Abstract
BACKGROUND & AIMS
Patients with celiac disease have been reported to be at increased risk for pancreatitis and pancreatic insufficiency, but the risk might have been overestimated because of patient selection and limited numbers of patients for analysis. Furthermore, no distinction has been made between patients with gallstone-related and non gallstone-related pancreatitis. We performed a nationwide study to determine the risk for any pancreatitis or subtype of pancreatitis among patients with biopsy-verified celiac disease.
METHODS
We analyzed data from patients in Sweden with celiac disease (n=28,908), identified based on small intestinal biopsy records from 28 pathology departments (those with villous atrophy, Marsh 3). Biopsies were performed from 1969 to 2008 and biopsy report data were collected from 2006 to 2008. Patients with pancreatitis were identified based on diagnostic codes in the Swedish Patient Register and records of pancreatic enzyme use in the Swedish Prescribed Drug Register. Data were matched with those from 143,746 individuals in the general population; Cox regression was used to estimate hazard ratios (HRs) for pancreatitis.
RESULTS
We identified 406 individuals with celiac disease who were later diagnosed with pancreatitis (and 143 with expected pancreatitis) (HR=2.85; 95% confidence interval [CI], 2.53–3.21). The absolute risk of any pancreatitis among patients with celiac disease was 126/100,000 person-years with an excess risk of 81/100,000 person-years. The HR for gallstone-related acute pancreatitis was 1.59 (95% CI, 1.06–2.40), for non-gallstone-related acute pancreatitis was 1.86 (1.52–2.26), for chronic pancreatitis was 3.33 (95% CI, 2.33–4.76), and for supplementation with pancreatic enzymes was 5.34 (95% CI, 2.99–9.53). The risk of any pancreatitis within 5 years of diagnosis was 2.76 (95% CI, 2.36–3.22).
CONCLUSIONS
Based on an analysis of medical records in Sweden, patients with celiac disease were at an almost 3-fold increase in risk of developing pancreatitis.
Keywords: Coeliac Disease, Celiac Disease, Cohort study, Inflammation, Pancreatitis, Epidemiology, retrospective analysis, gluten intolerance, complication
Introduction
Celiac disease is an autoimmune disorder that occurs in about 1% of the Western population. The disease is triggered by gluten exposure in genetically predisposed individuals1 and the only treatment is a lifelong gluten-free diet.
To our knowledge, the effect of celiac disease on pancreatic function is poorly understood. Macroamylasemia is five times more common in patients with active celiac disease than in healthy controls.2 Celiac disease is also overrepresented among patients with recurrent acute pancreatitis.3 Between 10 and 20% of patients with newly diagnosed celiac disease are likely to experience pancreatic insufficiency.4, 5 Supplementation of pancreatic enzymes in patients with celiac disease and chronic diarrhea expressing low fecal elastase indicating chronic pancreatic insufficiency, improved problems with diarrhea in 18 of 20 individuals.4 All of these patients experienced histopathological improvement.4, 6 In one study investigating the association between celiac disease and pancreatitis patients with celiac disease had a 20-fold elevated risk of chronic pancreatitis and a 3-fold higher risk of acute or chronic pancreatitis.7 However, that study included only hospitalized patients with celiac disease, potentially leaving out those diagnosed and treated on an outpatient basis. Furthermore, celiac disease was not validated histopathologically. Finally, no distinction was made between gallstone- and non-gallstone-related acute pancreatitis.
The main objective of this study was therefore to estimate the risk of any pancreatitis, including subtypes of pancreatitis, in patients with biopsy-verified celiac disease.
Methods
We linked data on biopsy reports with celiac disease to the Swedish Patient Register (containing both inpatient and hospital outpatient data) and the Swedish Prescribed Drug Register.
Celiac Disease
Patients with celiac disease were identified from biopsy reports performed between 1969 and 2008 in any of Sweden’s 28 pathology departments (Table 1). Celiac disease was defined as having villous atrophy (VA, equivalent to Marsh grade 3, see appendix).8 Although we did not request positive celiac disease serology for the diagnosis of celiac disease, in a random sample of patients undergoing patient chart reviews 88% (n=71/81) with available data on celiac disease serology were serologically positive before biopsy.9
TABLE 1.
Characteristics of the study participants.
| Matched reference individuals | Celiac disease | |
|---|---|---|
| Total | 143,746 | 28,908 |
| Age at study entry, years (median, range) | 30; 0–95 | 30; 0–95 |
| Age 10–19 (%) | 58,843 (40.9) | 11,799 (40.8) |
| Age 20–39 (%) | 26,290 (18.3) | 5,284 (18.3) |
| Age 40–59 (%) | 31,932 (22.2) | 6,404 (22.2) |
| Age ≥60 (%) | 26,681 (18.6) | 5,421 (18.8) |
| Entry year (median, range) | 1998; 1969–2008 | 1998; 1969–2008 |
| Follow-up#, years (median, range) | 10; 0–41 | 10; 0–41 |
| Follow-up#, years (mean±SD) | 11.4±6.5 | 11.2±6.5 |
| Females (%) | 89,204 (62.1) | 17,926 (62.0) |
| Males (%) | 54,542 (37.9) | 10,982 (38.0) |
| Calendar year | ||
| -1989 | 20,302 (14.1) | 4,088 (14.1) |
| 1990–99 | 59,553 (41.4) | 11,986 (41.5) |
| 2000- | 63,891 (44.4) | 12,834 (44.4) |
| Country of birth, Nordic | 135,549 (94.3) | 27,961 (96.7) |
Ages were rounded to the nearest year.
Follow-up time until diagnosis of pancreatitis, death, emigration, or 31 December 2009. In reference individuals follow-up can also end through small intestinal biopsy.
At each pathology department, IT personnel identified relevant biopsy reports from computerized databases and delivered data on biopsy date, personal identity number of the patient,10 morphology according to the Swedish SnoMed classification codes (Supplementary Table E1) and topography (duodenum and jejunum). Because searches were restricted to computerized databases, most of our biopsy report data originated from 1990 or later (Table 1).
This paper was based on the same celiac disease cohort as in our paper on mortality in celiac disease (n=29,096, Figure 1).11 Each of the 29,096 individuals with celiac disease was then matched with five reference individuals from the general population of Sweden (n=144,522)(matching on age, sex, calendar year and county of residence). Exclusions because of data irregularities are shown in Figure 1.
Figure 1. Flow chart of study participants.
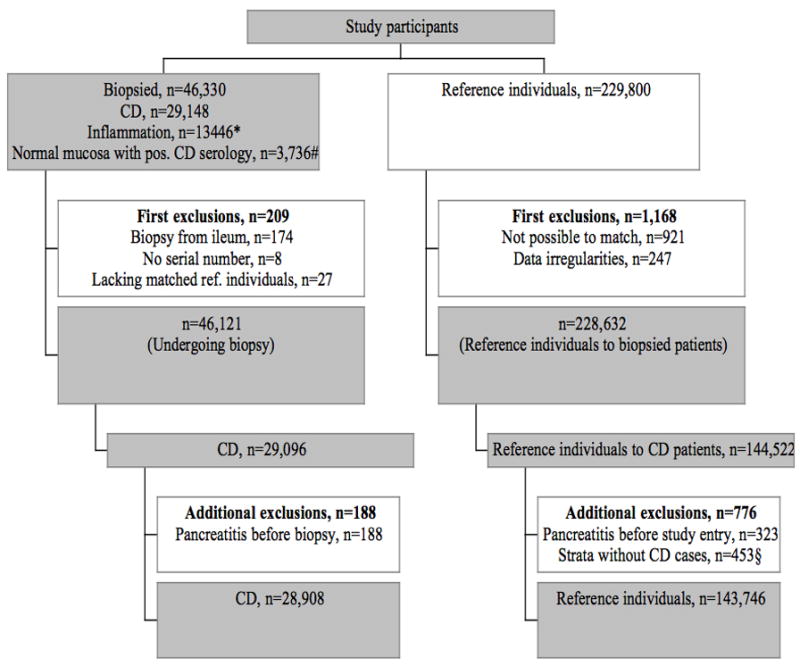
CD, Celiac disease
* Inflammation (intraepithelial lymphocytosis) equals Marsh 1–2
# Normal mucosa with positive IgA/IgG endomysium, transglutaminase or gliadin antibodies.
§ Because all calculations were performed stratum-wise, reference individuals whose index individual with celiac disease had been excluded were also excluded.
We then excluded individuals with a diagnosis of pancreatitis or a record of pancreatic enzyme supplementation before celiac disease diagnosis or study entry. The main analyses of this paper were based on 28,908 individuals with celiac disease and 143,746 matched reference individuals (Figure 1).
For comparative purposes, we also identified individuals undergoing small intestinal biopsy but without VA (inflammation, Marsh 1–2, n=13,306) and individuals with normal mucosa but positive IgA/IgG gliadin or endomysium or tissue transglutaminase antibodies (n=3,719, the majority of these individuals had tested positive for IgA gliadin). These two cohorts were identical to those in our paper on mortality in celiac disease.11 We then excluded anyone with a prior diagnosis of pancreatitis. Individuals undergoing small intestinal biopsy but without VA were used as secondary reference individuals.
Pancreatitis
We defined pancreatitis according to relevant International Classification of Disease (ICD) codes (see appendix). Pancreatitis was ascertained from both inpatient and hospital-based outpatient data (Swedish Patient Register)12, and the Swedish Prescribed Drug Register. Hence, in this study pancreatitis represents patients who were diagnosed with clinical pancreatitis plus those who received pancreatic enzymes presumably for pancreatic insufficiency.
We distinguished between gallstone-related acute pancreatitis, non-gallstone-related acute pancreatitis, and chronic pancreatitis. Gallstone-related pancreatitis was defined as having either a specific ICD-10 code (K85.1) or a combination of a code for acute pancreatitis (ICD7-10) and cholecystectomy, ERCP or cholecystotomy (see appendix). In this study patients who had gallstone-related pancreatitis at any stage were not included in the non-gallstone group. Use of pancreatic enzyme (ATC code A09AA02) was also considered proof of pancreatic insufficiency.
When looking specifically at celiac disease and the risk of supplementation of pancreatic enzymes, we restricted our analysis to patients entering the study (having a biopsy) on 1 July 2005 or later in that the Swedish Prescribed Drug Register began on this date. In the analysis of “any pancreatitis”, also these individuals who had a biopsy date before 1 July 2005 and later pancreatic enzyme supplementation were counted as positive for “any pancreatitis” since they had been at risk of a pancreatitis diagnosis also before 1 July 2005 (e.g. from gallstone-related pancreatitis).
Covariates
Any diabetes (type 1 or type 2) was identified through the Swedish Patient Register (see appendix for ICD codes). This register was also used to define alcohol-related disease and chronic obstructive lung disease (as a proxy for heavy smoking). Education was divided into four categories. Socio-economic status was ranked in line with the European Socio-economic Classification (ESeC, 7 categories).13 The ESeC version we used was based on occupation. Country of birth was classified as Nordic vs. non-Nordic.
Statistics
We used Cox regression to estimate HRs for pancreatitis (any, acute gallstone-related, acute non-gallstone-related, chronic pancreatitis and use of pancreatic enzymes). We used an internally stratified Cox regression model in which only individuals within each stratum were compared before a summary risk estimate was estimated. We could therefore eliminate the influence of age, sex, county of residence and calendar year because participants in each stratum were identical on these parameters.
Follow-up time started on the date of the first biopsy with VA (celiac disease diagnosis) and on the corresponding date in the matched reference individuals. It ended with a diagnosis of pancreatitis, death, emigration, or on 31 December 2009, whichever occurred first.
We also evaluated the risk of any pancreatitis (and our subtypes of pancreatitis) by time since diagnosis (<1 year, 1- <5 years and ≥5 years), sex, age at celiac disease diagnosis (10–19, 20–39, 40–59 and ≥60 years at first biopsy) and calendar period of the first biopsy with celiac disease (-1989, 1990–1999 and 2000). Incidence rates were calculated by dividing the number of person-years at risk with the number of first pancreatitis events and attributable risks (%) as (1-1/HR). We also examined the risk of pancreatitis after adjusting for diabetes mellitus, alcohol-related diagnosis codes, chronic obstructive lung disease, education, socio-economic position and country of birth.
Secondary Analyses
In a separate analysis, to account for the severity of acute pancreatitis, we restricted our outcome to those with a hospital stay ≥14 days or those with in-hospital mortality. In a subanalysis we also restricted our outcome of “any pancreatitis” to individuals with a diagnosis of any pancreatitis in the Patient Register (not counting pancreatic enzyme substitution as pancreatitis).
We compared the risk of any pancreatitis in biopsy-proven celiac disease vs. that in individuals without VA (13,106 with inflammation (Marsh 1–2)8; and 3,690 with normal mucosa (Marsh 0)8 but positive serology to gliadin, endomysium or tissue transglutaminase antibodies, n=3,690). These analyses were adjusted for age, sex and calendar period.
Lastly, we examined the proportion of celiac disease individuals and reference individuals having a diagnosis of pancreatitis occurring before celiac disease diagnosis and study entry. In this case-control study we used conditional logistic regression to calculate odds ratios (ORs) for an earlier pancreatitis diagnosis in celiac disease.
Statistical significance was defined as 95% confidence intervals (CIs) for risk estimates not including 1.0. SPSS 18.0 software was used for the statistical analysis.
Ethics
This project (2006/633-31/4) was approved by the Research Ethics Committee of the Karolinska Institute, Sweden (14 June 2006).
Results
Background data
The median age at first biopsy and celiac disease diagnosis was 30 years (range 0–95). Some 86% of individuals with celiac disease had been biopsied in 1990 or later. The majority of celiac disease patients were female (Table 1). We identified 406 individuals with celiac disease who were later diagnosed with pancreatitis (expected: n=143). Some 199 (49.0%) of these were identified through the Patient Register with a diagnosis code for acute or chronic pancreatitis, with another 207 (51%) through the Prescribed Drug Register, who received supplementation of pancreatic enzymes. Of patients with a diagnosis of any pancreatitis in the Patient Register, 144 (72%) had only an inpatient diagnosis, 16 (7.7%) only an outpatient diagnosis, and 39 (19.6%) both an inpatient and an outpatient diagnosis.
Celiac disease and future risk of any pancreatitis
Individuals with celiac disease were at increased risk of later pancreatitis (HR=2.85; 95% CI=2.53–3.21)(Table 2). The absolute risk of any pancreatitis was 126/100,000 person-years with an excess risk of 81/100,000 person-years. HRs for any pancreatitis were similar in men and women (p for interaction between celiac disease and sex=0.994)(Table 3). Although HRs for any pancreatitis were higher in patients diagnosed with celiac disease before age 40 years, the difference between age groups did not attain statistical significance (p for interaction between celiac disease and age=0.194).
TABLE 2.
Risk of pancreatitis based on time since celiac diagnosis (in individuals with celiac disease).
| Time since diagnosis | Observed events | Expected events# | HR; 95% CI | P-value | Absolute risk/ 100,000 PYAR | Excess risk/ 100,000 PYAR | Attributable percentage |
|---|---|---|---|---|---|---|---|
| Any pancreatitis | |||||||
| All | 406* | 143 | 2.85; 2.53–3.21 | <0.001 | 126 | 81 | 65 |
| Excluding first year | 360 | 133 | 2.71; 2.39–3.08 | <0.001 | 122 | 77 | 63 |
| Year <1 | 46 | 10 | 4.72; 3.23–6.89 | <0.001 | 161 | 127 | 79 |
| 1–4.99 | 114 | 43 | 2.63; 2.12–3.28 | <0.001 | 106 | 66 | 62 |
| ≥5 | 246 | 89 | 2.76; 2.36–3.22 | <0.001 | 131 | 84 | 64 |
| Gallstone-related acute pancreatitis | |||||||
| All | 30 | 19 | 1.59; 1.06–2.40 | 0.026 | 9 | 3 | 37 |
| Excluding first year | 28 | 18 | 1.55; 1.02–2.36 | 0.042 | 9 | 3 | 35 |
| Year <1 | 2 | 1 | 2.42; 0.48–12.11 | 0.282 | 7 | 4 | 59 |
| 1–4.99 | 9 | 5 | 1.65; 0.79–3.42 | 0.182 | 8 | 3 | 39 |
| ≥5 | 19 | 13 | 1.51; 0.90–2.53 | 0.120 | 10 | 3 | 34 |
| Non-gallstone-related acute pancreatitis | |||||||
| All | 133 | 72 | 1.86; 1.52–2.26 | <0.001 | 41 | 19 | 46 |
| Excluding first year | 113 | 65 | 1.74; 1.41–2.15 | <0.001 | 38 | 16 | 43 |
| Year <1 | 20 | 7 | 2.99; 1.74–5.11 | <0.001 | 70 | 47 | 66 |
| 1–4.99 | 37 | 23 | 1.58; 1.10–2.27 | 0.014 | 34 | 13 | 37 |
| ≥5 | 76 | 41 | 1.84; 1.42–2.40 | <0.001 | 40 | 18 | 46 |
| Chronic pancreatitis | |||||||
| All | 49 | 15 | 3.33; 2.33–4.76 | <0.001 | 15 | 11 | 70 |
| Excluding first year | 40 | 14 | 2.84; 1.93–4.18 | <0.001 | 14 | 9 | 65 |
| Year <1 | 9 | <1 | 22.55; 6.69–75.97 | <0.001 | 31 | 30 | 96 |
| 1–4.99 | 16 | 4 | 3.73; 1.98–7.04 | <0.001 | 15 | 11 | 73 |
| ≥5 | 24 | 10 | 2.32; 1.43–3.77 | 0.001 | 13 | 7 | 57 |
| Supplementation with pancreatic enzyme§ | |||||||
| All | 21* | 4 | 5.34; 2.99–9.53 | <0.001 | 203 | 165 | 81 |
| Year <1 | 10 | 2 | 6.19; 2.55–15.03 | <0.001 | 347 | 291 | 84 |
| 1–4.99 | 11 | 2 | 4.74; 2.19–10.25 | <0.001 | 148 | 117 | 79 |
HR, Hazard ratio; CI, Confidence interval; PYAR, Person-years at risk. Reference is general population comparator cohort.
The discrepancy in numbers (“total any pancreatitis”, n=406 is more than the sum of subgroups), is because in the main analysis (n=406) we also included patients biopsied before 1 July 2005 with supplementation with pancreatic enzymes (n=241).
Expected number of events in patients with celiac disease was derived from the observed number of events divided by the HR.
This analysis was restricted to individuals entering the study on 1 July 2005 or later.
TABLE 3.
Risk of any pancreatitis in relation to characteristics of patients with celiac disease.
| Subgroup | Observed events | Expected events* | HR; 95% CI | P-value | Absolute risk/ 100,000 PYAR | Excess risk/ 100,000 PYAR | Attributable percentage |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Females | 233 | 81 | 2.89; 2.47–3.39 | <0.001 | 115 | 75 | 65 |
| Males | 173 | 62 | 2.79; 2.32–3.36 | <0.001 | 143 | 92 | 64 |
| Age | |||||||
| 10–19 yrs | 39 | 10 | 3.80; 2.53–5.71 | <0.001 | 27 | 20 | 74 |
| 20–39 yrs | 69 | 21 | 3.33; 2.47–4.48 | <0.001 | 116 | 81 | 70 |
| 40–59 yrs | 146 | 58 | 2.53; 2.08–3.07 | <0.001 | 198 | 119 | 60 |
| ≥60 | 152 | 53 | 2.85; 2.33–3.48 | <0.001 | 355 | 230 | 65 |
| Calendar period | |||||||
| -1989 | 82 | 27 | 3.00; 2.27–3.97 | <0.001 | 96 | 64 | 67 |
| 1990–1999 | 181 | 66 | 2.74; 2.29–3.28 | <0.001 | 113 | 72 | 64 |
| 2000–2008 | 143 | 48 | 2.96; 2.42–3.61 | <0.001 | 184 | 122 | 66 |
HR, Hazard ratio; CI, Confidence interval; PYAR, Person-years at risk.
Reference is general population comparator cohort.
Expected number of events in patients with celiac disease was derived from the observed number of events divided by the HR.
Gallstone-related acute pancreatitis
Some 30 patients with celiac disease had a later diagnosis of gallstone-related acute pancreatitis (expected n=19)(Table 2), resulting in a HR of 1.59 (95% CI=1.06–2.40). The absolute risk of gallstone-related acute pancreatitis was 9/100,000 person-years with an excess risk of 3/100,000 person-years. Women with celiac disease were at a non-significant excess risk of gallstone-related acute pancreatitis (HR=1.37; 95% CI=0.83–2.28) compared with a 2.21-fold increased risk in men (95% CI=1.09–4.45)(Supplementary Table E2).
Non-gallstone-related acute pancreatitis
Some 133 patients with celiac disease had a later diagnosis of non-gallstone-related acute pancreatitis (expected n=72). The corresponding HR was 1.86 (95% CI=1.52–2.26)(Table 3). The absolute risk of non-gallstone-related acute pancreatitis was 41/100,000 person-years with an excess risk of 19/100,000 person-years. The highest HRs for non-gallstone-related acute pancreatitis were seen in celiac disease patients diagnosed before age 40 years (Supplementary Table E3).
Chronic pancreatitis
Patients with celiac disease were at increased risk of chronic pancreatitis (HR=3.33; 95% CI=2.33–4.76, based on 49 observed cases vs. 15 expected)(Table 2). The absolute risk of non-gallstone-related acute pancreatitis was 15/100,000 person-years with an excess risk of 11/100,000 person-years. See also Supplementary Table E4.
Supplementation with pancreatic enzyme
Restricting our dataset to patients entering the study (having a biopsy with VA) on 1 July 2005 or later, patients with celiac disease were at a 5.34-fold increased risk of receiving supplementation with pancreatic enzyme products after celiac disease diagnosis (95% CI=2.99–9.53, based on 21 observed cases vs. 4 expected)(Table 2). Given that this analysis was restricted to the years 2005–2009, we did not estimate calendar-specific HRs. Supplementary Table E5 shows additional data.
Secondary analyses
None of the above risk estimates changed after adjusting for country of birth, socioeconomic position, level of education, chronic obstructive lung disease or alcohol-related diseases (data not shown), except that the HR for chronic pancreatitis in celiac disease rose to 4.96 after adjustment (95% CI=3.18–7.76). Restricting our outcome of any pancreatitis to individuals with this diagnosis in the Patient Register the HR was 2.02 (95%CI=1.71–2.37).
Patients with celiac disease were also at increased risk of severe acute pancreatitis (gallstone-related: HR=3.18; 95% CI=1.46–6.90 and non-gallstone related: HR=2.00; 95% CI=1.16–3.44).
Individuals with celiac disease were at lower risk of any pancreatitis than individuals with inflammation but no villous atrophy (HR=0.73; 95% CI=0.55–0.92) and individuals with normal mucosa but positive celiac disease serology (HR=0.71; 95% CI=0.63–0.85). In a post-hoc analysis we found that these differences were due to large differences in the risk of pancreatitis in the first year after biopsy. The HR for any pancreatitis during the first year after celiac disease diagnosis was 12.03 (95% CI=8.15–17.75) in patients with inflammation (Marsh 1–2) and 9.81 (95% CI=5.16–18.62) in patients with normal mucosa but positive celiac disease serology. The relative risks of pancreatic enzyme supplementation were especially high. In the first year after biopsy patients with inflammation (Marsh 1–2) were at an almost 38-fold increased risk of being prescribed pancreatic enzymes compared with the general population (HR=37.67; 95% CI=15.23–93.16). Further, those with normal mucosa but positive celiac disease serology were at an almost 90-fold increased risk to receive such enzymes in the first year after biopsy (HR=88.88; 95% CI=18.89–418.08).
Pancreatitis occurring before celiac disease
Individuals with celiac disease were at a 2.59-fold increased risk of having pancreatitis before first biopsy with VA (95% CI=2.19–3.07). This excess risk was made up of earlier non-gallstone-related acute pancreatitis (OR=2.32; 95% CI=1.88–2.88), chronic pancreatitis (OR=3.79; 95% CI=2.78–5.16), but also an increased risk of earlier dietary supplementation with pancreatic enzymes (OR=5.02; 95% CI=2.74–9.20). In contrast, celiac disease patients were at no increased risk of earlier gallstone-related acute pancreatitis (OR=1.37; 95% CI=0.79–2.38).
Post-hoc analyses
Additional data are reported in the appendix.
Discussion
In the current study we have used data from histological reports, providing a robust classification of celiac disease. This also enabled us to identify individuals with a small intestinal biopsy without VA, who then served as secondary controls. The large sample size made it possible to study acute and chronic pancreatitis separately. Furthermore we were able to analyze the time trends in the occurrence of pancreatitis in relation to the diagnosis of celiac disease.
This study found a 2–3-fold increased risk of pancreatitis in patients with celiac disease. Risk estimates were low for gallstone-related acute pancreatitis and high for pancreatic insufficiency, necessitating pancreatic enzyme supplementation. The highest risks were generally noted in the first year of biopsy, especially for chronic pancreatitis and enzyme supplementation.
To our knowledge, only one population-based study has investigated the association between celiac disease and pancreatitis.7 In the current study we were able to stratify the analyses based on time since celiac disease diagnosis. Although surveillance bias may have contributed to our risk estimates, it is unlikely to explain the positive association between celiac disease and pancreatitis in that risk estimates were increased even more than 5 years after celiac disease diagnosis.
We distinguished between gallstone and non-gallstone related acute pancreatitis. As expected given the relatively young age of those with celiac disease diagnosis, non-gallstone related acute pancreatitis was more than 4 times more common as compared to the gallstone-related disease. There was however no difference in the relative risk of these two entities of acute pancreatitis. This might indicate a shared common cause in both entities of the disease in patients with celiac disease. The risk in excess of that expected from gallstone acute pancreatitis may also be related to celiac disease related pancreatic or papillary pathology.
Importantly, between 10 and 20% of newly diagnosed patients with celiac disease may experience pancreatic insufficiency.4, 5 Thus the observed risk estimates can be regarded as considerably lower than expected.
Several factors might contribute to the association between celiac disease and pancreatitis. Malnutrition is known to impair the pancreatic section and structural changes, including acinar atrophy, in the pancreas.14 VA is associated with pancreatic insufficiency and restored pancreatic enzyme levels are observed after introduction of a gluten-free diet.15 The altered levels of autoregulatory enteric hormones (such as cholecystokinin) might also be a contributing factor.15 Celiac disease also leads to papillary inflammation and stenosis that could sensitize the pancreas to develop acute pancreatitis.3 Persistent inflammation and irreversible changes to the pancreatic structure and function might contribute to the long-term increased risk of pancreatitis in patients with celiac disease. Finally, the two diseases may share immunological characteristics in the sense that Th1-associated cytokines are increased in both celiac disease and pancreatitis.16, 17
The main strength of this paper is its population-based design and size, which provided the statistical power to stratify the analyses by age, sex and calendar period. None of these factors, however, interacted with celiac disease regarding the risk of pancreatitis. We were also able to adjust our analysis for a number of potential confounders, including diabetes, alcohol-related disease, socio-economic position, education and country of birth. A second strength is our use of biopsy report data to identify cases with celiac disease. Biopsy reports with VA have a high positive predictive value for celiac disease (95%), and more than 96% of gastroenterologists and pediatricians in Sweden perform a biopsy in at least 9 of 10 patients with suspected celiac disease before diagnosis.9
Elevated levels of serum amylase might occur asymptomatically in patients with celiac disease.2 The diagnosis of acute pancreatitis in a general population has been recently validated in the Swedish Patient Register and the positive predictive value was 98%.18 Nevertheless, the positive predictive value of serum amylase or lipase in diagnosing acute pancreatitis among patients with celiac disease is unknown. However, because the diagnosis of acute pancreatitis is based on a combination of elevated serum amylase or lipase and clinical symptoms, including abdominal pain, it is unlikely that our results are biased by false-positive cases. Still it should be noted that the associations remained significantly higher than expected even after excluding pancreatic enzyme supplementation from our outcome. Furthermore, supplementation of pancreatic enzymes may be necessary initially in patients with CD due to reversible pancreatic insufficiency. This is resolved in most patients after introduction of gluten-free diet and is not per se be defined as chronic pancreatitis.4 This increased risk was evident even after excluding the first year of follow-up indicating that some patients might in fact have chronic pancreatitis.
Conclusion
In conclusion, we found a moderately increased risk of pancreatitis in patients with celiac disease. This statistically significant association was seen even after excluding patients identified though prescribed drug register. Future studies should focus on potential mechanisms underlying the association between celiac disease and pancreatitis.
Supplementary Material
Acknowledgments
Funding:
OSA was supported by grants from Swedish Society of Medicine, Karolinska Institute, Olle Engkvist Byggmästare foundation and Signe and Olof Wallenius Foundation.
JAM: The National Institutes of Health – DK057892.
JFL was supported by grants from The Swedish Society of Medicine, the Swedish Research Council – Medicine (522-2A09-195), the Swedish Celiac Society, and the Fulbright Commission.
Independence (role of the sponsors): None of the funders had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Guarantor: JFL had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Abbreviations used in this article
- CI
Confidence Interval
- HR
Hazard Ratio
- VA
Villous Atrophy
Footnotes
Details of ethics approval: This project (2006/633-31/4) was approved by the Research Ethics Committee of the Karolinska Institute, Sweden on June 14, 2006.
Writing Assistance: None.
Copyright: Please note that since Dr Murray is a Mayo clinic employee the following applies to the copyright transfer on his part: “This transfer is subject to applicable Mayo terms located on the following page: http://www.mayo.edu/copyright/.”
Conflicts of interest/Disclosure summary
DS has received an educational and research grant from Solvay (formerly involved with Creon).
JAM: Grant support: Alba Therapeutics (>$50,000); Advisory board: Alvine Pharmaceuticals, Inc. (<$10,000), Nexpep (<$10,000), Consultant (none above 10,000 USD): Ironwood, Inc., Flamentera, Actogenix, Ferring Research Institute inc., Bayer Healthcare Pharmaceuticals, Vysera Biomedical, 2G Pharma, Inc, ImmunosanT, Inc and Shire US Inc.
The other authors (OSA and JFL) declare that they have no conflicts of interest.
Author contributions:
ICMJE criteria for authorship read and met: JFL, OSA, DSS, JAM.
Agree with the manuscript’s results and conclusions: JFL, OSA, DSS, JAM
Designed the experiments/the study: JFL.
Collected data: JFL
Analyzed the data: JFL
Wrote the first draft of the paper: JFL and OSA.
Contributed to study design, interpretation of data and writing: JFL, OSA, DSS, JAM.
Interpretation of data; approved the final version of the manuscript: JFL, OSA, DSS, JAM
Responsible for data integrity: JFL.
Obtained funding: JFL.
References
- 1.Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;18:53–81. doi: 10.1146/annurev.immunol.18.1.53. [DOI] [PubMed] [Google Scholar]
- 2.Rabsztyn A, Green PH, Berti I, Fasano A, Perman JA, Horvath K. Macroamylasemia in patients with celiac disease. Am J Gastroenterol. 2001;96:1096–100. doi: 10.1111/j.1572-0241.2001.03746.x. [DOI] [PubMed] [Google Scholar]
- 3.Patel RS, Johlin FC, Jr, Murray JA. Celiac disease and recurrent pancreatitis. Gastrointest Endosc. 1999;50:823–7. doi: 10.1016/s0016-5107(99)70166-5. [DOI] [PubMed] [Google Scholar]
- 4.Leeds JS, Hopper AD, Hurlstone DP, Edwards SJ, McAlindon ME, Lobo AJ, Donnelly MT, Morley S, Sanders DS. Is exocrine pancreatic insufficiency in adult coeliac disease a cause of persisting symptoms? Aliment Pharmacol Ther. 2007;25:265–71. doi: 10.1111/j.1365-2036.2006.03206.x. [DOI] [PubMed] [Google Scholar]
- 5.Carroccio A, Iacono G, Montalto G, Cavataio F, Di Marco C, Balsamo V, Notarbartolo A. Exocrine pancreatic function in children with coeliac disease before and after a gluten free diet. Gut. 1991;32:796–9. doi: 10.1136/gut.32.7.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans KE, Leeds JS, Morley S, Sanders DS. Pancreatic insufficiency in adult celiac disease: do patients require long-term enzyme supplementation? Dig Dis Sci. 2010;55:2999–3004. doi: 10.1007/s10620-010-1261-y. [DOI] [PubMed] [Google Scholar]
- 7.Ludvigsson JF, Montgomery SM, Ekbom A. Risk of pancreatitis in 14,000 individuals with celiac disease. Clin Gastroenterol Hepatol. 2007;5:1347–53. doi: 10.1016/j.cgh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue') Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 9.Ludvigsson JF, Brandt L, Montgomery SM, Granath F, Ekbom A. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009;9:19. doi: 10.1186/1471-230X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–67. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302:1171–8. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 12.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Otterblad Olausson P. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose D, Harrison E. Social Class in Europe: An introduction to the European Socio-economic Classification. Abingdon(Oxon); Routledge: 2010. [Google Scholar]
- 14.Brooks SE, Golden MH. The exocrine pancreas in kwashiorkor and marasmus. Light and electron microscopy. West Indian Med J. 1992;41:56–60. [PubMed] [Google Scholar]
- 15.Nousia-Arvanitakis S, Karagiozoglou-Lamboudes T, Aggouridaki C, Malaka-Lambrellis E, Galli-Tsinopoulou A, Xefteri M. Influence of jejunal morphology changes on exocrine pancreatic function in celiac disease. J Pediatr Gastroenterol Nutr. 1999;29:81–5. doi: 10.1097/00005176-199907000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Pietruczuk M, Dabrowska MI, Wereszczynska-Siemiatkowska U, Dabrowski A. Alteration of peripheral blood lymphocyte subsets in acute pancreatitis. World J Gastroenterol. 2006;12:5344–51. doi: 10.3748/wjg.v12.i33.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvati VM, MacDonald TT, Bajaj-Elliott M, Borrelli M, Staiano A, Auricchio S, Troncone R, Monteleone G. Interleukin 18 and associated markers of T helper cell type 1 activity in coeliac disease. Gut. 2002;50:186–90. doi: 10.1136/gut.50.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razavi D, Ljung R, Lu Y, Andren-Sandberg A, Lindblad M. Reliability of acute pancreatitis diagnosis coding in a National Patient Register: a validation study in Sweden. Pancreatology. 2011;11:525–32. doi: 10.1159/000331773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


