Abstract
Background:
To identify patient and general practice (GP) characteristics associated with emergency (unplanned) first admissions for cancer in secondary care.
Methods:
Patients who had a first-time admission with a primary diagnosis of cancer during 2007/08 to 2009/10 were identified from administrative hospital data. We modelled the associations between the odds of these admissions being unplanned and various patient and GP practice characteristics using national data sets, including the Quality and Outcomes Framework (QOF).
Results:
There were 639 064 patients with a first-time admission for cancer, with 139 351 unplanned, from 7957 GP practices. The unplanned proportion ranged from 13.9% (patients aged 15–44 years) to 44.9% (patients aged 85 years and older, P<0.0001), with large variation by ethnicity (highest in Asians), deprivation, rurality and cancer type. In unadjusted analyses, all included patient and practice-level variables were statistically significant predictors of the admissions being unplanned. After adjustment, patient area-level deprivation was a key factor (most deprived compared with least deprived quintile OR 1.36, 95% CI 1.32–1.40). Higher total QOF performance protected against unplanned admission (OR 0.94 per 100 points; 95% CI 0.91–0.97); having no GPs with a UK primary medical qualification (OR 1.08, 95% CI 1.04–1.11) and being less able to offer appointments within 48 h were associated with higher odds.
Conclusion:
We have identified some patient and practice characteristics associated with a first-time admission for cancer being unplanned. The former could be used to help identify patients at high risk, while the latter raise questions about the role of practice organisation and staff training.
Keywords: hospital admissions, primary care, socio-economic deprivation, access
Cancer survival in the United Kingdom is poorer than in many other European countries (Berrino et al, 2001; Coleman et al, 2008) and is not adequately explained by artefact such as death registrations (Woods et al, 2011). Strategies to improve cancer care and patient survival focus on early diagnosis (Department of Health, 1995; 2000; 2007; 2011). Raine et al (2010) showed how hospital data could be used to look at late, unplanned presentation for three common cancers. Shawihdi et al (2011) found that unplanned presentation was associated with poorer outcomes for oesophagogastric cancers and suggested that it could be a quality indicator for local services.
Cancer indicators based on admission rates will be strongly affected by variation in prevalence, but a proportional approach that takes first admissions for cancer as a marker of incidence minimises this problem. Alongside tailored general practice cancer profiles devised in association with the Association of Public Health Observatories, National Cancer Intelligence Network (NCIN) showed that, nationally, 25% of cancers are diagnosed via the emergency route (National Cancer Intelligence Network, 2010). As late diagnosis is a key factor in poorer cancer survival in the United Kingdom, it is important to understand how patient and general practice (GP) characteristics might influence late presentation. We investigated the associations between first-time unplanned (emergency) admissions for cancer, patient factors and practice characteristics using established national databases.
Materials and Methods
Data sources
The index admission for cancer for each patient was derived from 3 years of Hospital Episodes Statistics (HES) data (financial years 2007/8 to 2009/10). This administrative database covers all admissions and outpatient appointments in NHS (public) hospitals in England. Eligible admissions were selected from records where the primary diagnosis was cancer (World Health Organization, 1992). We tracked back 3 years (3 × 365 days) from the admission date of the patient’s first admission within the 3-year study period (in-patients, day cases or regular day/night attenders) and excluded any patients who had a prior admission with a primary diagnosis of cancer (ICD10 codes C00–C96, excluding C44 and C97). Although we had access to data for outpatient attendances, these data contained very little diagnostic information, and the use of oncology specialty codes would have identified patients diagnosed with a minority of cancers and only those patients who were principally managed medically. We therefore did not use outpatient data.
All patients who had a prior admission with a primary diagnosis of cancer within the 3 years preceding their index admission were excluded from our analyses; tracking back just 1 year was insufficient. Cancers were grouped by ICD10 codes according to NCIN’s set of 22 broad groupings (National Cancer Intelligence Network, 2010). Hospital Episodes Statistics classifies admissions as ‘elective’ (from waiting list, booked or planned), ‘emergency’ (via the Accident and Emergency Department, emergency GP referral and so on), maternity, elective transfer and other. The outcome measure used throughout this study had a value of 1 if the admission was unplanned (‘emergency’ in HES) and 0 otherwise.
Population factors
We assigned an Index of Multiple Deprivation 2007 deprivation score for each patient via the patient’s postcode; these were averaged and assigned to GP practices via the practice postcode (Department for Communities and Local Government, 2008). Rural/urban classification of patients’ places of residence was made using the National Statistics Postcode Directory from November 2010, available from the Office for National Statistics (2010). The rurality of patients’ homes was mapped to Lower Super Output Areas based on postcodes. The GeoConvert online tool at MIMAS was used for classifying the rural/urban status of GP practices (University of Manchester, 2011).
Practice characteristics and Quality and Outcomes Framework scores
For 2010, we obtained numbers of GPs (excluding GP Retainers and GP Registrars) as full-time equivalents in total and broken down by age group, sex and country of primary medical qualification from the NHS Information Centre for Health and Social Care (2011). Practices with unknown list size or <500 registered patients were excluded. Our practice sample size was therefore 7957 (out of 8305 practices in England with Quality and Outcomes Framework (QOF) data).
Quality and Outcomes Framework is a national pay for performance scheme for GPs introduced in 2004 (Ashworth and Millett, 2008). Each GP practice receives payments depending on its performance in >100 indicators in four domains: clinical, organisational, patient experience and additional services. Of 1000 points available in total, the clinical domain accounts for between 650 and 697. The QOF total practice score summarises overall performance, with higher scores indicating better performance. The two QOF cancer indicators of interest measured whether practices keep a register of patients diagnosed with any cancer, except non-melanotic skin cancers, from 1 April 2003 (‘Cancer01’) and the percentage of patients with cancer diagnosed within the last 18 months and who had a review within 6 months of confirmed diagnosis (‘Cancer03’) (NHS Information Centre for Health and Social Care, 2011). Quality and Outcomes Framework scores were averaged over the study years. Performance for the two patient experience indicators on access were averaged over the two available years: providing appointments within 48 h (PE07) and providing advance booking more than 2 days ahead (PE08).
Analysis strategy
We explored bivariate (crude) associations between each of the six patient factors and the outcome measure using χ2-tests. The selected patient factors were age group (seven categories), sex, ethnicity (White, Black, Mixed, Asian and other), broad cancer type (22 groups), deprivation and rurality. We will refer to these crude proportions as ‘unplanned proportions’. Associations between the outcome and each practice characteristic were assessed using Pearson’s/Spearman’s correlation coefficient or t-tests/analysis of variance, as appropriate.
To investigate the independent effects of the patient and practice variables, we fitted a logistic regression model containing all available variables; to adjust for the clustering of patients within practices, we also fitted Generalised Estimating Equations. Deprivation was considered first as a linear term, and second as five categories representing equal population. All analyses used SAS Version 9.2 (SAS Institute, Cary, NC, USA).
Results
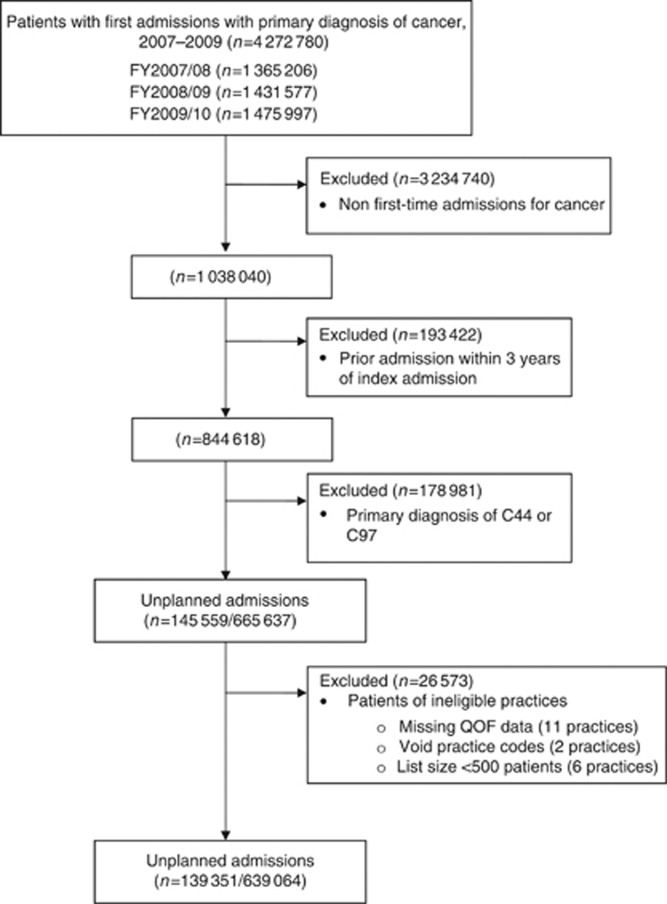
Figure 1 shows the selection process for the study. During the 3 study years, there were 4 272 780 admissions to hospital with a primary diagnosis of cancer. Of these, 21.9% were unplanned (n=665 637 patients had a valid first-time admission). After excluding patients from practices with <500 patients or missing practice identification codes, 21.8% of patients had unplanned admissions (n=139 351 of 639 064 patients with a first-time admission for cancer). Most practices (98.5%, n=7957) had 10 or more included patients, while 62.9% of practices had 50 or more included patients. Unplanned admissions had a median stay of 10 days, with two-thirds having a diagnostic (mostly scans or endoscopies) or other procedure (e.g., drainage of ascites) recorded.
Figure 1.
Patient sample selection process.
The unplanned proportion was higher among the youngest, oldest and those living in deprived or non-rural areas (Table 1). There was wide variation across the 22 types of cancer, with a small decrease over the study period.
Table 1. Crude proportions of first-time unplanned admissions for cancer.
| Patient characteristic | Number of patients with first-time cancer admission | Number with first-time unplanned admission for cancer | Percentage of admissions that were unplanned (%) | P -value |
|---|---|---|---|---|
| Age group (years) | <0.0001 | |||
| 0–4 | 2044 | 783 | 38.3 | |
| 5–14 | 2503 | 814 | 32.5 | |
| 15–44 | 50 666 | 7041 | 13.9 | |
| 45–64 | 211 785 | 32 054 | 15.1 | |
| 65–74 | 175 011 | 34 225 | 19.6 | |
| 75–84 | 147 269 | 42 067 | 28.6 | |
| 85 and over | 49 786 | 22 367 | 44.9 | |
| Sex | <0.0001 | |||
| Male | 312 951 | 71 349 | 22.8 | |
| Female | 326 113 | 68 002 | 20.9 | |
| Ethnic group | <0.0001 | |||
| White | 531 657 | 117 837 | 22.2 | |
| Mixed | 2063 | 401 | 19.4 | |
| Asian | 11 389 | 2486 | 21.8 | |
| Black | 9385 | 2092 | 22.3 | |
| Other | 5978 | 1455 | 24.3 | |
| Not known | 78 592 | 15 080 | 19.2 | |
| Cancer type | <0.0001 | |||
| Pancreas | 13 225 | 7436 | 56.2 | |
| Brain and central nervous system | 13 170 | 6484 | 49.2 | |
| Acute leukaemia | 8336 | 4087 | 49.0 | |
| Lung | 62 442 | 24 803 | 39.7 | |
| Other | 96 961 | 37 398 | 38.6 | |
| Ovary | 12 079 | 3 493 | 28.9 | |
| Multiple myeloma | 9654 | 2674 | 27.7 | |
| Stomach | 13 970 | 3684 | 26.4 | |
| Chronic leukaemia | 7192 | 1716 | 23.9 | |
| Kidney | 13 653 | 3157 | 23.1 | |
| Non-Hodgkin’s lymphoma | 23 541 | 5318 | 22.6 | |
| Colorectal | 80 508 | 17 285 | 21.5 | |
| Oesophagus | 18 946 | 3407 | 18.0 | |
| Larynx | 4764 | 661 | 13.9 | |
| Cervix | 5964 | 779 | 13.1 | |
| Prostate | 55 275 | 6487 | 11.7 | |
| Testis | 4732 | 445 | 9.4 | |
| Bladder | 48 333 | 3696 | 7.6 | |
| Oral | 9863 | 721 | 7.3 | |
| Uterus | 16 017 | 1036 | 6.5 | |
| Breast | 101 506 | 4170 | 4.1 | |
| Melanoma | 18 933 | 414 | 2.2 | |
| Deprivation quintile, derived from patient’s postcode | <0.0001 | |||
| 1 (least deprived) | 131 224 | 25 373 | 19.3 | |
| 2 | 136 924 | 27 519 | 20.1 | |
| 3 | 133 580 | 28 537 | 21.4 | |
| 4 | 122 964 | 28 304 | 23.0 | |
| 5 (most deprived) | 113 717 | 29 436 | 25.9 | |
| 6 (unknown) | 655 | 182 | 27.8 | |
| Rurality of residence | <0.0001 | |||
| Urban >10 K | 496 040 | 111 039 | 22.4 | |
| Town and fringe | 72 445 | 14 897 | 20.6 | |
| Village, hamlet and isolated dwellings | 70 170 | 13 297 | 18.9 | |
| Not resident in England | 409 | 118 | 28.9 | |
| Year of diagnosis | <0.0001 | |||
| 2007 | 206 656 | 46 421 | 22.5 | |
| 2008 | 214 097 | 46 713 | 21.8 | |
| 2009 | 218 311 | 46 217 | 21.2 |
By cancer type, the lowest unplanned proportions were for patients diagnosed with melanoma (2.2%) and breast cancer (4.1%), and highest for brain and central nervous system cancers (49.2%) and pancreatic cancer (56.2%). There was a small fall in the outcome rate during the 3 years of interest.
Approximately one in seven practices included in the study was single-handed, defined as one full- or part-time GP at the practice (Table 2). Nearly half of all GPs were aged 50 years and older (47%), with 19% of practices consisting of GPs all aged 50 years and older. Over two-thirds of GPs gained their primary medical qualification in the United Kingdom (68%) and one in six practices did not have any UK-qualified GPs (17.6%). Less than 5% of practices had only female GPs and 21.0% of practices did not have any female GPs.
Table 2. Summary of practice characteristics (7957 practices with complete data).
| Characteristic | Measure | Results |
|---|---|---|
| Practice list size | Median (IQR) | 5974 (3511–9230) |
| GPs (full-time equivalents) per practice | Mean (s.d.) | 4 (3) |
| Single-handed practices | N (%) | 1100 (13.8) |
| FTE GPs per 10 000 patients | Median (IQR) | 5.7 (4.5–6.5) |
| GPs aged 50 years and older | Percentage | 47 |
| None | N (%) | 991 (12.5%) |
| Some | N (%) | 5484 (68.9%) |
| All | N (%) | 1482 (18.6%) |
| GPs qualified in the United Kingdom | Percentage | 68 |
| None | N (%) | 1399 (17.6%) |
| Some | N (%) | 3225 (40.5%) |
| All | N (%) | 3333 (41.9%) |
| Female GPs | Percentage | 40 |
| None | N (%) | 1667 (21.0%) |
| Some | N (%) | 5937 (74.6%) |
| All | N (%) | 353 (4.4%) |
| Deprivation quintile, derived from mean score for the practice | ||
| 1–4 | N (%) | 6366 (80.0%) |
| 5 (most deprived) | N (%) | 1591 (20.0%) |
| Rurality of practicea | ||
| Urban >10 K | N (%) | 6717 (84.4%) |
| Town and fringe | N (%) | 909 (11.4%) |
| Village, hamlet and isolated dwellings | N (%) | 321 (4.0%) |
|
QOF performance
| ||
| QOF total practice performance score (maximum of 1000 points) | Median (IQR) | 967.3 (947.7–981.5) |
| CANCER 01 indicator (recording of new diagnoses of cancer) | ||
| Always recorded | N (%) | 7943 (99.8%) |
| Sometimes/never recorded | N (%) | 14 (0.2%) |
| CANCER 03 indicator (review of patients diagnosed with cancer in previous 18 months) | ||
| Patients always reviewed | N (%) | 5347 (67.2%) |
| Patients sometimes/never reviewed | N (%) | 2610 (32.8%) |
| Patient experience (PE07): providing appointments within 48 h (figures are % of total points available) | Median (IQR) | 85.6 (77.2–91.8) |
| Patient experience (PE08): providing advance booking (figures are % of total points available) | Median (IQR) | 78.5 (65.1–88.7) |
Abbreviations: FTE=full-time equivalent; GR=general practice; IQR=interquartile range; QOF=Quality and Outcomes Framework.
Missing data for 10 practices.
According to QOF data, almost all practices always recorded new diagnoses of cancer. In contrast, only two-thirds of practices always reviewed patients who had been diagnosed with cancer in the past 18 months.
In the unadjusted analysis, all examined patient and most practice-level variables were statistically significant predictors of the first-time admission for cancer being unplanned. In the adjusted analysis, there were statistically significant relationships between the outcome measure and all patient and many practice-level characteristics (Table 3).
Table 3. Associations between admissions, patient and practice characteristics.
|
Unadjusted analysis
|
Adjusted analysis
|
|||
|---|---|---|---|---|
| Variable | Odds ratio (95% CI) | P -value | Odds ratio (95% CI) | P -value |
| Patient variables | ||||
| Age group (years) | <0.0001 | <0.0001 | ||
| 0–4 | 0.76 (0.70–0.83) | <0.0001 | 0.20 (0.18–0.22) | <0.0001 |
| 5–14 | 0.59 (0.54–0.64) | <0.0001 | 0.17 (0.15–0.19) | <0.0001 |
| 15–44 | 0.20 (0.19–0.20) | <0.0001 | 0.15 (0.14–0.15) | <0.0001 |
| 45–64 | 0.22 (0.21–0.22) | <0.0001 | 0.20 (0.19–0.20) | <0.0001 |
| 65–74 | 0.30 (0.29–0.30) | <0.0001 | 0.26 (0.25–0.26) | <0.0001 |
| 75–84 | 0.49 (0.48–0.50) | <0.0001 | 0.43 (0.42–0.44) | <0.0001 |
| 85 and over | 1 | — | 1 | — |
| Sex | ||||
| Male | 1 | — | 1 | — |
| Female | 0.89 (0.88–0.90) | <0.0001 | 1.07 (1.05–1.08) | <0.0001 |
| Ethnicity | <0.0001 | <0.0001 | ||
| White | 1 | — | 1 | — |
| Mixed | 0.83 (0.81–0.85) | <0.0001 | 0.87 (0.85–0.89) | <0.0001 |
| Asian | 1.13 (1.06–1.20) | <0.0001 | 1.16 (1.08–1.24) | <0.0001 |
| Black | 1.01 (0.96–1.06) | 0.828 | 1.12 (1.05–1.18) | 0.241 |
| Other | 0.98 (0.94–1.03) | 0.404 | 1.03 (0.98–1.08) | 0.212 |
| Not known | 0.85 (0.76–0.94) | 0.003 | 0.93 (0.83–1.04) | <0.0001 |
| Cancer type | <0.0001 | <0.0001 | ||
| Acute leukaemia | 1.53 (1.46–1.60) | <0.0001 | 1.78 (1.69–1.87) | <0.0001 |
| Bladder | 0.13 (0.13–0.14) | <0.0001 | 0.10 (0.10–0.10) | <0.0001 |
| Brain and CNS | 1.55 (1.49–1.60) | <0.0001 | 1.99 (1.92–2.07) | <0.0001 |
| Breast | 0.07 (0.07–0.07) | <0.0001 | 0.07 (0.07–0.08) | <0.0001 |
| Cervix | 0.24 (0.22–0.26) | <0.0001 | 0.30 (0.28–0.32) | <0.0001 |
| Chronic leukaemia | 0.50 (0.47–0.53) | <0.0001 | 0.48 (0.45–0.51) | <0.0001 |
| Colorectal | 0.44 (0.43–0.45) | <0.0001 | 0.37 (0.36–0.38) | <0.0001 |
| Kidney | 0.48 (0.46–0.50) | <0.0001 | 0.50 (0.48–0.52) | <0.0001 |
| Larynx | 0.26 (0.24–0.28) | <0.0001 | 0.26 (0.23–0.28) | <0.0001 |
| Lung | 1.05 (1.03–1.07) | <0.0001 | 0.95 (0.93–0.98) | <0.0001 |
| Melanoma | 0.04 (0.03–0.04) | <0.0001 | 0.04 (0.03–0.04) | <0.0001 |
| Multiple myeloma | 0.61 (0.58–0.64) | <0.0001 | 0.55 (0.52–0.58) | <0.0001 |
| Non-Hodgkin’s lymphoma | 0.47 (0.45–0.48) | <0.0001 | 0.47 (0.45–0.49) | <0.0001 |
| Oesophagus | 0.35 (0.34–0.36) | <0.0001 | 0.30 (0.28–0.31) | <0.0001 |
| Oral | 0.13 (0.12–0.14) | <0.0001 | 0.13 (0.12–0.14) | <0.0001 |
| Ovary | 0.65 (0.62–0.68) | <0.0001 | 0.68 (0.65–0.71) | <0.0001 |
| Pancreas | 2.05 (1.97–2.12) | <0.0001 | 1.91 (1.84–1.99) | <0.0001 |
| Prostate | 0.21 (0.21–0.22) | <0.0001 | 0.20 (0.19–0.20) | <0.0001 |
| Stomach | 0.57 (0.55–0.59) | <0.0001 | 0.45 (0.43–0.47) | <0.0001 |
| Testis | 0.17 (0.15–0.18) | <0.0001 | 0.30 (0.27–0.33) | <0.0001 |
| Uterus | 0.11 (0.10–0.12) | <0.0001 | 0.11 (0.10–0.11) | <0.0001 |
| Other | 1 | — | 1 | — |
| Deprivation quintile | <0.0001 | <0.0001 | ||
| 1 (least deprived) | 1 | — | 1 | — |
| 2 | 1.05 (1.03–1.07) | <0.0001 | 1.04 (1.02–1.07) | <0.0001 |
| 3 | 1.13 (1.11–1.16) | <0.0001 | 1.12 (1.09–1.15) | <0.0001 |
| 4 | 1.25 (1.22–1.27) | <0.0001 | 1.20 (1.17–1.23) | <0.0001 |
| 5 (most deprived) | 1.46 (1.43–1.49) | <0.0001 | 1.36 (1.32–1.40) | <0.0001 |
| 6 (unknown) | 1.61 (1.35–1.91) | <0.0001 | 1.46 (1.12–1.89) | <0.0001 |
| Rurality of residence | <0.0001 | <0.0001 | ||
| Urban >10 K | 1.12 (1.09–1.14) | <0.0001 | 1.04 (1.01–1.06) | 0.002 |
| Town and fringe | 1 | — | 1 | |
| Village, hamlet and isolated dwellings | 0.90 (0.88–0.93) | <0.0001 | 0.96 (0.93–0.99) | 0.003 |
| Not resident in England | 1.57 (1.26–1.96) | <0.0001 | 1.03 (0.72–1.48) | 0.876 |
| Year of diagnosis | <0.0001 | <0.0001 | ||
| 2007 | 1 | — | 1 | — |
| 2008 | 0.96 (0.95–0.98) | <0.0001 | 0.96 (0.94–0.97) | <0.0001 |
| 2009 | 0.93 (0.91–0.94) | <0.0001 | 0.91 (0.90–0.93) | <0.0001 |
| Practice variables | ||||
| List size per 10 000 patients | 0.94 (0.92–0.95) | <0.0001 | 0.97 (0.95–0.99) | 0.014 |
| FTE per 10 000 patients | 0.98 (0.97–0.99) | <0.0001 | 0.99 (0.98–1.00) | 0.001 |
| Single-handed practices | ||||
| Single GP | 1.16 (1.12–1.19) | <0.0001 | 1.01 (0.96–1.06) | 0.628 |
| More than one GP | 1 | — | 1 | — |
| GPs aged 50 years and over | <0.0001 | 0.486 | ||
| None | 1 | — | 1 | — |
| Some | 0.97 (0.95–0.99) | 0.015 | 1.00 (0.97–1.02) | 0.797 |
| All | 1.10 (1.06–1.14) | <0.0001 | 1.02 (0.98–1.06) | 0.343 |
| GPs qualified in the United Kingdom | <0.0001 | <0.0001 | ||
| None | 1.23 (1.19–1.26) | <0.0001 | 1.08 (1.04–1.11) | <0.0001 |
| Some | 1.06 (1.04–1.08) | <0.0001 | 1.04 (1.02–1.06) | 0.001 |
| All | 1 | — | 1 | — |
| Female GPs | <0.0001 | 0.310 | ||
| None | 1 | — | 1 | — |
| Some | 0.88 (0.86–0.90) | <0.0001 | 0.98 (0.95–1.01) | 0.205 |
| All | 0.98 (0.93–1.04) | 0.553 | 1.02 (0.96–1.08) | 0.594 |
| Practice deprivation average scorea | 1.01 (1.01–1.01) | <0.0001 | 1.00 (1.00–1.00) | 0.002 |
| Practice deprivation quintilea | ||||
| <5 | 1 | — | 1 | — |
| 5 (most deprived) | 1.23 (1.21–1.26) | <0.0001 | 1.01 (0.98–1.03) | 0.576 |
| Practice deprivation quintilea | <0.0001 | <0.0001 | ||
| 1 (least deprived) | 1 | — | 1 | — |
| 2 | 1.03 (1.01–1.05) | 0.011 | 0.95 (0.93–0.98) | <0.0001 |
| 3 | 1.08 (1.06–1.11) | <0.0001 | 0.92 (0.90–0.95) | <0.0001 |
| 4 | 1.16 (1.14–1.19) | <0.0001 | 0.91 (0.88–0.93) | <0.0001 |
| 5 (most deprived) | 1.31 (1.27–1.34) | <0.0001 | 0.93 (0.90–0.96) | <0.0001 |
| Rurality of practiceb | <0.0001 | 0.330 | ||
| Urban >10 K | 0.95 (0.90–0.99) | 0.010 | 1.02 (0.99–1.06) | 0.192 |
| Town and fringe | 1 | — | 1 | — |
| Village, hamlet and isolated dwellings | 1.13 (1.11–1.16) | <0.0001 | 0.99 (0.95–1.04) | 0.809 |
| QOF total practice performance score (per 100 points) | 0.85 (0.83–0.88) | <0.0001 | 0.94 (0.91–0.97) | <0.0001 |
| QOF CANCER01 | ||||
| Diagnosis always recorded | 1 | — | 1 | — |
| Diagnosis sometimes or never recorded | 0.90 (0.61–1.32) | 0.576 | 0.75 (0.55–1.01) | 0.052 |
| QOF CANCER03 | ||||
| Patient always reviewed | 1 | — | 1 | — |
| Patient sometimes or never reviewed | 1.02 (1.00–1.03) | 0.060 | 1.01 (0.99–1.02) | 0.567 |
| QOF PE07: providing 48 h appointments | 0.72 (0.68–0.77) | <0.0001 | 0.85 (0.79–0.92) | <0.0001 |
| QOF PE08: providing advance booking | 0.83 (0.79–0.87) | <0.0001 | 0.98 (0.92–1.04) | 0.520 |
Abbreviations: CI=confidence interval; CNS=central nervous system; FTE=full-time equivalent; GP=general practice; QOF=Quality and Outcomes Framework.
Only one of these deprivation variables was included at a time.
Missing data for 1013 patients at 10 practices.
After adjustment, practice rurality, having only one GP, having all GPs aged 50 or older or having all female GPs were no longer significant. Patient area deprivation remained more important than practice deprivation (most deprived compared with least deprived quintile: OR 1.36; 95% CI 1.32–1.40). Patients of practices with more GPs per 10 000 population were slightly less likely to use the unplanned route (OR 0.97; 95% CI 0.95–0.99). The unplanned route remained more common for patients of practices where no GP gained their primary medical qualification in the United Kingdom (OR 1.08; 95% CI 1.04–1.11).
For the QOF measures, the adjusted odds was inversely associated with higher average QOF total performance (OR 0.94 per extra 100 points; 95% CI 0.91–0.97). In contrast, there was no statistically significant relation with either QOF cancer indicator. Higher performance for 48 h appointments was associated with lower odds, but the relation with advance booking was no longer statistically significant after adjustment.
We found various two-way interactions to be statistically significant at the 5% level. These suggest, for instance, that the adjusted odds is higher with large lists in towns, but lower with large lists in villages. For clarity, however, we have not shown these.
Discussion
Summary of results
We assessed the associations between the odds of a patient’s first-time admission for cancer being unplanned rather than planned and various patient and practice characteristics. We found large variation in this measure by age, sex and ethnicity. The highest outcome rates were among Asian patients, those aged 85 years and over and for females. There was also wide variation for different types of cancer, which was affected by deprivation and rurality of patients’ places of residence. After adjusting for patient factors and deprivation, higher unplanned proportions were significantly associated with smaller list size and the country of qualification of the GP. In contrast, there were only weak associations with the QOF indicators considered. The strongest associations found were the protective effects of higher QOF total performance scores and the proportion of patients obtaining appointments within 48 h (indicator PE07).
If this outcome is a useable indicator of practice quality (which we discuss below), then one would hope to see correlations with other performance measures such as QOF indicators. We did observe relations with total QOF score and patient experience measures but not with cancer-specific indicators after adjustment. The relation was significant with GPs per 10 000 patients, with a 1% drop in odds per GP per 10 000 patients, as inconsistently found with other indicators (Saxena et al, 2007). There are also significant relations between quality of care (e.g., QOF or prescribing) and the age, sex and country of origin or qualification of GPs (Baker, 1996; Tsimtsiou et al, 2009; Ashworth et al, 2011), although these findings were also inconsistent and sociodemographic factors usually appear to be more important (Baker, 1996; Tsimtsiou et al, 2009). We found an inverse relation between patients obtaining appointments within 2 working days and the odds of unplanned admissions, suggesting that timely primary-care access is important.
We observed 16% higher unadjusted odds of the admission being unplanned in single-handed practices, which became 1% and not significant after adjusting for area-level factors and deprivation. Definitions of the term ‘single-handed’ differ (Smith, 2004), although we found similar results by applying full-time equivalents or headcounts. Other studies have documented difficulties faced by practices with only one GP (Ashworth et al, 2011), although these practices can still achieve good patient ratings, for example, in terms of patient access and consultation time (Campbell et al, 2001; van den Hombergh et al, 2005; Vamos et al, 2011).
The analysis presented here is far from exhaustive, but suggests what is possible with current data. We now discuss the strengths and limitations of this study before considering policy implications and further research.
Strengths of this study
We have used two established national data sets, HES and QOF, and a range of geographical and practice information with good completeness.
Limitations
These may be divided into definition, data and analysis issues.
Definition issues
The outcome measure relies on being able to identify a patient’s first admission for cancer. Tracking back 3 years incurred less misclassification than the 1 year look-back adopted by Raine et al (2010), although any method depends on the availability of unique patient identifiers (no method is perfect). We used only the primary diagnosis rather than the first four diagnoses, opting for greater specificity. Owing to missing diagnostic information, we were unable to use outpatient records, and thus were unable to determine precisely where the patient is along the pathway. Cancer registration data contain an ‘anniversary’ (diagnosis) date which could be compared with the date of admission, but these dates need to be linked to HES and we did not have access. Even with successful linkage of dates, because many practices have fairly few cancer patients, this prevents assessment of practice performance on the measure.
Data issues
We excluded practices with <500 registered patients, missing QOF data or practice identification codes, but these exclusions should not affect our findings. As this study was exploratory, we have not performed sensitivity analyses or imputation. We considered only several practice variables as national practice-level information is limited.
We also considered a small number of patient factors, and more might be made of HES data (see future work). We did not have information on patient behaviour including patient preferences (Raine et al, 2010), diet, smoking, exercise, exposure to other carcinogens and tumour stage, i.e., factors partly or largely beyond the GPs’ control that may lead to poorer outcomes. Sociodemographic information was at area-level only.
Analysis issues
We found a number of statistically significant interactions, but for clarity have not shown these. We fitted deprivation first as a linear term and second in fifths. Although plots of residuals suggested that adjustment for deprivation was incomplete even after trying polynomials, we reported the effects just by deprivation fifth for simplicity, noting that the choice made little difference to the other coefficients. Also for clarity, we categorised several continuous variables, including rurality and the proportions of GPs aged 50 or over or who were female.
Policy and research implications
The Kings Fund recommended that an integral part of quality improvement will be to gather data to evaluate care in broader terms and to compare inter-practice and temporal performance (King’s Fund, 2010). The Royal College of GPs has produced a cancer diagnosis audit tool for practices and cancer networks to help with this (Royal College of General Practitioners, 2011); referral rates are known to vary across England (National Audit Office, 2010). The measure used in our study has been proposed as an indicator of practice quality. The key assumption is that unplanned admission is a poor outcome (which has support from other studies) and follows on from presentation to A&E with more advanced symptoms. Some types of cancer rarely present with symptoms when a GP consultation might make a difference to early diagnosis. Some unplanned admissions will also be unavoidable. Although numbers of patients with cancer can be small at practice level (nearly half of practices had <10 patients in 3 years), the indicator may function better for GP consortia or other larger practices. Access to and use of diagnostic facilities is not uniform across the United Kingdom, and some referrals for private treatment or investigation are not captured in HES (Baughan et al, 2009).
There are striking differences in admissions by age, cancer type and deprivation. The findings could therefore help to identify patient groups who are susceptible to late diagnosis, including the very young or very old, patients diagnosed with brain or pancreatic tumours and patients living in deprived areas.
Conclusion
We have identified some patient and practice characteristics that are associated with a first-time admission for cancer being unplanned rather than planned. The former could be used to help identify patients at high risk of the outcome, while the latter raise questions about the role of organisation of practices and staff training. Specialists also have a role in preventing unplanned admissions. For example, rapid access to diagnostic services and cancer specialists could lead to earlier diagnosis. Methods for general practitioners to obtain advice and support from specialist services for their patients when they experience complications would also help reduce unplanned admissions. In-depth study of practices with very low or high first-time emergency admission rates for cancer may provide lessons that are more widely applicable to improve cancer detection, referral delay and survival.
Acknowledgments
The Department of Primary Care & Public Health at Imperial College London is grateful for support from the NIHR Collaboration for Leadership in Applied Health Research & Care scheme, and the NIHR Biomedical Research Centre scheme.
Author contributions
AB, AM, MS and PA conceived the study. AB, CT and MP performed the analysis. AB and CT drafted the manuscript and all authors critically reviewed it.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The Dr Foster Unit at Imperial is principally funded through a research grant by Dr Foster Intelligence, an independent healthcare information company and joint venture with the Information Centre of the NHS. The Dr Foster Unit at Imperial is affiliated with the Imperial Centre for Patient Safety and Service Quality at Imperial College Healthcare NHS Trust, which is funded by the National Institute of Health Research.
Role of the funding source
The funders had no role in the design, analysis, write-up or decision to submit for publication.
Ethics
We hold Section 251 (formerly Section 60) National Information Governance Board for Health and Social Care permission to hold these data for research purposes. We hold South East Local Research Ethics Committee approval to analyse the data.
10/04/2012
This paper has been modified since advance online publication, an acknowledgement has been added
References
- Ashworth M, Millett C (2008) Quality improvement in UK primary care: the role of financial incentives. J Ambulatory Care Manage 31: 220–225 [DOI] [PubMed] [Google Scholar]
- Ashworth M, Schofield P, Seed P, Durbaba S, Kordowicz M, Jones R (2011) Identifying poorly performing general practices in England: a longitudinal study using data from the quality and outcomes framework. J Health Serv Res Policy 16: 21–27 [DOI] [PubMed] [Google Scholar]
- Baker R (1996) Characteristics of practices, general practitioners and patients related to levels of patients' satisfaction with consultations. Br J Gen Pract 46: 601–605 [PMC free article] [PubMed] [Google Scholar]
- Baughan P, O'Neill B, Fletcher E (2009) Auditing the diagnosis of cancer in primary care: the experience in Scotland. Br J Cancer 101: S87–S91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrino F, Gatta G, Sant M, Capocaccia R (2001) The EUROCARE study of survival of cancer patients in Europe: aims, current status, strengths and weaknesses. Eur J Cancer 37: 673–677 [DOI] [PubMed] [Google Scholar]
- Campbell JL, Ramsay J, Green J (2001) Practice size: impact on consultation length, workload, and patient assessment of care. Br J Gen Pract 51: 644–650 [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Quaresma M, Berrino F, Lutz J-M, De Angelis R, Capocaccia R, Baili P, Rachet B, Gatta G, Hakulinen T, Micheli A, Sant M, Weir HK, Elwood JM, Tsukuma H, Koifman S, e Silva GA, Francisci S, Santaquilani M, Verdecchia A, Storm HH, Young JL (2008) Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 9: 730–756 [DOI] [PubMed] [Google Scholar]
- Department for Communities and Local Government (2008) The English Indices of Deprivation 2007. The Stationery Office: London [Google Scholar]
- Department of Health (1995) A Report by the Expert Advisory Group on Cancer to the Chief Medical Officers of England and Wales. A Policy Framework for Commissioning Cancer Services (The Calman-Hine Report). Department of Health: London [Google Scholar]
- Department of Health (2000) The NHS Cancer Plan. A plan for investment. a plan for reform. Her Majesty's Stationery Office: Norwich [Google Scholar]
- Department of Health (2007). Cancer Reform Strategy Department of Health: London [Google Scholar]
- Department of Health (2011) Improving outcomes: a strategy for cancer. Department of Health: London [Google Scholar]
- King's Fund (2010) Getting the measure of quality. Opportunities and challenges. King's Fund: London [Google Scholar]
- National Audit Office (2010) Delivering the Cancer Reform Strategy. The Stationery Office: London [Google Scholar]
- National Cancer Intelligence Network (2010) Routes to diagnosis – Technical supplement. National Cancer Intelligence Network
- NHS Information Centre for Health and Social Care (2011) Number of GPs per GP Practice by gender, ageband and country of qualification area in England as at 30 September 2010. NHS Information Centre for Health and Social Care
- Office for National Statistics (2010) Postcode Directories ESRC/JISC Census Programme. Census Dissemination Unit, Mimas (University of Manchester)/Census Geography Data Unit (UKBORDERS)
- Raine R, Wong W, Scholes S, Ashton C, Obichere A, Ambler G (2010) Social variations in access to hospital care for patients with colorectal, breast, and lung cancer between 1999 and 2006: retrospective analysis of hospital episode statistics. Br Med J 340: b5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal College of General Practitioners (2011) National Audit of Cancer Diagnosis in Primary Care. Royal College of General Practitioners: London [Google Scholar]
- Saxena S, Car J, Eldred D, Soljak M, Majeed A (2007) Practice size, caseload, deprivation and quality of care of patients with coronary heart disease, hypertension and stroke in primary care: national cross-sectional study. BMC Health Serv Res 7: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawihdi M, Stern N, Thompson E, Sturgess R, Kapoor N, Pearson MG, Bodger K (2011) Emergency admission as a route for oesophagogastric cancer diagnosis: a marker of poor outcome and a candidate quality indicator for local services. 60: A30–A31 [Google Scholar]
- Smith DJ (2004) The Shipman Inquiry—Fifth Report. Safeguarding Patients: Lessons from the Past—Proposals for the Future. Cm 6394. Her Majesty's Stationery Office: Norwich [Google Scholar]
- Tsimtsiou Z, Ashworth M, Jones R (2009) Variations in anxiolytic and hypnotic prescribing by GPs: a cross-sectional analysis using data from the UK Quality and Outcomes Framework. Br J Gen Pract 59: e191–e198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Manchester (2011) Geoconvert. Access available at URL http://geoconvert.mimas.ac.uk/ (accessed July 2012)
- Vamos EP, Pape UJ, Bottle A, Hamilton FL, Curcin V, Ng A, Molokhia M, Car J, Majeed A, Millett C (2011) Association of practice size and pay-for-performance incentives with the quality of diabetes management in primary care. CMAJ 183: E809–E816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hombergh P, Engels Y, van den Hoogen H, van Doremalen J, van den Bosch W, Grol R (2005) Saying 'goodbye' to single-handed practices; what do patients and staff lose or gain? Fam Pract 22: 20–27 [DOI] [PubMed] [Google Scholar]
- Woods LM, Coleman MP, Lawrence G, Rashbass J, Berrino F, Rachet B (2011) Evidence against the proposition that ‘UK cancer survival statistics are misleading’: simulation study with National Cancer Registry data. Br Med J 342(342): d3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (1992) International Statistical Classification of Diseases and Related Health Problems, 10th Revision [PubMed]