (BD Biosciences) in an 8-μm pore-size transwell (Corning). Basal medium containing 1% FBS in the presence or absence of the aforementioned N-cadherin and α9-integrin-blocking antibodies was added to each well and incubated at 37 °C for 6, 12 and 48 h (for RH30, CW9019 and HTB82 cell lines, respectively). The control wells were treated with 20 μg ml−1 anti-α-tubulin monoclonal antibody (Cell Signaling). Remaining cells were removed from the upper chamber with a cotton swab, and cells migrated to the lower surface were stained with 0.2% crystal violet, lysed in 10% acetic acid and the absorbance at 590 nm was determined as a value proportional to the number of cells on the lower surface of each membrane. All analyses were made in triplicate.
Wound-healing assay
Cells were seeded on a 24-well plate (3 × 105 cells per well) and incubated in the presence or absence of 20 μg ml−1 of either N-cadherin- or α9-integrin-blocking antibodies as described above. The control wells were treated with 20 μg ml−1 anti-α-tubulin monoclonal antibody as isotypic control (Cell Signaling). A day later, cell monolayers were scratched with a pipette tip and placed in complete growth medium with or without the suitable blocking antibody. The initial scratched areas were measured using the ImageJ software (NIH, freely available at http://rsb.info.nih.gov/ij/) and serial images of random selected fields were acquired until the healing of the wounds was complete. Healing velocity was calculated as a quotient between the scratched area and the time required for the wound to heal. All analyses were made in triplicate.
Chromatin immunoprecipitation (ChIP) assay
Chromatin immunoprecipitation was performed following the manufacturer’s (Millipore) instructions using 5 μg of polyclonal rabbit antibodies against Hes1 AB5702 (Millipore) and Notch1 sc-6014 (Santa Cruz Biotechnology). The same amount of rabbit immunoglobulin (IgG) was used as a control (Abcam). The co-immunoprecipitated DNAs were used for PCR to amplify N-cadherin and α9-integrin promoter regions. The primers used were as follows: N-cadherin promoter (forward 5′-ACCCAGAGATCAAGGAGCTG-3′ reverse 5′-CTCCACTTCCACCTCCACAT-3′) and α9-integrin promoter (forward 5′-GAGCTCAAAAGTGCCCTCTC-3′ reverse 5′-TGAGGGAGGAAAAAGAAGCA-3′).
Results
Pharmacological inhibition of Notch signalling impairs cell adhesion in RMS cell lines
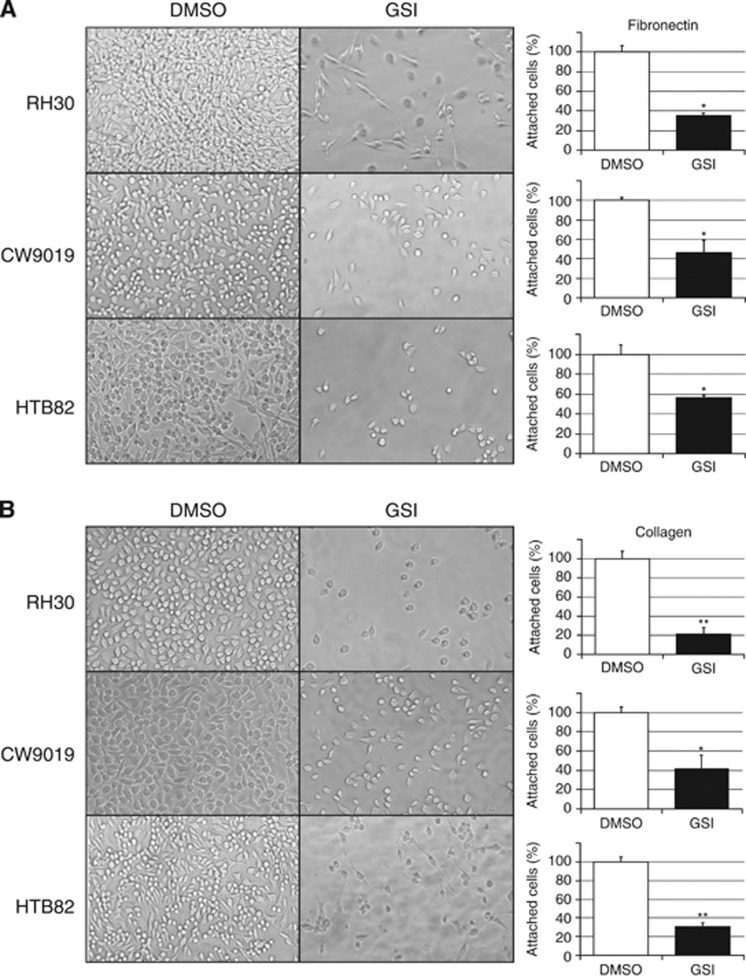
Cells treated with GSI showed a clear reduction in cell adhesiveness detected in either collagen- or fibronectin-coated plates (Figure 1). The effect produced by GSI-XXI on cell adhesiveness varied among the cell lines analysed and according to the coating of the plate surface; however, in general, treated cells showed a significant reduction in adhesion that ranged from 20 to 50% in fibronectin-coated plates (Figure 1A) and from 30 to 60% in collagen-coated plates (Figure 1B). Cell viability was assessed by flow cytometry with no substantial differences observed between GSI-treated and untreated (DMSO) cells (Supplementary Figure S1). These observations fitted our hypothesis that Notch pathway may be able to regulate some of the molecular processes that lead to cell adhesion and migration. However, they did not rule out the possibility that the effects observed may have been caused by effects of the drug on other pathways or directly on cell adhesion molecules.
Figure 1.
Cell adhesion to fibronectin- or collagen-coated plates. Images showing a representative field of attached cells for each cell line in the presence (GSI) or absence (DMSO) of γ-secretase inhibitor and plots representing the quantification of cells attached to fibronectin- (A) or collagen-coated (B) plates. All samples were evaluated in triplicate. Student’s t-test significance: *P<0.05, **P<0.005.
Pharmacological inhibition of Notch signalling significantly reduces N-cadherin and α9-integrin expression
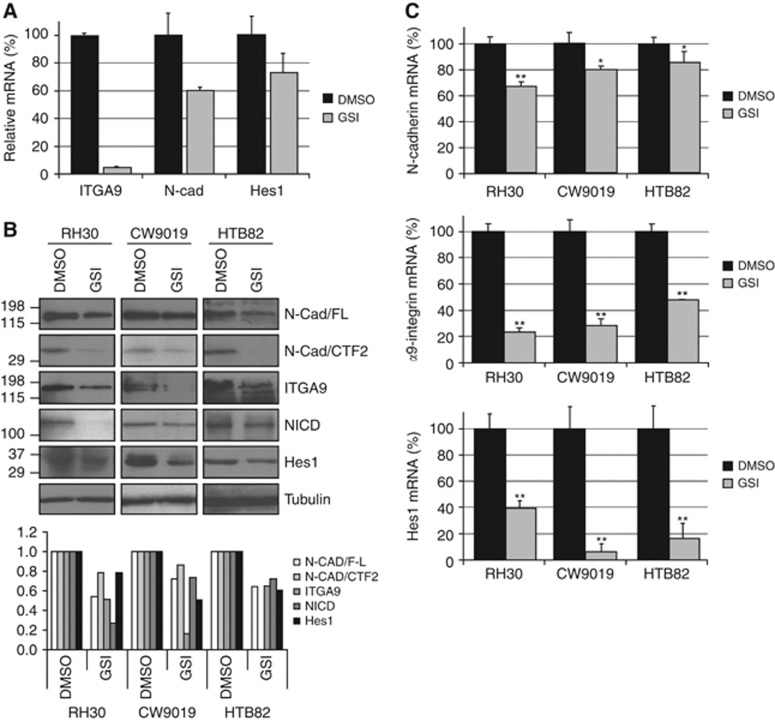
A cDNA microarray permitted preliminary identification of adhesion proteins downregulated under GSI treatment. Neuronal cadherin and α9-integrin were selected for their downregulation under GSI treatment, their significant expression (other proteins downregulated but barely expressed in control samples were ruled out) and their previously well-established involvement in cell adhesion, motility and metastasis (Figure 2A). With the aim of confirming the results obtained in the cDNA microarray, we specifically analysed the expression of the two selected genes by western blotting and qRT–PCR. Western blotting (Figure 2B) revealed a reduction in both full-length N-cadherin and the CTF2 fragment levels. The CTF1 fragment was not detected in the RMS cell lines analysed (not shown), thereby suggesting a negligible role of this isoform in RMS. Levels of α9-integrin were also reduced in cells treated with GSI. Levels of β1-integrin, the usual partner of α9-integrin, showed no changes (not shown). The reduction in Hes1, as a marker of effective Notch inhibition in treated cells, is also shown in Figure 2B. Similarly, mRNA levels of both N-cadherin and α9-integrin showed statistically significant reductions in the three cell lines, down to between 60 and 85% for N-cadherin and to 20–50% for α9-integrin, compared with untreated cells (100%). The mRNA expression of Hes1 was also analysed as a marker of correct Notch inhibition and showed a statistically significant reduction, as expected, in cells treated with GSI (Figure 2C). Although pharmacological Notch inhibition was not complete, reductions in N-cadherin and α9-integrin expressions were significant, particularly for α9-integrin.
Figure 2.
Pharmacological inhibition of Notch signalling significantly reduced N-cadherin and α9-integrin expression. (A) Complementary DNA (cDNA) expression microarray showing the relative levels of α9-integrin (ITGA9), N-cadherin (N-Cad) and Hes1 expression in GSI-treated samples (GSI) compared with the control (DMSO). (B) Panel of western blots showing the effects of GSI on full-length N-cadherin (N-Cad/FL) and CTF2 fragment, α9-integrin (ITGA9), NICD and Hes1 levels compared with untreated samples (DMSO). Alpha-tubulin was used as a loading control. Below the western blot composition, a plot depicting densitometric analysis of each band of the western blot. (C) Relative mRNA levels evaluated by quantitative PCR in the presence (GSI) or absence (DMSO) of γ-secretase inhibitor. Neuronal cadherin, α9-integrin and Hes1 mRNA levels were evaluated in triplicate. Student’s t-test significance: *P<0.05, **P<0.005.
Although these results confirmed the N-cadherin and α9-integrin reduction in cells treated with GSI, they failed to demonstrate unequivocally that the effects observed could be exclusively Notch-dependent.
Genetic modification of Notch pathway activation gives rise to a parallel regulation of N-cadherin and α9-integrin
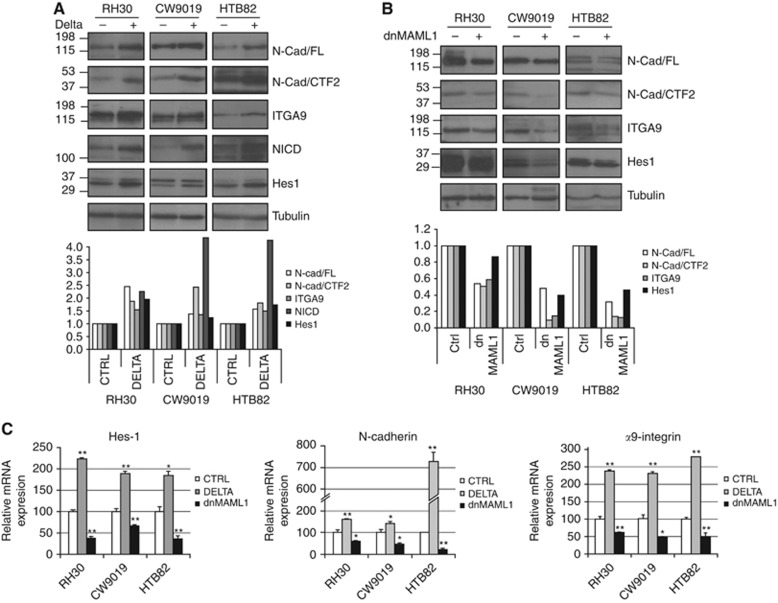
Seeking to rule out the possibility of the observed effects on cells treated with Notch inhibitors being attributable to the involvement of other pathways or to a direct effect of the drugs on N-cadherin or α9-integrin – and therefore demonstrate that these effects resulted from specific Notch activation – we genetically manipulated Notch signalling activity by transfecting constructs containing the Notch ligand Delta1 (Delta) and a dominant negative form of MAML1 (dnMAML1). Activation of the Notch pathway by transfection of Delta promotes N-cadherin and α9-integrin increases in both protein and mRNA levels (Figures 3A and C). Conversely, inhibition of the pathway by overexpression of the dominant negative dnMAML1 produces a parallel reduction in protein and mRNA levels of N-cadherin and α9-integrin (Figures 3B and C). Western blot revealed the presence of full-length N-cadherin (N-Cad FL) and the CTF2 fragment, whereas the CTF1 fragment was not detected (not shown). Both forms of N-cadherin detected (N-Cad FL and CTF2) and α9-integrin showed induction when the Notch pathway was activated (overexpression of Delta) and a clear reduction when repressed (overexpression of dnMAML1) (Figures 3A and B). No changes were observed in the amount of β1-integrin, the usual partner of α9-integrin (not shown).
Figure 3.
Genetic modifications of the Notch pathway influenced N-cadherin and α9-integrin expression. Western blots showing the concomitant variations of Notch pathway activation (NICD and Hes1) and N-cadherin (N-Cad/FL and CTF2) and α9-integrin (ITGA9) expression in RMS cells transfected with Delta (A) or dnMAML1 (B). Below each western blot composition, a plot depicting densitometric analysis of each band of the western blot. The variations associated with genetic modification of the Notch pathway (Delta and MAML1 transfections) observed in protein are also seen in the mRNAs of N-cadherin, α9-integrin and Hes1 determined by quantitative PCR (C). All RNA measurements were taken in triplicate. Student’s t-test significance: *P<0.05, **P<0.005.
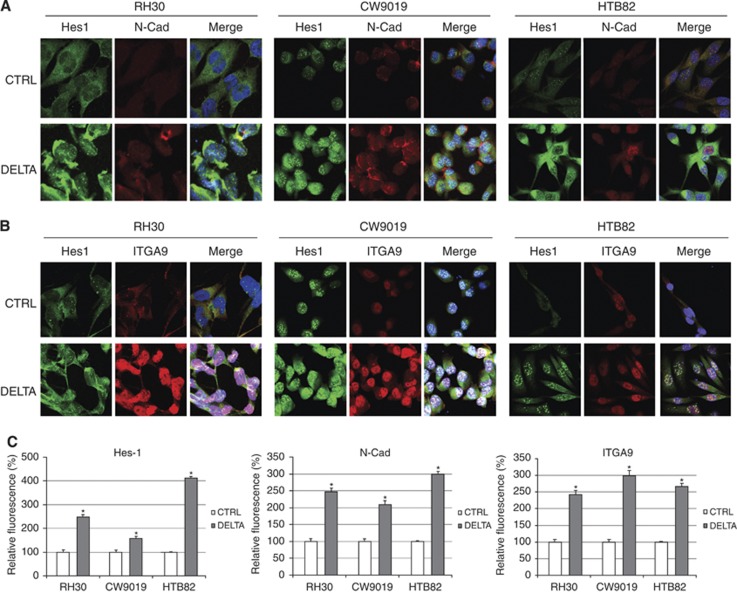
Immunofluorescence
Fluorescent immunocytochemistry against N-cadherin revealed moderate expression of this protein in non-transfected cells (Figures 4A and C). When cells underwent overactivation of the Notch pathway (by Delta1 transfection), N-cadherin expression was significantly increased in the three cell lines analysed and conserved the localisation pattern of the endogenous protein in each cell line. The staining was localised in cytoplasm and cell membrane in RH30 and CW9019 cell lines, but more prominently in the nucleus in HTB82, which suggests a major presence of the CTF2 form in this cell line (also observed by western blot, Figure 3).
Figure 4.
N-cadherin and α9-integrin immunocytochemistry. Immunocytochemistry with anti-Hes1 and N-cadherin (N-Cad) antibodies (A) or Hes1 and α9-integrin (ITGA9) antibodies (B) in cells transfected with Delta or control vector (CTRL). Staining was as follows: Hes1 (green), N-cadherin and α9-integrin (red) and nuclei (blue). (C) Relative fluorescence quantification for Hes1, N-cadherin and α9-integrin. Student’s t-test significance: *P<0.05.
The distribution of α9-integrin staining was general and showed prominent nuclear and cytoplasmic staining. As in the results found with N-cadherin transfections, RMS cells transfected with Delta also showed a statistically significant increase in α9-integrin staining compared with controls (Figures 4B and C).
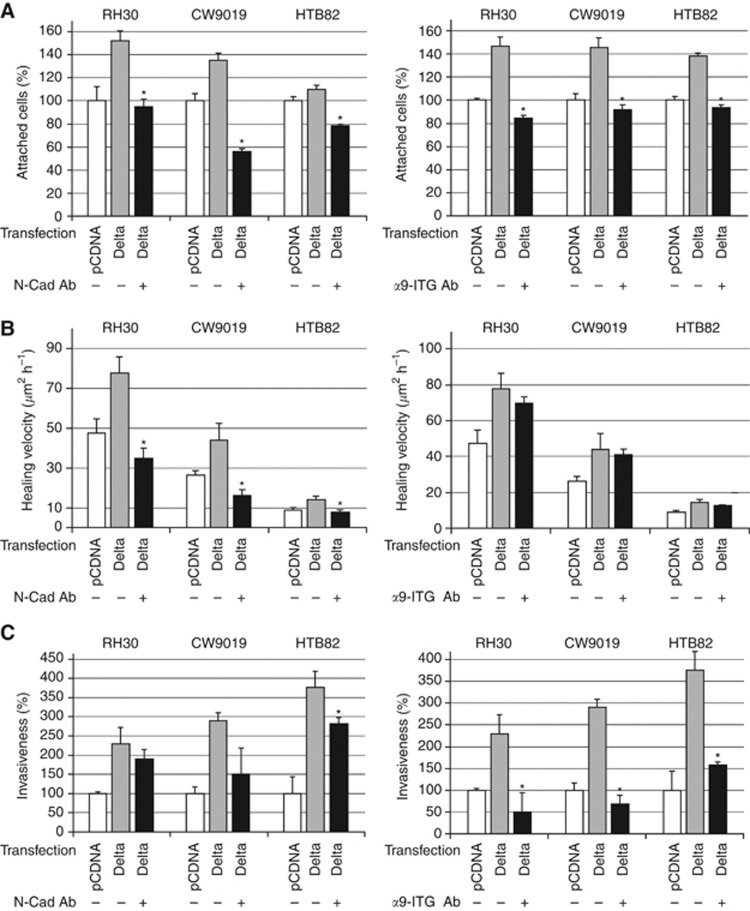
Specific anti-N-cadherin- and anti-α9-integrin-blocking antibodies reduce invasive properties of RMS cells
Seeking to demonstrate that the role of N-cadherin and α9-integrin is critical for the Notch-mediated increase in RMS cell capability to migrate and invade, we performed adhesion, wound-healing and invasion assays in the presence or absence of specific blocking antibodies against these two proteins. The main aim of these experiments was to demonstrate whether inhibition of N-cadherin and/or α9-integrin suffices to compensate for the increases in adhesion, wound-healing and invasiveness detected in cells with constitutive Notch activation. Cells transfected with Delta or NICD showed an increase in their adhesion to fibronectin-coated plates, which could be reverted using either anti-N-cadherin- or anti-α9-integrin-blocking antibodies (Figure 5A). The effects of the blocking antibodies on healing velocity differed for N-cadherin and α9-integrin. Thus, while N-cadherin-blocking antibody gave rise to a significant reduction in healing velocity in the three cell lines analysed, the anti-α9-integrin-blocking antibody did not promote any significant decrease in the capability of cells to heal a wound (Figure 5B). Finally, the effects observed with both antibodies on cell invasiveness in a matrigel/transwell assay also differed (Figure 5C). Interestingly, the α9-integrin-blocking antibody produced marked invasiveness impairment in this assay, whereas only a very weak effect of N-cadherin-blocking antibody on invasiveness was observed.
Figure 5.
Anti-N-cadherin- and anti-α9-integrin-blocking antibodies reduced invasive properties induced by Notch activation. Three RMS cell lines were transfected with the constructions indicated below the bottom of each bar (pCDNA, Delta) in the presence (+) or absence (−) of either N-cadherin-blocking antibody (N-Cad Ab) or α9-integrin-blocking antibody (α9-ITG Ab) as indicated in the figure. (A) Adhesion assay on fibronectin-coated plates. The percentage of cells attached was quantified per cell line and condition. (B) Wound-healing assay. All bars indicate the healing velocity of each cell line, transfection and treatment (C) Matrigel/Transwell assay. Invasiveness is represented as a percentage of cells in the lower compartment, compared with the control. The three assays were performed in three independent wells per cell line and condition. Student’s t-test significance: *P<0.05.
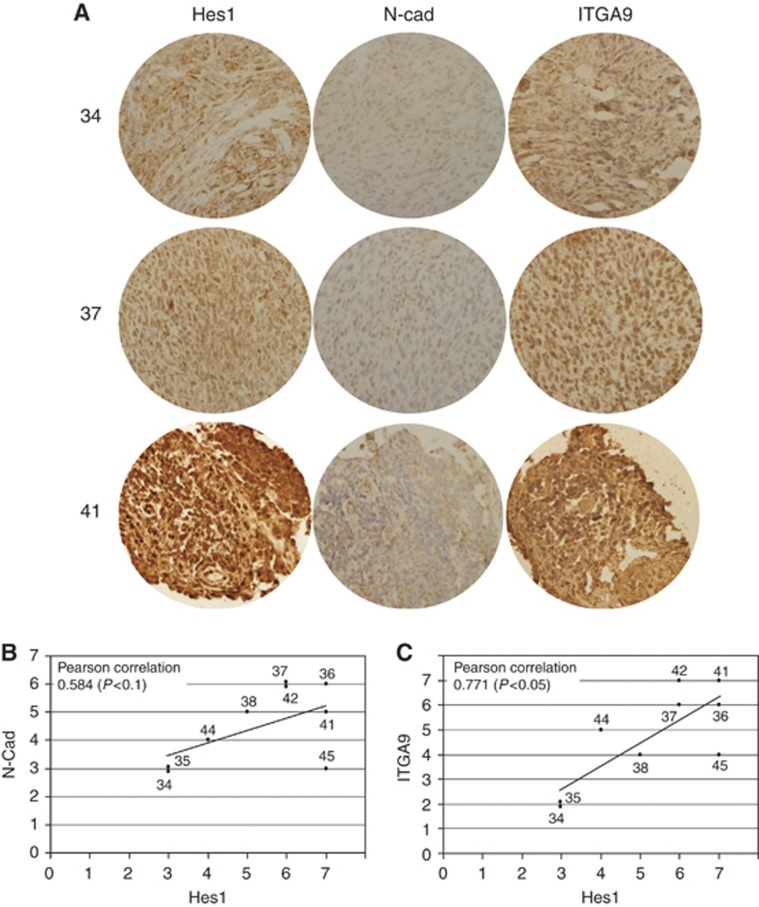
Correlation among Hes1, N-cadherin and α9-integrin in RMS archival tumour samples
Patient sections were stained using antibodies against Hes1, N-cadherin and α9-integrin with the aim of demonstrating the correlation among these proteins observed in cultured RMS cell lines. The intensity of labelling was evaluated using a semiquantitative method as described above. The results obtained showed a correlation between the expression of Hes1 with both N-cadherin and α9-integrin (Figure 6). Although the number of samples was limited (n=9), the correlation between Hes1 and N-cadherin yielded a Pearson correlation of 0.584 (P<0.1) and the correlation for Hes1 and α9-integrin a value of 0.771 (P<0.05), thereby suggesting a dependency of Hes1 expression on N-cadherin and α9-integrin. This correlation among these proteins confirms, in patients, the aforementioned findings in cultured cells and suggests that Notch activation may also influence the expression of these two adhesion proteins in tumours.
Figure 6.
The expression of Hes1, N-cadherin and α9-integrin correlate in archival RMS tumour samples. (A) Representative sections of three patients (34, 37 and 41) stained with anti-Hes1, N-Cad and α9-integrin antibodies. (B and C) Plots depicting the correlations between Hes1 and both N-cadherin and α9-integrin semiquantitative scores. Pearson correlation value and significance are shown in the upper left corner in each plot.
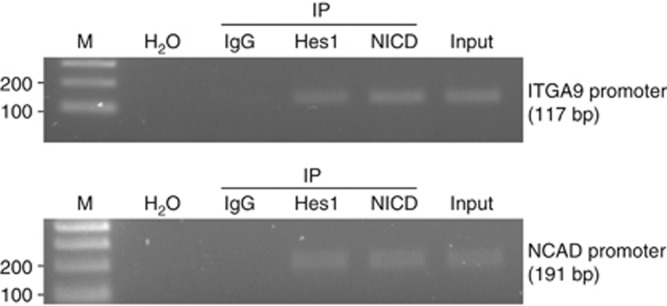
NICD and Hes1 bind to N-cadherin and α9-integrin promoters
Chromatin immunoprecipitation assay revealed co-immunoprecipitation of both NICD and Hes1 with the N-cadherin and α9-integrin promoter sequences. The control IgGs rendered no amplification (Figure 7). These results pointed to a direct binding of these Notch downstream effectors to the promoter regions of N-cadherin and α9-integrin genes.
Figure 7.
Binding of NICD and Hes1 to N-cadherin and α9-integrin promoters. Lysates from RH30 cells were analysed by ChIP assay using specific antibodies against Hes1, NICD and rabbit IgG as a control. Immunoprecipitated chromatin was amplified by PCR using N-cadherin and α9-integrin promoter region-specific primers. IgG: negative control rabbit IgG; Input: 5% of the sonicated cell lysate used for IP; H2O: PCR negative control; M: 100 bp DNA ladder.
Discussion
Rhabdomyosarcoma patients with metastatic disease continue to have poor prognosis and the major cause of death in these patients is the formation of distant metastases. The identification of molecular components and mechanisms that control metastasis in RMS may be useful for developing targeted therapies focused on reducing metastasis in this neoplasia. Although the mechanism by which increased N-cadherin expression promotes malignancy is not fully understood, it is thought to promote adhesion of malignant cells to N-cadherin-expressing stromal or endothelial cells, thereby facilitating invasion and migration of tumour cells to distant sites (Sandig et al, 1997; Qi et al, 2005). Although several works have demonstrated that N-cadherin expression suffices to promote metastasis in some cancers (Nieman et al, 1999; Hazan et al, 2000; Li et al, 2009) and an aberrant N-cadherin expression has been described in RMS (Soler et al, 1993), no previous publications have shown the importance of N-cadherin for RMS invasiveness. Herein, a central role of N-cadherin in RMS cell adhesion and motility is demonstrated; furthermore, a new mechanism of N-cadherin induction, mediated by Notch pathway activation, is also proposed. Likewise, α9-integrin – forming a heterodimer with β1-integrin – has previously been implicated in processes, such as cell migration, involving dynamic interaction among cells and their microenvironment (Gupta and Vlahakis, 2009; Oommen et al, 2011). However, its possible role in RMS remains unknown. Therefore, this work represents the first description of a pro-invasive role of α9-integrin in RMS.
We and others recently implicated the Notch pathway in paediatric sarcoma motility and invasiveness (Zhang et al, 2008; Roma et al, 2011); however, the molecular connection between the Notch pathway and the process that leads to cell adhesion remains largely obscure. A previous work described the requirement of Notch activation in the epithelial-to-mesenchymal transition of epithelial cells, which finally rendered a disassembly of E-cadherin junctions, thereby permitting an increase in cell motility (Zavadil et al, 2004). The results herein presented point to the existence of a Notch-mediated mechanism able to positively modulate the expression of adhesion proteins, such as N-cadherin and α9-integrin, which may drive motility and invasiveness in neoplasic cells, thereby permitting cancer progression. The three cell lines studied belong to different RMS subtypes and are known to have great biological differences. Two contain PAX/FOXO1 translocations and one bears no translocation. The fact that we consistently found a relationship between Notch and N-cadherin and α9-integrin in such different cell lines suggests that the mechanism here described is a general mechanism in RMS – and perhaps in other neoplasias – and not a mere characteristic of a particular RMS subtype. Therefore, Notch-controlled changes in the levels of proteins implicated in tumour cell spread, such as a downregulation of E-cadherin in epithelial tumours (Zavadil et al, 2004) and an upregulation of N-cadherin and α9-integrin in a mesenchymal tumour such as RMS, support the role of Notch as an upstream key signalling pathway in the control of cancer dissemination. Furthermore, the promoter-binding study, herein provided, pointed to a direct interaction of Hes1 and NICD to N-cadherin and α9-integrin gene regulatory regions. NICD and Hes1 are known to be proteins able to bind directly (Hes1) or indirectly (NICD) to DNA, thereby influencing gene transcription. The binding of NICD to the N-cadherin and α9-integrin promoter regions strongly suggests a role in their transcriptional activation, as NICD is an archetypal transcriptional activator. On the other hand, Hes1 is a known transcriptional repressor; however, it may also act as a transcriptional activator of certain genes (Yan et al, 2002). It is not clear whether Hes1 can exert a repressive or activating role in the promoters of the two genes included in this study. It is also conceivable that the relative amounts of both NICD and Hes1 may cooperatively regulate – inducing or repressing, depending on their relative proportion – the expression of the N-cadherin and α9-integrin genes.
The use of pharmacological Notch pathway inhibitors, such as GSIs, has been proposed as a therapeutic alternative in several cancers, focused essentially on reducing cell proliferation. Several GSIs have been actively studied as potential inhibitors of the generation of the β-amyloid peptide associated with Alzheimer’s disease (Selkoe and Kopan, 2003). More recently, some GSIs have begun to be studied in phase I–II trials for patients with advanced breast cancer and acute T-cell leukaemias (Deangelo et al, 2006; Krop et al, 2006). Beyond the use of GSIs as anti-proliferation agents, the results of our experiments point to GSIs as compounds able to reduce the invasive features of tumour cells and, therefore, suitable for use in the fight against metastasis.
Taken together, the results of this work reveal that the Notch pathway has a significant role in the regulation of N-cadherin and α9-integrin expression in RMS and, therefore, the Notch pathway should be considered a key regulator of the mechanisms, such as cell motility and invasiveness, which lead to tumour progression. Hence, Notch-inhibiting molecules can be considered as possible therapeutic agents against metastasis. Furthermore, the importance of N-cadherin and α9-integrin as players directly involved in cell attachment, migration and invasiveness in RMS is also demonstrated, consequently pointing to these proteins as new candidates for target-specific therapies focused on reducing metastasis in this neoplasia.
Acknowledgments
The authors wish to thank Ms Marta Rebull and Mr Isaac Vidal for laboratory technical support, Ms Marta Valeri for technical support with the in vivo wound-healing assay and confocal microscopy, Ms Paqui Gallego for technical support with the real-time PCR assays, Mr Ricardo Gonzalo for cDNA microarrays hybridisation and Ms Christine O’Hara for help with the English version of this manuscript. This work was supported by grants from Institut Català d’Oncologia (ICO), Instituto de Salud Carlos III (RD06/0020/1021 and PI11/00740), Fundació la Marató de TV3, Asociación Española Contra el Cáncer, Fundació SMALL and Fundació A. BOSCH.
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
10/04/2012
This paper has been modified since advance online publication, an acknowledgement has been added
Supplementary Material
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 268: 225–232 [DOI] [PubMed] [Google Scholar]
- Barr FG, Gallili N, Holich J, Biegel JA, Rovera G, Emanuel BS (1993) Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcomas. Nat Genet 3: 113–117 [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Schaffhauser B, Christofori G (2002) Cadherins and the tumour progression: is it all in a switch? Cancer Lett 176(2): 123–128 [DOI] [PubMed] [Google Scholar]
- Chigurupati S, Venkataraman R, Barrera D, Naganathan A, Madan M, Paul L, Pattisapu JV, Kyriazis GA, Sugaya K, Bushnev S, Lathia JD, Rich JN, Chan SL (2010) Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer Res 70(1): 418–427 [DOI] [PubMed] [Google Scholar]
- Christofori G. Metastatic colon cancer cells negotiate the intravasation notch (2011) Cancer Cell 19(1): 6–8 [DOI] [PubMed] [Google Scholar]
- Davis RJ, D’Cruz CM, Lowell MA, Biegel JA, Barr FG (1994) Fusion of PAX7 to the FOXO1 by the variant t(1;13) (p36;q14) translocation in alveolar rhabdomyosarcomas. Cancer Res 54: 2869–2872 [PubMed] [Google Scholar]
- Deangelo DJ, Stone RM, Silverman LB, Stock W, Attar EC, Fearen I, Dallob A, Matthews C, Stone J, Freedman SJ, Aster J (2006) A phase I clinical trial of the Notch inhibitor MK-0752 in patients with T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) and other leukemias. J Clin Oncol. ASCO Annual meeting Proceedings 24(18S): abstract 6585 [Google Scholar]
- Engin F, Bertin T, Ma O, Jiang MM, Wang L, Sutton RE, Donehower LA, Lee B (2009) Notch signaling contributes to the pathogenesis of human osteosarcomas. Hum Mol Genet 18(8): 1464–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, Eberhart CG (2006) Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res 66: 7445–7452 [DOI] [PubMed] [Google Scholar]
- Fiorilli P, Partridge D, Staniszewska I, Wang JY, Grabacka M, So K, Marcinkiewicz C, Reiss K, Khalili K, Croul SE (2008) Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation. Lab Invest 88(11): 1143–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi Y, Hernandez SL, Das I, Ahn A, Huang J, Vorontchikhina M, Sharma A, Kanamaru E, Borisenko V, Desilva DM, Suzuki A, Wang X, Shawber CJ, Kandel JJ, Yamashiro DJ, Kitajewski J (2008) A Notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis. Cancer Res 68: 4727–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahan D, Callahan R (1997) The mouse mammary tumor associated gene INT3 is a unique member of the NOTCH gene family (NOTCH4). Oncogene 14: 1883–1890 [DOI] [PubMed] [Google Scholar]
- Gupta SK, Vlahakis NE (2009) Integrin alpha9beta1 mediates enhanced cell migration through nitric oxide synthase activity regulated by Src tyrosine kinase. J Cell Sci 122(12): 2043–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA (2000) Exogenous expression of N-Cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 148(4): 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan RB, Qiao R, Keren R, Badano I, Suyama K (2004) Cadherin switch in tumor progression. Ann N Y Acad Sci 1014: 155–163 [DOI] [PubMed] [Google Scholar]
- Krop IE, Kosh M, Fearen I, Savoie J, Dallob A, Matthews C, Stone J, Winer E, Freedman SJ, Lorusso P (2006) Phase I pharmacokinetic (PK), and pharmacodynamic (PD) trial of the novel oral Notch inhibitor MK-0752 in patients (pts) with advanced breast cancer (BC) and other solid tumors. J Clin Oncol. ASCO Annual meeting Proceedings 24(18S): abstract 10574 [Google Scholar]
- Li K, He W, Lin N, Wang X, Fan QX (2009) N-Cadherin knock-down decreases invasiveness of esophageal squamous cell carcinoma in vitro. World J Gastroenterol 15(6): 697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Xiao M, Balint K, Smalley KS, Brafford P, Qiu R, Pinnix CC, Li X, Herlyn M (2006) Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res 66(8): 4182–4190 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT method. Methods 25(4): 402–408 [DOI] [PubMed] [Google Scholar]
- Luo D, Renault VM, Rando TA (2005) The regulation of notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol 16: 612–622 [DOI] [PubMed] [Google Scholar]
- Mariotti A, Perotti A, Sessa C, Rüegg C (2007) N-Cadherin as a therapeutic target in cancer. Expert Opin Investig Drugs 16(4): 451–465 [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Osborne BA, Miele L (2003) Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene 22: 6598–6608 [DOI] [PubMed] [Google Scholar]
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ (1999) N-Cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol 147(3): 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oommen S, Gupta SK, Vlahakis NE (2011) Vascular endothelial growth factor A (VEGF-A) induces endothelial and cancer cell migration through direct binding to integrin alpha9beta1: identification of a specific alpha9beta1 binding site. J Biol Chem 286(2): 1083–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang RT, Leung CO, Ye TM, Liu W, Chiu PC, Lam KK, Lee KF, Yeung WS (2010) MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinogenesis 31(6): 1037–1044 [DOI] [PubMed] [Google Scholar]
- Park JT, Li M, Nakayama K, Mao TL, Davidson B, Zhang Z, Kurman RJ, Eberhart CG, IeM Shih, Wang TL (2006) Notch3 gene amplification in ovarian cancer. Cancer Res 66: 6312–6318 [DOI] [PubMed] [Google Scholar]
- Qi J, Chen N, Wang J, Siu CH (2005) Transendothelial migration of melanoma cells involves N-cadherin-mediated adhesion and activation of the beta-catenin signaling pathway. Mol Biol Cell 16(9): 4386–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M (1999) Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10: 547–558 [DOI] [PubMed] [Google Scholar]
- Roma J, Masià A, Reventós J, Sánchez de Toledo J, Gallego S (2011) Notch pathway inhibition significantly reduces rhabdomyosarcoma invasiveness and mobility in vitro. Clin Cancer Res 17(3): 505–513 [DOI] [PubMed] [Google Scholar]
- Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J (2006) TM4 microarray software suite. Methods Enzymol 411: 134–193 [DOI] [PubMed] [Google Scholar]
- Sandig M, Voura EB, Kalnins VI, Siu CH (1997) Role of cadherins in the transendothelial migration of melanoma cells in culture. Cell Motil Cytoskeleton 38(4): 351–364 [DOI] [PubMed] [Google Scholar]
- Schreiber TD, Steinl C, Essl M, Abele H, Geiger K, Müller CA, Aicher WK, Klein G (2009) The integrin alpha9beta1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation. Haematologica 94(11): 1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D, Kopan R (2003) Notch and presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci 26: 565–597 [DOI] [PubMed] [Google Scholar]
- Singh P, Chen C, Pal-Ghosh S, Stepp MA, Sheppard D, Van De Water L (2009) Loss of integrin alpha9beta1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. J Invest Dermatol 129(1): 217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler AP, Johnson KR, Wheelock MJ, Knudsen KA (1993) Rhabdomyosarcoma-derived cell lines exhibit aberrant expression of the cell-cell adhesion molecules N-CAM, N-Cadherin, and cadherin-associated proteins. Exp Cell Res 208(1): 84–93 [DOI] [PubMed] [Google Scholar]
- Takada Y, Ye X, Simon S (2007) The integrins. Genome Biol 8(5): 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Setoguchi T, Hirotsu M, Gao H, Sasaki H, Matsunoshita Y, Komiya S (2009) Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation. Br J Cancer 100: 1957–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Holt CM, Xu C, Ridley C, P O Jones R, Baron M, Trump D (2007) Notch3 activation modulates cell growth behaviour and cross-talk to Wnt/TCF signaling pathway. Cell Signal 19(12): 2458–2467 [DOI] [PubMed] [Google Scholar]
- Yan B, Raben N, Plotz PH (2002) Hes-1, a known transcriptional repressor, acts as a transcriptional activator for the human acid alpha-glucosidase gene in human fibroblast cells. Biochem Biophys Res Commun 291(3): 582–587 [DOI] [PubMed] [Google Scholar]
- Zavadil J, Cermak L, Soto-Nieves N, Böttinger EP (2004) Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 23(5): 1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayzafoon M, Abdulkadir SA, McDonald JM (2004) Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J Biol Chem 279(5): 3662–3670 [DOI] [PubMed] [Google Scholar]
- Zhang P, Yang Y, Nolo R, Zweidler-McKay PA, Hughes DP (2010) Regulation of NOTCH signaling by reciprocal inhibition of HES1 and Deltex1 and its role in osteosarcoma invasiveness. Oncogene 29(20): 2916–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Yang Y, Zweidler-McKay PA, Hughes DP (2008) Critical role of notch signalling in osteosarcoma invasion and metastasis. Clin Cancer Res 14(10): 2962–2969 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.