Abstract
Background:
Adaptive servoventilation (ASV) has demonstrated efficacy in treating sleep-disordered breathing (SDB) in patients with heart failure (HF), but large randomized trials are lacking. We, therefore, sought to perform a systematic review and meta-analysis of existing data.
Methods:
A systematic search of the PubMed database was undertaken in March 2012. Publications were independently assessed by two investigators to identify studies of ≥ 1-week duration that compared ASV to a control condition (ie, subtherapeutic ASV, continuous or bilevel pressure ventilation, oxygen therapy, or no treatment) in adult patients with SDB and HF. Mean, variability, and sample size data were extracted independently for the following outcomes: apnea-hypopnea index (AHI), left ventricular ejection fraction (LVEF), quality of life (SF-36 Health Survey; Medical Outcomes Trust), 6-min walk distance, peak oxygen consumption (o2) % predicted, and ventilatory equivalent ratio for CO2 (e/co2) slope measured during exercise. Random effects meta-analysis models were applied.
Results:
Fourteen studies were identified (N = 538). Comparing ASV to control conditions, the weighted mean difference in AHI (−14.64 events/h; 95% CI, −21.03 to −8.25) and LVEF (0.40; 95% CI, 0.08-0.71) both significantly favored ASV. ASV also improved the 6-min walk distance, but not peak o2 % predicted, e/co2 slope, or quality of life, compared with control conditions.
Conclusions:
In patients with HF and SDB, ASV was more effective than control conditions in reducing the AHI and improving cardiac function and exercise capacity. These data provide a compelling rationale for large-scale randomized controlled trials to assess the clinical impact of ASV on hard outcomes in these patients.
The prevalence of sleep-disordered breathing (SDB) in patients with heart failure (HF) is as high as 47% to 76% in those with a reduced ejection fraction1 and 55% in those with preserved ejection fraction.2 Both central sleep apnea with Cheyne-Stokes breathing (CSA-CSB) and obstructive sleep apnea (OSA) are seen in HF. CPAP therapy reduces the apnea-hypopnea index (AHI),3‐10 improves the left ventricular ejection fraction (LVEF),3,4,7,8,10 and reduces sympathetic activity in HF.3 However, the only major prospective randomized trial to date (Canadian Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial [CANPAP]) comparing patients with CSB and HF randomized to CPAP therapy vs usual medical care observed no major difference in transplant-free survival.11 In this trial, CPAP was ineffective in suppressing CSA in almost one-half of the patients; in addition, some patients with HF and with CSA-CSB had trouble tolerating conventional CPAP or bilevel pressure ventilation (BPV) support. Recent technological modifications include adaptive servoventilation (ASV), which adjusts the delivered pressure support according to the needs of the patient. Manufacturers vary in their strategies, but the overall goal is to stabilize minute ventilation by targeting either airflow or tidal volume. Thus, ventilation can vary gradually and naturally over the course of the night. The end-expiratory pressure can be adjusted to eliminate obstructive respiratory events and the back-up rate aborts any impending apneas. ASV can effectively treat complex sleep apnea emerging after CPAP treatment,12 although most data suggest that this condition is transient and self limited,13 and also opioid-induced SDB.14
Large randomized studies demonstrating the effectiveness of ASV in treating SDB and other clinical outcomes in HF are lacking. A meta-analysis of studies published before June 2010, included in a recent practice-parameters document published by Aurora et al,15 found that ASV significantly improved LVEF and the AHI. However, only baseline/end-trial data in groups of patients administered ASV were analyzed and did not incorporate data from control groups. Thus, only a few studies were available, and the number of patients contributing to the summary statistics of these studies was also small. By performing an updated systematic review and meta-analysis, we sought to test the hypothesis that adult patients with SDB and stable HF would benefit from ASV compared with a control condition (subtherapeutic ASV, CPAP, BPV, oxygen, or no treatment), using end-points of SDB severity, LVEF, exercise capacity, cardiopulmonary exercise testing, and quality of life measured over ≥ 1 week.
Materials and Methods
This report consists of a review of published literature. Ethical review is, therefore, not required.
Systematic Literature Search
A systematic literature search was undertaken on March 24, 2012, using the PubMed database. The following search string was used to identify clinical trials comparing ASV to a control group: (((((((((((((“Sleep Apnea Syndromes”[Mesh])) OR (Apn*)) OR (Hypopn*)) OR (Sleep apn*)) OR (Obstructive sleep apn*)) OR (OSA)) OR (OSAS)) OR (OSAHS)) OR (SAHS)) OR (“Cheyne-Stokes Respiration”[Mesh])) OR (Cheyne-Stokes)) OR (Cheyne Stokes)) AND ((((((“Heart Failure”[Mesh])) OR (Congestive heart failure)) OR (Heart failure)) OR (CHF)) OR (HF)) AND ((((Adaptive servo ventilation)) OR (Adaptive ventilation)) OR (ASV)). Papers or peer-reviewed abstracts referenced in these papers, or other papers known to the authors, were also included and subjected to the selection criteria.
Study Eligibility Criteria
Two authors (B. K. S. and J. P. B.) independently excluded all qualitative reviews, letters, case studies, and meta-analyses, and then applied the following exclusion criteria in order by participants, intervention, comparator, outcome(s), and study design16: patients aged < 18 years, patients without a diagnosis of HF, patients with decompensated HF, ASV not used as the intervention, no use of a comparator, no measure of at least one outcome of interest, and study duration < 1 week. Publication in a language other than English was not grounds for exclusion. The primary outcome measure of interest was the severity of SDB expressed as total AHI measured either by polysomnography or the ASV device. Secondary outcomes of interest included central apnea index (CAI), obstructive apnea index (OAI), arousal index, LVEF, quality of life expressed as role-physical and vitality components of the SF-36 Health Survey (Medical Outcomes Trust),17 exercise capacity measured by 6-min walk distance,18 maximal oxygen consumption (o2) (measured as peak o2 % predicted) and ventilatory drive (ventilatory equivalent ratio for CO2 [e/co2] slope) measured during exercise testing. Finally, all studies meeting these criteria were compared for common methodology and data to ensure that the study samples were not duplicated. If duplicates were found, the paper with the oldest publication date was excluded.
Data Extraction
Data were extracted independently by authors B. K. S., D. G. M., and J. P. B. Descriptive data included study structure, duration of study arms, duration of washout (if applicable), type of control used, HF inclusion criteria, SDB inclusion criteria, proportion of male patients, and mean age. For outcome data in crossover studies, the end-trial mean and variability in the ASV arm and control arm were extracted. For outcome data in parallel studies, data describing the mean and variability of the change in each variable over the ASV arm and control arm were extracted (end-trial minus baseline). The change in SD was calculated assuming a paired correlation of r=0.5 as previously described,19 if this value was not reported in the publication. If any of these data were absent or unclear, we attempted to contact the first, last, and corresponding authors of the relevant publications.
Data Synthesis and Statistical Analysis
Analyses were conducted by authors B. K. S. and J. P. B. using Review Manager (RevMan) version 5.1 software (Nordic Cochrane Centre). Parallel and crossover studies were analyzed separately with random effects models using DerSimonian and Laird methodology.20 A forest plot was constructed using the weighted mean difference of the outcome variable between the ASV and control arm of each study. Subgroup analyses of our primary outcome (AHI) were planned for studies using CPAP as the control condition, and studies recruiting patients with predominantly CSA. The Q statistic was calculated when at least three studies were available, and considered significant when P ≤ .05, indicating heterogeneity. The I2 statistic was also calculated when at least three studies were available, to estimate the percentage of the observed variability due to heterogeneity rather than chance. Sensitivity analyses were performed for each meta-analysis including at least three studies, by removing one study at a time and observing the effect this had on the overall result.21 Finally, publication bias for each analysis was assessed visually using a funnel plot.
Results
Identification and Description of Included Studies
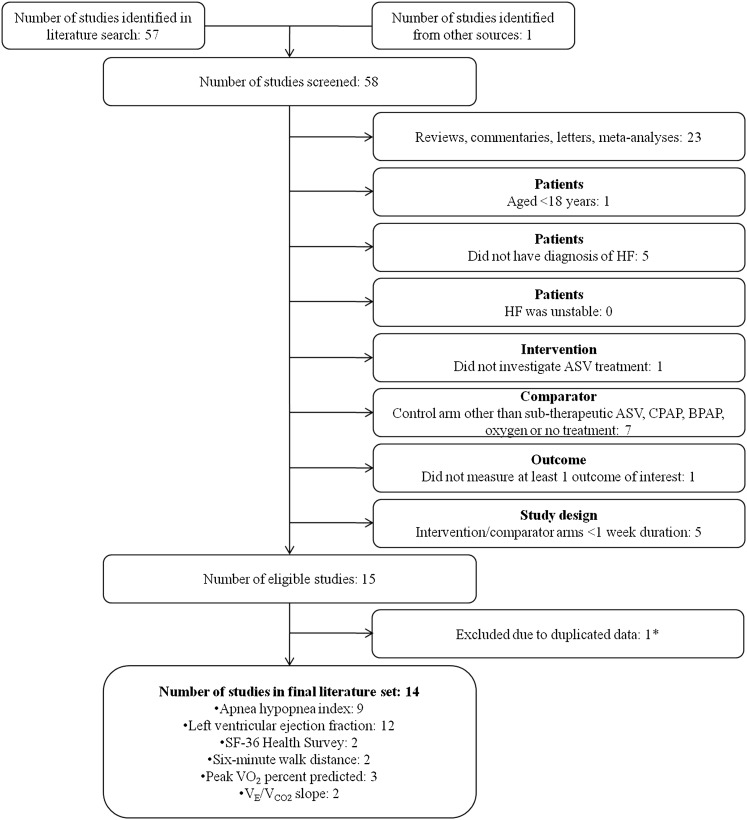
The systematic literature search returned 57 full articles; one additional article was known to the authors. After the exclusion criteria were applied, 14 studies comparing ASV to a control condition in adult patients with stable HF for ≥ 1 week including at least one outcome measure of interest remained (Fig 1).22‐35
Figure 1.
Literature exclusion flowchart. Each publication was independently assessed and excluded in order by participants, intervention, comparator, outcome(s), and study design, with 14 studies remaining. ASV = adaptive servoventilation; BPAP = bilevel pressure ventilation; HF = heart failure; VE/VCO2 = ventilatory equivalent ratio for CO2; VO2 = oxygen consumption.
The design and patient characteristics of the included studies are summarized in Table 1, the effect size and 95% CI for each meta-analyzed variable are summarized in Table 2, and the authors’ judgment as to the risk of bias in each study is summarized in Table 3. The included studies had a combined sample size of 538 patients. Control groups were no treatment (four studies),28,30,32,35 rejection of or poor compliance with ASV (three studies),26,33,34 subtherapeutic ASV (one study),22 CPAP (three studies),23,29,31 BPV (one study),25 and nasal oxygen (two studies).24,27 A parallel design was adopted in 12 studies; the two studies using nasal oxygen in the control arm were both crossover studies. Additional data were obtained from studies by Pepperell et al,22 Campbell et al,24 and Oldenburg et al.33
Table 1.
—Descriptive Characteristics of Identified Studies
| Study/Year | Study Design | Duration | Control Type | HF Inclusion Criteria | SDB Inclusion Criteria | Patients at Beginning of Trial, No. | Patients at Trial Completion, No. | Male Patients, % | Age (Mean ± SD), y |
| Pepperell et al22/2003 | Parallel; randomized | 1 mo | Subtherapeutic ASV | Stable HF, NYHA class ≥ II | 3% ODI > 10 events/h during PSG, > 50% central events | ASV: 15Control subjects: 15 | ASV: 15Control subjects: 11 | ASV: 100Control subjects: 93 | ASV: 71.4 ± 8.6Control subjects: 70.9 ± 7.9 |
| Philippe et al29/2006 | Parallel; randomized | 6 mo | CPAP | Stable HF, NYHA class ≥ II, LVEF ≤ 45% | AHI > 15 events/h, > 80% central events | ASV: 12Control subjects: 13 | ASV: 9Control subjects: 8 | ASV: 100Control subjects: 100 | ASV: 64.2 ± 15.5Control subjects: 60.3 ± 11.5 |
| Zhang et al27/2006 | Crossover; nonrandomized | 2 wk (washout 2 wk) | Nasal oxygen | Stable HF, no further details | “Predominant CSR on PSG”, obstructive AHI ≤ 10 events/h | Total: 14 (crossover trial) | Total: 14 (crossover trial) | Total: 57 (crossover trial) | Range, 39-58, no further details |
| Fietze et al25/2008 | Parallel; randomized | 6 wk | BPV | Stable HF, NYHA class II-III, LVEF < 45% | RDI > 15 events/h, < 20% obstructive events | ASV: 17Control subjects: 20 | 30 completed, no further details | ASV: 88Control subjects: 95 | ASV: 61.9 ± 9.1Control subjects: 56.4 ± 10.9 |
| Bitter et al26/2010 | Parallel; nonrandomized | 12 mo | Rejection of ASV or ASV use < 4 h/night | Stable HF, NYHA class II-III | AHI > 15 events/h, > 50% central events | ASV: 46Control subjects: 39 | ASV: 39Control subjects: 21 | ASV: 85Control subjects: 86 | ASV: 67.4 ± 8.5Control subjects: 69.7 ± 7.9 |
| Hastings et al30/2010 | Parallel; nonrandomized | 6 mo | No treatment | Stable HF, NYHA class II-III, LVEF < 45% | AHI > 15 events/h, no further details | ASV: 14Control subjects: 8 | ASV: 11Control subjects: 8 | ASV: 100Control subjects: 100 | ASV: 61.3 ± 10Control subjects: 64.5 ± 7.8 |
| Kasai et al31/2010 | Parallel; randomized | 3 mo | CPAP | Stable HF, NYHA class ≥ II, LVEF < 50% | Total AHI ≥ 15 events/h, obstructive AHI ≥ 5 events/h | ASV: 16Control subjects: 15 | ASV: 15Control subjects: 15 | ASV: 100Control subjects: 100 | ASV: 56.9 ± 14.3Control subjects: 56.5 ± 12.6 |
| Koyama et al32/2010 | Parallel; nonrandomized | 4 wk | No treatment | Stable HF, NYHA class II-III, LVEF < 55% | AHI ≥ 15 events/h, majority central events | ASV: 10Control subjects: 7 | ASV: 10Control subjects: 7 | ASV: 80Control subjects: 57 | ASV: 68.4 ± 4.0Control subjects: 71.4 ± 7.6 |
| Haruki et al35/2011 | Parallel; nonrandomized | 5 mo | No treatment | Stable HF, NYHA class ≥ II, LVEF < 50% | AHI > 15 events/h, obstructive AHI ≤ 5 events/h | ASV: 15Control subjects: 15 | ASV: 15Control subjects: 11 | ASV: 73Control subjects: 73 | ASV: 67 ± 11Control subjects: 67 ± 14 |
| Oldenburg et al33/2011 | Parallel; nonrandomized | 7 mo | Rejection of ASV or ASV use < 50% of nights or ASV use < 4 h/night | Stable HF, NYHA class ≥ II, LVEF ≤ 40% | AHI ≥ 15 events/h, > 80% central events | ASV: 66Control subjects: 62 | ASV: 56Control subjects: 59 | ASV: 96Control subjects: 88 | ASV: 67.7 ± 9.5Control subjects: 62.5 ± 11.8 |
| Koyama et al34/2011 | Parallel; nonrandomized | 12 mo | Rejection of ASV or insufficient use of ASV, no further details | Stable HF, NYHA class II-III, LVEF < 55% | AHI ≥ 15 events/h, no further details. | ASV: 27Control subjects: 16 | ASV: 27Control subjects: 16 | ASV: 85Control subjects: 81 | ASV: 74.8 ± 7.6Control subjects: 75.4 ± 6.4 |
| Yoshihisa et al28/2011 | Parallel; nonrandomized | 6 mo | No treatment | Stable HF, NYHA class ≥ II | AHI > 15 events/h, central sleep apnea | ASV: 23Control subjects: 37 | ASV: 23Control subjects: 37 | ASV: 87Control subjects: 78 | ASV: 60.8 ± 13.7Control subjects: 60.5 ± 16.7 |
| Campbell et al24/2011 | Crossover; randomized | 8 wk (washout 3 wk) | Nasal oxygen | Stable HF, LVEF < 50% | AHI > 15 events/h, >50% central events | Total: 10 (crossover trial) | Total: 7 (crossover trial) | Total: 100 (crossover trial) | Total: 64 ± 6.8 |
| Randerath et al23/2012 | Parallel; randomized | 12 mo | CPAP | Stable HF, NYHA class II-III, LVEF ≥ 20% | AHI ≥ 15 events/h, ≤ 80% central events and 20%-50% obstructive events | ASV: 36Control subjects: 34 | ASV: 26Control subjects: 25 | ASV: 86Control subjects: 94 | ASV: 65.3 ± 10.0Control subjects: 67.4 ± 8.1 |
AHI = apnea-hypopnea index; ASV = adaptive servoventilation; BPV = bilevel pressure ventilation; CSR = Cheyne-Stokes respiration; HF = heart failure; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; ODI = oxygen desaturation index; PSG = polysomnography; SDB = sleep-disordered breathing.
Table 2.
—Effect Sizes in Individual Studies of All Meta-Analyzed Variables Comparing ASV and Control Arms
| Study/Year | AHI,a Events/h | CAI, Events/h | OAI, Events/h | ArI, Events/h | LVEFb | SF-36 Energy-Vitality Subscalec (/100) | 6-Min Walk Distance,d m | Peak o2 Predicted,e % | e/co2 Slopef (Fraction) |
| Pepperell et al22/2003 | −10.70 (−18.93 to −2.47) | DU | DU | DU | 0.13 (−0.58-0.85) | 5.60 (−2.42-13.62) | DU | DU | DU |
| Philippe et al29/2006 | DU | DU | DU | DU | DU | DU | DU | DU | DU |
| Zhang et al27/2006 | −21.30 (−25.62 to −16.98) | DU | DU | −7.40 (−11.77 to −3.03) | 4.00 (0.57-7.43) | DU | DU | DU | DU |
| Fietze et al25/2008 | −2.00 (−12.59-8.59) | DU | −0.20 (−0.79-0.39) | DU | −0.39 (−1.12-0.33) | DU | DU | DU | DU |
| Bitter et al26/2010 | DU | DU | DU | DU | DU | DU | DU | 20.10 (9.84-30.36) | −0.70 (−3.47-2.07) |
| Hastings et al30/2010 | DU | DU | DU | DU | 0.89 (−0.08-1.85) | DU | DU | 0.50 (−7.17-8.17) | DU |
| Kasai et al31/2010 | −12.20 (−23.79 to −0.61) | DU | DU | DU | 0.83 (0.08-1.59)g | 19.30 (2.87-35.73) | 43.70 (2.16-85.24) | DU | DU |
| Koyama et al32/2010 | −32.70 (−49.81 to −15.59) | −14.70 (−26.63 to −2.77) | −4.30 (−8.46 to −0.14) | −13.50 (−34.02-7.02) | 1.21 (0.14-2.28) | DU | DU | DU | DU |
| Haruki et al35/2011 | DU | DU | DU | DU | 0.62 (−0.18-1.42) | DU | DU | DU | DU |
| Oldenburg et al33/2011 | −27.40 (−35.93 to −18.87) | DU | DU | DU | 0.52 (0.15-0.89) | DU | 23.00 (−16.45-62.45) | 11.10 (4.38-17.82) | −3.80 (−6.37 to −1.23) |
| Koyama et al34/2011 | DU | DU | DU | DU | 0.83 (0.11-1.54) | DU | DU | DU | DU |
| Yoshihisa et al28/2011 | −12.20 (−17.55 to −6.85) | DU | DU | DU | 0.38 (−0.15-0.90) | DU | DU | DU | DU |
| Campbell et al24/2011 | −14.40 (−26.93 to −1.87) | DU | DU | −1.00 (−10.38-8.38) | 4.20 (−10.62-19.02)g | DU | DU | DU | DU |
| Randerath et al23/2012 | −11.90 (−20.79 to −3.01) | −0.30 (−3.88-3.28) | −4.70 (−8.14 to −1.26) | −1.60 (−8.91-5.71) | −0.44 (−0.99-0.12) | DU | DU | DU | DU |
All data effect size (95% CI), unless otherwise indicated. Effect sizes and CIs are reported in the units of each variable. ArI = arousal index; CAI = central apnea index; DU = data unavailable; OAI = obstructive apnea index; e/co2 = ventilatory equivalent ratio for CO2; o2 = oxygen consumption. See Table 1 legend for expansion of other abbreviations.
AHI: negative effect sizes favor ASV.
LVEF: positive effect sizes favor ASV.
SF-36: positive effect sizes favor ASV.
6-min walk distance: positive effect sizes favor ASV.
Peak o2 predicted: positive effect sizes favor ASV.
e/co2 slope: negative effect sizes favor ASV.
Indicates primary outcome measure.
Table 3.
—Authors’ Judgment as to Risk of Bias in Identified Studies
| Study/Year | Adequate Sequence Generation? | Allocation Concealment? | Blinding (All Outcomes)? | Free of Selective Reporting? | Free of Incomplete Outcome Data?a |
| Pepperell et al22/2003 | Yes | Yes | Yes (double) | Yes | Yes |
| Philippe et al29/2006 | Not stated | Not stated | No | Reported some data in graphical form only | Yes |
| Zhang et al27/2006 | No | No | No | Yes | N/A |
| Fietze et al25/2008 | Not stated | Not stated | No | Yes | No |
| Bitter et al26/2010 | No | No | No | Incomplete data for control group | N/A |
| Hastings et al30/2010 | No | No | No | Incomplete data for control group | N/A |
| Kasai et al31/2010 | Not stated | Not stated | No | Yes | Yes |
| Koyama et al32/2010 | No | No | No | Yes | N/A |
| Haruki et al35/2011 | No | No | No | Yes | N/A |
| Oldenburg et al33/2011 | No | No | No | Incomplete data for control group | N/A |
| Koyama et al34/2011 | No | No | No | Yes | N/A |
| Yoshihisa et al28/2011 | No | No | No | Incomplete data for control group | N/A |
| Campbell et al24/2011 | Not stated | Not stated | No | Yes | Yes |
| Randerath et al23/2012 | Not stated | Yes | Yes (single) | Yes | Yes |
N/A = not applicable.
Not applicable for nonrandomized studies.
Meta-analyses
Severity of Sleep-Disordered Breathing:
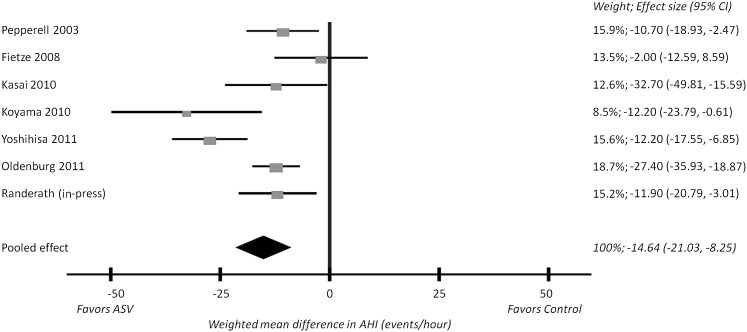
The changes in AHI in the ASV and control arms were available from seven parallel studies (n = 352).22,23,25,28,31‐33 The weighted mean difference was −14.64 events/h favoring ASV (95% CI, −21.03 to −8.25; P = .0001), as shown in Figure 2. Significant between-studies heterogeneity was evident (Q[6] = 20.36; P = .002) and the I2 was 71%, indicating that 71% of the observed variance came from real differences between the studies rather than chance. All but one individual study31 reported a significant difference in AHI between ASV and CPAP of ≥ 10 events/h. Sensitivity analysis indicated that no single study, when removed from the meta-analysis, changed the overall statistical significance of the model. In the two crossover studies,24,27 the weighted mean difference in AHI between ASV and oxygen therapy was −20.46 events/h favoring ASV (95% CI, −24.88 to −16.04; P = .0001).
Figure 2.
Forest plot of AHI data in parallel studies. Each publication is represented by a square, the horizontal position of which represents the effect size, and error bars, which represent the 95% CI. All squares lie on the left of the null effect vertical line (mean difference of 0), indicating that all studies found a greater reduction in AHI with ASV compared with the control arm. The size of each square is proportional to the weight of each study in the pooled analysis, also listed as a percentage. The diamond represents the meta-analysis: the apex is the weighted mean difference (−14.64 events/h), and the width is the 95% CI (−21.03-−8.25 events/h). The width of the diamond does not cross the null effect vertical line, so the difference is statistically significant (P = .0001). AHI = apnea-hypopnea index. See Figure 1 legend for expansion of other abbreviation.
Two subgroup analyses were planned for our primary outcome (AHI) in parallel trials. Comparing ASV and CPAP, the weighted mean difference in AHI significantly favored ASV in two studies23,31 (−0.65 events/h; 95% CI, −1.06 to −0.25; P = .002). Then, comparing ASV and all control conditions in only the five studies recruiting patients with predominantly CSA,22,25,28,32,33 the weighted mean difference in AHI significantly favored ASV (−0.99 events/h; 95% CI, −1.50 to −0.48; P = .0001).
In addition to the total AHI, two parallel studies23,32 (n = 47) reported the CAI and three parallel studies23,25,32 (n = 117) reported the OAI separately. There was no significant difference in either of these indices between the ASV and control arms (weighted mean difference in CAI: −6.33 events/h, nonsignificantly favoring ASV; 95% CI, −20.25-7.59; P = .37; and weighted mean difference in OAI: −2.67 events/h, nonsignificantly favoring ASV; 95% CI −6.18-0.83; P = .14). Significant between-studies heterogeneity and substantial variance were evident in the OAI analysis (Q[2] = 9.84; P = .007), I2 was 80%; and when one study25 was removed, the weighted mean difference between ASV and control arms became significant (−4.54 events/h favoring ASV; 95% CI, −7.19 to −1.89; P = .0008).
Arousal index data were available from two parallel studies (n = 87)23,32 and both crossover studies (n = 21).24,27 The difference between ASV and the control arms was nonsignificant in both analyses (weighted mean difference in parallel studies −3.35 events/h, nonsignificantly favoring ASV; 95% CI, −12.12-5.07; P = .42; and weighted mean difference for crossover studies: −5.6 events/h, nonsignificantly favoring ASV; 95% CI, −11.24-0.04; P = .06).
Left Ventricular Ejection Fraction:
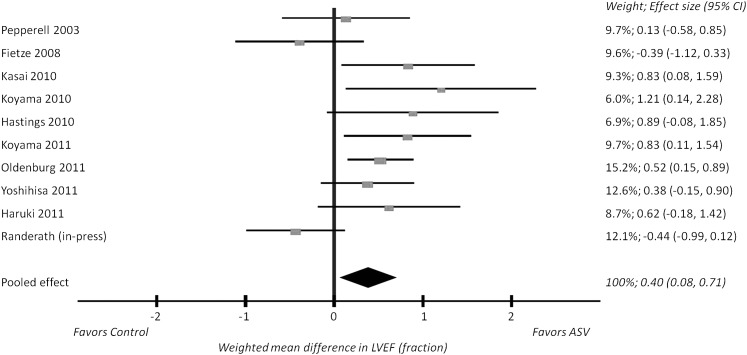
Meta-analysis of LVEF data from 10 parallel studies (n = 385)22,23,25,28,30,32‐36 resulted in a weighted mean difference of 0.40 favoring ASV (95% CI, 0.08-0.71; P = .01), as shown in Figure 3. Significant between-studies heterogeneity was evident (Q[9] = 20.26; P = .02), and the I2 was 56%. When removed from the pooled analysis individually, no study caused the weighted mean difference to become nonsignificant (P > .05). The effect size of individual studies ranged from 1.2 favoring ASV32 to 0.44 (nonsignificantly) favoring CPAP.23 In the two crossover studies, the weighted mean difference in LVEF between ASV and oxygen therapy was 4.01 favoring ASV (95% CI, 0.67-7.35; P = .02).
Figure 3.
Forest plot of LVEF data in parallel studies. Each publication is represented by a square, the horizontal position of which represents the effect size, and error bars, which represent the 95% CI. Squares lying to the right of the null effect vertical line (mean difference of 0) are those that found a greater increase in LVEF with ASV compared with the control arm. The size of each square is proportional the weight of each study in the pooled analysis, also listed as a percentage. The diamond represents the meta-analysis: the apex is the weighted mean difference (0.40), and the width is the 95% CI (0.08-0.71). The width of the diamond does not cross the null effect vertical line, so the difference is statistically significant (P = .01). LVEF = left ventricular ejection fraction. See Figure 1 legend for expansion of other abbreviation.
Quality of Life:
Two parallel studies (n = 60)22,31 used the SF-36 Health Survey; however, only one of the two subscales we intended to analyze was reported in both papers. There was no significant difference in the change in SF-36 energy-vitality scores between the ASV and control arms (weighted mean difference was 10.50, nonsignificantly favoring ASV; 95% CI, −2.37-23.36; P = .11); the I2 was 54%.
Exercise Capacity:
Two parallel studies (n = 145)31,33 described the change in 6-min walk distance after treatment with ASV and the control arm. The weighted mean difference in 6-min walk distance was 32.82, favoring ASV (95% CI, 4.21-61.42; P = .02); the I2 was 0%.
Cardiopulmonary Exercise Testing:
Changes in peak o2, expressed as peak o2 % predicted, were available from three parallel studies (n = 194).26,30,33 There was no significant difference in the change in peak o2 between ASV and the control arms (weighted mean difference: 10.14, nonsignificantly favoring ASV; 95% CI, −0.09-20.38; P = .051). The I2 was 79%, and significant heterogeneity was evident (Q[2] = 9.58; P = .008). When one study was removed during sensitivity analysis,30 the weighted mean difference became significant (weighted mean difference was 14.73, favoring ASV; 95% CI, 6.08-23.38; P = .0008).
There was also no significant difference in the change in ventilatory drive, expressed as e/co2 slope, comparing the ASV and control arms of two parallel studies (n = 175)26,33 (weighted mean difference: −2.30, nonsignificantly favoring ASV; 95% CI, −5.33-0.74; P = 0.14). The I2 was 61%.
Assessment of Publication Bias
We found no evidence of publication bias when funnel plots were inspected for each meta-analysis. Additional unpublished studies may exist.
Discussion
This systematic review and meta-analysis showed that ASV was more effective than control conditions (combined) and CPAP treatment (subgroup analysis) in treating SDB in HF. LVEF and exercise capacity also showed greater improvement with ASV compared with control conditions. These data provide a compelling rationale for large-scale, randomized, controlled trials to assess the clinical impact of ASV on hard outcomes for patients with SDB and HF. None of the controlled studies identified in our literature search specifically recruited patients with opioid-induced SDB, suggesting that this, too, is an avenue of research worthy of further attention because preliminary studies suggest efficacy of these devices.14 Our results build on those of Aurora et al,15 which reported uncontrolled changes with ASV only, by locating eight additional studies for inclusion and investigating a greater number of outcomes, including exercise capacity, quality of life, and cardiopulmonary exercise testing. ASV has previously been shown to reduce SDB22,28,30,31,37 and systemic inflammation,32 and improve LVEF,22,26,27,31‐33,37 quality of life,31,37 exercise capacity,31,33 and respiratory instability33 in patients with HF and CSA. ASV was also more effective than CPAP in improving parameters like SDB, LVEF, and treatment compliance in patients with both systolic HF and SDB.27
The need to treat SDB in patients with HF has been debated. While some data suggest poor outcomes for these patients,38 others have suggested no major impact from SDB.39 Such studies are challenging to interpret because SDB may be a marker of the aggressiveness of medical therapy,40 and thus residual confounding is likely to occur in many of these prognostic studies. As such, the focus has shifted to interventional studies to assess whether treating SDB truly improves clinical outcomes in patients with HF.
Some authors had initially made a major distinction between CSA-CSB in these patients in contrast to OSA; others have suggested that these two diseases exist on a continuum with similar underlying mechanisms. Thus, many have adopted the concept of using the term SDB, recognizing that there are likely components of OSA and CSA in many patients with HF. Early reports had shown suggestion of benefit to treating both CSB5 and OSA,10 but the more definitive CANPAP trial found no benefit to treating CSB in patients with HF.11 Post hoc analyses assessed subgroups of patients in whom CSB was eliminated compared with those who had persistence of CSB despite CPAP therapy. These data suggested that elimination of CSB indeed showed benefit compared with patients in whom CSB persisted,41 consistent with the concept that eliminating CSB is beneficial to patients with HF. Therefore, focus has shifted toward newer technology, which is purported to eliminate SDB more effectively than standard CPAP. Our analyses are, in fact, consistent with this concept and thus provide a rationale for large-scale rigorous studies assessing hard clinical outcomes of treating SDB in HF. Such efforts are ongoing.42,43
This meta-analysis has some limitations despite the strengths of our methodologic approach. First, we observed substantial between-studies heterogeneity in terms of both study design and the effect sizes of each end point. We acknowledge that despite our choice of random effects models, the pooling of disparate studies may not accurately summarize the overall effect of ASV treatment, and also limits the generalizability of our results. Second, the number of studies available was reasonably small, and thus we are still likely underpowered to see treatment benefits based on this literature. The small number of studies also limited our ability to investigate the observed heterogeneity incorporating covariates such as age and sex. Third, our risk of bias assessment suggested that several studies were of poor methodologic quality, leaving open the potential for bias and confounding. Fourth, based on the existing literature, we were unable to assess the impact of ASV on myocardial infarction, sudden cardiac death, and hospitalizations. Thus, further data are clearly required. Fifth, secular trends in HF therapy have been occurring over the past decade (for example, cardiac resynchronization therapy and increased use of implantable defibrillators); these newer therapies may affect SDB and its outcomes,44,45 and, thus, results could be different if the studies were undertaken today. As such, we acknowledge that our results are clearly limited to the populations studied. Despite these limitations, we view our findings as an important addition to the literature because they provide a compelling rationale for further research and represent the best available evidence until more data are available.
Conclusions
In conclusion, ASV is more effective than control conditions in reducing SDB severity and improving cardiac function and exercise capacity in patients with SDB and HF. We support large-scale comparative effectiveness research in this area.
Acknowledgments
Author contributions: Dr Sharma had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.Dr Sharma: contributed to the study design; data collection, interpretation, and statistical analysis; and manuscript preparation.
Dr Bakker: contributed to the study design; data collection, interpretation, and statistical analysis; and manuscript preparation.
Dr McSharry: contributed to data collection and interpretation and manuscript preparation.
Dr Desai: contributed to data interpretation and manuscript preparation.
Dr Javaheri: contributed to data interpretation and manuscript preparation.
Dr Malhotra: contributed to study design, data interpretation, and manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Bakker has received previous funding from Philips Respironics. Dr Desai has consulted for Boston Scientific; Intel; Novartis AG; and Relypsa. Dr Javaheri has consulted for ResMed and received previous funding from Philips Respironics. Dr Malhotra is a consultant for Philips Respironics; Sleep HealthCenters; Sleep Group Solutions, Inc; Apnicure, Inc; Apnex Medical, Inc; and Pfizer, Inc. Drs Sharma and McSharry have reported that no conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The authors’ funding sponsors were not involved in this investigation and had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We thank Angela Campbell, PhD; Olaf Oldenburg, MD; and Justin Pepperell, MD, for supplying additional data for these analyses.
Abbreviations
- AHI
apnea-hypopnea index
- BPV
bilevel pressure ventilation
- CAI
central apnea index
- CSA
central sleep apnea
- CSB
Cheyne-Stokes breathing
- HF
heart failure
- LVEF
left ventricular ejection fraction
- OAI
obstructive apnea index
- OSA
obstructive sleep apnea
- SDB
sleep-disordered breathing
- e/co2
ventilatory equivalent ratio for CO2
- o2
oxygen consumption
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Funding/Support: Dr. Bakker does not have any current funding to disclose. Dr McSharry is supported by the American Heart Association (AHA) [11POST5660004]. Dr Malhotra is supported by the US National Institutes of Health [Grants 5R01HL085188-04, 5R01HL090897-03, 5K24HL093218-03, and 1P01HL095491-01A1] and the AHA [grant 0840159N], but has relinguished all outside personal income since May 2012. No financial support was obtained for this investigation.
References
- 1.Sharma B, Owens R, Malhotra A. Sleep in congestive heart failure. Med Clin North Am. 2010; 94(3):447-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J, Sanderson J, Chan W, et al. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest. 1997; 111(6):1488-1493 [DOI] [PubMed] [Google Scholar]
- 3.Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995; 152(2):473-479 [DOI] [PubMed] [Google Scholar]
- 4.Granton JT, Naughton MT, Benard DC, Liu PP, Goldstein RS, Bradley TD. CPAP improves inspiratory muscle strength in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1996; 153(1):277-282 [DOI] [PubMed] [Google Scholar]
- 5.Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation. 2000; 102(1):61-66 [DOI] [PubMed] [Google Scholar]
- 6.Tkacova R, Rankin F, Fitzgerald FS, Floras JS, Bradley TD. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. 1998; 98(21):2269-2275 [DOI] [PubMed] [Google Scholar]
- 7.Egea CJ, Aizpuru F, Pinto JA, et al. ; Spanish Group of Sleep Breathing Disorders Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: a multicenter study. Sleep Med. 2008; 9(6):660-666 [DOI] [PubMed] [Google Scholar]
- 8.Johnson CB, Beanlands RS, Yoshinaga K, et al. Acute and chronic effects of continuous positive airway pressure therapy on left ventricular systolic and diastolic function in patients with obstructive sleep apnea and congestive heart failure. Can J Cardiol. 2008; 24(9):697-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004; 169(3):361-366 [DOI] [PubMed] [Google Scholar]
- 10.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003; 348(13):1233-1241 [DOI] [PubMed] [Google Scholar]
- 11.Bradley TD, Logan AG, Kimoff RJ, et al. ; CANPAP Investigators Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005; 353(19):2025-2033 [DOI] [PubMed] [Google Scholar]
- 12.Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007; 30(4):468-475 [DOI] [PubMed] [Google Scholar]
- 13.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med. 2009; 5(3):205-211 [PMC free article] [PubMed] [Google Scholar]
- 14.Javaheri S, Malik A, Smith J, Chung E. Adaptive pressure support servoventilation: a novel treatment for sleep apnea associated with use of opioids. J Clin Sleep Med. 2008; 4(4):305-310 [PMC free article] [PubMed] [Google Scholar]
- 15.Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012; 35(1):17-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151(4):264-269 [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual & Interpretation Guide. Lincoln, RI:QualityMetric Inc;2000 [Google Scholar]
- 18.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166(1):111-117 [DOI] [PubMed] [Google Scholar]
- 19.Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to Meta-Analysis. Chichester, England:John Wiley & Sons Ltd;2009 [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7(3):177-188 [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327(7414):557-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pepperell JC, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003; 168(9):1109-1114 [DOI] [PubMed] [Google Scholar]
- 23.Randerath WJ, Nothofer G, Priegnitz C, et al. Long-term auto-servoventilation or constant positive pressure in heart failure and coexisting central with obstructive sleep apnea. Chest. 2012; 142(2):440-447 [DOI] [PubMed] [Google Scholar]
- 24.Campbell AJ, Ferrier K, Neill AM. The effect of oxygen versus adaptive pressure support servo-ventilation in patients with central sleep apnoea-Cheyne Stokes respiration and congestive heart failure [published online ahead of print October 27, 2011]. Intern Med J. doi:10.1111/j.1445-5994.2011.02623.x [DOI] [PubMed] [Google Scholar]
- 25.Fietze I, Blau A, Glos M, Theres H, Baumann G, Penzel T. Bi-level positive pressure ventilation and adaptive servo ventilation in patients with heart failure and Cheyne-Stokes respiration. Sleep Med. 2008; 9(6):652-659 [DOI] [PubMed] [Google Scholar]
- 26.Bitter T, Westerheide N, Faber L, et al. Adaptive servoventilation in diastolic heart failure and Cheyne-Stokes respiration. Eur Respir J. 2010; 36(2):385-392 [DOI] [PubMed] [Google Scholar]
- 27.Zhang XL, Yin KS, Li XL, Jia EZ, Su M. Efficacy of adaptive servoventilation in patients with congestive heart failure and Cheyne-Stokes respiration. Chin Med J (Engl). 2006; 119(8):622-627 [PubMed] [Google Scholar]
- 28.Yoshihisa A, Shimizu T, Owada T, et al. Adaptive servo ventilation improves cardiac dysfunction and prognosis in chronic heart failure patients with Cheyne-Stokes respiration. Int Heart J. 2011; 52(4):218-223 [DOI] [PubMed] [Google Scholar]
- 29.Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006; 92(3):337-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hastings PC, Vazir A, Meadows GE, et al. Adaptive servo-ventilation in heart failure patients with sleep apnea: a real world study. Int J Cardiol. 2010; 139(1):17-24 [DOI] [PubMed] [Google Scholar]
- 31.Kasai T, Usui Y, Yoshioka T, et al. ; JASV Investigators Effect of flow-triggered adaptive servo-ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respiration. Circ Heart Fail. 2010; 3(1):140-148 [DOI] [PubMed] [Google Scholar]
- 32.Koyama T, Watanabe H, Kobukai Y, et al. Beneficial effects of adaptive servo ventilation in patients with chronic heart failure. Circ J. 2010; 74(10):2118-2124 [DOI] [PubMed] [Google Scholar]
- 33.Oldenburg O, Bitter T, Lehmann R, et al. Adaptive servoventilation improves cardiac function and respiratory stability. Clin Res Cardiol. 2011; 100(2):107-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koyama T, Watanabe H, Terada S, et al. Adaptive servo-ventilation improves renal function in patients with heart failure. Respir Med. 2011; 105(12):1946-1953 [DOI] [PubMed] [Google Scholar]
- 35.Haruki N, Takeuchi M, Kaku K, et al. Comparison of acute and chronic impact of adaptive servo-ventilation on left chamber geometry and function in patients with chronic heart failure. Eur J Heart Fail. 2011; 13(10):1140-1146 [DOI] [PubMed] [Google Scholar]
- 36.Kasai T, Narui K, Dohi T, et al. First experience of using new adaptive servo-ventilation device for Cheyne-Stokes respiration with central sleep apnea among Japanese patients with congestive heart failure: report of 4 clinical cases. Circ J. 2006; 70(9):1148-1154 [DOI] [PubMed] [Google Scholar]
- 37.Oldenburg O, Schmidt A, Lamp B, et al. Adaptive servoventilation improves cardiac function in patients with chronic heart failure and Cheyne-Stokes respiration. Eur J Heart Fail. 2008; 10(6):581-586 [DOI] [PubMed] [Google Scholar]
- 38.Jilek C, Krenn M, Sebah D, et al. Prognostic impact of sleep disordered breathing and its treatment in heart failure: an observational study. Eur J Heart Fail. 2011; 13(1):68-75 [DOI] [PubMed] [Google Scholar]
- 39.Roebuck T, Solin P, Kaye DM, Bergin P, Bailey M, Naughton MT. Increased long-term mortality in heart failure due to sleep apnoea is not yet proven. Eur Respir J. 2004; 23(5):735-740 [DOI] [PubMed] [Google Scholar]
- 40.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999; 99(12):1574-1579 [DOI] [PubMed] [Google Scholar]
- 41.Arzt M, Floras JS, Logan AG, et al. CANPAP Investigators Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation. 2007; 115(25):3173-3180 [DOI] [PubMed] [Google Scholar]
- 42. National Institutes of Health Clinical Center. Chronic heart failure-Cheyne Stokes respiration- CS2 (3C-Study). NCT00563693. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2007. http://clinicaltrials.gov/ct2/show/NCT00563693. Accessed March 5, 2012.
- 43. National Institutes of Health Clinical Center. Effect of adaptive servo ventilation (ASV) on survival and hospital admissions in heart Failure (ADVENT-HF). NCT01128816. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2010. http://clinicaltrials.gov/ct2/show/NCT01128816. Accessed March 5, 2012.
- 44.Sinha AM, Skobel EC, Breithardt OA, et al. Cardiac resynchronization therapy improves central sleep apnea and Cheyne-Stokes respiration in patients with chronic heart failure. J Am Coll Cardiol. 2004; 44(1):68-71 [DOI] [PubMed] [Google Scholar]
- 45.Stanchina ML, Ellison K, Malhotra A, et al. The impact of cardiac resynchronization therapy on obstructive sleep apnea in heart failure patients: a pilot study. Chest. 2007; 132(2):433-439 [DOI] [PMC free article] [PubMed] [Google Scholar]