Abstract
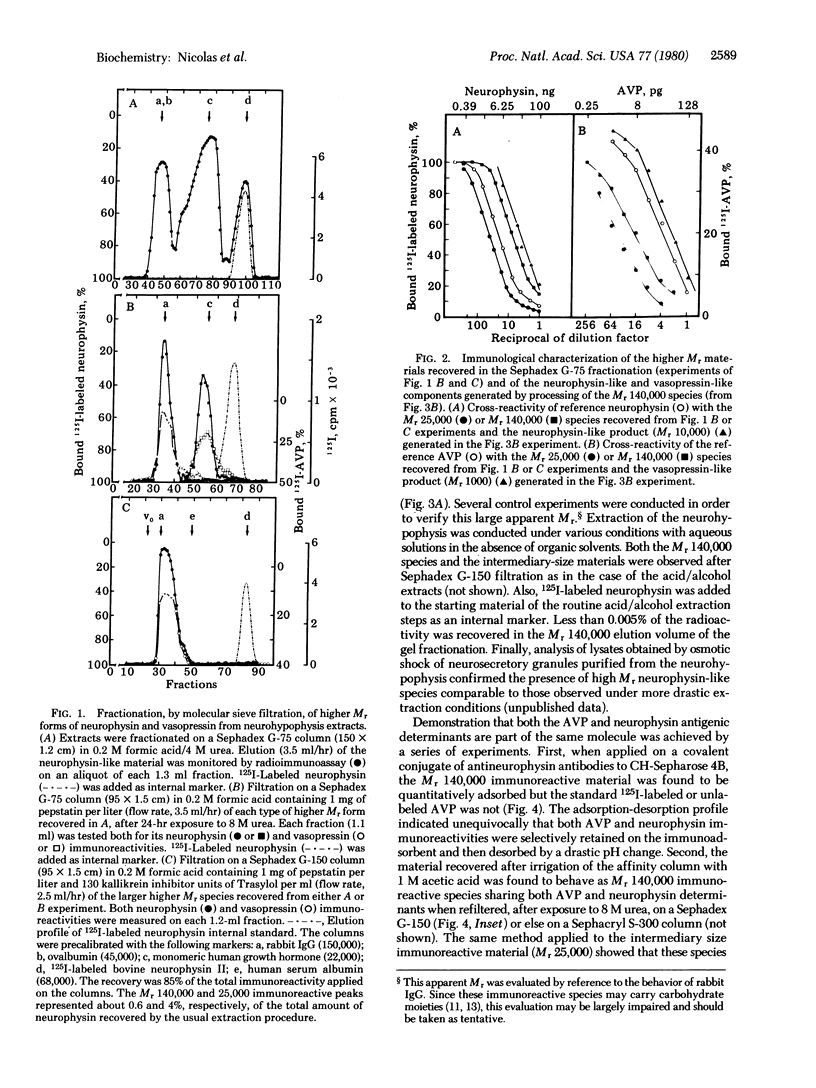
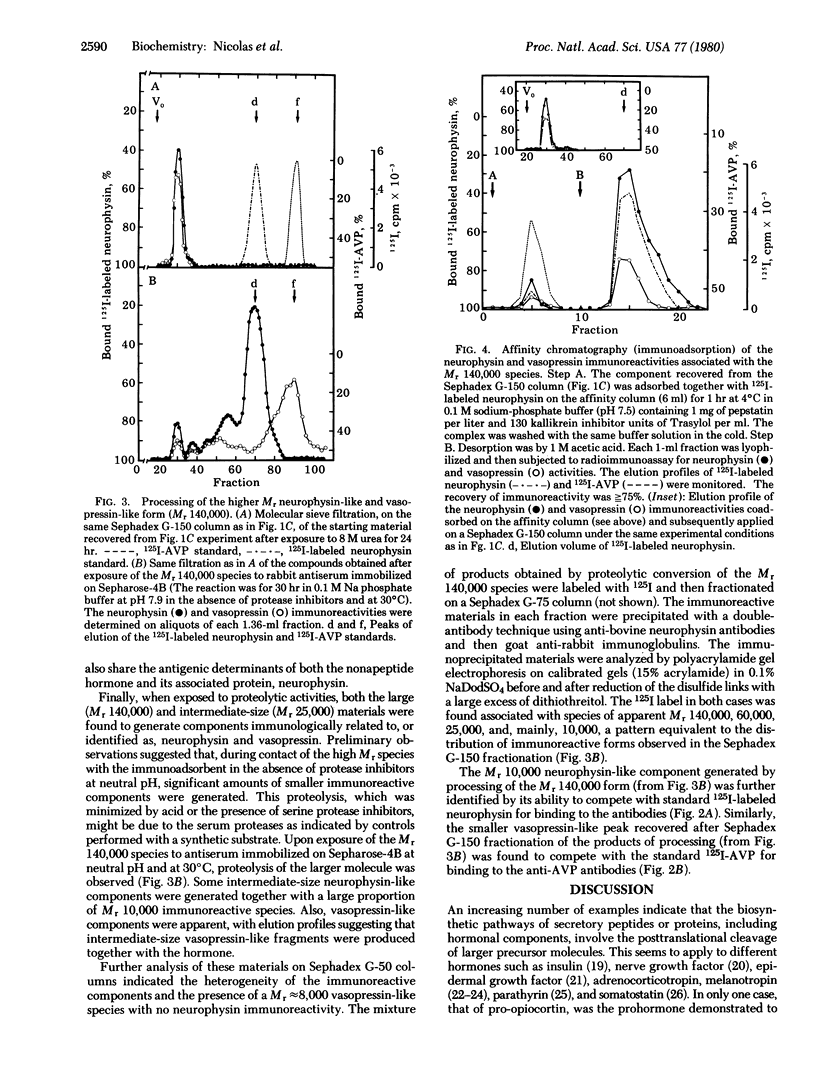
Extracts of bovine neurohypophysis made in acid/ethanol solution containing protease inhibitors were fractionated by two successive filtrations on Sephadex G-75 columns equilibrated in the presence and then in the absence of 4 M urea. Analysis of the pattern of neurophysin-like immunoreactivity in the eluate, with two different antibodies, indicated the presence of high Mr forms of neurophysin (apparent sizes, [unk]70,000 and 20,000-25,000, respectively) besides the Mr 10,000 neurophysin. [8-Arginine]vasopressin-like immunoreactivity was also detected, coeluting with the neurophysin-like species, in the material recovered in the exclusion and Mr 20,000-25,000 elution volumes of the same molecular sieve fractionation of neurohypophyseal extracts. Upon subsequent Sephadex G-150 filtration, the immunoreactive material recovered in the exclusion volume of the Sephadex G-75 filtration showed an apparent Mr of approximately 140,000. Both neurophysin-like and vasopressin-like immunoreactivities coeluted in the same volume. The elution profile of this Mr 140,000 material was unmodified when reanalyzed by the same molecular sieve filtration after exposure to 8 M urea. When these Mr 140,000 immunoreactive forms of vasopressin and neurophysin were submitted to affinity chromatography on anti-neurophysin antibodies immobilized on Sepharose, both immunoreactivities were selectively coadsorbed to the immunoadsorbent. Similarly, the neurophysin and vasopressin immunoreactivities associated with Mr≈25,000 were retained together on the same anti-neurophysin immunoadsorbent. The Mr 140,000 and Mr 25,000 species having both neurophysin and [8-arginine]vasopressin antigenic determinants generated the two neurosecretory components when exposed to proteolytic activities. This in vitro processing was inhibited in acid medium, at low temperature, and in the presence of a mixture of protease inhibitors. It is concluded that these two large forms of proteins containing both neurophysin and vasopressin may represent common biosynthetic precursors of these two neurohypophyseal components.
Keywords: prohormones, radioimmunoassay, affinity chromatography, proteolytic enzymes, neurohypophysis
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger E. A., Shooter E. M. Evidence for pro-beta-nerve growth factor, a biosynthetic precursor to beta-nerve growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3647–3651. doi: 10.1073/pnas.74.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow E. Chemistry and biology of the neurophysins. Annu Rev Biochem. 1979;48:251–274. doi: 10.1146/annurev.bi.48.070179.001343. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Gainer H. Neurophysin biosynthesis in normal rats and in rats with hereditary diabetes insipidus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4046–4049. doi: 10.1073/pnas.74.9.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camier M., Lauber M., Möhring J., Cohen P. Evidence for higher molecular weight immunoreactive forms of vasopressin in the mouse hypothalamus. Relationships with putative proneurophysins. FEBS Lett. 1979 Dec 15;108(2):369–373. doi: 10.1016/0014-5793(79)80566-9. [DOI] [PubMed] [Google Scholar]
- Cohen P., Nicolas P., Camier M. Biochemical aspects of neurosecretion: neurophysin--neurohypophyseal hormone complexes. Curr Top Cell Regul. 1979;15:263–318. doi: 10.1016/b978-0-12-152815-7.50011-9. [DOI] [PubMed] [Google Scholar]
- Fawcett C. P., Powell A. E., Sachs H. Biosynthesis and release of neurophysin. Endocrinology. 1968 Dec;83(6):1299–1310. doi: 10.1210/endo-83-6-1299. [DOI] [PubMed] [Google Scholar]
- Frey P., Forand R., Maciag T., Shooter E. M. The biosynthetic precursor of epidermal growth factor and the mechanism of its processing. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6294–6298. doi: 10.1073/pnas.76.12.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer H., Sarne Y., Brownstein M. J. Neurophysin biosynthesis: conversion of a putative precursor during axonal transport. Science. 1977 Mar 25;195(4284):1354–1356. doi: 10.1126/science.65791. [DOI] [PubMed] [Google Scholar]
- Giudice L. C., Chaiken I. M. Immunological and chemical identification of a neurophysin-containing protein coded by messenger RNA from bovine hypothalamus. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3800–3804. doi: 10.1073/pnas.76.8.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Potts J. T., Jr, Rich A. Proparathyroid hormone: identification of a biosynthetic precursor to parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Mar;69(3):643–647. doi: 10.1073/pnas.69.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug T. L., Adelman R. C. Evidence for a large thyrotropin and its accumulation during aging in rats. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1431–1437. doi: 10.1016/s0006-291x(77)80139-3. [DOI] [PubMed] [Google Scholar]
- Lauber M., Camier M., Cohen P. Higher molecular weight forms of immunoreactive somatostatin in mouse hypothalamic extracts: evidence of processing in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):6004–6008. doi: 10.1073/pnas.76.11.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber M., Camier M., Cohen P. Immunological and biochemical characterization of distinct high molecular weight forms of neurophysin and somatostatin in mouse hypothalamus extracts. FEBS Lett. 1979 Jan 15;97(2):343–347. doi: 10.1016/0014-5793(79)80118-0. [DOI] [PubMed] [Google Scholar]
- Legros J. J., Franchimont P. Comparison between radio-immunological behavior of purified human, bovine and porcine neurophysins. Ann Endocrinol (Paris) 1974 Mar-Apr;35(2):189–194. [PubMed] [Google Scholar]
- Lin C., Joseph-Bravo P., Sherman T., Chan L., McKelvy J. F. Cell-free synthesis of putative neurophysin precursors from rat and mouse hypothalamic poly (A)-RNA. Biochem Biophys Res Commun. 1979 Aug 13;89(3):943–950. doi: 10.1016/0006-291x(79)91869-2. [DOI] [PubMed] [Google Scholar]
- Liu T. C., Ax R. L., Jackson G. L. Characterization of luteinizing hormone synthesized and released by rat pituitaries in vitro: dissociation of immunological and biological activities. Endocrinology. 1979 Jul;105(1):10–15. doi: 10.1210/endo-105-1-10. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhring B., Möhring J. Plasma ADH in normal Long-Evans rats and in Long-Evans rats heterozygous and homozygous for hypothalamic diabetes insipidus. Life Sci. 1975 Oct 15;17(8):1307–1314. doi: 10.1016/0024-3205(75)90143-5. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Nicolas P., Camier M., Dessen P., Cohen P. Interactions of oxytocin and vasopressin with bovine neurophysins I and II. Effects of hormone binding on the protein quaternary structure: a simple model. J Biol Chem. 1976 Jul 10;251(13):3965–3971. [PubMed] [Google Scholar]
- Reichert L. E., Jr, Ramsey R. B. Evidence for the existence of a large molecular weight protein in human pituitary tissue having follicle stimulating hormone activity. J Clin Endocrinol Metab. 1977 Mar;44(3):545–552. doi: 10.1210/jcem-44-3-545. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: identification of beta-lipotropin peptides and their arrangement relative to corticotropin in the precursor synthesized in a cell-free system. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5300–5304. doi: 10.1073/pnas.74.12.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. T., Brownstein M. J., Gainer H. Trypsin liberates an arginine vasopressin-like peptide and neurophysin from a Mr 20,000 putative common precursor. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6086–6090. doi: 10.1073/pnas.76.12.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACHS H., TAKABATAKE Y. EVIDENCE FOR A PRECURSOR IN VASOPRESSIN BIOSYNTHESIS. Endocrinology. 1964 Dec;75:943–948. doi: 10.1210/endo-75-6-943. [DOI] [PubMed] [Google Scholar]
- Schmale H., Leipold B., Richter D. Cell-free translation of bovine hypothalamic mRNA. Synthesis and processing of the prepro-neurophysin I and II. FEBS Lett. 1979 Dec 15;108(2):311–316. doi: 10.1016/0014-5793(79)80553-0. [DOI] [PubMed] [Google Scholar]
- Stachura M. E., Frohman L. A. Growth hormone: independent release of big and small forms from rat pituitary in vitro. Science. 1975 Feb 7;187(4175):447–449. doi: 10.1126/science.1111113. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKABATAKE Y., SACHS H. VASOPRESSIN BIOSYNTHESIS. 3. IN VITRO STUDIES. Endocrinology. 1964 Dec;75:934–942. doi: 10.1210/endo-75-6-934. [DOI] [PubMed] [Google Scholar]



