Abstract
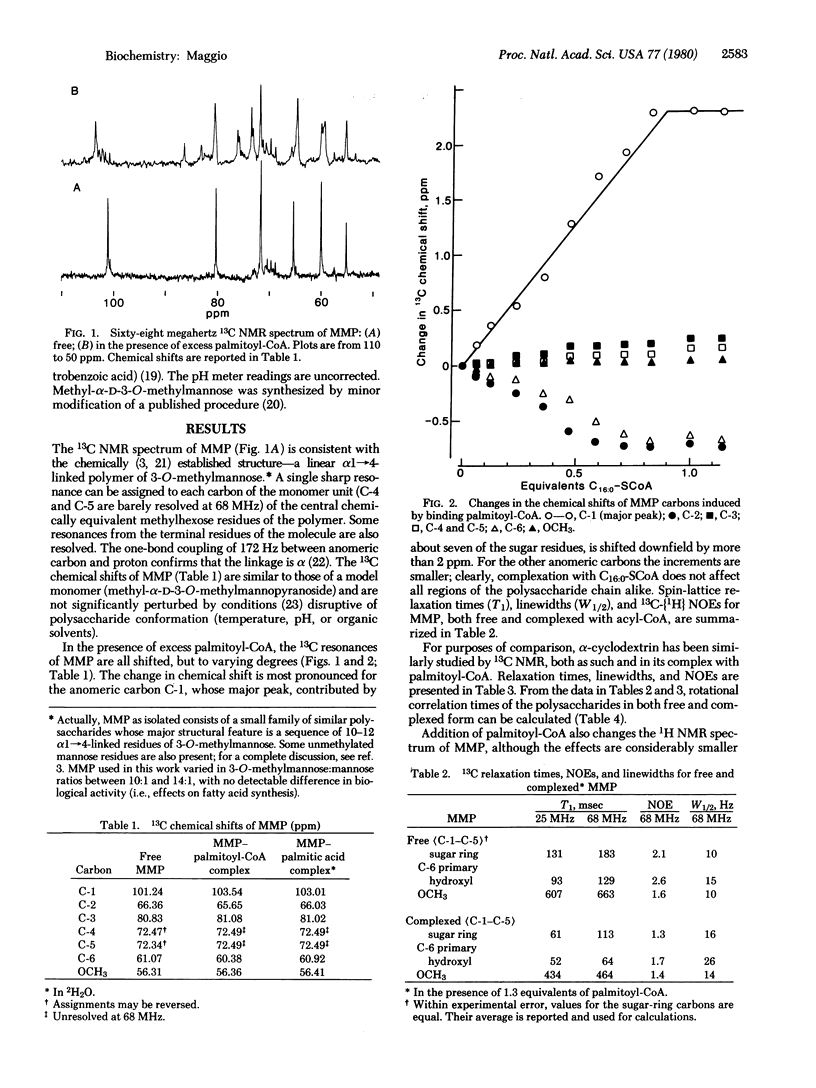
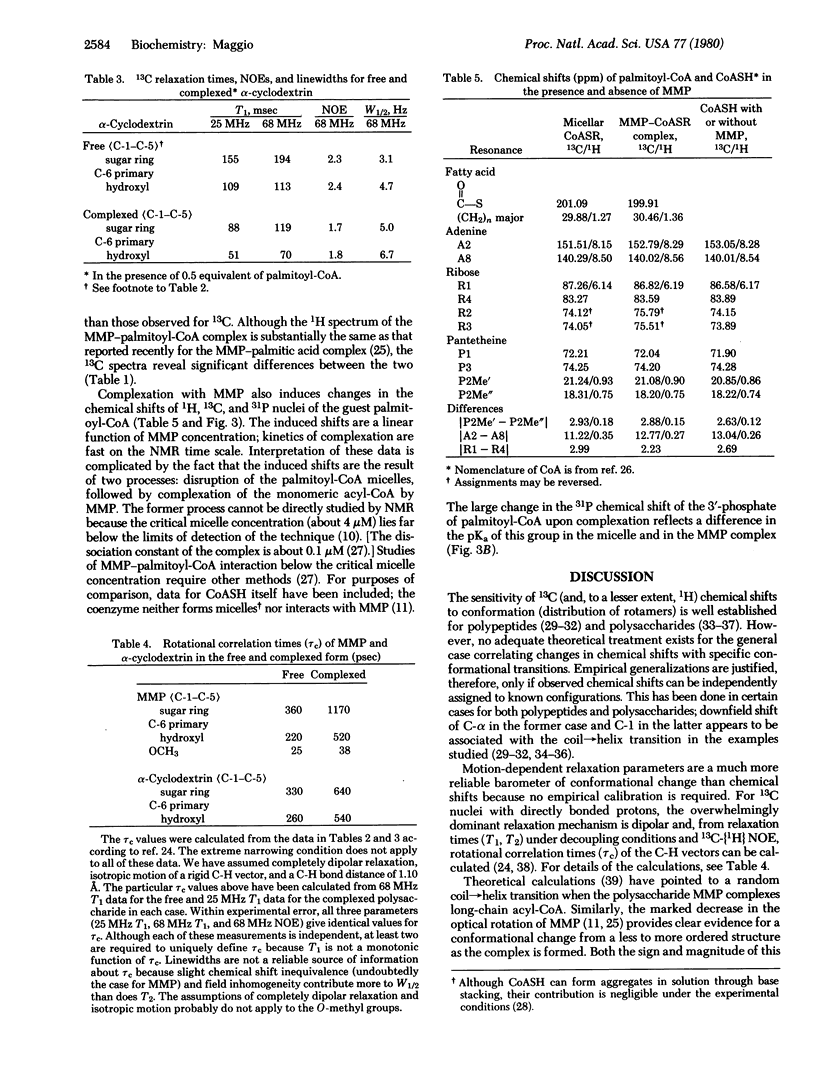
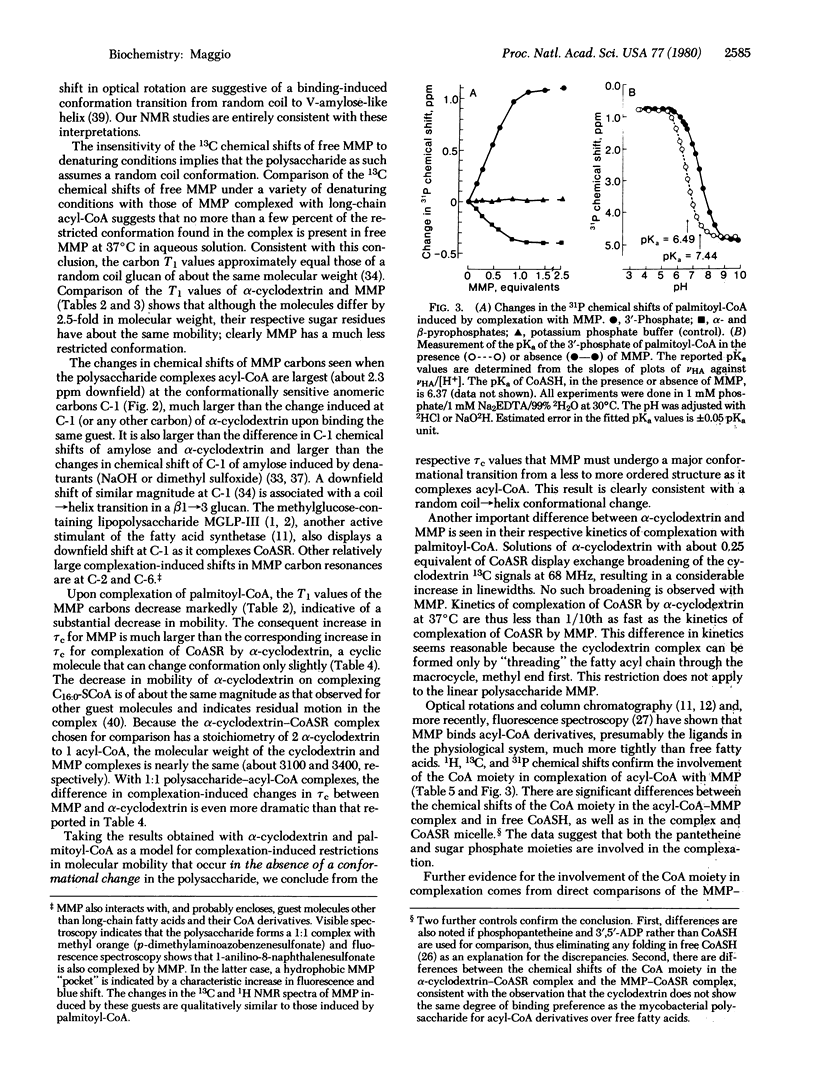
MMP, a linear alpha 1 leads to 4 linked polymer of 3-O-methylmannose, regulates the fatty acid synthetase from Mycobacterium smegmatis by forming stoichiometric complexes with the long-chain acyl-CoA synthetase products. In agreement with previous proposals [Bloch, K. (1977) in Advances in Enzymology and Related Areas of Molecular Biology, ed. Meister, A. (Wiley, New York), Vol. 45, pp. 1-84], nuclear magnetic resonance studies show that the polysaccharide, a random coil in its free form, undergoes a major conformational transition upon enclosing long-chain acyl-CoA. The polysaccharide, probably in helical conformation in the complexed form, interacts with both the paraffinic chain and the CoA moieties of the included fatty acyl thioester.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Arif A., Blecher M. Synthesis of fatty acyl CoA and other thiol esters using N-hydroxysuccinimide esters of fatty acids. J Lipid Res. 1969 May;10(3):344–345. [PubMed] [Google Scholar]
- Allerhand A., Oldfield E. Determination of rotational mobilities of backbone and side-chain carbons of poly(gamma-benzyl L-glutamate) in the helical and random-coil states from measurements of carbon-13 relaxation times and nuclear Overhauser enhancements. Biochemistry. 1973 Aug 28;12(18):3428–3433. doi: 10.1021/bi00742a011. [DOI] [PubMed] [Google Scholar]
- Bergeron R., Machida Y., Bloch K. Complex formation between mycobacterial polysaccharides or cyclodextrins and palmitoyl coenzyme A. J Biol Chem. 1975 Feb 25;250(4):1223–1230. [PubMed] [Google Scholar]
- Boccalon G., Verdini A. S., Giacometti G. Helix-coil transition of a synthetic polypeptide monitored by fourier transform carbon-13 nuclear magnetic resonance. J Am Chem Soc. 1972 May 17;94(10):3639–3641. doi: 10.1021/ja00765a070. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Bloch K. Purification and properties of fatty acyl thioesterase I from Escherichia coli. J Biol Chem. 1972 May 25;247(10):3123–3133. [PubMed] [Google Scholar]
- Candy D. J., Baddiley J. 3-O-methyl-D-mannose from Streptomyces griseus. Biochem J. 1966 Jan;98(1):15–18. doi: 10.1042/bj0980015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P., Jennings H. J., Smith I. C. Composition, sequence, and conformation of polymers and oligomers of glucose as revealed by carbon-13 nuclear magentic resonance. J Am Chem Soc. 1974 Dec 25;96(26):8081–8087. doi: 10.1021/ja00833a038. [DOI] [PubMed] [Google Scholar]
- Flick P. K., Bloch K. Vitro alterations of the product distribution of the fatty synthetase from Mycobacterium phlei. J Biol Chem. 1974 Feb 25;249(4):1031–1036. [PubMed] [Google Scholar]
- Gray G. R., Ballou C. E. Isolation and characterization of a polysaccharide containing 3-O-methyl-D-mannose from Mycobacterium phlei. J Biol Chem. 1971 Nov 25;246(22):6835–6842. [PubMed] [Google Scholar]
- Ilton M., Jevans A. W., McCarthy E. D., Vance D., White H. B., 3rd, Bloch K. Fatty acid synthetase activity in Mycobacterium phlei: regulation by polysaccharides. Proc Natl Acad Sci U S A. 1971 Jan;68(1):87–91. doi: 10.1073/pnas.68.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoche H., Esders T. W., Koths K., Bloch K. Palmityl coenzyme A inhibition of fatty acid synthesis. Relief by bovine serum albumin and mycobacterial polysaccharides. J Biol Chem. 1973 Apr 10;248(7):2317–2322. [PubMed] [Google Scholar]
- Lee C-H, Sarma R. H. Inverstigation of the solution conformation of coenzyme A and its derivatives by hydrogen-1 and phosphorus-31 fast Fourier transform nuclear magnetic resonance spectroscopy. J Am Chem Soc. 1975 Mar 5;97(5):1225–1236. doi: 10.1021/ja00838a043. [DOI] [PubMed] [Google Scholar]
- Machida Y., Bergeron R., Flick P., Bloch K. Effects of cyclodextrins on fatty acid synthesis. J Biol Chem. 1973 Sep 10;248(17):6246–6247. [PubMed] [Google Scholar]
- Machida Y., Bloch K. Complex formation between mycobacterial polysaccharides and fatty acyl-CoA derivatives. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1146–1148. doi: 10.1073/pnas.70.4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra S. K., Ballou C. E. Heterogeneity and refined structtures of 3-O-methyl-D-mannose polysaccharides from Mycobacterium smegmatis. J Biol Chem. 1977 Apr 25;252(8):2459–2469. [PubMed] [Google Scholar]
- Saier M. H., Jr, Ballou C. E. The 6-O-methylglucose-containing lipopolysaccharide of Mycobacterium phlei. Structure of the reducing end of the polysaccharide. J Biol Chem. 1968 Aug 25;243(16):4319–4331. [PubMed] [Google Scholar]
- Saitô H., Ohki T., Sasaki T. A 13C nuclear magnetic resonance study of gel-forming (1 goes to 3)-beta-d-glucans. Evidence of the presence of single-helical conformation in a resilient gel of a curdlan-type polysaccharide 13140 from Alcaligenes faecalis var. myxogenes IFO 13140. Biochemistry. 1977 Mar 8;16(5):908–914. doi: 10.1021/bi00624a015. [DOI] [PubMed] [Google Scholar]
- Saitô H., Smith I. C. Carbon-13 nuclear magnetic resonance studies of polyamino acids: the helix-coil transition of poly-L-lysine. Arch Biochem Biophys. 1973 Sep;158(1):154–163. doi: 10.1016/0003-9861(73)90608-5. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Ballou C. E. The 6-O-methylglucose-containing lipopolysaccharides of Mycobacterium phlei. Locations of the neutral and acidic acyl groups. J Biol Chem. 1973 Oct 25;248(20):7118–7125. [PubMed] [Google Scholar]
- Yabusaki K. K., Ballou C. E. Interaction of mycobacterial polymethylpolysaccharides with paranaric acid and palmitoyl-coenzyme A: structural specificity and monomeric dissociation constants. Proc Natl Acad Sci U S A. 1978 Feb;75(2):691–695. doi: 10.1073/pnas.75.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabusaki K. K., Cohen R. E., Ballou C. E. Conformational changes associated with complex formation between a mycobacterial polymethylpolysaccharide and palmitic acid. J Biol Chem. 1979 Aug 10;254(15):7282–7286. [PubMed] [Google Scholar]
- Zahler W. L., Barden R. E., Cleland W. W. Some physical properties of palmityl-coenzyme A micelles. Biochim Biophys Acta. 1968 Sep 2;164(1):1–11. doi: 10.1016/0005-2760(68)90065-9. [DOI] [PubMed] [Google Scholar]



