Abstract
Objective
Recently, a growing number of studies have revealed a pro-thrombotic and cytotoxic role for extracellular chromatin. Cerebral ischemia/reperfusion injury is characterized by a significant amount of cell death and neutrophil activation, both of which may result in the release of chromatin. The goal of this study was to assess the effect of extracellular chromatin in ischemic stroke using a mouse model of transient middle cerebral artery occlusion.
Methods and results
Similar to reports in stroke patients, we observed increased levels of circulating nucleosomes and DNA after ischemic stroke in mice. In addition, we observed that general hypoxia also augmented extracellular chromatin. We hypothesized that targeting extracellular chromatin components would be protective in ischemic stroke. Indeed, treatment with recombinant human DNase 1 significantly improved stroke outcome. Neutralization of histones using an anti-histone antibody was also protective as evidenced by smaller infarct volumes, whereas increasing levels of extracellular histones via histone infusion exacerbated stroke outcome by increasing infarct size and worsening functional outcome.
Conclusions
Our results indicate that extracellular chromatin is generated and is detrimental during cerebral ischemia/reperfusion in mice. Targeting DNA and/or histones may be a new therapeutic strategy to limit injury resulting from ischemic stroke.
Keywords: stroke, chromatin, histones, DNase 1
Introduction
Stroke is a leading cause of death and permanent disability worldwide.1 It is primarily caused by obstruction of cerebral arteries.2 Currently, early thrombolysis with tissue plasminogen activator (tPA) is the only available therapeutic option for acute thromboembolic stroke. However, tPA-mediated thrombolysis is only recommended in the limited time window of up to 3 hours after the onset of stroke symptoms as later applications are associated with the risk of severe intracerebral hemorrhage.3 Various trials testing new thrombolytics, platelet aggregation inhibitors or anticoagulants failed to improve treatment of ischemic stroke patients.4-6 Thus, in order to develop safer and more effective stroke therapeutics, a better understanding of the pathogenic mechanisms of thrombotic stroke development and the resulting cerebral injury is warranted.
Tissue damage following cerebral ischemia is caused by the interaction of complex pathophysiological processes, including platelet and leukocyte recruitment upon reperfusion, promoting both thrombosis and inflammation.7 In the ischemic area, cell death and/or neutrophil activation may lead to the release of nuclear chromatin consisting of DNA and histones. Our group recently showed that these extracellular DNA traps represent a new link between inflammation/infection and thrombosis.8 These DNA traps provide a stimulus and scaffold for thrombus formation.8 Extracellular nucleosomes were recently shown to promote coagulation and intravascular thrombus formation.9 Furthermore, histones are potent mediators of platelet activation and aggregation8,10 and were shown to be cytotoxic.11 Nucleosome levels are known to be elevated in many conditions where cells are stressed, such as trauma, cancer and auto-immune disease, all of which have thrombotic complications associated with the disease progression.12 Recently, markers of extracellular DNA traps were detected in the thrombus and plasma of mice and baboons subjected to deep vein thrombosis, an example of inflammation-enhanced thrombosis.8,13 Interestingly, significantly elevated concentrations of DNA and nucleosomes have also been found in stroke patients.14-17 DNase 1 is present in plasma where it can facilitate chromatin breakdown after cell death.18,19 Serum levels of DNase 1 were reported to be elevated in the clinical setting of myocardial ischemia20 and a polymorphism resulting in a less active DNase 1 is associated with myocardial infarction21 indicating that this endonuclease could play a protective role in cardiovascular disease.
In this study, we examined the generation of extracellular chromatin by hypoxic conditions and in ischemic stroke using a mouse model of transient middle cerebral artery occlusion (tMCAO). We show that markers of extracellular DNA traps are elevated in both models and we provide evidence that extracellular chromatin is a potential therapeutic target in ischemic stroke.
Materials and Methods
Animals
Wild-type C57BL/6 (WT) mice were from Jackson Laboratory (Bar Harbor, ME). All animals were 8-10 weeks old males except for the hypoxia experiments in which female animals were also used. Animals had free access to standard chow and water and were kept on a light/dark cycle of 12 h. All experimental procedures were approved by the Animal Care and Use Committee of the Immune Disease Institute.
Materials
Recombinant human DNase 1 (Dornase alpha, Pulmozyme®) was purchased from Genentech Inc. (San Francisco, CA). Calf thymus histones were purchased from Worthington Biochemical Corp. (Lakewood, NJ). Antibodies against histone H2A/H4 were isolated from cell culture supernatants of hybridoma clone BWA3 by affinity chromatography on protein G columns as described.22 Purified antibodies were dialyzed against saline and were characterized by ELISA as described (Figure S1).
Determination of nucleosome levels in plasma
To measure nucleosome and DNA levels in plasma, blood was collected from the retro-orbital sinus (49:1 v/v of blood:0.5M EDTA). Plasma was prepared by centrifuging anticoagulated whole blood for 5 min at 2300 x g. Plasma supernatant was carefully removed and centrifuged again for 5 min at 2300 x g to remove any remaining blood cells. Plasma was stored at −80°C until analysis. Nucleosome levels were measured using the Cell Death Detection ELISA or Cell Death Detection ELISAPLUS (Roche, Indianapolis, IN). This assay allows for the relative quantification of histone-complexed DNA fragments (mono- and oligonucleosomes).
Quantification of plasma DNA
Plasma was diluted 1:10 in phosphate buffered saline (PBS) and 50 μl of diluted plasma was mixed with 50 μl SytoxGreen in PBS (2 μM, Invitrogen) to label DNA and fluorescence was recorded in a fluorometer (Fluoroskan, Thermo Fisher Scientific, Waltham, MA). Auto-fluorescence was determined in samples mixed with phosphate buffered saline without SytoxGreen and subtracted.
Hypoxia treatment
Mice were exposed to normobaric hypoxia at 6% oxygen for 24 hours or were housed at normal air room pressure. For hypoxia treatment, animals were placed in a controlled atmosphere animal chamber (A-15274-P, Biospherix, Lacona, NY). Hypoxia was achieved by substituting nitrogen for oxygen using a Pro:ox model 110 compact oxygen controller (Biospherix). Mice were given food and water ad libitum.
Induction of cerebral ischemia
Focal cerebral ischemia was induced by 60 or 120 min transient middle cerebral artery occlusion (tMCAO). Mice were anesthetized with 2% isoflurane/oxygen mixture. Following a midline skin incision in the neck, the proximal common carotid artery and the external carotid artery were ligated, and a standardized silicon rubber-coated 6.0 nylon monofilament (6021; Doccol Corp., Redlands, CA) was inserted and advanced via the right internal carotid artery to occlude the origin of the right MCA. Operation time per animal did not exceed 15 mins. The intraluminal suture was left in situ during the complete occlusion time. Then animals were re-anesthetized, and the occluding monofilament was withdrawn to allow reperfusion. In animals undergoing a sham treatment, the exact same procedure was followed except that the monofilament was only inserted very briefly (less than 2 seconds) into the common carotid artery without advancement into the right internal carotid artery. Some animals were exclusively used for laser-Doppler flowmetry (Periflux 5000, Perimed, Kings Park, NY) to monitor regional cerebral blood flow (rCBF) in the MCA territory (6 mm lateral and 2 mm posterior from bregma). rCBF consistently decreased to less than 10% of baseline values upon occlusion of the MCA by the monofilament. Upon filament withdrawal, rCBF was restored to approximately 75% of baseline flow (Figure S2). Sham surgeries did not cause a drop in rCBF (not shown). Arterial blood oxygenation was measured using a small rodent oxymeter thigh sensor (Mouse OX, STARR Life Sciences, Oakmont, PA) and did not change during the course of the surgery and the 24 h reperfusion period (not shown).
DNase 1 or histone treatment
Treatment of WT animals with recombinant human DNase 1 was performed by an intra-peritoneal administration of 50 μg of rhDNase 1 15 min before surgery. Five minutes before reperfusion, 10 μg of rhDNase 1 was given via retro-orbital intravenous administration. To maintain rhDNase 1 levels, a second intra-peritoneal dose of 50 μg was given 12 h after the first dose. A delayed rhDNase 1 treatment regimen was also used, in which mice were injected 1 h after the start of reperfusion with 50 μg rhDNase 1 i.p. plus 10 μg rhDNase 1 i.v., followed by 50 μg i.p. 12 h later. The half life of rhDNase 1 in circulation is approximately 22 h after infusion into DNase KO mice.23
Treatment with anti-histone H4 antibody (BWA3) consisted of a single intravenous retro-orbtial bolus injection of antibody (BWA3 or IgG1 isotype control antibody) at a concentration of 10 mg/kg 5 min before reperfusion.
For treatment with histones, mice received 10 mg/kg of calf thymus histones by retro-orbital intravenous administration, immediately after the start of reperfusion.
Assessment of infarct volume
Mice were sacrificed 24 h after initiation of tMCAO. Brains were quickly removed and cut into 2 mm-thick coronal sections using a mouse brain slice matrix. The slices were stained with 2% 2,3,5-triphenyl-tetrazolium chloride (TTC; Sigma-Aldrich, St. Louis, MO) in PBS to visualize healthy tissue and unstained infarctions. Sections were photographed with a digital Nikon D70 camera and infarct areas (white) were measured blindly using Image J software (National Institutes of Health; http://rsb.info.nih.gov/ij.html). Edema formation was measured by comparing the volume of the ischemic hemisphere with the volume of the control hemisphere. None of the experiments resulted in significant edema formation that could bias infarct volume.
Bederson score
Neurological function was assessed, blinded for the mouse genotype, 24 h after initiation of tMCAO, using the modified Bederson score.24 This test determines global neurological function according to the following scoring system: 0, no deficit; 1, forelimb flexion; 2, decreased resistance to lateral push; 3, unidirectional circling; 4, longitudinal spinning; 5, no movement.
Grip test
The grip test was performed as described.25 A mouse was placed on a wooden bar (3 mm in diameter, 40 cm long) attached to two vertical supports 40 cm above a flat surface. When placing the mouse on the bar midway between the supports, the experiment was rated according to the following system: 0, falls off; 1, hangs onto bar by two forepaws; 2, same as for 1, but attempts to climb onto bar; 3, hangs onto bar by two forepaws plus one or both hindpaws; 4, hangs onto bar by all four paws plus tail wrapped around bar; 5, escape (mouse able to reach one of the supports).
Corner test
For the corner test26, a mouse was placed on a flat surface between two vertical boards arranged at a 30° angle with a small opening along the joint between the two boards to encourage entry into the corner. The mouse was placed between the two angled boards facing the corner and half way to the corner. When entering deep into the corner, the mouse rears forward and upward, then turns back to face the open end. The non-ischemic mouse turns either left or right, but the ischemic mouse preferentially turns toward the non-impaired, ipsilateral (right) side. The turns in one versus the other direction were recorded from ten trials for each test. Turning movements that were not part of a rearing movement were not scored. Results are expressed as the number of right turns of 10 valid trials. Mice that were not able to walk (Bederson score 4 or 5) were excluded.
Statistical analysis
Data are expressed as mean plus or minus SEM. All statistical analysis was performed using Prism 4 (version 4.0b, Graphpad Software, Inc., La Jolla, CA). To compare groups, the unpaired 2-tailed Student t test was used. P values less than .05 were considered statistically significant.
Results
Hypoxia elevates circulating levels of nucleosomes
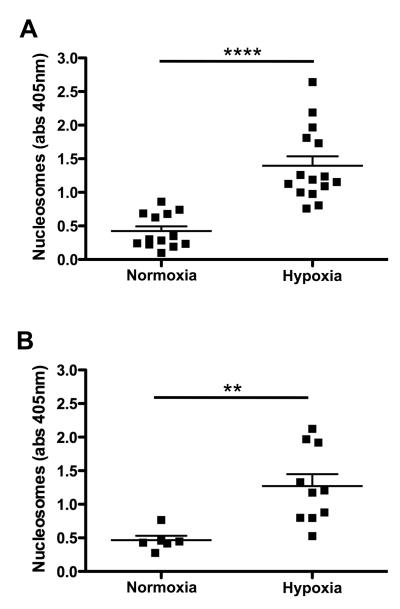
It is well established that conditions associated with hypoxia, such as exposure to high altitude or long-haul air travel, are associated with a hypercoagulable state and form a risk factor for venous thrombosis.27,28 Since extracellular DNA traps promote thrombosis and coagulation,8,9 we investigated the effect of hypoxia on circulating markers of extracellular chromatin. Mice were exposed to normobaric hypoxia at 6% oxygen for 24 hours. Control mice were housed at normal room air oxygen concentration. Immediately after exposure, blood was collected to determine nucleosome levels in plasma (Figure 1). We performed four independent experiments and in each experiment, mice that experienced hypoxia showed significantly higher plasma levels of nucleosomes when compared to the normoxic mice. Overall, plasma nucleosome levels in mice that received hypoxia treatment (1.35 ± 0.11, n=25) were more than 3-fold increased over the values of normoxic mice (0.44 ± 0.05, n=19, p<0.0005). No difference was observed between male and female animals. These results indicate that hypoxia is associated with the liberation of extracellular chromatin components, which could contribute to an increased risk for thrombosis.
Figure 1. Nucleosome levels are increased after hypoxia.
Female (A) and male (B) wild-type animals were kept under normoxic (room air) or hypoxic (hypoxia chamber, 6% 02) conditions for 24 h, after which plasma samples were collected. Nucleosome levels were determined in the plasma samples and expressed as nucleosome specific absorbance levels (Abs 405). Hypoxia significantly increased the levels of circulating nucleosomes. (** p<0.005; **** p<0.0001)
Extracellular chromatin markers are increased after stroke
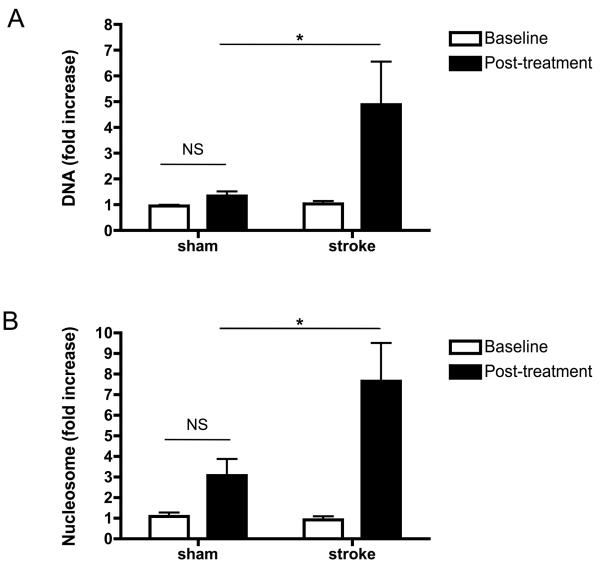
Based on our observation that general hypoxia leads to increased levels of extracellular chromatin markers, we decided to investigate the effect of cerebral ischemia/reperfusion injury on circulating levels of extracellular chromatin components. Mice were subjected to 2 h of tMCAO, followed by 22 hrs of reperfusion. Before the surgical procedure and after reperfusion, blood samples were collected and plasma was prepared. Sham-operated animals underwent the same surgery but without occlusion of the right MCA. Levels of circulating DNA and nucleosomes were determined in the plasma samples (Figure 2). While not statistically significant, the sham procedure showed a trend towards an increase of both parameters over baseline levels, which may be related to tissue damage of the invasive surgery. Nevertheless, in animals that experienced stroke, levels of circulating DNA and nucleosomes were increased several-fold over baseline levels (Figure 2, p<0.05). These data indicate that cerebral ischemia/reperfusion injury leads to generation of extracellular chromatin, resulting in a significant elevation in the amount of these cell death markers in the circulation.
Figure 2. DNA and nucleosome levels are increased after cerebral ischemia/reperfusion.
Mice were subjected to 2 h of tMCAO, followed by 22 h of reperfusion. Before the surgical procedure (baseline) and after 22 h of reperfusion (post-treatment), blood samples were collected and plasma was prepared. Sham-operated animals underwent the same surgical procedures but without occlusion of the right MCA. DNA levels (A, n=9 for each group) and nucleosome levels (B, n=6 for each group) were measured and normalized over baseline levels of sham-treated mice. Both DNA and nucleosome levels slightly increased in some animals after sham treatment but this was not significant (NS). DNA and nucleosome levels were significantly higher in all animals that experienced stroke. (* p<0.05)
Infusion of recombinant DNase 1 improves ischemic stroke outcome in mice
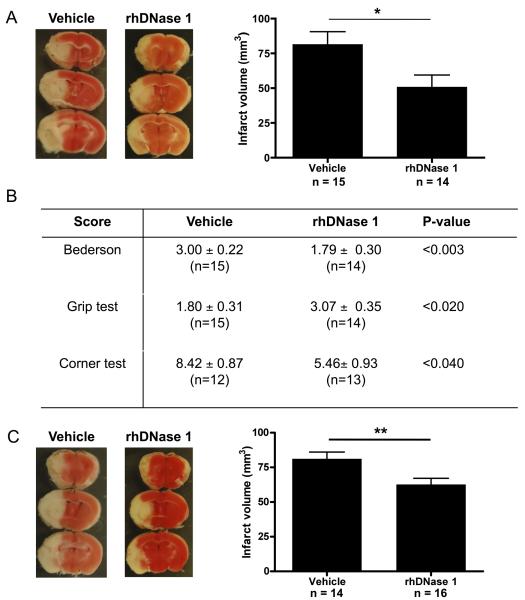
Our group has previously shown that DNase 1 can cleave chromatin/platelet strings, thereby limiting the extent of extracellular DNA trap-mediated thrombosis in vitro.8 We hypothesized that this enzyme may have a beneficial impact on stroke progression by limiting excessive platelet recruitment and activation by extracellular chromatin generated upon cerebral ischemia/reperfusion injury. To test this, we treated mice with recombinant human DNase 1 (rhDNase 1, 50 μg i.p. before surgery, followed by 10 μg i.v. before the start of reperfusion and 50 μg i.p. 12 h after the first i.p. dose). Compared to vehicle-treated animals, mice that received rhDNase 1 developed approximately 40% smaller infarcts (81.45 ± 9.08 mm3 versus 50.67 ± 8.74 mm3, p<0.03, Figure 3A). Treatment of mice with rhDNase 1 also led to a dramatic improvement of their functional outcome as shown by three separate tests assessing neurologic and motoric behavior. Compared to vehicle-treated mice, the Bederson test score (1.79 ± 0.30 versus 3.00 ± 0.22 for vehicle versus rhDNase 1 treated animals respectively, p<0.03), the grip test score (1.80 ± 0.31 versus 3.07 ± 0.35 for vehicle versus rhDNase 1 treated animals respectively, p<0.02) and the corner test result (8.41 ± 0.87 versus 5.46 ± 0.93 for vehicle versus rhDNase 1 treated animals respectively, p<0.04) were all significantly better in mice treated with rhDNase 1 (Figure 3B). To investigate whether the protective effect of rhDNase 1 is still maintained when rhDNase 1 treatment is delayed, we began to administer rhDNase 1 treatment 1 h after the start of reperfusion (50 μg i.p. plus 10 μg i.v. followed by 50 μg i.p. 12 h later). Interestingly, we observed that animals that received rhDNase 1 at this late time point developed smaller infarcts (62.68 ± 4.40 mm3) compared to the control group, treated with vehicle (81.19 ± 4.88 mm3, p<0.009, Figure 3C). When comparing early rhDNase 1 treatment (Figure 3A) with delayed rhDNase 1 treatment (Figure 3C), the decrease in infarct volumes was lower in the latter, but still highly statistically significant (p<0.009). Functional outcome scores were however not significantly improved in the delayed treatment group.
Figure 3. rhDNase 1 improves outcome after ischemic stroke.
Wild-type mice treated with rhDNase 1 or vehicle were subjected to 1 h of tMCAO and 23 h of reperfusion after which mice behavior was tested and brains analyzed. (A) Early treatment with rhDNase 1 (started before reperfusion). Representative 2,3,5-triphenyl tetrazolium chloride stains of 3 consecutive coronal brain sections (left), on which ischemic infarctions appear white. Brain infarct volumes as measured by planimetry (right). Mice receiving early treatment with rhDNase 1 developed significantly smaller infarctions than control (vehicle) mice. (B) Neurological Bederson score, grip test score and corner test results of the experiment shown in A. On all three parameters, mice treated early with rhDNase 1 showed a significantly better neurologic outcome when compared to control mice (* p<0.05). (C) Delayed treatment with rhDNase 1 (started 1 h after reperfusion). Representative 2,3,5-triphenyl tetrazolium chloride stains of 3 consecutive coronal brain sections (left). Brain infarct volumes as measured by planimetry (right). Mice receiving the delayed treatment with rhDNase 1 also developed significantly smaller infarctions than control (vehicle) mice.
Targeting histones is protective in ischemic stroke
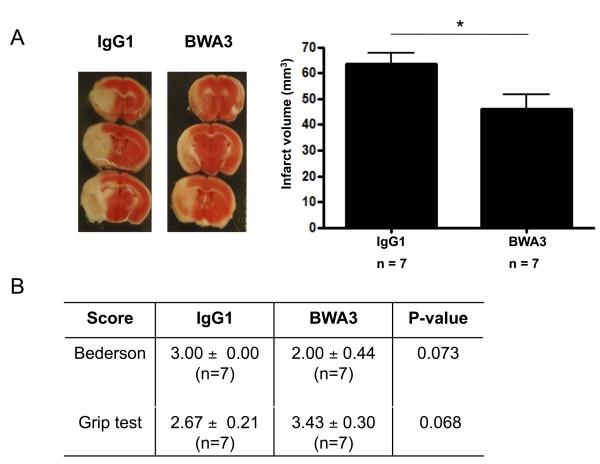
Nucleosomes are segments of DNA that are wound around a protein core of histones. It has been shown that extracellular histones are highly cytotoxic toward endothelium, induce intravascular thrombosis and even cause death when administered at very high doses (75 mg/kg).11 Furthermore, sub-lethal concentrations of histones aggregate platelets and cause thrombocytopenia in a dose-dependent manner in mice.10 To assess whether extracellular histones are of pathological significance in the progression of ischemic stroke, we infused 10 mg/kg anti-histone H2A/H4 antibody (BWA3)22 5 min before reperfusion (Figure 4). This antibody was previously shown to rescue mice from extracellular histone-mediated death in a mouse model of sepsis.11 Administration of histone-neutralizing antibody resulted in a protective effect when compared to animals treated with IgG1 isotype control antibody. The infarct volumes of BWA3-treated mice were ~30% smaller than in IgG1 treated mice (46.00 ± 5.82 mm3 versus 63.43 ± 4.29 mm3, p<0.05, Figure 4A). Neurologic/motoric outcome as measured by the Bederson and grip test scores were generally better in BWA3-treated mice although this was not significant (Figure 4B).
Figure 4. Histone neutralization is protective in ischemic stroke.
Wild-type mice treated with a histone-neutralizing antibody (BWA3) or isotype control antibody (IgG1) were subjected to 1 h of tMCAO and 23 h of reperfusion after which mice behavior was tested and brains analyzed. (A) Representative 2,3,5-triphenyl tetrazolium chloride stains of 3 consecutive coronal brain sections (left), on which ischemic infarctions appear white. Brain infarct volumes as measured by planimetry (right). Mice treated with BWA3 developed significantly smaller infarctions in comparison to mice treated with the isotype control (* p<0.05). (B) Functional Bederson and grip test scores. Neutralization of histones (BWA3) showed a tendency for a better neurologic outcome when compared to IgG1 control treatment. Treatment with vehicle gave the same results as treatment with IgG1 antibody (not shown, p=0.12).
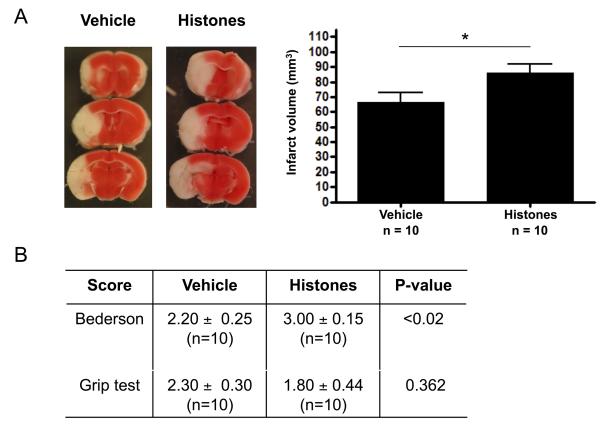
Elevated concentrations of extracellular histones exacerbate ischemic stroke
To further confirm the detrimental role of histones in cerebral ischemia/reperfusion injury, we infused mice that underwent tMCAO with a low dose (10 mg/kg) of calf thymus histones immediately after the start of reperfusion. Control animals received sterile saline. This low dose did not affect platelet counts of the treated mice (Figure S3) nor blood pH (not shown). We observed significantly larger infarcts in mice that were treated with histones compared to control mice (86.10 ± 5.57 mm3 versus 66.65 ± 6.16 mm3 respectively, p<0.05, Figure 5A). This increase was functionally relevant as the Bederson score was significantly worse in histone-treated mice (p<0.05, Figure 5B). Taken together, our results indicate that histones and extracellular chromatin are major contributors to reperfusion injury after stroke.
Figure 5. Infusion of histones exacerbates ischemic stroke.
Wild-type mice treated with histones or saline (vehicle) were subjected to 1 h of tMCAO and 23 h of reperfusion after which mice behavior was tested and brains analyzed. (A) Representative 2,3,5-triphenyl tetrazolium chloride stains of 3 consecutive coronal brain sections (left), on which ischemic infarctions appear white. Brain infarct volumes as measured by planimetry (right). Mice treated with histones developed significantly larger infarctions in comparison to mice treated with vehicle (* p<0.05). (B) Functional Bederson and grip test scores. Infusion of histones led to worsening of neurologic outcome, as seen by increased Bederson score, when compared to control treatment.
Discussion
In this study, we demonstrate that markers of extracellular chromatin are increased in hypoxic conditions and after ischemic stroke. Treatment of mice submitted to experimental stroke with rhDNase 1 or histone-neutralizing antibodies has a protective effect indicating an important role for extracellular chromatin in the development of ischemic stroke and thus suggesting that DNA and histones may be relevant therapeutic targets for this condition.
Extracellular chromatin markers are increased after hypoxia and ischemic stroke
We observed that general hypoxia leads to an increase of nucleosome levels in plasma. The hypoxia level used in our study is similar to that found at extreme altitudes such as experienced by mountaineers. High altitude is known to be associated with thrombotic complications.28 Recently, it was reported that spending time even at moderate altitude was a potential risk factor for thrombosis.29 The origin of circulating nucleosomes released by hypoxia is unknown, but because chromatin is both pro-coagulant and pro-thrombotic,8,9 it is plausible that it contributes to the higher incidence of thrombosis in hypoxic conditions. This observation led us to postulate that ischemia may similarly result in the generation of extracellular chromatin that in the case of stroke may worsen brain injury. We therefore examined whether tMCAO would have an effect on circulating levels of extracellular chromatin. We observed that experimental ischemic stroke indeed results in a significant increase of both DNA and nucleosome levels in the circulation. These experimental results in mice are in agreement with clinical observations that DNA/nucleosome levels are elevated in stroke patients. With ischemic cell damage being a dynamic process with considerable inter-individual variation, the striking association between circulating DNA/nucleosome levels and stroke severity has always been surprising.30 Indeed, these levels strongly correlate with stroke severity and are associated with morbidity, mortality and degree of disability.14-17 In one study, assessment of nucleosomes, S100 protein, neuron-specific enolase, C-reactive protein and leukocytes three days after stroke revealed that only nucleosomes provided independent prognostic information concerning the one-year recovery period.14 Liberation of chromatin and its degradation products from damaged cells seems a plausible mechanism for increased DNA/nucleosome levels during and after stroke. It is difficult to identify the cellular origin of the circulating DNA/nucleosomes. Undoubtedly, dying neurons release chromatin, which could cross the disrupted blood-brain barrier. Given the well-established role of neutrophils in the pathophysiology of ischemic stroke7, it is also tempting to speculate that neutrophils could lead to significant chromatin release through formation of NETs. NETs promote thrombosis and bind red blood cells, which could further hinder efficient reperfusion of ischemic areas.8 We recently demonstrated that extracellular chromatin, likely originating from neutrophils, contributes to the pathogenesis of DVT in mice.13 Interestingly, besides neutrophils, mast cells, monocytes and eosinophils also release extracellular DNA traps in response to inflammatory stimuli and reactive oxygen species.31-33
rhDNase 1 is protective in ischemic stroke
After tMCAO, both platelet-dependent thrombus formation and coagulation play a crucial role in stroke progression, implying that new components inhibiting thrombosis and/or coagulation could become beneficial in stroke treatment.34 We previously showed in vitro that both thrombolytic (tPA) and DNase activity were needed to dissolve clots containing chromatin and that DNase 1 can cleave chromatin/platelet strings, thereby limiting the extent of extracellular DNA trap-mediated thrombosis in vitro.8 rhDNase 1 is a readily available drug that is currently used for the treatment of cystic fibrosis where extracellular chromatin is generated.35 In this study we show that rhDNase 1 has a protective effect on cerebral ischemia/reperfusion injury, even when administered 1 h after onset of reperfusion. Based on our previous findings, a plausible explanation for our observation of the protective effect of rhDNase 1 could be that rhDNase 1 removes excessive chromatin material that could act as thrombogenic DNA traps blocking the microcirculation. Our findings in ischemic stroke are in accordance with our observations in a mouse model of deep vein thrombosis (DVT), where infusion of rhDNase 1 protected mice from flow restriction-induced DVT, most probably by cleaving extracellular DNA traps. This notion is further supported by recent findings by von Brühl et al. who described that neutrophil extracellular DNA traps promote mouse DVT growth via a mechanism that is dependent on platelet glycoprotein (GP) Ib and factor XII.36 Also in this study, rhDNase 1 reduced DVT. Interestingly, GPIb and FXII have been shown to be important mediators of experimental stroke, suggesting that similar mechanisms might also apply in cerebral ischemia/reperfusion injury.37-40 Moreover, polyphosphate polymers potently initiate fibrin formation via the factor XII-driven intrinsic pathway and hence are potent activators of coagulation, possibly by acting as templates to assemble clotting proteins.41-43 Recently, it was shown that this blood coagulation-triggering activity positively correlates with polyphosphate polymer size and that short polymers are even able to antagonize the blood clotting capacity of longer polymers by acting as competitors keeping clotting proteins from assembling effectively together on longer polymers.44 Thus, rhDNase 1 could also attenuate blood clotting by cleaving long DNA polymers into smaller, less pro-coagulant fragments. In addition, we recently showed that von Willebrand factor (VWF) also interacts with extracellular DNA traps and that VWF plays a crucial pathophysiological role in both DVT and stroke.8,45-48 Whether VWF binding to extracellular DNA further promotes brain injury (that could be reduced by rhDNase 1) remains to be established.
Histones contribute to ischemic stroke progression
Our results show that the other important chromatin component, histones, also contributes to cerebral ischemia/reperfusion injury and that targeting of histones results in a protective effect. These data are in line with our previous findings that histones also promoted DVT in mice.13 Histones promote platelet activation and aggregation,8,10 which could promote secondary thrombus formation during the reperfusion phase after ischemic stroke. Also, histones have been shown to be cytotoxic to endothelium, which could affect the blood brain barrier.11,49 Consistent with our findings, Huang et al. reported that histones exacerbate liver ischemia/reperfusion injury and that histone neutralization (using the same antibody as used in our study) protects against injury.50 Interestingly, these authors demonstrated a cytotoxic effect of histones, mediated by TLR9, suggesting that histones serve as new link between tissue damage and activation of innate immunity. Similarly, histones were shown to be an important mediator of death in sepsis11 and after acetaminophen-induced toxicity. 51 In both studies, blocking histones, using the same antibody used in our work, protected mice from death. Recently, it was shown that extracellular histones also enhance plasma thrombin generation by impairing protein C activation,52 a mechanism that could contribute to microvascular thrombosis after stroke. Given that activated protein C can cleave histones, thereby reducing their cytotoxicity in sepsis,11 it is interesting to note that activated protein C is also protective in a murine ischemic stroke model.53 Interestingly, APC mutants with reduced anticoagulant activity were shown to still have a protective effect in ischemic stroke.54 This further suggests that other mechanisms besides promoting thrombosis could mediate the detrimental effects of histones in cerebral ischemia/reperfusion injury, including cytotoxic, inflammatory or apoptotic activities or disruption of the blood brain barrier.
Further studies are now needed to clarify the exact pathogenic mechanisms of histone induced brain injury and to establish whether histone/chromatin release could also contribute to the cognitive decline observed after invasive procedures such as coronary artery bypass surgeries.55
In conclusion, our results indicate that extracellular chromatin and/or its components are important mediators of ischemic stroke injury. It will be important to further elucidate the exact mechanisms of stroke exacerbation by extracellular chromatin. We propose that anti-chromatin therapy could lead to new strategies in stroke management. Cleavage of DNA by DNase 1 and/or neutralization of histones using anti-histone antibodies may complement current thrombolytic therapy to limit reperfusion injury. Further studies are needed to investigate the potential synergistic effects of combined therapies targeting different substrates, such as the combination of DNase 1 with tPA.
Supplementary Material
Acknowlegdements
We thank Dr. Hans Georg Mannherz for the protocol for DNase 1 administration and helpful discussions and Lesley Cowan for help with the preparation of the manuscript.
Sources of funding This research was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health Grants R01 HL041002 and R01 HL102101 (to D.D.W.) and a fellowship from the Deutsche Forschungsgemeinschaft, Germany (FU 742/1-1) (to T.A.F.). S.F.D.M. is a post-doctoral fellow of the Research Foundation Flanders (Fonds voor Wetenschappelijk Onderzoek Vlaanderen).
Footnotes
Disclosure of conflicts None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 3.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: A science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams HP, Effron MB, Torner J, Dávalos A, Frayne J, Teal P, Leclerc J, Oemar B, Padgett L, Barnathan ES, Hacke W. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: Results of an international phase iii trial: Abciximab in emergency treatment of stroke trial (abestt-ii) Stroke. 2008;39:87–99. doi: 10.1161/STROKEAHA.106.476648. [DOI] [PubMed] [Google Scholar]
- 5.Paciaroni M, Agnelli G, Micheli S, Caso V. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: A meta-analysis of randomized controlled trials. Stroke. 2007;38:423–430. doi: 10.1161/01.STR.0000254600.92975.1f. [DOI] [PubMed] [Google Scholar]
- 6.Hacke W, Furlan AJ, Al-Rawi Y, Davalos A, Fiebach JB, Gruber F, Kaste M, Lipka LJ, Pedraza S, Ringleb PA. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion–diffusion weighted imaging or perfusion CT (dias-2): A prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J. Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massberg S, Grahl L, von Bruehl M-L, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs TA, Bhandari A, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;107:15880–15885. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holdenrieder S, Stieber P. Clinical use of circulating nucleosomes. Crit. Rev. Clin. Lab. Sci. 2009;46:1–24. doi: 10.1080/10408360802485875. [DOI] [PubMed] [Google Scholar]
- 13.Brill A, Fuchs TA, Savchenko A, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2011 Nov 1; doi: 10.1111/j.1538-7836.2011.04544.x. epub ahead of print, doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiger S, Holdenrieder S, Stieber P, Hamann GF, Bruening R, Ma J, Nagel D, Seidel D. Nucleosomes in serum of patients with early cerebral stroke. Cerebrovasc. Dis. 2006;21:32–37. doi: 10.1159/000089591. [DOI] [PubMed] [Google Scholar]
- 15.Lam NY-L, Rainer TH, Wong LK-S, Lam W, Lo Y-MD. Plasma DNA as a prognostic marker for stroke patients with negative neuroimaging within the first 24 h of symptom onset. Resuscitation. 2006;68:71–78. doi: 10.1016/j.resuscitation.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Rainer TH, Wong LKS, Lam W, Yuen E, Lam NYL, Metreweli C, Lo YMD. Prognostic use of circulating plasma nucleic acid concentrations in patients with acute stroke. Clin. Chem. 2003;49:562–569. doi: 10.1373/49.4.562. [DOI] [PubMed] [Google Scholar]
- 17.Tsai N-W, Lin T-K, Chen S-D, Chang W-N, Wang H-C, Yang T-M, Lin Y-J, Jan C-R, Huang C-R, Liou C-W, Lu C-H. The value of serial plasma nuclear and mitochondrial DNA levels in patients with acute ischemic stroke. Clin. Chim. Acta. 2011;412:476–479. doi: 10.1016/j.cca.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 18.Mannherz HG, Peitsch MC, Zanotti S, Paddenberg R, Polzar B. A new function for an old enzyme: The role of DNase 1 in apoptosis. Curr. Top. Microbiol. Immunol. 1995;198:161–174. doi: 10.1007/978-3-642-79414-8_10. [DOI] [PubMed] [Google Scholar]
- 19.Napirei M, Wulf S, Mannherz HG. Chromatin breakdown during necrosis by serum DNase 1 and the plasminogen system. Arthritis Rheum. 2004;50:1873–1883. doi: 10.1002/art.20267. [DOI] [PubMed] [Google Scholar]
- 20.Kawai Y, Yoshida M, Arakawa K, Kumamoto T, Morikawa N, Masamura K, Tada H, Ito S, Hoshizaki H, Oshima S, Taniguchi K, Terasawa H, Miyamori I, Kishi K, Yasuda T. Diagnostic use of serum deoxyribonuclease 1 activity as a novel early-phase marker in acute myocardial infarction. Circulation. 2004;109:2398–2400. doi: 10.1161/01.CIR.0000129232.61483.43. [DOI] [PubMed] [Google Scholar]
- 21.Kumamoto T, Kawai Y, Arakawa K, Morikawa N, Kuribara J, Tada H, Taniguchi K, Tatami R, Miyamori I, Kominato Y, Kishi K, Yasuda T. Association of gln222arg polymorphism in the deoxyribonuclease I (DNase 1) gene with myocardial infarction in japanese patients. Eur. Heart J. 2006;27:2081–2087. doi: 10.1093/eurheartj/ehl177. [DOI] [PubMed] [Google Scholar]
- 22.Monestier M, Fasy TM, Losman MJ, Novick KE, Muller S. Structure and binding properties of monoclonal antibodies to core histones from autoimmune mice. Mol. Immunol. 1993;30:1069–1075. doi: 10.1016/0161-5890(93)90153-3. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig S, Mannherz HG, Schmitt S, Schaffer M, Zentgraf H, Napirei M. Murine serum deoxyribonuclease 1 (DNase 1) activity partly originates from the liver. Int. J. Biochem. Cell Biol. 2009;41:1079–1093. doi: 10.1016/j.biocel.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 25.Moran PM, Higgins LS, Cordell B, Moser PC. Age-related learning deficits in transgenic mice expressing the 751-amino acid isoform of human beta-amyloid precursor protein. Proc. Natl. Acad. Sci. U. S. A. 1995;92:5341–5345. doi: 10.1073/pnas.92.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J. Neurosci. Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- 27.Bradford A. The role of hypoxia and platelets in air travel-related venous thromboembolism. Curr. Pharm. Des. 2007;13:2668–2672. doi: 10.2174/138161207781662966. [DOI] [PubMed] [Google Scholar]
- 28.Wheatley K, Creed M, Mellor A. Haematological changes at altitude. J. R. Army Med. Corps. 2011;157:38–42. doi: 10.1136/jramc-157-01-07. [DOI] [PubMed] [Google Scholar]
- 29.Smallman DP, McBratney CM, Olsen CH, Slogic KM, Henderson CJ. Quantification of the 5-year incidence of thromboembolic events in U.S. Air force academy cadets in comparison to the U.S. Naval and Military Academies. Mil. Med. 2011;176:209–213. doi: 10.7205/milmed-d-10-00144. [DOI] [PubMed] [Google Scholar]
- 30.Geiger S, Holdenrieder S, Stieber P, Hamann GF, Bruening R, Ma J, Nagel D, Seidel D. Nucleosomes as a new prognostic marker in early cerebral stroke. J. Neurol. 2007;254:617–623. doi: 10.1007/s00415-006-0407-5. [DOI] [PubMed] [Google Scholar]
- 31.von Köckritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 32.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon H-U. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 33.Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: Novel insights and targets for treatment. Blood. 2008;112:3555–3562. doi: 10.1182/blood-2008-04-144758. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman J. Dornase aerosol effect on sputum viscosity in cases of cystic fibrosis. JAMA : The Journal of the American Medical Association. 1968;205:312–313. [PubMed] [Google Scholar]
- 36.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012 Mar 26; doi: 10.1084/jem.20112322. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Meyer SF, Schwarz T, Schatzberg D, Wagner DD. Platelet glycoprotein Ib alpha is an important mediator of ischemic stroke in mice. Exp Transl Stroke Med. 2011;3:9. doi: 10.1186/2040-7378-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Meyer SF, Schwarz T, Deckmyn H, Denis CV, Nieswandt B, Stoll G, Vanhoorelbeke K, Kleinschnitz C. Binding of von Willebrand factor to collagen and glycoprotein Ib alpha, but not to glycoprotein IIb/IIIa, contributes to ischemic stroke in mice. Arterioscler. Thromb. Vasc. Biol. 2010;30:1949–1951. doi: 10.1161/ATVBAHA.110.208918. [DOI] [PubMed] [Google Scholar]
- 39.Kleinschnitz C, Pozgajova M, Pham M, Bendszus M, Nieswandt B, Stoll G. Targeting platelets in acute experimental stroke: Impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- 40.Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, Renne C, Gailani D, Nieswandt B, Renne T. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J. Exp. Med. 2006;203:513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller F, Renne T. Platelet polyphosphates: The nexus of primary and secondary hemostasis. Scand. J. Clin. Lab. Invest. 2011;71:82–86. doi: 10.3109/00365513.2010.550312. [DOI] [PubMed] [Google Scholar]
- 42.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SA, Choi SH, Davis-Harrison R, Huyck J, Boettcher J, Reinstra CM, Morrissey JH. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116:4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Köllnberger M, Wakefield TW, Lämmle B, Massberg S, Wagner DD. Von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2010;117:1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Meyer SF, Stoll G, Wagner DD, Kleinschnitz C. Von Willebrand factor: An emerging target in stroke therapy. Stroke. 2012;43:599–606. doi: 10.1161/STROKEAHA.111.628867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleinschnitz C, De Meyer SF, Schwarz T, Austinat M, Vanhoorelbeke K, Nieswandt B, Deckmyn H, Stoll G. Deficiency of von Willebrand factor protects mice from ischemic stroke. Blood. 2009;113:3600–3603. doi: 10.1182/blood-2008-09-180695. [DOI] [PubMed] [Google Scholar]
- 48.Zhao B-Q, Chauhan AK, Canault M, Patten IS, Yang JJ, Dockal M, Scheiflinger F, Wagner DD. Von willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–3334. doi: 10.1182/blood-2009-03-213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, Liao X, Billiar T, Xu J, Esmon CT, Tsung A. Endogenous histones function as alarmins in sterile inflammatory liver injury through toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J. Immunol. 2011;187:2626–2631. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9:1795–1803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez JA, Xu X, Liu D, Zlokovic BV, Griffin JH. Recombinant murine-activated protein C is neuroprotective in a murine ischemic stroke model. Blood Cells. Mol. Dis. 2003;30:271–276. doi: 10.1016/s1079-9796(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 54.Guo H, Wang Y, Singh I, Liu D, Fernandez JA, Griffin JH, Chow N, Zlokovic BV. Species-dependent neuroprotection by activated protein C mutants with reduced anticoagulant activity. J. Neurochem. 2009;109:116–124. doi: 10.1111/j.1471-4159.2009.05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stroobant N, Van Nooten G, Van Belleghem Y, Vingerhoets G. The effect of CABG on neurocognitive functioning. Acta Cardiol. 2010;65:557–564. doi: 10.1080/ac.65.5.2056243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.