Abstract
Objective:
The purpose of our study was to examine in patients hospitalized with community-acquired pneumonia (CAP) the association between abnormal Paco2 and ICU admission and 30-day mortality.
Methods:
A retrospective cohort study was conducted at two tertiary teaching hospitals. Eligible subjects were admitted with a diagnosis of CAP. Arterial blood gas analyses were obtained with measurement of Paco2 on admission. Multivariate analyses were performed using 30-day mortality and ICU admission as the dependent measures.
Results:
Data were abstracted on 453 subjects with a documented arterial blood gas analysis. One hundred eighty-nine patients (41%) had normal Paco2 (35-45 mm Hg), 194 patients (42%) had a Paco2 < 35 mm Hg (hypocapnic), and 70 patients (15%) had a Paco2 > 45 mm Hg (hypercapnic). In the multivariate analysis, after adjusting for severity of illness, hypocapnic patients had greater 30-day mortality (OR = 2.84; 95% CI, 1.28-6.30) and a higher need for ICU admission (OR = 2.88; 95% CI, 1.68-4.95) compared with patients with normal Paco2. In addition, hypercapnic patients had a greater 30-day mortality (OR = 3.38; 95% CI, 1.38-8.30) and a higher need for ICU admission (OR = 5.35; 95% CI, 2.80-10.23). When patients with COPD were excluded from the analysis, the differences persisted between groups.
Conclusion:
In hospitalized patients with CAP, both hypocapnia and hypercapnia were associated with an increased need for ICU admission and higher 30-day mortality. These findings persisted after excluding patients with CAP and with COPD. Therefore, Paco2 should be considered for inclusion in future severity stratification criteria to appropriate identified patients who will require a higher level of care and are at risk for increased mortality.
Community-acquired pneumonia (CAP) is the leading infectious cause of death in the United States.1 It affects 5 million people each year and leads to 1 million hospital admissions.2 Of those admitted to the hospital with CAP, approximately 10% to 20% will require admission to the ICU,3,4 where mortality may be as high as 50%,5,6 especially when mechanical ventilation is needed.7,8 The most frequent cause of mortality in hospitalized patients with CAP is respiratory failure.9
Respiratory failure is defined as a series of gas exchange abnormalities severe enough to deprive vital organs of oxygen (hypoxemic respiratory failure) or cause acidosis secondary to hypercapnia (hypercapnic respiratory failure).10 Hypoxemic respiratory failure is well recognized as a prognostic marker in different severity-of-illness scores to predict poor clinical outcomes in hospitalized patients with CAP.11‐14 By contrast, ventilatory abnormalities reflected by an alteration in Paco2 have not been considered a poor prognostic marker unless arterial pH changes are observed.13,15 Paco2 is widely accepted as an indicator of ventilator adequacy. Abnormally high levels may indicate severe respiratory fatigue and impending cardiopulmonary arrest.16 However, limited data are available on the value of abnormal Paco2 as a predictor of severity in hospitalized patients with CAP. Therefore, our aim was to examine the association between abnormal Paco2 and the need for invasive mechanical ventilation, ICU admission, and 30-day mortality in patients hospitalized with CAP.
Materials and Methods
This is a retrospective cohort study of hospitalized patients with CAP at two academic teaching tertiary care hospitals in San Antonio, Texas. The Institutional Review Board of the University of Texas Health Science Center at San Antonio approved the research protocol with exempt status.
Study Sites/Inclusion and Exclusion Criteria
We identified all patients admitted to the study hospitals between January 1, 1999, and December 1, 2002, with a primary discharge diagnosis of pneumonia (International Classification of Diseases, Ninth Revision codes 480.0-483.99 or 485-487.0) or a secondary discharge diagnosis of pneumonia with a primary diagnosis of respiratory failure (518.81) or sepsis (038.xx). Inclusion criteria were (1) ≥ 18 years of age, (2) admission diagnosis of CAP, (3) a confirmed infiltrate or other finding consistent with CAP on chest radiograph or CT scan of the chest obtained within 24 h of admission, and (4) completing an arterial blood gas analysis within 24 h of admission.
Exclusion criteria included (1) discharge from an acute care facility within 14 days of admission, (2) transfer from another acute care hospital, or (3) having “comfort measures only” status during the admission. If a subject was admitted more than once during the study period, only the first hospitalization was abstracted.
Study Groups
Based on the Paco2 values from the arterial blood gas measured in the first 24 h of admission with CAP, the patients were stratified into 3 groups: normal Paco2 (35-45 mm Hg), hypocapnic (Paco2 < 35 mm Hg), and hypercapnic (Paco2 > 45 mm Hg). The definition of COPD was based on clinical data obtained during chart review, as discuss previously.17 Owing to the absence of pulmonary function test results, terms including “chronic obstructive pulmonary disease,” “emphysema,” and “chronic bronchitis” were used as proxy measures for COPD. Missing values or data were assumed to be normal and not show COPD. This strategy is widely used in the application of prognostic prediction rules and reflects the methods used in the original pneumonia severity index (PSI) score studies13,18
Data Abstraction
Chart review data included demographics, comorbid conditions, physical examination findings, laboratory data, and chest radiographic reports. Comorbid conditions were identified from either the admission note or the chart problem list. In addition, data on the CAP process of care measures were abstracted. These included timing of first dose of antibiotics, collection of blood cultures prior to antibiotic administration, and obtaining blood cultures and oxygen saturation measurement within 24 h of presentation. Antimicrobial therapy was considered guideline concordant if it agreed with the American Thoracic Society guidelines.11
Risk Adjustment
The PSI score was used to assess severity of illness at presentation. The PSI is a validated prediction rule for 30-day mortality in patients with CAP.13 Patients were classified into five risk classes with 30-day mortality ranging from 0.1% for class 1 to 27% for class 5 for patients enrolled in the Patient Outcomes Research Team (PORT) cohort study.14
Outcomes
The primary outcome was 30-day mortality. Secondary outcomes were ICU admission, need for invasive mechanical ventilation, and hospital length of stay (LOS). Mortality was assessed using information from the Texas Department of Health and Department of Veteran Affairs clinical database. Mortality status was assessed through the end of December 2002.
Statistical Analyses
Univariate statistics were used to test the association of demographic and clinical characteristics with Paco2 levels. Categorical variables were analyzed using the χ2 test, and continuous variables were analyzed using Student t test.
We used Cox proportional hazards model to produce survival curves after adjusting for the PSI score.13,19 We performed a logistic regression analysis using ICU admission and 30-day mortality as the dependent variables and Paco2 as the independent variable. A subgroup analysis was repeating the univariate and multivariate analysis excluding patients with a diagnosis of COPD. A secondary analysis was performed using Paco2, tachypnea (respiratory rate > 30/min) and arterial pH (acidosis [pH < 7.35] or alkalosis [pH > 7.45]) as independent variables using a Cox proportional hazard model to predict 30-day mortality. All analyses were performed using SPSS version 19.0 for Windows (IBM).
Results
Four hundred fifty-three patients who satisfied the inclusion criteria were identified during the study period. The distribution among groups was 189 patients (41.7%) with normal Paco2 (35-45 mm Hg), 194 patients (42.8%) with hypocapnia (Paco2 < 35 mm Hg), and 70 patients with hypercapnia (15.5%) (Paco2 > 45 mm Hg).
Patient Characteristics
Table 1 shows patient demographic characteristics by study groups. Hypocapnic patients were more likely to have been younger; to have had multilobar infiltrates, metabolic alkalosis, and chronic liver disease; and to have presented with tachycardia. They were less likely to have COPD as compared with patients with normal Paco2 levels. However, in comparison with normocapnic patients, hypercapnic patients had more preexisting comorbid conditions, such as congestive heart failure, prior stroke, and COPD. Furthermore, hypercapnic patients were more likely to have altered mental status, hypoxemia, acidosis, and a higher PSI scores.
Table 1.
—Comparison of Demographic and Clinical Characteristics Among Patients With CAP According to Levels of Paco2
| Variables | Paco2 < 35 mm Hg (n = 194) | Paco2 35-45 mm Hg (n = 189) | Paco2 > 45 mm Hg (n = 70) |
| Age (SD), y | 56 (15.0)a | 60 (15.3) | 60 (17.1) |
| Men | 150 (77.7) | 136 (72.0) | 61 (87.1)a |
| Comorbid conditions | |||
| COPD | 33 (17.0)a | 57 (30.2) | 36 (51.4)a |
| Congestive heart failure | 21 (10.8) | 26 (13.8) | 18 (25.7)a |
| History of stroke | 19 (9.8) | 19 (10.1) | 15 (21.4)a |
| History of malignancy | 15 (7.7) | 15 (7.9) | 6 (8.6) |
| Chronic liver disease | 31 (16.0)a | 16 (8.5) | 10 (14.3) |
| Renal insufficiency | 21 (10.8) | 23 (12.2) | 4 (5.7) |
| Physiologic parameters | |||
| Respiratory rate > 30/min | 28 (14.4) | 21 (11.1) | 14 (20.0) |
| Heart rate > 125/min | 43 (22.2)a | 23 (12.2) | 14 (20.0) |
| Systolic BP < 90 mm Hg | 7 (3.6) | 4 (2.1) | 2 (2.9) |
| Temperature < 95°F (< 30°C) or > 104°F (> 40°C) | 8 (4.1) | 2 (1.1) | 2 (2.9) |
| Altered mental status | 29 (14.9) | 19 (10.1) | 18 (25.7)a |
| Arterial pH < 7.35 | 13 (6.7) | 11 (5.8) | 25 (35.7)a |
| BUN > 30 mg/dL | 49 (25.3) | 44 (23.3) | 9 (12.9) |
| Pao2 < 60 mm Hg | 56 (28.9) | 44 (23.3) | 39 (55.7)a |
| Hematocrit < 30% | 21 (10.8) | 14 (7.4) | 5 (7.1) |
| Multilobar infiltrates | 97 (50.0)a | 72 (38.5) | 29 (42.6) |
| Pleural effusion | 58 (29.9) | 52 (27.5) | 17 (24.3) |
| PSI score, mean (SD) | 92 (37.7) | 90 (34.7) | 109 (39.2)a |
Values are given as No. (%) unless otherwise indicated. CAP = community-acquired pneumonia; PSI = pneumonia severity index.
P < .05 compared with reference group (Paco2 35-45 mm Hg).
Clinical Outcomes
Overall 30-day mortality was higher in patients with hypocapia and hypercapnia compared with the reference group with normal Paco2 levels (13.4% and 20.0% vs 5.3%, respectively; P = .01). Both groups (hypocapnic and hypercapnic) also had significantly higher ICU admissions. However, there were no statistically significant differences among groups for invasive mechanical ventilation and mean hospital LOS (Table 2).
Table 2.
—Comparison of Outcomes Among All Patients With CAP According to Levels of Paco2
| Outcome | Paco2 < 35 mm Hg (n = 194) | Paco2 35-45 mm Hg (n = 189) | Paco2 > 45 mm Hg (n = 70) |
| 30-d mortality | 26 (13.4)a | 10 (5.3) | 14 (20.0)a |
| ICU admission | 62 (32.0)a | 29 (15.3) | 38 (54.3)a |
| Invasive mechanical ventilation | 24 (12.4) | 16 (8.5) | 11 (15.7) |
| Length of stay, mean (SD) | 10 (27.4) | 7 (9.6) | 9.6 (9.5) |
Values are given as No. (%) unless otherwise indicated. See Table 1 legend for expansion of abbreviation.
P < .05 compared with reference group (Paco2 35-45 mm Hg).
Table 3 shows univariate and multivariable analysis of specific variables according to 30-day mortality, ICU admission, and invasive mechanical ventilation. In the univariate analysis, both hypocapnia and hypercapnia were associated with 30-day mortality and need for ICU admission. However, these conditions were not associated with mechanical ventilation and LOS. The same results were found in the multivariable analysis after adjusting for severity of illness (PSI).
Table 3.
—Comparison of 30-d Mortality, ICU Admission, and Mechanical Ventilation Among Hypocapnic (Paco2 < 35 mm Hg) and Hypercapnic (Paco2 > 45 mm Hg) Patients With CAP vs Normal Group
| Univariable |
Multivariable |
|||||
| Outcome | OR | 95% CI | P Value | HR | 95% CI | P Value |
| Hypocapnic group | ||||||
| 30-d mortality | 2.77 | 1.30-5.92 | .009 | 2.84 | 1.28-6.30 | .01 |
| ICU admission | 2.59 | 1.58-4.26 | < .001 | 2.88 | 1.68-4.95 | < .001 |
| Invasive mechanical ventilation | 1.53 | 0.78-2.97 | .2 | 1.49 | 0.75-2.95 | .2 |
| Hypercapnic group | ||||||
| 30-d mortality | 4.47 | 1.88-10.63 | .001 | 3.38 | 1.38-8.30 | .008 |
| ICU admission | 6.55 | 3.54-12.11 | < .001 | 5.35 | 2.80-10.23 | < .001 |
| Invasive mechanical ventilation | 2.02 | 0.89-4.59 | .09 | 1.82 | 0.78-4.25 | .17 |
Multivariable analysis adjusted for pneumonia severity of index score. HR = hazard ratio. See Table 1 legend for expansion of other abbreviation.
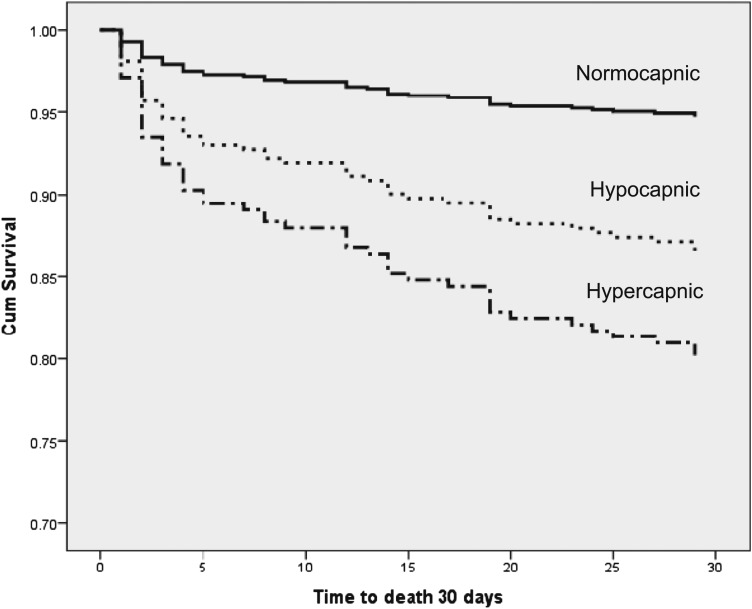
Figure 1 shows the survival curves based on the Cox proportional hazard model. It demonstrates that both hypocapnia and hypercapnia had significantly increased mortality compared with the reference group with normal Paco2.
Figure 1.
Cox survival curves of patients with hypocapnia (Paco2 < 35 mm Hg) and hypercapnia (Paco2 > 45 mm Hg) vs those with normocapnia (Paco2 35-45 mm Hg) at admission. Cum = cumulative.
We evaluated the Paco2 interaction with pH level and respiratory rate to determine an association with 30-day mortality. We found that hypocapnia was independently associated with higher 30-day mortality (hazard ratio [HR] = 2.99; 95% CI, 1.23-7.25; P = .015), but there was not an association with alkalosis (HR = 1.18; 95% CI, 0.55-2.55; P = .7) or tachypnea (HR = 1.77; 95% CI, 0.71-4.40; P = .2). In addition, hypocapnia and acidosis were independently associated with higher 30-day mortality (HR = 2.63; 95% CI, 1.27-5.46; P = .009 and HR = 3.81; 95% CI, 1.66-8.78; P = .002, respectively) but not with tachypnea (OR = 1.71; 95% CI, 0.77-3.77; P = .2). Hypercapnia was independently associated with higher 30-day mortality (OR = 3.36; 95% CI, 1.35-8.40; P = .009) but not with acidosis (OR = 2.11; 95% CI, 0.81-5.50; P = .1) or tachypnea (OR = 0.19; 95% CI, 0.02-1.41; P = .1).
Subgroup Analysis
To assess the influence of COPD on outcomes, we performed an additional analysis excluding patients with a history of COPD (n = 126 [27.8%]). In this subgroup (n = 327), 30-day mortality and ICU admission were also greater among patients with hypocapnia and hypercapnia as compared with the reference group. Furthermore, higher need for invasive mechanical ventilation was observed among hypercapnic patients without COPD (Table 4).
Table 4.
—Comparison of Outcomes Among Patients With CAP Without COPD According to Levels of Paco2
| Outcome | Paco2 < 35 mm Hg (n = 161) | Paco2 35-45 mm Hg (n = 132) | Paco2 > 45 mm Hg (n = 34) |
| 30-d mortality | 22 (13.7)a | 4 (3.0) | 9 (26.5)a |
| ICU admission | 48 (29.8)a | 19 (14.4) | 17 (50.0)a |
| Invasive mechanical ventilation | 16 (9.9) | 8 (6.1) | 7 (20.6)a |
Values are given as No. (%), unless otherwise indicated. See Table 1 legend for expansion of abbreviation.
P < .05 compared with reference group (Paco2 35-45 mm Hg).
Table 5 shows the univariate and multivariable analyses after adjusting for severity of illness (PSI) in subjects without COPD. Both hypocapnia and hypercapnia were associated with higher 30-day mortality and ICU admission in the univariate and multivariate analyses. Patients without COPD and hypercapnia also have an increased risk for invasive mechanical ventilation in the univariate analysis.
Table 5.
—Subgroup Analysis of Patients With CAP After Excluding Those With COPD: Comparison of 30-d Mortality, ICU Admission, and Mechanical Ventilation Among Hypocapnic and Hypercapnic Patients With CAP vs Normal Group
| Univariable |
Multivariable |
|||||
| Outcome | OR | 95% CI | P Value | HR | 95% CI | P Value |
| Hypocapnic (non-COPD) | ||||||
| 30-d mortality | 5.06 | 1.70-15.1 | .004 | 4.91 | 1.61-14.99 | .005 |
| ICU admission | 2.53 | 1.40-4.57 | .002 | 2.55 | 1.34-4.84 | .004 |
| Invasive mechanical ventilation | 1.71 | 0.71-4.13 | .2 | 1.56 | 0.63-3.88 | .3 |
| Hypercapnic (non-COPD) | ||||||
| 30-d mortality | 11.52 | 3.29-40.34 | < .001 | 8.98 | 2.45-32.92 | .001 |
| ICU admission | 5.95 | 2.59-16.63 | < .001 | 4.25 | 1.72-10.52 | .002 |
| Invasive mechanical ventilation | 4.02 | 1.34-12.03 | .01 | 3.08 | 0.97-9.80 | .056 |
Discussion
The main findings of our study are that patients hospitalized with CAP with abnormal Paco2 levels (hypercapnia or hypocapnia) at the clinical presentation to the hospital were more likely to die within 30 days of admission and require ICU care when compared with those with normal Paco2. These findings remain after adjusting for severity of illness and excluding patients with COPD from the cohort. This suggests that Paco2 may play an important role in the clinical outcomes in hospitalized patients with CAP.
ICU admission is an important decision that affects outcomes, treatment, and costs.20‐22 For several decades, a number of predictor severity tools, such as PSI,13 CURB-65,23 and Infectious Diseases Society of America/American Thoracic Society guidelines,11,12 have been developed to predict 30-day mortality, but they also have been recommended to identify patients for consideration of the need for ICU admission. A recent meta-analysis suggests that these severity score systems are not accurate to predict ICU admission.24 These scores include several demographic, comorbid condition, physiologic, laboratory or radiologic variables, but none consider Paco2 levels as criteria of severity. In addition, recent new severity scores, such as SCAP25 or SMART-COP,15 have included low arterial pH as criteria, but Paco2 has not been used as a predictive variable. It is well known that the levels of Paco2 determine arterial pH value, but often pH values can be compensated by bicarbonate levels, especially in patients with chronic disease, and may not reflect an abnormal Paco2 value. Our results suggest that abnormal Paco2 levels should be considered in severity of illness scores and require further validation.
Additionally, our study shows that patients admitted for CAP with hypocapnia and hypercapnia have a higher risk of mortality within 30 days of admission when compared with normal Paco2 levels. Compared with normocapnia, hypocapnia was associated with increase in mortality of more than threefold in general and nearly fivefold in an analysis that excluded patients with COPD. Similarly, hypercapnia was associated with increased mortality of nearly threefold in general and nearly ninefold if patients with COPD were excluded. These results are consistent with data previously published by Sin et al,26 which showed that hospitalized patients with CAP had higher in-hospital mortality when they had abnormal Paco2 levels at admission.
Both hypocapnia and hypercapnia are independently associated with a greater tendency toward respiratory failure. Hypocapnia can cause or aggravate cellular ischemia by inducing a leftward shift in the oxyhemoglobin dissociation curve and reducing oxygen delivery to tissues.27 It is common in several diseases (eg, high-altitude pulmonary edema, lung injury, or hepatic failure) and a criterion for systemic inflammatory response syndrome.28 In respiratory disorders such as pneumonia, hypocapnia can worsen ventilation-perfusion matching and gas exchange in the lung via a number of mechanisms, including bronchoconstriction, reduction in collateral ventilation, reduction in parenchymal compliance, and attenuation of hypoxic pulmonary vasoconstriction and increased intrapulmonary shunting.29 Additionally, hypercapnia can increase sympathetic neural drive, cardiac output, heart rate, and systemic and pulmonary BP.30 In patients with respiratory disorders, mainly associated with hypoxemia, hypercapnia can increase pulmonary vascular resistance, enhance hypoxic vasoconstriction, mediate large airway constriction, impair contractility of vascular smooth muscles, and limit gas exchange.31‐33 In our study, hypocapnia and hypercapnia were independently associated with 30-day mortality with no relationship to respiratory rate or pH level, suggesting that they are likely to be intrinsically involved in the poor prognosis of these patients.
Our study has several limitations. First, this is a retrospective study involving only two centers with a relatively small sample size. Second, our sample was predominately male because of the higher population of veterans enrolled in this cohort. It is unknown if abnormal Paco2 levels in women would have similar results. Third, our cohort did not have information regarding certain variables that may influence the association of hypocapnia or hypercapnia, such as obtaining an arterial blood gas analysis, the presence of chronic hypercapnia prior to hospitalization, lactate levels, cause(s) of death, and the use of noninvasive ventilation. Finally, we used the decision of ICU admission as the gold standard, because this reflected the actual clinical practice. However, the variability of clinical judgment and bed availability may play a role and influenced the site-of-care decisions.
In conclusion, our study demonstrates significantly higher ICU admissions and increased 30-day mortality among patients with hypocapnia and hypercapnia hospitalized with CAP. Further prospective studies are needed to confirm this important finding and establish a role for Paco2 levels in predictive schemes to asses risk stratification and need for ICU care in patients with CAP.
Acknowledgments
Author contributions: Dr Restrepo is guarantor of the study. He had full access to the data and will vouch for the integrity of the data analysis.
Dr Laserna: contributed to the analysis and interpretation of the data and preparation of the manuscript and gave final approval of the manuscript.
Dr Sibila: contributed to the analysis and interpretation of the data and preparation of the manuscript and gave final approval of the manuscript.
Dr Aguilar: contributed to the interpretation of the data and preparation of the manuscript and gave final approval of the manuscript.
Dr Mortensen: contributed to originating and coordinating the study, obtaining funding, coordinating acquisition of the data, and preparing the manuscript and gave final approval of the manuscript.
Dr Anzueto: contributed to the design of the study, acquisition of the data, analysis of the data, and preparation of the manuscript and gave final approval of the manuscript.
Dr Blanquer: contributed to the interpretation of the data and preparation of the manuscript and gave final approval of the manuscript.
Dr Sanz: contributed to the interpretation of the data and preparation of the manuscript and gave final approval of the manuscript.
Dr Rello: contributed to the interpretation of the data and preparation of the manuscript and gave final approval of the manuscript.
Dr Marcos: contributed to the interpretation of the data and preparation of the manuscript and gave final approval of the manuscript.
Dr Velez: contributed to the interpretation of the data and preparation of the manuscript and gave final approval of the manuscript.
Dr Aziz: contributed to the interpretation of the data and preparation of the manuscript and gave final approval of the manuscript.
Dr Restrepo: contributed to coordinating all the steps, including obtaining funding, coordinating acquisition of data, and preparing the manuscript, and gave final approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Restrepo participated in advisory boards for Theravance, Inc; Trius Therapeutics; Forest Laboratories, Inc; Johnson & Johnson (until 2011); and a research development advisory board for Novartis AG. He served as a consultant for Theravance, Inc; Trius Therapeutics; and Pfizer, Inc (Wyeth). Drs Laserna, Sibila, Aguilar, Mortensen, Anzueto, Blanquer, Sanz, Rello, Marcos, Velez, and Aziz have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Abbreviations
- CAP
community-acquired pneumonia
- HR
hazard ratio
- LOS
length of stay
- PSI
pneumonia severity index
Footnotes
Funding/Support: This research was supported by a Howard Hughes Medical Institute faculty start-up grant [00378-001] and a Department of Veteran Affairs Veterans Integrated Service Network 17 new faculty grant. Drs Laserna and Sibila are supported by Sociedad Espanola de Neumologia y Cirugia Toracica, Societat Catalana de Pneumologia, and Fundacio Catalana de Pneumologia. Dr Sibila is supported by Instituto de Salud Carlos III [Grant BAE11/00102]. Dr Restrepo’s time is partially protected by an award from the National Heart, Lung, and Blood Institute [K23HL096054].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Kozak LJ, DeFrances CJ, Hall MJ. National hospital discharge survey: 2004 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2006;162(162):1-209 [PubMed] [Google Scholar]
- 2.Trotter CL, Stuart JM, George R, Miller E. Increasing hospital admissions for pneumonia, England. Emerg Infect Dis. 2008;14(5):727-733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renaud B, Santin A, Coma E, et al. Association between timing of intensive care unit admission and outcomes for emergency department patients with community-acquired pneumonia. Crit Care Med. 2009;37(11):2867-2874 [DOI] [PubMed] [Google Scholar]
- 4.Ewig S, Woodhead M, Torres A. Towards a sensible comprehension of severe community-acquired pneumonia. Intensive Care Med. 2011;37(2):214-223 [DOI] [PubMed] [Google Scholar]
- 5.Restrepo MI, Anzueto A. Severe community-acquired pneumonia. Infect Dis Clin North Am. 2009;23(3):503-520 [DOI] [PubMed] [Google Scholar]
- 6.Woodhead M, Welch CA, Harrison DA, Bellingan G, Ayres JG. Community-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit Care. 2006;10(suppl 2):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus DC, Marrie TJ, Obrosky DS, et al. Severe community-acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717-723 [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez A, Mendia A, Sirvent JM, et al. ; CAPUCI Study Group Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35(6):1493-1498 [DOI] [PubMed] [Google Scholar]
- 9.Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 2002;162(9):1059-1064 [DOI] [PubMed] [Google Scholar]
- 10.Kruse JA, Fink MP, Carlsson RW. Saunders Manual of Critical Care. 1st ed.Philadelphia, PA: Elsevier Science;2003:17-18 [Google Scholar]
- 11.Niederman MS, Mandell LA, Anzueto A, et al. ; American Thoracic Society Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730-1754 [DOI] [PubMed] [Google Scholar]
- 12.Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America ; American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243-250 [DOI] [PubMed] [Google Scholar]
- 14.Fine MJ, Hough LJ, Medsger AR, et al. The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157(1):36-44 [PubMed] [Google Scholar]
- 15.Charles PG, Wolfe R, Whitby M, et al. ; Australian Community-Acquired Pneumonia Study Collaboration SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47(3):375-384 [DOI] [PubMed] [Google Scholar]
- 16.Caruana-Montaldo B, Gleeson K, Zwillich CW. The control of breathing in clinical practice. Chest. 2000;117(1):205-225 [DOI] [PubMed] [Google Scholar]
- 17.Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006;28(2):346-351 [DOI] [PubMed] [Google Scholar]
- 18.Fine MJ, Singer DE, Hanusa BH, Lave JR, Kapoor WN. Validation of a pneumonia prognostic index using the MedisGroups Comparative Hospital Database. Am J Med. 1993;94(2):153-159 [DOI] [PubMed] [Google Scholar]
- 19.Lee ET, Wang JW. Statistical Methods for Survival Data Analysis: Wiley Series in Probability and Statistics. 3rd ed.New York, NY: Wiley;2003 [Google Scholar]
- 20.Restrepo MI, Mortensen EM, Rello J, Brody J, Anzueto A. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest. 2010;137(3):552-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niederman MS, McCombs JS, Unger AN, Kumar A, Popovian R. The cost of treating community-acquired pneumonia. Clin Ther. 1998;20(4):820-837 [DOI] [PubMed] [Google Scholar]
- 22.Guest JF, Morris A. Community-acquired pneumonia: the annual cost to the National Health Service in the UK. Eur Respir J. 1997;10(7):1530-1534 [DOI] [PubMed] [Google Scholar]
- 23.Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax. 2000;55(3):219-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalmers JD, Mandal P, Singanayagam A, et al. Severity assessment tools to guide ICU admission in community-acquired pneumonia: systematic review and meta-analysis. Intensive Care Med. 2011;37(9):1409-1420 [DOI] [PubMed] [Google Scholar]
- 25.España PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249-1256 [DOI] [PubMed] [Google Scholar]
- 26.Sin DD, Man SF, Marrie TJ. Arterial carbon dioxide tension on admission as a marker of in-hospital mortality in community-acquired pneumonia. Am J Med. 2005;118(2):145-150 [DOI] [PubMed] [Google Scholar]
- 27.Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med. 2002;347(1):43-53 [DOI] [PubMed] [Google Scholar]
- 28.Bone RC, Balk RA, Cerra FB, et al. ; The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644-1655 [DOI] [PubMed] [Google Scholar]
- 29.Curley G, Laffey JG, Kavanagh BP. Bench-to-bedside review: carbon dioxide. Crit Care. 2010;14(2):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bigatello LM, Patroniti N, Sangalli F. Permissive hypercapnia. Curr Opin Crit Care. 2001;7(1):34-40 [DOI] [PubMed] [Google Scholar]
- 31.Feihl F, Eckert P, Brimioulle S, et al. Permissive hypercapnia impairs pulmonary gas exchange in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;162(1):209-215 [DOI] [PubMed] [Google Scholar]
- 32.Smith GL, Austin C, Crichton C, Wray S. A review of the actions and control of intracellular pH in vascular smooth muscle. Cardiovasc Res. 1998;38(2):316-331 [DOI] [PubMed] [Google Scholar]
- 33.Ingram RH., Jr Effects of airway versus arterial CO2 changes on lung mechanics in dogs. J Appl Physiol. 1975;38(4):603-607 [DOI] [PubMed] [Google Scholar]